ABSTRACT
Purpose: To describe previously reported locomotor muscle and whole-body composition factors related to mobility in older individuals.
Methods: A narrative review of the literature, including a combination of search terms related to muscle and whole-body composition factors and to mobility in older individuals, was carried out. Statistical measures of association and risk were consolidated to summarize the common effects between studies.
Results: Fifty-three studies were reviewed. Muscle and whole-body factors accounted for a substantial amount of the variability in walking speed, with coefficients of determination ranging from 0.30 to 0.47. Muscle power consistently accounted for a greater percentage of the variance in mobility than did strength. Risks associated with high fat mass presented a minimum odds ratio (OR) of 0.70 and a maximum OR of 4.07, while the minimum and maximum ORs associated with low lean mass were 0.87 and 2.30 respectively. Whole-body and regional fat deposits accounted for significant amounts of the variance in mobility.
Conclusion: Muscle power accounts for a greater amount of the variance in the level of mobility in older individuals than does muscle strength. Whole-body fat accounts for a greater amount of the variance in level of mobility than does whole-body lean tissue. Fat stored within muscle also appears to increase the risk of a mobility limitation in older individuals.
Key Words: disability, fat, mobility, muscle, power, sarcopenia, strength
RÉSUMÉ
Objectif : Décrire les facteurs préalablement observés de composition des muscles locomoteurs et de composition corporelle liés à la mobilité des personnes âgées.
Méthode : Examen narratif de la documentation, y compris une combinaison des critères de recherche liés à des facteurs de composition des muscles et de l'ensemble du corps, et à la mobilité des personnes âgées. Les mesures statistiques d'association et de risques ont été consolidées afin de résumer les effets communs aux différentes études.
Résultats : Au total, 53 études ont été examinées. Les facteurs liés aux muscles et au corps dans son ensemble comptaient pour une part importante de la variabilité de la vitesse de marche, avec des coefficients de détermination variant de 0,30 à 0,47. La puissance musculaire se retrouve constamment en une plus forte proportion que la force dans la variation de la mobilité. Les risques associés à une masse grasse élevée présentent un rapport d'incidence rapproché de 0,70, jusqu'à un maximum de 4,07, alors que les rapports minimum et maximum associés à une faible masse maigre sont de 0,87 à 2,30, respectivement. Le corps dans son ensemble et les dépôts de graisse localisés ont un rôle considérable à jouer dans la variation de la mobilité.
Conclusion : La puissance musculaire joue un rôle plus important que la force des muscles dans la variation du degré de mobilité chez les personnes âgées. La quantité totale de gras corporel a des effets plus importants sur la variation du degré de mobilité que l'ensemble des tissus maigres. Le gras dans les muscles semble aussi accroître les risques de limitation de la mobilité chez les individus plus âgés.
Mots clés: force, gras, incapacité, mobilité, muscles, puissance, sarcopénie
INTRODUCTION
Maintaining locomotor muscle structure and function into old age is thought to preserve mobility.1–5 Sarcopenic changes, the age-associated decrease in lean mass,6–11 muscle strength,12–38 and power15,17–20,35–37,39–43 are thought to be related to mobility limitations; however, the relationships are variable,25,28,41,42 and in some cases conflicting.6,7,44 On the other hand, there is a sizeable body of research to support the notion that increasing fat depots, at both whole-body10,12,33,34,38,44–58 and regional levels,6,9,11,32,44,51 constitute an additional set of risk factors for mobility limitation.
Sarcopenia, along with increased whole-body and regional fat deposits, is a normal manifestation of old age.4 In an effort to optimize rehabilitative countermeasures (e.g., resistance and aerobic exercise protocols), it is important to clearly understand how muscle size, strength, and power, as well as whole-body and regional composition, affect mobility in older populations. While exercise training is an effective countermeasure,4,59–61 it is not clear which structural or functional changes should be targeted with these rehabilitation efforts. Furthermore, a collective synthesis and review of the critical cross-sectional and longitudinal studies that identify the impact of muscle and body composition on mobility has not been performed. The purpose of this narrative review, therefore, was to catalogue and synthesize the previously reported relationships between muscle and whole-body factors and mobility in older individuals.
METHODS
Search Method/Criteria
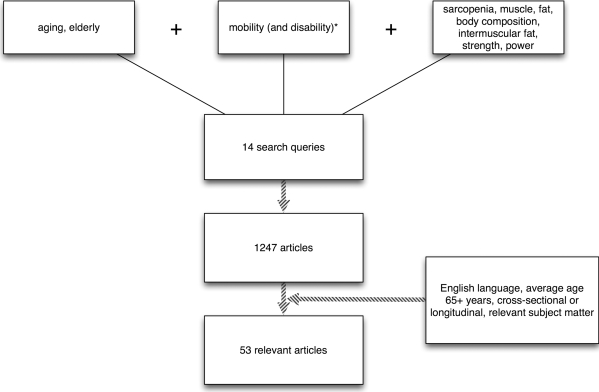
The literature search was limited to the Medline, CINAHL, PEDro, and SCIRUS databases and the private libraries of the authors. The search was restricted to cross-sectional and longitudinal research papers published in English that included subject cohorts with a mean age of 65 years and older. All search terms used stemmed from one of three categories: (1) age; (2) mobility; or (3) muscle and body composition parameters (see Figure 1). Search words and phrases were listed respective to the aforementioned categories: (1) elderly, aging; (2) mobility; and (3) sarcopenia, body composition, muscle, fat, intermuscular fat, strength, power. Search strings producing more than 300 hits were constrained by adding the word disability to the search string.
Figure 1.
Search methods.
Strength/Power and Mobility Relationships
For studies assessing the relationship between strength/power and mobility and reporting correlation coefficients (Pearson's r), the correlation coefficient was transformed to a coefficient of determination (r2) by squaring the reported r-value. This transformation was performed in order to provide consistency in summarizing the data in a tabular format. Gait speed was used as a surrogate for mobility because of the large number of studies that used gait speed as the exemplar mobility construct; gait speed has also been validated as a predictor of disability against the Estimated Population for the Epidemiologic Study of the Elderly performance battery (EPESE).62
Leg muscle strength was determined by the knee extensor and/or hip extensor isometric and/or isokinetic force- or torque-producing ability. Strength measures were limited to these muscle groups as a means to compare strength relationships to power relationships, given that muscle power was consistently determined by a leg-press action, which predominantly involves combined hip and knee extension. Muscle power was defined in two ways: (1) high-force, defined as efforts ≥70% of the maximal weight the person would be able to move through a complete range of motion (one repetition maximum, or 1RM) as quickly as possible; and (2) low-force, defined as moving a weight equivalent to 40% 1RM as quickly as possible.39
Whole-Body and Regional Composition and Risk of Mobility Limitation
Cumulatively, odds ratios (ORs), relative odds ratios (RORs), hazard ratios (HRs), and relative risks (RRs) were consolidated in order to simplify results. Several studies reported tiers of risk based on both the categorization of the independent variable and the number of covariates included in the model.7,8,10,12,32,34,44,46–49,52,54,55,57 All risks reported in this analysis are based on results for the highest risk category and the model accounting for the most covariates, as a means of preventing any overlapping effect of the covariate.
Because of the varied and inconsistent descriptions of sarcopenia,7,12,52,54,57 reported risk was separated into three categories based on varying operational definitions for sarcopenia. Skeletal muscle index (SMI) and appendicular skeletal muscle index (ASMI) were defined as lean mass divided by total body mass in kilograms and lean appendicular mass divided by height squared, respectively. Sarcopenic obesity was characterized as elevated fat mass and low lean mass, quantified independently by each study. Specifically, sarcopenic obesity was calculated either based on the highest tiers of body fat and lowest tiers of lean tissue12,57 or via a residuals method.52,54 Residuals were calculated as the difference of the actual value of appendicular lean mass (aLM) versus the predicted value based on a prediction model that incorporates fat mass as a predictor of a sarcopenic obese individual.52,54 In this predictive model, a positive residual identifies a muscular individual while a negative residual identifies a sarcopenic obese individual.
High fat mass and low lean mass are described in both relative and absolute terms. Absolute measures include a unit of mass (i.e., kg) or cross-sectional area (i.e., cm2) while relative measures are expressed as the value in question (fat or lean mass in kg) relative to either total body mass (kg) or height (m). Any exceptions to these definitions are addressed specifically in the text.
Determination of Cut-Points for Dichotomized and Continuous Variables
Cut-points for dichotomized or otherwise categorized continuous variables are described in the Results section below. Cut-points for composition measures were determined almost exclusively via the distribution of values across tertiles, quartiles, or quintiles, and labelling the most extreme categories of the respective variable as high or low.9,10,12,13,32,34,44–46,48,49,52,55,57,63 Studies using the sarcopenic obesity construct via the residuals method determined cut-points using regression analysis.52,54 The regression between calculated residuals and aLM/Ht2 (r = 0.88 (men); r = 0.71 (women)) was dissected by lines marking the 20th percentile of the x (aLM/Ht2) and y (residuals) axes, and all points below or to the left of these markers were considered sarcopenic or sarcopenic obese, respectively.52,54 Other studies separated sarcopenia into two classes based on severity, defining Class I sarcopenia as muscle mass more than one standard deviation below a young healthy mean and Class II sarcopenia as muscle mass equal to more than two standard deviations below a young healthy mean.8,12 In cases where authors differentiated between Class I and Class II sarcopenia, the latter is reported in our data, in order to maintain consistency with those studies that simply reported sarcopenia as two standard deviations or more below a young healthy mean.7
The parameters defining disability vary from the results of physical performance tests to those of questionnaires assessing perception of function and may include a combination of the two. Performance tests included gait speed, for which disability is defined as less than 1.2 m/s (Table 4),34 and the EPESE physical battery, for which disability is defined as a score of less than 10 (scale = 0–12).52 Questionnaire studies typically used variations of the Activities of Daily Living (ADL) scale and/or the Instrumental Activities of Daily Living (IADL) scale.12,47,49,57 In these studies, answers were dichotomized into perceived difficulty or no perceived difficulty in performing itemized tasks. Values assigned to these answers (in the form of 1 = no difficulty, 0 = difficulty) were summed, and a “disability” score resulted when a majority of answers reported difficulty performing the listed tasks. Some studies used a two-item questionnaire in which a report of any difficulty in walking a quarter-mile or climbing 10 steps resulted in a disability classification.9,10,13,32,44,46,48,55,63 Yet other studies combined performance tests and questionnaires, assigning performance test results a value of 1 or 0 based on the individual's ability to complete the task.8,55
Table 4.
Reported “Risk” (Odds Ratio and Relative Odds Ratio) for Mobility-Related Functional Limitations and Disabilities in Relation to Whole-Body and Regional Composition of Fat and Lean Tissue
| Cut-Offs | Whole-Body Composition* | BMI* | Regional Composition* | Covariates | Functional Limitations and Disability Criteria | |
|---|---|---|---|---|---|---|
| Koster et al. (2008)46 | Cut-off: High Fat M: >31.3% F: >43.7% Cut-off: High BMI ≥30 |
HR: High Fat M: 1.31 (0.93–1.84) F: 2.03 (1.51–2.72) |
HR: High BMI M: 1.64 (1.18–2.27) F: 2.19 (1.65–2.89) |
Age, site, marital status, education, smoking (pack-years), SPPB score, self-rated health, heart disease, cerebrovascular disease, Peripheral Arterial Disease, diabetes mellitus, lung disease, osteoarthritis, cancer, depression, cognitive impairment | Self-report: Difficulty walking ¼ mile or climbing 10 steps | |
| Stenholm et al. (2008)34 | Cut-off: High Fat M: >29.2% F: >40.1% Cut-off: High BMI ≥35 |
OR: High Fat M and F combined: 2.53 (1.70–3.77) |
OR: High BMI Fat M and F combined: 5.43 (3.46–8.50) |
BMI: Age, sex High Fat: Age, sex, education, smoking, alcohol consumption, hypertension, cardiovascular disease, pulmonary disease, joint disease, diabetes mellitus, anti-inflamatory drug use, estrogen, C-reactive protein, hand-grips strength, height |
Gait speed: <1.2m/s = limitation | |
| Visser et al. (2005)32 | Cut-off: High Fat None reported Cut-off: Intramyocellular Fat M: <34 HU F: <30 HU Cut-off: Lean CSA M: < 235cm2 F: < 166cm2 |
OR: High Fat M: 1.18 (0.77–1.80) F: 2.73 (1.91–3.91) Note: Fat reported in absolute fat mass (kg) |
OR: Intramyocellular Fat M: 1.79 (1.22–2.65) F: 1.55 (1.10–2.17) OR: Lean CSA M: 1.45 (0.92–2.27) F: 1.34 (0.95–1.88) |
Age, race, study site, height, total-body fat mass, education, alcohol consumption, smoking status, physical activity, prevalent disease, self-rated health, depression, cognitive status, strength, muscle CSA, muscle attenuation | Self-report: Difficulty walking ¼ mile or climbing 10 steps | |
| Zoico et al. (2004)12 | Cut-off: High Fat F: >45.6% Cut-off: Low Lean F: < 5.3kg/m2 Cut-off: High BMI F: >30 |
OR: High Fat F: 3.07 (1.02–9.25) OR: Low Lean F: 2.30 (0.79–6.66) |
OR: High BMI F: 4.56 (1.51–13.77) |
Age, heart disease, hypertension, diabetes, arthritis | Self-report: Difficulty performing majority of ADLs/IADLs | |
| Davison et al.(2002)57 | Cut-off: High Fat (absolute) None reported (relative) M: 40.15–57.15% F: 43.48–67.07% Cut-off: Low Lean (absolute) None reported (relative) M: 5.88–8.46 kg/m2 F: 3.73–5.96 kg/m2 Cut-off: High BMI M/F: ≥35 Cut-off: Low BMI M/F: < 18.5 |
OR: High Fat (absolute) M: 1.24 (0.76–2.01) F: 2.60 (1.89–3.59) OR: High Fat (relative) M: 1.87 (1.19–2.96) F: 2.74 (1.43–5.25) OR: Low Lean (absolute) M: 1.25 (0.71–2.20) F: 1.09 (0.62–1.93) OR: Low Lean (relative) M: 1.20 (0.61–2.35) F: 0.87 (0.43–1.77) |
OR: High BMI M: 2.26 (1.04–4.94) F: 4.81 (2.33–9.91) OR: Low BMI M: 1.85 (0.70–4.89) F: 3.44 (1.31–9.06) |
M: Age, ethnicity, education, diabetes mellitus, hypertension, heart disease, stroke, hip fracture, arthritis F: Age, ethnicity, education, heart disease, stroke, hip fracture, arthritis |
Self-report: Difficulty on the majority of walking ¼ mile, climbing 10 steps, carrying 10 pounds, crouching, and rising from chair | |
| Sternfeld et al. (2002)47 | Cut-off: High Fat M/F: > 90th percentile fat mass (kg) Cut-off: Low Lean M/F: < 10th percentile lean mass (kg) |
OR: High Fat M: 1.09 (1.05–1.12) F: 1.08 (1.06–1.10) OR: Low Lean M: 0.97 (0.90–1.04) F: 1.00 (0.93–1.07) |
Age, comorbidity, self-reported physical activity, smoking status | Self-report: “A lot of difficulty” performing or “unable to perform” stooping/crouching/kneeling, carrying more than 10 pounds, walking up and down stairs | ||
| Broadwin et al. (2001)10 | Cut-off: High Fat M: 23.8–46.0% F: 33.1–55.0% Cut-off: Low Lean M: 35.5–75.6% F: 45.6–67.0% |
OR: High Fat M: 0.7 (0.1–9.9) F: 1.9 (0.7–5.2) OR: Low Lean M: 1.1 (0.1–10.0) F: 1.6 (0.6–4.2) |
Age, chronic diseases, alcohol use, education, depression, smoking, current estrogen use | Self-report: Difficulty walking ¼ mile or climbing 10 steps | ||
| Friedman et al. (2001)45 | Cut-off: High BMI M/F: ≥40 |
ROR: High BMI M: 4.21 (1.76–10.03) F: 4.68 (2.68–8.16) |
Age, polypharmacy, depression | Self-report: Difficulty performing majority of ADLs/IADLs | ||
| Visser, Harris, et al. (1998)44 | Cut-off: High Fa M: > 32.0% F: > 43.7% Cut-off: Low Lean (total) M: < 51.2kg F: < 35.2kg (lower extremity) M: < 16.8kg F: < 11.3kg |
OR: High Fat M: 3.04 (1.09–8.50) F: 4.07 (2.00–8.28) OR: Low Lean (total) M: 1.61 (0.50–2.43) F: 0.39 (0.18–0.85) |
OR: Low lean (lower extremity) M: 2.01 (0.62–6.53) F: 0.53 (0.25–1.14) |
Age, education self-rated health, chronic illness, physical activity, smoking, alcohol use, estrogen use (women only), height | Self-report: “A lot of difficulty” performing or “unable to perform” stooping/crouching/kneeling, standing for 15 minutes, or ½ mile walk | |
| Visser, Langlois, et al. (1998)55 | Cut-off: High Fat M: >37.5 kg F: >42.4 kg Cut-off: Low Lean M: <43.5kg F: < 29kg |
OR: High Fat M: 2.77 (1.82–4.23) F: 3.04 (2.18–4.25) OR: Low Lean M: 0.79 (0.53–1.18) F: 0.79 (0.57–1.08) |
Age, education, depression, chronic illness, edema, physical activity, recent weight loss, smoking, alcohol use, study site, chronic illness during follow-up | Self-report: Difficulty walking ¼ mile or climbing 10 steps | ||
| Launer et al. (1994)49 | Cut-off: High BMI F: >28.1 | OR: High BMI F: 1.78 (1.06–3.01) |
Aging, smoking status, socio-economic status as measured by years of education, study time | Self-report: Difficulty walking 400m; walking across a room; climbing 2 steps; doing heavy chores; running errands; bending to the floor; or transferring from a car, bed, bath, or toilet | ||
| LaCroix et al. (1993)48 | Cut-off: High BMI M/F: > 80th percentile Cut-off: Low BMI M/F: < 20th percentile |
RR: High BMI M: 1.0 (0.9–1.2) F: 1.0 (0.9–1.1) RR: Low BMI M: 1.2 (1.0–1.5) F: 1.4 (1.2–1.6) |
Community, age, behavioural risk factors, chronic disease | Self-report: Difficulty walking ¼ mile or climbing 10 steps or descending 10 steps |
HU = Houndsfield units; HR = hazard ratio; OR = odds ratio; ROR = relative odds ratio; RR = relative risk; BMI = body mass index; SPPB = Short Physical Performance Battery; CSA = cross-sectional area; M = Male; F = Female
Indices of “Risk” are reported as OR, HR, RR, or ROR (95% CI).
RESULTS
Search Results
In total, 53 studies of older individuals (≥65 years) were assessed. Of those studies, 34 described lower-extremity strength and power relationships with mobility;11–28,31–43,47,50,63 30 examined relationships between body composition and mobility;6–10,12,13,31–34,38,44–58,63–65 and 9 had overlapping parameters.12,13,31–34,38,50,63 Of the aforementioned studies, 3 addressed regional fat composition of the lower extremities and its relationship to mobility.9,32,51 Subject cohorts consisted predominantly of elderly non-Hispanic white men and women (mean age = 74); the sample sizes ranged from as many as 7,120 individuals45 to as few as 1627 (see Table 1).
Table 1.
Study Demographics
| Study | Population Size | Age* mean ± SD / range |
|---|---|---|
| Bohannon (2008)38 | 687 participants | 73.6 ± 6.1 years |
| Jankowski et al. (2008)58 | 109 participants | 60 + years |
| Koster et al. (2008)46 | 2,982 participants: 1,527 women, 1,455 men; 41% African Americans | 74.2 ± 2.9 years |
| Puthoff et al. (2008)15 | 30 participants: 25 women, 5 men | 77.3 ± 7.0 years |
| Reid et al. (2008)6 | 57 participants: 31 women, 26 men | 74.2 ± 7.0 years |
| Stenholm et al. (2008)34 | 2,099 participants: 1,175 women, 924 men | 66.6 ± 0.3 years |
| Bouchard et al. (2007)56 | 904 participants: 467 women, 437 men | 74.1 ± 4.2 years |
| Bean et al. (2007)40 | 138 participants | 75.4 ± 6.9 years |
| Buchman et al. (2007)16 | 886 participants: 664 women, 222 men | 80.5 ± 6.87 years |
| Delmonico et al. (2007)54 | 2,976 participants: 1,548 women, 1,428 men; 41% African Americans | 73.8 ± 2.9 years |
| Estrada et al. (2007)11 | 189 women | 67.5 ± 4.8 years |
| Misic et al. (2007)33 | 55 participants: 36 women, 19 men | 69.3 ± 5.5 years |
| Puthoff & Nielsen (2007)18 | 30 participants: 25 women, 5 men | 77.3 ± 7.0 years |
| Perry et al. (2007)17 | 44 non-fallers, 34 fallers | 76.2 ± 0.7 years |
| Schrager et al. (2007)53 | 871 participants: 493 women, 378 men | 74.0 ± 7.1 years |
| Sergi et al. (2007)65 | 1,672 participants: 1,236 women, 1,436 men | 73.2 ± 5.6 years |
| Lebrun et al. (2006)50 | 396 women | 66.3 ± 3.8 years |
| Marsh et al. (2006)19 | 720 participants: 384 women, 336 men | 73.0 ± 6.1 years |
| Sayers et al. (2005)35 | 101 participants: 64 women, 37 men | 80.7 ± 0.6 years |
| Herman et al. (2005)39 | 37 participants: 24 women, 13 men | 75.6 ± 6.6 years |
| Visser et al. (2005)32 | 3,075 participants: 1,345 women, 1,286 men; 34% African Americans | 73.5 ± 2.9 years |
| Cuoco et al. (2004)20 | 47 participants: 41 women, 6 men | 72.7 ± 0.8 years |
| Ostchega et al. (2004)21 | 1,499 participants | Age range = 50–70 years |
| Song et al. (2004)64 | 26 women | 75.5 ± 5.1 years |
| Zoico et al. (2004)12 | 167 women | 71.7 ± 2.4 years |
| Bean et al. (2003)41 | 1,032 participants: 557 women, 475 men | 74.2 years |
| Newman et al. (2003)52 | 2,984 participants: 1,552 women, 1,432 men; 41% African Americans | 73.6 ± 2.9 years |
| Bean et al. (2002)37 | 45 participants: 34 women, 11 men | 72.7 ± 4.6 years |
| Davison et al. (2002)57 | 2,917 participants: 1,526 women, 1,391 men | 76.8 ± 2.0 years |
| Ferrucci et al. (2002)23 | 620 older women | 65 + years of age |
| Janssen et al. (2002)8 | 4,504 participants: 2,278 women, 2,224 men | 70.5 ± 7 years |
| Ploutz-Snyder et al. (2002)22 | 100 participants | 73.0 ± 0.9 years |
| Visser et al. (2002)9 | 3,075 participants: 1,537 women, 1,442 men; 40% African Americans | 73.6 ± 2.9 years |
| Broadwin et al. (2001)10 | 1,051 participants: 634 women, 417 men | 70.7 years (range: 55–92) |
| Friedman et al. (2001)45 | 7,120 participants: 3,312 women, 3808 men | 71.7 ± 5.7 years |
| Sternfeld et al. (2002)47 | 1,655 participants: 947 women, 708 men | 69.4 years (range: 55–95.5) |
| Foldvari et al. (2000)36 | 197 women | 74.8 ± 5.0 years |
| Visser, Newman, et al. (2000)63 | 3,075 participants: 1,537 women, 1,442 men; 40% African Americans | 73.6 ± 2.9 years |
| Visser, Deeg, et al. (2000)13 | 449 participants: 233 women, 216 men | 75.4 ± 6.4 years |
| Rantanen et al. (1999)26 | 1,002 women | 78.3 ± 8.1 years |
| Zamboni et al. (1999)51 | 144 women | 72.0 ± 2.2 years |
| Baumgartner et al. (1998)7 | 808 participants: 382 women, 426 men | 73.7 ± 6.0 years |
| Payette et al. (1998)31 | 30 women | 81.5 ± 7 years |
| Visser, Langlois, et al. (1998)55 | 5,201 participants: 2,714 women, 2,095 men | 72.9 ± 5.6 years |
| Visser, Harris, et al. (1998)44 | 753 participants: 478 women, 275 men | 78.2 ± 0.4 |
| Rantenen et al. (1996)25 | 458 participants: 315 women, 143 men | All participants were either 75 or 80 years of age |
| Brown et al. (1995)27 | 16 participants | 80.9 years (range: 75–88) |
| Launer et al. (1994)49 | 426 women | 66.1 ± 3.6 years |
| Skelton et al. (1994)42 | 100 healthy men and women | 77.3 years (range: 65–89) |
| Rantanen et al. (1994)24 | 295 participants: 191 women, 104 women | 75 years |
| LaCroix et al. (1993)48 | 6,981 participants: 3,935 women, 3,046 men | 65 + years |
| Bassey et al. (1992)43 | 26 participants: 13 women, 13 men | 187 ± 1.6 years |
| Hyatt et al. (1990)14 | 92 participants: 64 women, 28 men | 77 ± 6.4 years |
| Danneskiold-Samsee et al. (1984)28 | 52 participants: 29 women, 23 men | 80 years (range: 78–81) |
Mean ± SD or a range reported when available
Strength/Power and Mobility Relationships
The relationships of muscle strength and muscle power, respectively, with gait speed are reported in Table 2.
Table 2.
Relationship between Power and Gait Speed and between Strength and Gait Speed in Older Individuals (>65 years of age)
| Mean Gait Speed | Gait Speed Protocol | High-Force, Low-Velocity Power and Gait Speed r2 |
Low-Force, High-Velocity Power and Gait Speed r2 |
Strength and Gait Speed r2 (Strength-testing Method) |
||
|---|---|---|---|---|---|---|
| Puthoff et al. (2008)15 | 0.72 m/s | Intensity: Habitual Course: 4 m |
0.38 | 0.39 (Isotonic leg press) | ||
| Puthoff & Nielsen (2007)18 | 0.97 m/s | Intensity: Habitual Course: 4 m |
0.35 | 0.31 | 0.31 (Isotonic leg press) | |
| Bean et al. (2007)40 | 0.93 m/s | Intensity: Not indicated Course: 4 m |
0.34 (70% 1RM) | 0.34 | n/a | |
| Marsh et al. (2006)19 | 1.2 m/s | Intensity: “steady pace” Course: 400 m |
0.58 | n/a | 0.53 (Isometric sum score of hip, knee and ankle) | |
| Misic et al. (2007)33 | No mean reported | Intensity: Habitual Course: 7 m |
n/a | n/a | 0.21 (sum of isokinetic knee extension and flexion) |
|
| Herman et al. (2005)39 | 1.21 m/s | Intensity: Habitual Course: 4 m |
0.27 | 0.26 | n/a | |
| Sayers et al. (2005)35 | 0.87 m/s | Intensity: “self-paced” Course: 400 ms |
n/a | 0.18 | 0.06 (Isotonic leg press) | |
| Cuoco et al. (2004)20 | 1.12 m/s | Intensity: Habitual Course: 2+m |
0.26 (70% 1RM) | 0.35 | 0.07 (Isotonic leg press) | |
| Ostchega et al. (2004)21 | 0.95 m/s | Intensity: Habitual Course: 20 feet |
n/a | n/a | 0.20 (Isokinetic knee extension) | |
| Bean et al. (2003)41 | 1.08 m/s | Intensity: Habitual Course: 4 m |
0.41 | n/a | 0.38 (Isometric Hip extension) 0.36 (Isometric knee extension) |
|
| Bean et al. (2002)37 | 1.18 m/s (habitual) Note: Maximal mean gait speed not reported |
Intensity: Habitual and maximal Course: 2+ meters |
Habitual: 0.61 Maximal: 0.56 |
n/a | Habitual: 0.57 (Isotonic leg press) Maximal: 0.56 (Isotonic leg press) |
|
| Ploutz-Snyder et al. (2002)22 | 70% had no difficulty walking 1.22 m/s |
Intensity: Maximal Course: 25 feet |
n/a | n/a | 0.27 (Isometric knee extension) | |
| Brown et al. (1995)27 | Min.–Max. =0.5–1.5 m/s | Intensity: Habitual Course: Not reported |
n/a | n/a | 0.20 (Isometric knee extension) | |
| Bassey et al (1992)43 | Male: 2.2 m/s Female: 1.37 m/s Combined: 1.79 |
Intensity: Habitual | Course: 6.1 m | Female: 0.93 Male: 0.58 Combined: 0.80 |
n/a | n/a |
*Muscles tested: hip—abductors, adductors, flexors, extensors; knee—flexors, extensors; ankle—dorsiflexors, plantarflexors
“self paced” = 400 m walk effort in which gate speed is neither maximal or habitual but, rather, paced at the speed the individual felt he or she could maintain for the entire 400 m
Minimum and maximum r2 values with respect to gait speed and power or strength are as follows: r2 = 0.18, 0.38 when power was measured as low force; r2 = 0.27, 0.93 when power was measured as high force; and r2 = 0.06, 0.57 when the independent variable was strength. Of those studies that reported both strength and power relationships with mobility using a variety of mobility constructs, all but two35,42 demonstrated that power explains more variance in mobility than strength,15,17–20,36,37,41 and these two investigations observed a stronger “strength” relationship only in men. When men and women were pooled, however, the variance associated with power was double that associated with strength (r2 = 0.16 vs. 0.08).35
Mobility measures other than gait speed (stair climb, walking distance, step mounting, sit-to-stand, tandem stand, Short Physical Performance Battery score, and self-report) demonstrated similar associations with strength (r2 = 0.06 to 0.41) and power (r2 = 0.07 to 0.83),15,18,23–28,37,38,40–43 although there was some inconsistency with respect to the strength of the relationships between muscle function and stair-climbing ability.25,28,42 General surveys of mobility limitations were also associated (r2 = 0.06 to 0.18) with quadriceps strength,14,36 though power best discriminated subjects characterized as fallers from those characterized as non-fallers.17
Whole-Body and Regional Composition and Risk of Mobility Limitation
The risks of a mobility limitation associated with sarcopenia and sarcopenic obesity are reported in Table 3. Risk indices across and within studies for mobility limitations demonstrated a minimum OR of 0.47 and a maximum OR of 4.58 for SMI, while ASMI yielded a minimum HR of 0.84 and a maximum HR of 4.08. Sarcopenic obesity demonstrated a minimum HR of 0.91 and a maximum OR of 2.04. Estrada et al.11 defined relative sarcopenia and absolute sarcopenia as appendicular lean mass divided by total mass and lean mass divided by height squared, respectively. This group concluded that relative sarcopenia (mean r2 = 0.13) is a better predictor of functional limitations than absolute sarcopenia (mean r2 = 0.06).11
Table 3.
Reported “Risk” (Odds and Hazard) Ratios for Mobility-Related Functional Limitations and Disabilities in Relation to Various Indices of Sarcopenia
| Sarcopenia Cut-offs / Definitions | SMI* | ASMI* | SO* | Covariates | Functional Limitations and Disability Criteria | ||
|---|---|---|---|---|---|---|---|
| Delmonico et al. (2007)54 | ASMI: M: < 7.25 kg/ht2 F: < 5.67 kg/ht2 SO: 20th percentile of residuals distribution in relation to ASMI |
HR: M: 0.84 (0.66–1.08) F: 1.04 (0.82–1.31) |
HR: M: 0.91 (0.73–1.15) F: 1.34 (1.11–1.61) |
ASMI: Age, race, comorbidity, lower-extremity function, and interim hospitalization (SO additionally adjusted for physical activity and body mass) |
Self-report: Difficulty walking ¼ mile or climbing 10 steps | ||
| Zoico et al. (2004)12 | ASMI: < 4.7 kg/ht2 SMI: < 23.1% SO: < 5.3kg/m2 lean and >45.6% fat |
OR: M/F: 3.86 (1.01–14.87) |
OR: M/F: 0.98 (0.35–2.78) |
OR: M/F: 2.16 (0.73–6.42) |
Age, heart disease, hypertension, diabetes and arthritis, BMI, ASMI, and SMI | Self-report: Difficulty performing majority of ADLs/IADLs | |
| Newman et al. (2003)52 | ASMI and SO: Lower 20th percentile respective to distribution of each method | OR: M: 1.5 (1.1–2.1) F: 0.9 (0.7–1.2) |
OR: M: 1.8 (1.3–2.5) F: 1.9 (1.4–2.5) |
Age, race, drinking, smoking, physical activity, and comorbidity | Performance: EPESE physical battery score < 10 | ||
| Davison et al. (2002)57 | SO: Lowest 2 quintiles lean and highest 2 quintiles fat | OR: M: 1.49 (0.87–2.36) F: 2.04 (0.92–4.53) |
Age, ethnicity, education, hypertension, heart disease, stroke, hip fracture, and arthritis (Type II diabetes mellitus for women) | Self-report: Difficulty on majority of walking ¼ mile, climbing 10 steps, carrying 10 pounds, crouching, and rising from chair | |||
| Janssen et al. (2002)8 | SMI: M: < 31.5% F: < 22.1% |
OR: M: 0.47–4.58** F: 0.30–3.96** |
Age, race, BMI, health behaviours, and comorbidity | Performance: Inability to complete 8 ft walk, 5 chair stands, or tandem stand | Self-report: Reported difficulty on majority of walking ¼ mile, climbing 10 steps, carrying 10 pounds, crouching, and rising from chair | ||
| Baumgartner et al. (1998)7 | ASMI: M: < 7.26 kg/ht2 F: < 5.45 kg/ht2 |
OR: M: 3.66 (1.42–10.02) F: 4.08 (1.52–11.31) |
Age, ethnicity, obesity, income, alcohol intake, physical activity, current smoking, and comorbidity | Self-report: New Mexico Elderly Health Survey—report of ≥3 disabilities |
SMI = skeletal muscle index (total lean mass (kg)/total mass (kg)×100); ASMI = appendicular skeletal muscle index (total lean appendicular mass (kg)/Height2); SO = sarcopenic obesity (inclusion of high fat + low lean mass, which is individually described by each study); ADL = activities of daily living; IADL = instrumental activities of daily living; EPESE = Estimated Population for the Epidemiologic Study of the Elderly; OR = odds ratio; HR = hazard ratio; BMI = body mass index; M = male; F = female
Indices of “Risk” are reported as OR or HR (95% CI).
No single definition of disability was reported. Therefore a range is presented based on the individual ORs associated with various self report and functional measures.
The risks associated with low whole-body relative or absolute lean mass and high whole-body relative or absolute fat mass (including both high and low BMIs) are reported in Table 4. Minimum and maximum values of risk indices for disability with respect to high whole-body fat mass are as follows: OR = 0.70, 4.07 for relative measures of adiposity versus OR = 1.08, 3.04 for absolute measures of adiposity. With the exception of one study,47 the risk associated with both relative and absolute fat mass is higher for women than for men. Risks associated with low whole-body lean mass demonstrate a minimum OR of 0.97 and a maximum OR of 2.30 for relative measure of lean mass versus a minimum OR of 0.87 and a maximum OR of 1.60 for absolute measures of lean mass. Meanwhile, minimum and maximum values of risk associated with a high BMI were OR = 1.0, 5.43, while the risk values for those with low BMI were OR = 1.20, 3.44. Similar gender differences in risk profiles were observed with respect to BMI.
Only two cross-sectional studies9,32 have examined the association of increased intramyocellular fat of the thigh on mobility. It should be noted that both of these studies measured muscle density via computed tomography, which is an indirect assessment of intramyocellular fat. One reported a small but independent risk (1.67 (95% CI: 1.16–2.41); see Table 4).32 The other reported a small but significant (p < 0.05) association between intramyocellar fat and lower-extremity function in men and women (r2 = 0.07 and 0.03, respectively).9 Using dual x-ray absorptiometry (DXA) to quantify regional fat mass, another study51 reported that leg fat mass discriminated between those with and without disability (p = 0.01).
Regional lower-extremity muscle mass consistently yields a small but not always independent relationship with mobility. Visser et al.32 found that low lean muscle mass of the thigh demonstrated an increased risk (1.79 (95% CI: 1.25–2.58)) of mobility limitation, though this risk no longer existed when strength was entered as a covariate (1.40 (95% CI: 0.94–2.08)), suggesting that lean mass is mediated by strength.32 Estrada et al.11 also found that low lower-extremity lean mass was inversely related to mobility disability, and a more recent study suggested that for every 1 kg increase in lower-extremity lean mass there was a 53% reduction in mobility limitation.6 Furthermore, the same study demonstrated a notably larger lean mass in individuals scoring >7 on the Short Physical Performance Battery (SPPB) (scale = 0–12) than in those scoring below this threshold of mobility.6
DISCUSSION
It seems intuitive that muscle is intricately linked to an older individual's level of mobility; however, there is debate as to which specific muscle parameters are most influential. A review of the literature on muscle strength, power, and composition reveals strong trends suggesting that muscle power has a stronger association with mobility than does muscle strength,15,17–20,36,37,39,41 and that high whole-body fat mass is more influential than low whole-body lean mass with respect to mobility.12,32,44,46,47,50–52,54,55,57,58,63
The association between strength and mobility is discrete, in that if an individual is not capable of producing the force required to functionally ambulate, rise from a chair, or negotiate stairs, the consequences are obvious. However, most, if not all, daily activities are performed at sub-maximal intensities, and in most cases there is a spectrum of ability; the outcomes cannot simply be reduced to “able” or “not able.” Along the continuum of the mobility–strength relationship, the association becomes less discrete as confounding influences enter into this relationship, as represented by the moderate coefficients of determination presented in Table 2. Power correlates better than strength with all mobility measures,15 which has piqued some investigators' interest in assessing power by emphasizing its constituent parts.15,18,20,39,40 Distinguishing the power generated by a muscle at a high percentage (90–100%) of 1RM from that generated by a muscle at a low percentage (40%) of 1RM emphasizes the force component and the velocity component of power, respectively. Since power represents force per unit time, some have proposed that power produced under the low-load, high-velocity condition would best predict mobility, since time is the differentiating factor between strength and power.20,40 Contrary to this hypothesis, it has been shown that power measured under the high-load, low-velocity condition best correlates with gait speed and other mobility measures,15,18 while others have found little or no difference between the measures.39,40 Although the speed of contraction cannot be discounted, it does appear that the force component of power is the predominant piece of the power–mobility relationship, which may have therapeutic implications. Indeed, traditional resistance training, which focuses on force enhancement, has been shown to improve power in the elderly population to a similar extent as power-specific exercises.59
Normalizing strength to muscle cross-sectional area, frequently referred to as “muscle quality,” improves the strength–mobility relationship to a similar extent as power alone.33 Specifically, Misic et al.33 described an improvement from an r2 of 0.29 (p < 0.05) for strength and mobility to 0.42 (p < 0.05) for muscle quality and mobility.33 Unfortunately, no studies to date have assessed power normalized to muscle size in relation to mobility in the elderly, so it is not known whether the power relationship would further improve if expressed as power per unit of muscle size. Some evidence exists, however, that suggests the plausibility of this relationship.66 Future studies should assess power produced per unit of muscle size as it pertains to mobility, since this could either underscore power-specific muscle factors or demonstrate that power and muscle quality are equally good predictors of mobility.
Studies of whole-body composition have emphasized the impact of fat mass rather than lean mass on disability. Every study included in this review found that increased fat mass was a significant predictor of either current mobility limitation9,12,32,44,46,47,50–52,54,55,57,58,63 or future disability,10,49,54 while few demonstrated an influence of lean mass on mobility (see Table 3 and Table 4).6–11 Bouchard et al.56 measured fat mass and lean mass in relation to physical capacity and found that only fat mass had an influence.56 This group cautioned against using BMI as a body-composition assessment, noting that it is increasingly invalid in older populations as an assessment of composition. Despite these cautionary remarks, Jankowski et al.58 demonstrated that BMI was nearly as good of a predictor of mobility limitation as a fat index (fat mass/total body mass) via poor performances on the Continuous Scale Physical Function Performance test (CS-PFP) (r2 = 0.50 vs. R2 = 0.54) and the Short Form-36 (SF-36) (r2 = 0.34 vs. r2 = 0.37). Several other studies have supported the notion that BMI is a valid surrogate for body fatness in the elderly with respect to disability risk and that this assessment is cost effective and feasible.12,34,38,45,46,48,57,65 Other studies investigating the relationship between body composition and mobility are difficult to present in total, since various other fat and mobility measures were employed.31,50,51 In general, these disparate studies suggest that increasing fat mass (both total body and regional) may affect functional mobility as much as or more than lean mass.31,50,51 For example, no relationship has been reported between lean mass and a timed up-and-go test.31 Further, an additional 10 kg of fat mass can result in a reduction in physical activity and physical performance50 and can serve to discriminate between those with and those without disability.51
Contrary to the above observations, some studies have demonstrated that lean tissue affects mobility in the elderly population.6–11 Some of these studies use indices such as the percentage of lean mass; therefore, the effect of fat cannot be discounted.8,10 Visser et al. determined that low lean mass was not predictive of disability13,44,55,63 but years later described muscle mass as being predictive of disability.9 Most recently, Visser et al.32 concluded that muscle mass predicts disability but that the relationship is mediated by strength.32 On the other hand, Baumgartner et al.7 found an approximately fourfold increased risk for mobility limitation in those with the lowest lean mass, as assessed by appendicular lean mass/height;2,7 however, these authors did not measure strength, and, therefore, the possibility of this mediating relationship cannot be discarded.7 Of course, the possibility that strength mediates the relationship between lean mass and mobility does not negate the importance of lean mass as a predictor of mobility. It does, however, highlight the fact that other intrinsic muscle factors, irrespective of muscle size, are important strength-training outcomes and that strength and power may be more clinically important endpoints than hypertrophy.
There is an increasing body of literature suggesting that regional fat mass affects both muscle quality67–69and measures of mobility and physical performance in older individuals.9,32,51 The role of leg fat, as measured via DXA, is associated with an increased risk of mobility limitation,51 though the role of low lean mass in the legs is questionable.6,51 Although Reid et al.6 recently demonstrated a decreasing OR (0.47 (95% CI: 0.22, 0.89)) for disability with every 1 kg increase in leg-muscle mass, this group admittedly acknowledged a limitation in that they did not investigate the role of regional fat mass and, in particular, fat infiltration of skeletal muscle.6 Only one study to date has identified intramyocellular fat as increasing the risk of mobility limitation independent of strength or whole-body fat mass,32 although recently others have suggested that fat inside and outside the muscle cells of calf muscles can adversely affect physical performance in older, obese individuals.69 More research is warranted to determine whether or not this relationship persists. Recently, we have identified intramuscular fat (a total of intra- and extramyocellular fat independent of subcutaneous fat) of the thigh musculature as being inversely associated with the number of steps taken per day and the distance walked in 6 minutes, though knee-extension force was most related to the timed up-and-go manoeuvre and to negotiating stairs.70
Limitations exist in narrative reviews of the literature that can restrict their usefulness. A major limitation in reviewing only cohort cross-sectional studies is that causal relationships cannot be determined. Evaluation of the findings for the control arm of even a few longitudinal cohort (and ideally randomized) studies would permit better understanding of the temporal relationship between body composition and mobility. In this review we have attempted to minimize these limitations by providing a transparent outline of the search strategy and terms, constraining the studies to those whose aim was to determine association and risk, and providing adequate detail on each study so that readers can decide for themselves the impact of muscle-function and composition factors on mobility in older individuals. Despite an effort to constrain the criteria for inclusion for each paper, the literature cited within this review varies extensively in terms of cohort demographics, outcome measures, measurement tools, statistical methods, and definitions of terms. With more than 50 included studies, however, we feel that our narrative review strongly represents the relationships between and the associated risks of sarcopenic and age-related changes in body composition. Furthermore, we feel that limiting the manuscript to cross-sectional and longitudinal studies was necessary to mitigate the potential confounding influence of rehabilitation countermeasures. We have added substantial detail from the included studies, but warn the reader that efforts to determine and integrate complex interactions within and across studies can never be conclusive. Although different methodological approaches across studies may partially explain the variability in outcomes, we found relative consistency among the outcomes, which lends credence to the generalizability of our narrative conclusions.
CONCLUSION
Muscle composition and function are subject to change during the latter third of life. Of particular concern are age-related declines in strength and power, which are likely affected by increasing total fat mass as well as by fat stored within whole muscle and the muscle cell. Fat mass also appears to have some independent effect on mobility, aside from its role in decreasing strength and power. Loss of lean muscle tissue is also related to mobility, but not to the same extent as muscle function and whole-body fat composition. Lastly, other intrinsic factors of muscle appear to be of more concern than lean mass with respect to mobility, which suggests that in this population, gains in muscle force and power production may be more influential than muscle hypertrophy as it relates to mobility.
CLINICALLY RELEVANT SUMMARY
Age-related changes in muscle function and regional and whole-body composition are modifiable in older populations.4,59–61,71,72Countermeasures aimed at enhancing muscle power will likely have a positive impact on mobility.35 While it seems intuitive that simply increasing muscle mass is important, increases in lean tissue and decreases in regional and whole-body fat deposits with rehabilitation countermeasures are often coupled to the muscle-growth response.71 Recent evidence also suggests that resistance exercise may be a mode of exercise that can positively affect muscle and whole-body composition changes in older individuals.71 This review highlights the clinically important role of muscle and whole-body composition in mobility among older individuals and suggests that, moving forward, clinicians should include in their tests of effectiveness a description of changes in muscle function as well as clinically feasible measures of regional and whole-body composition.
Kidde J, Marcus R, Dibble L, Smith S, LaStayo P. Regional muscle and whole-body composition factors related to mobility in older individuals: a review. Physiother Can. 2009;61:197–209.
REFERENCES
- 1.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–43. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 2.Rantanen T. Muscle strength, disability and mortality. Scand J Med Sci Sport. 2003;13:3–8. doi: 10.1034/j.1600-0838.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- 3.Functional outcomes for clinical trials in frail older persons: time to be moving. J Gerontol A Biol Sci Med Sci. 2008;63:160–4. doi: 10.1093/gerona/63.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouwer B, Olney S. Aging skeletal muscle and the impact of resistance exercise. Physiother Can. 2004;56:23–32. [Google Scholar]
- 5.Evans WJ. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993;123:465–8. doi: 10.1093/jn/123.suppl_2.465. [DOI] [PubMed] [Google Scholar]
- 6.Reid KF, Naumova EN, Carabello RJ, Phillips EM, Fielding RA. Lower extremity muscle mass predicts functional performance in mobility-limited elders. J Nutr Health Aging. 2008;12:493–8. doi: 10.1007/BF02982711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 8.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 9.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 10.Broadwin J, Goodman-Gruen D, Slymen D. Ability of fat and fat-free mass percentages to predict functional disability in older men and women. J Am Geriatr Soc. 2001;49:1641–5. doi: 10.1046/j.1532-5415.2001.t01-1-49273.x. [DOI] [PubMed] [Google Scholar]
- 11.Estrada M, Kleppinger A, Judge JO, Walsh SJ, Kuchel GA. Functional impact of relative versus absolute sarcopenia in healthy older women. J Am Geriatr Soc. 2007;55:1712–9. doi: 10.1111/j.1532-5415.2007.01436.x. [DOI] [PubMed] [Google Scholar]
- 12.Zoico E, Di Francesco V, Guralnik JM, Mazzali G, Bortolani A, Guariento S, et al. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int J Obes Relat Metab Disord. 2004;28:234–41. doi: 10.1038/sj.ijo.0802552. [DOI] [PubMed] [Google Scholar]
- 13.Visser M, Deeg DJ, Lips P, Harris TB, Bouter LM. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J Am Geriatr Soc. 2000;48:381–6. doi: 10.1111/j.1532-5415.2000.tb04694.x. [DOI] [PubMed] [Google Scholar]
- 14.Hyatt RH, Whitelaw MN, Bhat A, Scott S, Maxwell JD. Association of muscle strength with functional status of elderly people. Age Ageing. 1990;19:330–6. doi: 10.1093/ageing/19.5.330. [DOI] [PubMed] [Google Scholar]
- 15.Puthoff ML, Janz KF, Nielson D. The relationship between lower extremity strength and power to everday walking behaviors in older adults with functional limitations. J Geriatr Phys Ther. 2008;31:24–31. doi: 10.1519/00139143-200831010-00005. [DOI] [PubMed] [Google Scholar]
- 16.Buchman AS, Wilson RS, Boyle PA, Tang Y, Fleischman DA, Bennett DA. Physical activity and leg strength predict decline in mobility performance in older persons. J Am Geriatr Soc. 2007;55:1618–23. doi: 10.1111/j.1532-5415.2007.01359.x. [DOI] [PubMed] [Google Scholar]
- 17.Perry MC, Carville SF, Smith IC, Rutherford OM, Newham DJ. Strength, power output and symmetry of leg muscles: effect of age and history of falling. Eur J Appl Physiol. 2007;100:553–61. doi: 10.1007/s00421-006-0247-0. [DOI] [PubMed] [Google Scholar]
- 18.Puthoff ML, Nielsen DH. Relationships among impairments in lower-extremity strength and power, functional limitations, and disability in older adults. Phys Ther. 2007;87:1334–47. doi: 10.2522/ptj.20060176. [DOI] [PubMed] [Google Scholar]
- 19.Marsh AP, Miller ME, Saikin AM, Rajeski J, Hu N, Lauretani F, et al. Lower extremity strength and power are associated with 400-meter walk time in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2006;61:1186–93. doi: 10.1093/gerona/61.11.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuoco A, Callahan DM, Sayers S, Frontera WR, Bean J, Fielding RA. Impact of muscle power and force on gait speed in disabled older men and women. J Gerontol A Biol Sci Med Sci. 2004;59:1200–6. doi: 10.1093/gerona/59.11.1200. [DOI] [PubMed] [Google Scholar]
- 21.Ostchega Y, Dillon CF, Lindle R, Carroll M, Hurley BF. Isokinetic leg muscle strength in older americans and its relationship to a standardized walk test: data from the national health and nutrition examination survey 1999–2000. J Am Geriatr Soc. 2004;52:977–82. doi: 10.1111/j.1532-5415.2004.52268.x. [DOI] [PubMed] [Google Scholar]
- 22.Ploutz-Snyder LL, Manini T, Ploutz-Snyder RJ, Wolf DA. Functionally relevant thresholds of quadriceps femoris strength. J Gerontol A Biol Sci Med Sci. 2002;57:B144–52. doi: 10.1093/gerona/57.4.b144. [DOI] [PubMed] [Google Scholar]
- 23.Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–54. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 24.Rantanen T, Era P, Heikkinen E. Maximal isometric strength and mobility among 75-year-old men and women. Age Ageing. 1994;23:132–7. doi: 10.1093/ageing/23.2.132. [DOI] [PubMed] [Google Scholar]
- 25.Rantanen T, Era P, Heikkinen E. Maximal isometric knee extension strength and stair-mounting ability in 75- and 80-year-old men and women. Scand J Rehabil Med. 1996;28:89–93. [PubMed] [Google Scholar]
- 26.Rantanen T, Guralnik JM, Sakari-Rantala R, Leveille S, Simonsick EM, Ling S, et al. Disability, physical activity, and muscle strength in older women: the Women's Health and Aging Study. Arch Phys Med Rehabil. 1999;80:130–5. doi: 10.1016/s0003-9993(99)90109-0. [DOI] [PubMed] [Google Scholar]
- 27.Brown M, Sinacore DR, Host HH. The relationship of strength to function in the older adult. J Gerontol A Biol Sci Med Sci. 1995;50:55–9. doi: 10.1093/gerona/50a.special_issue.55. [DOI] [PubMed] [Google Scholar]
- 28.Danneskiold-Samsoe B, Kofod V, Munter J, Grimby G, Schnohr P, Jensen G. Muscle strength and functional capacity in 78–81-year-old men and women. Eur J Appl Physiol Occup Physiol. 1984;52:310–4. doi: 10.1007/BF01015216. [DOI] [PubMed] [Google Scholar]
- 29.Kwon IS, Oldaker S, Schrager M, Talbot LA, Fozard JL, Metter EJ. Relationship between muscle strength and the time taken to complete a standardized walk-turn-walk test. J Gerontol A Biol Sci Med Sci. 2001;56:B398–404. doi: 10.1093/gerona/56.9.b398. [DOI] [PubMed] [Google Scholar]
- 30.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 31.Payette H, Hanusaik N, Boutier V, Morais JA, Gray-Donald K. Muscle strength and functional mobility in relation to lean body mass in free-living frail elderly women. Eur J Clin Nutr. 1998;52:45–53. doi: 10.1038/sj.ejcn.1600513. [DOI] [PubMed] [Google Scholar]
- 32.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 33.Misic MM, Rosengren KS, Woods JA, Evans EM. Muscle quality, aerobic fitness and fat mass predict lower-extremity physical function in community-dwelling older adults. Gerontology. 2007;53:260–6. doi: 10.1159/000101826. [DOI] [PubMed] [Google Scholar]
- 34.Stenholm S, Rantanen T, Heliovaara M, Koskinen S. The mediating role of C-reactive protein and handgrip strength between obesity and walking limitation. J Am Geriatr Soc. 2008;56:462–9. doi: 10.1111/j.1532-5415.2007.01567.x. [DOI] [PubMed] [Google Scholar]
- 35.Sayers SP, Guralnik JM, Thombs LA, Fielding RA. Effect of leg muscle contraction velocity on functional performance in older men and women. J Am Geriatr Soc. 2005;53:467–71. doi: 10.1111/j.1532-5415.2005.53166.x. [DOI] [PubMed] [Google Scholar]
- 36.Foldvari M, Clark M, Laviolette LC, Bernstein MA, Kaliton D, Castaneda C, et al. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55:M192–9. doi: 10.1093/gerona/55.4.m192. [DOI] [PubMed] [Google Scholar]
- 37.Bean JF, Kiely DK, Herman S, Leveille SG, Mizer K, Frontera WR, et al. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50:461–7. doi: 10.1046/j.1532-5415.2002.50111.x. [DOI] [PubMed] [Google Scholar]
- 38.Bohannon R. Knee extension strength and adiposity explain some of older adults' self-reported difificulty with mobility. J Geriatr Phys Ther. 2008;31:101–4. doi: 10.1519/00139143-200831030-00004. [DOI] [PubMed] [Google Scholar]
- 39.Herman S, Kiely DK, Leveille S, O'Neill E, Cyberey S, Bean JF. Upper and lower limb muscle power relationships in mobility-limited older adults. J Gerontol A Biol Sci Med Sci. 2005;60:476–80. doi: 10.1093/gerona/60.4.476. [DOI] [PubMed] [Google Scholar]
- 40.Bean JF, Kiely DK, LaRose S, Alian J, Frontera WR. Is stair climb power a clinically relevant measure of leg power impairments in at-risk older adults? Arch Phys Med Rehabil. 2007;88:604–9. doi: 10.1016/j.apmr.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci. 2003;58:728–33. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 42.Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing. 1994;23:371–7. doi: 10.1093/ageing/23.5.371. [DOI] [PubMed] [Google Scholar]
- 43.Bassey EJ, Fiatarone MA, O'Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 1992;82:321–7. doi: 10.1042/cs0820321. [DOI] [PubMed] [Google Scholar]
- 44.Visser M, Harris TB, Langlois J, Hannan MT, Roubenoff R, Felson DT, et al. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998;53:M214–21. doi: 10.1093/gerona/53a.3.m214. [DOI] [PubMed] [Google Scholar]
- 45.Friedmann JM, Elasy T, Jensen GL. The relationship between body mass index and self-reported functional limitation among older adults: a gender difference. J Am Geriatr Soc. 2001;49:398–403. doi: 10.1046/j.1532-5415.2001.49082.x. [DOI] [PubMed] [Google Scholar]
- 46.Koster A, Patel KV, Visser M, van Eijk JThM, Kanaya AM, de Rekeneire N, et al. Joint effects of adiposity and physical activity on incident mobility limitation in older adults. J Am Geriatr Soc. 2008;56:636–43. doi: 10.1111/j.1532-5415.2007.01632.x. [DOI] [PubMed] [Google Scholar]
- 47.Sternfeld B, Ngo L, Satariano WA, Tager IB. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol. 2002;156:110–21. doi: 10.1093/aje/kwf023. [DOI] [PubMed] [Google Scholar]
- 48.LaCroix AZ, Guralnik JM, Berkman LF, Wallace RB, Satterfield S. Maintaining mobility in late life. II. Smoking, alcohol consumption, physical activity, and body mass index. Am J Epidemiol. 1993;137:858–69. doi: 10.1093/oxfordjournals.aje.a116747. [DOI] [PubMed] [Google Scholar]
- 49.Launer LJ, Harris T, Rumpel C, Madans J. Body mass index, weight change, and risk of mobility disability in middle-aged and older women: the epidemiologic follow-up study of NHANES I. J Am Med Assoc. 1994;271:1093–8. [PubMed] [Google Scholar]
- 50.Lebrun CE, van der Schouw YT, de Jong FH, Grobbee DE, Lamberts SW. Fat mass rather than muscle strength is the major determinant of physical function and disability in postmenopausal women younger than 75 years of age. Menopause. 2006;13:474–81. doi: 10.1097/01.gme.0000222331.23478.ec. [DOI] [PubMed] [Google Scholar]
- 51.Zamboni M, Turcato E, Santana H, Maggi S, Harris TB, Pietrobelli A, et al. The relationship between body composition and physical performance in older women. J Am Geriatr Soc. 1999;47:1403–8. doi: 10.1111/j.1532-5415.1999.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 52.Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–9. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 53.Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102:919–25. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–74. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 55.Visser M, Langlois J, Guralnik JM, Cauley JA, Kronmal RA, Robbins J, et al. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr. 1998;68:584–90. doi: 10.1093/ajcn/68.3.584. [DOI] [PubMed] [Google Scholar]
- 56.Bouchard DR, Beliaeff S, Dionne IJ, Brochu M. Fat mass but not fat-free mass is related to physical capacity in well-functioning older individuals: nutrition as a determinant of successful aging (NuAge)—the Quebec Longitudinal Study. J Gerontol A Biol Sci Med Sci. 2007;62:1382–8. doi: 10.1093/gerona/62.12.1382. [DOI] [PubMed] [Google Scholar]
- 57.Davison KK, Ford ES, Cogswell ME, Dietz WH. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc. 2002;50:1802–9. doi: 10.1046/j.1532-5415.2002.50508.x. [DOI] [PubMed] [Google Scholar]
- 58.Jankowski CM, Gozansky WS, Van Pelt RE, Schenkman ML, Wolfe P, Schwartz RS, et al. Relative contributions of adiposity and muscularity to physical function in community-dwelling older adults. Obesity (Silver Spring) 2008;16:1039–44. doi: 10.1038/oby.2007.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henwood TR, Riek S, Taaffe DR. Strength versus muscle power-specific resistance training in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2008;63:83–91. doi: 10.1093/gerona/63.1.83. [DOI] [PubMed] [Google Scholar]
- 60.Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–75. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 61.Sayers SP. High velocity power training in older adults. Curr Aging Sci. 2008;1:62–7. doi: 10.2174/1874609810801010062. [DOI] [PubMed] [Google Scholar]
- 62.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 63.Visser M, Newman AB, Nevitt MC, Kritchevsky SB, Stamm EB, Goodpaster BH, et al. Reexamining the sarcopenia hypothesis: muscle mass versus muscle strength. Ann NY Acad Sci. 2000;904:456–61. [PubMed] [Google Scholar]
- 64.Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004;79:874–80. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 65.Sergi G, Perissinotto E, Toffanello ED, Maggi S, Manzato E, Buja A, et al. Lower extremity motor performance and body mass index in elderly people: the Italian Longitudinal Study on Aging. J Am Geriatr Soc. 2007;55:2023–9. doi: 10.1111/j.1532-5415.2007.01460.x. [DOI] [PubMed] [Google Scholar]
- 66.Runge M, Rittweger J, Russo CR, Schiessl H, Felsenberg D. Is muscle power output a key factor in the age-related decline in physical performance? A comparison of muscle cross section, chair-rising test and jumping power. Clin Physiol Funct Imaging. 2004;24:335–40. doi: 10.1111/j.1475-097X.2004.00567.x. [DOI] [PubMed] [Google Scholar]
- 67.Newman AB, Haggerty CL, Goodpaster B, Harris T, Kritchevsky S, Nevitt M, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–30. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 68.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–64. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 69.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the J elderly: the Health ABC Study. J Appl Physiol. 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 70.LaStayo PC, Marcus RL, Smith S, Kidde J, Butler C, Hill M. Is there a relationship between muscle, mobility and physical activity in elderly cancer survivors? J Nutr Health Aging. 2008;12:419–26. [Google Scholar]
- 71.Marcus RL, Smith S, Morrell G, Addison O, Dibble LE, Wahoff-Stice D, et al. Comparison of combined aerobic and high-force eccentric resistance exercise with aerobic exercise only for people with type 2 diabetes mellitus. Phys Ther. 2008;88:1345–54. doi: 10.2522/ptj.20080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prior SJ, Joseph LJ, Brandauer J, Katzel LI, Hagberg JM, Ryan AS. Reduction in midthigh low-density muscle with aerobic exercise training and weight loss impacts glucose tolerance in older men. J Clin Endocrinol Metab. 2007;92:880–6. doi: 10.1210/jc.2006-2113. [DOI] [PubMed] [Google Scholar]