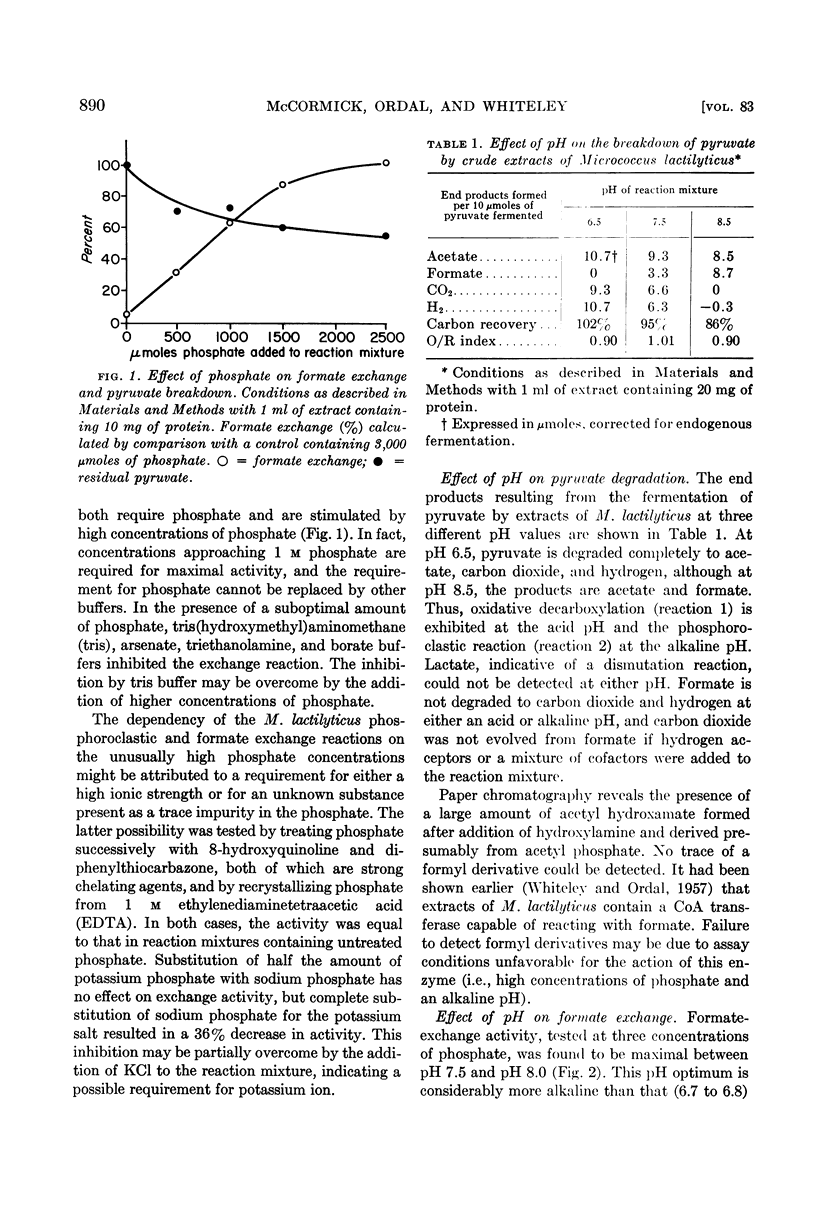
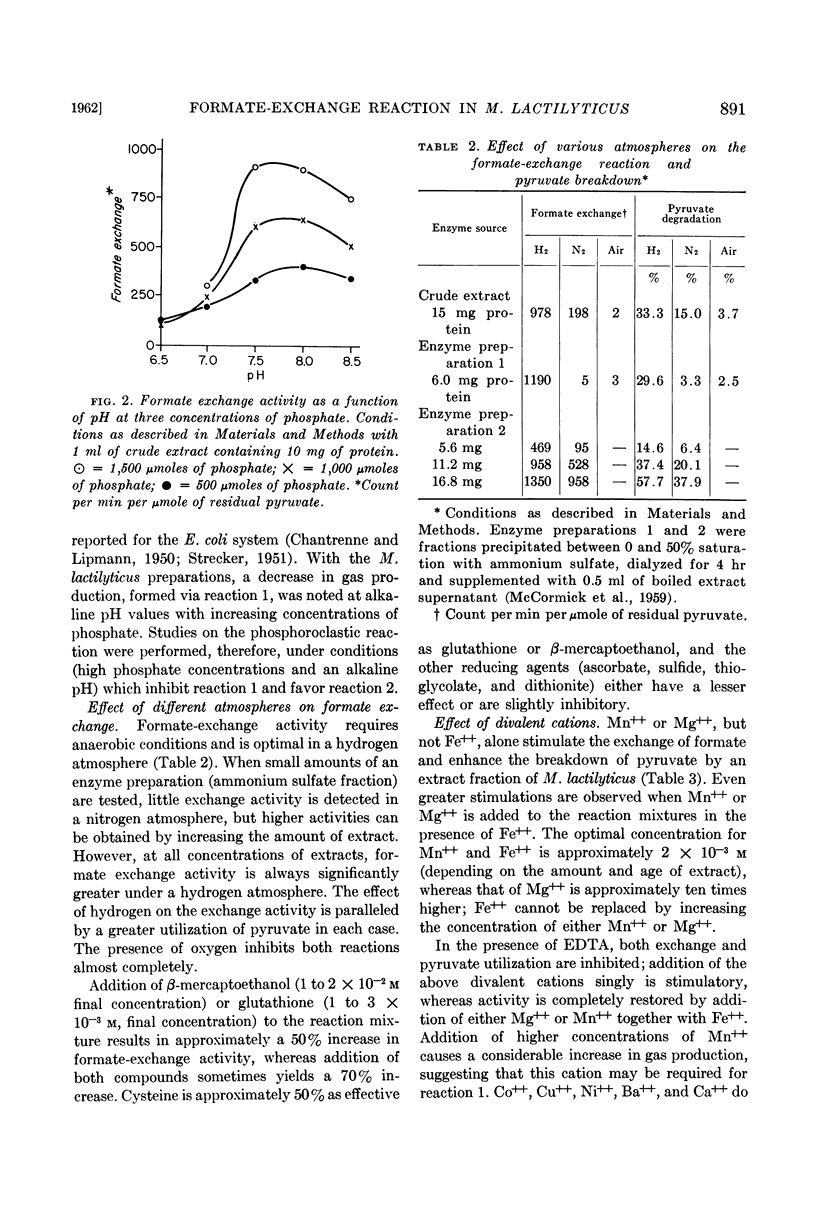
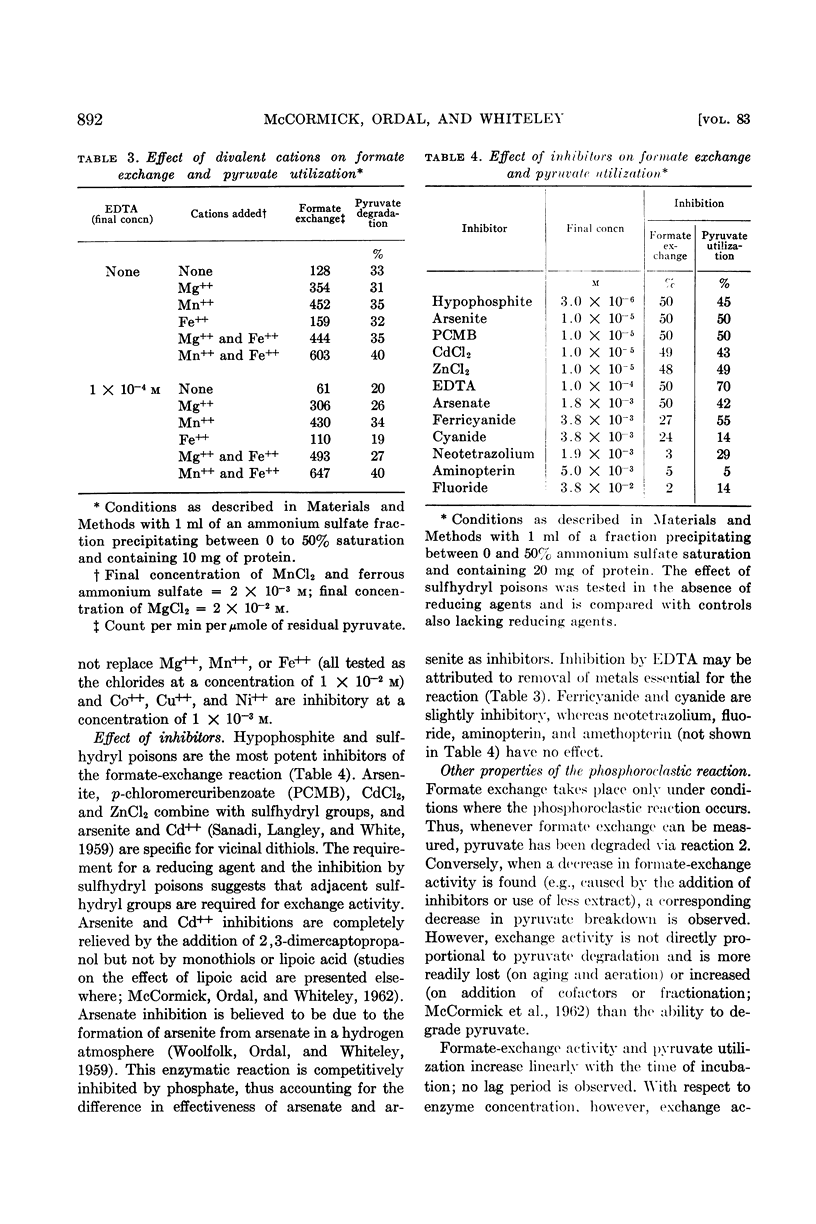
Abstract
McCormick, N. G. (University of Washington, Seattle), E. J. Ordal, and H. R. Whiteley. Degradation of pyruvate by Micrococcus lactilyticus. I. General properties of the formate-exchange reaction (J. Bacteriol. 83:887–898. 1962.—At an alkaline pH, extracts of Micrococcus lactilyticus2 catalyze the phosphoroclastic degradation of pyruvate to formate and acetyl phosphate and the rapid exchange of formate into the carboxyl group of pyruvate. At an acid pH, hydrogen, carbon dioxide, and acetyl phosphate are produced, and carbon dioxide is exchanged into the carboxyl group of pyruvate. A concentration of approximately 1 m phosphate is required for the phosphoroclastic reaction and formate exchange; the production of carbon dioxide and hydrogen is greatly inhibited by high concentrations of phosphate. Formate exchange requires a divalent metal ion and is stimulated by reducing agents and an atmosphere of hydrogen. Inhibition by p-chloromercuribenzoate, Zn++, Cd++, and arsenite indicates that sulfhydryl groups on the enzyme are involved in the reaction; the inhibition by arsenite and Cd++ may be relieved by 2,3-dimercaptopropanol, suggesting that vicinal dithiols may be required. Inhibition by hypophosphite may reflect a competition with formate for a site on the enzyme.
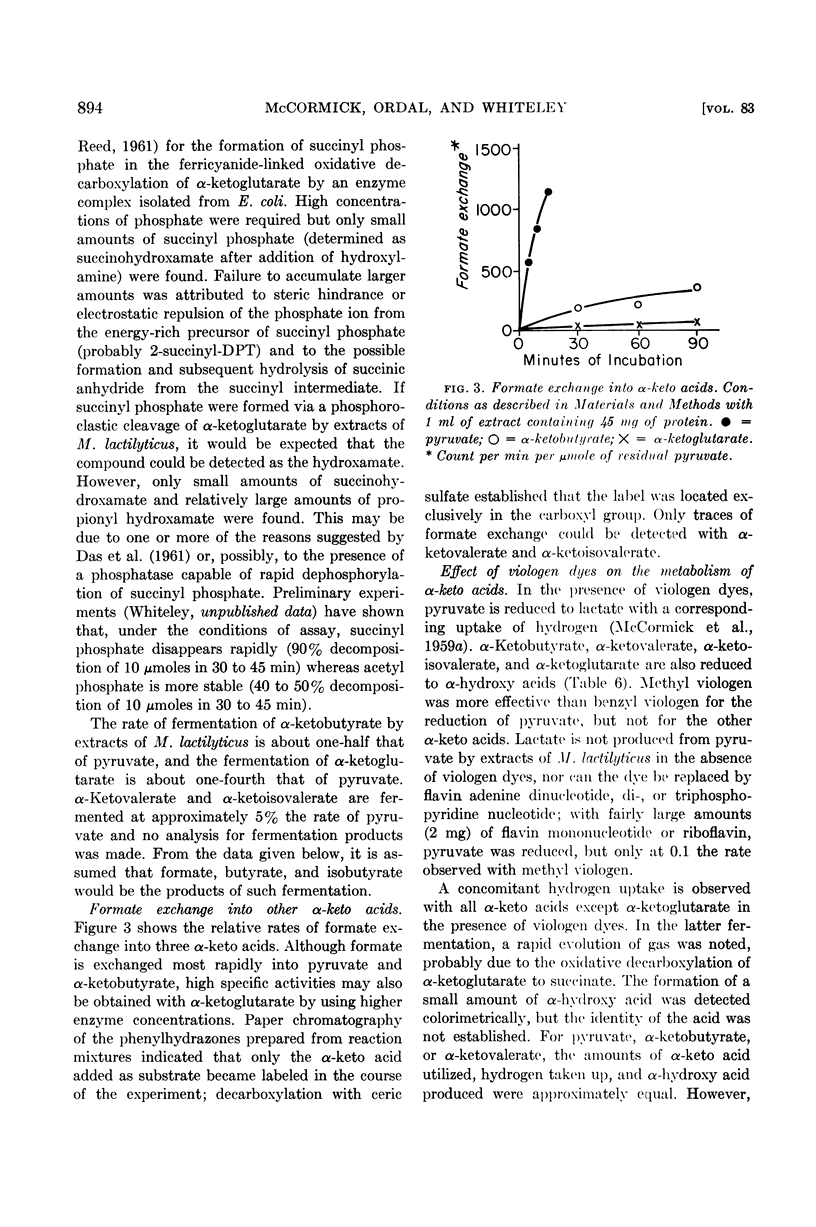
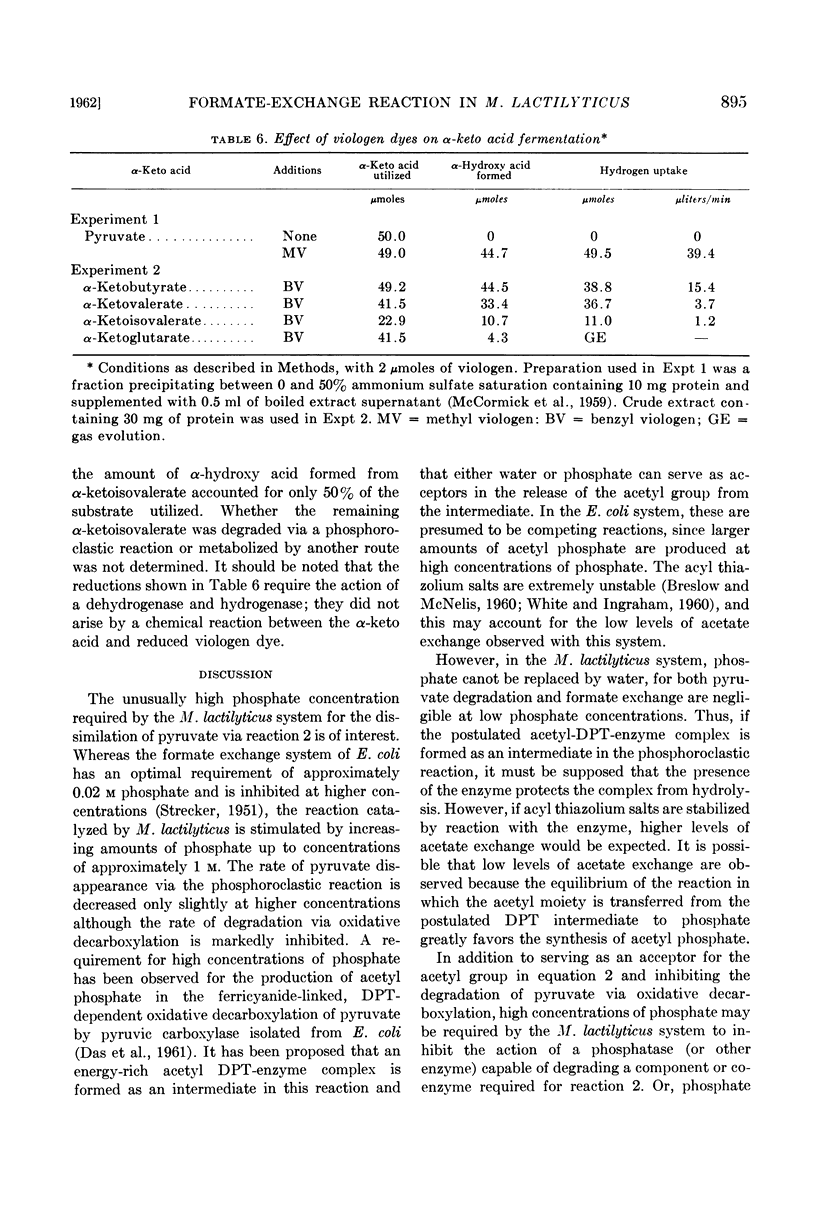
At an alkaline pH, α-ketobutyrate is degraded to propionate and formate, whereas α-ketoglutarate is fermented to succinate, propionate, carbon dioxide, hydrogen, and formate. Formate is exchanged into the carboxyl groups of α-ketobutyrate and α-ketoglutarate under these conditions. Only traces of α-ketovalerate and α-ketoisovalerate are fermented at an alkaline pH and the exchange of formate into these compounds is very low.
The addition of viologen dyes under the conditions used for formate exchange causes a reduction of pyruvate, α-ketobutyrate, α-ketovalerate, and α-ketoisovalerate to the corresponding α-hydroxy acids.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASNIS R. E., FRITZ M., GLICK M. C. Some observations on the phosphoroclastic dissimilation of pyruvate by cell-free extracts of Escherichia coli. Biochim Biophys Acta. 1956 Dec;22(3):578–579. doi: 10.1016/0006-3002(56)90074-9. [DOI] [PubMed] [Google Scholar]
- CHANTRENNE H., LIPMANN F. Coenzyme A dependence and acetyl donor function of the pyruvate-formate exchange system. J Biol Chem. 1950 Dec;187(2):757–767. [PubMed] [Google Scholar]
- DAS M. L., KOIKE M., REED L. J. On the role of thiamine pyrophosphate in oxidative decarboxylation of alpha-keto acids. Proc Natl Acad Sci U S A. 1961 Jun 15;47:753–759. doi: 10.1073/pnas.47.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EATON N. R., KLEIN H. P. Studies on the aerobic degradation of glucose by Saccharomyces cerevisiae. Biochem J. 1957 Nov;67(3):373–381. doi: 10.1042/bj0670373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foubert E. L., Douglas H. C. Studies on the Anaerobic Micrococci: I. Taxonomic Considerations. J Bacteriol. 1948 Jul;56(1):25–34. [PMC free article] [PubMed] [Google Scholar]
- GRUNBERG-MANAGO, SZULMAJSTER J., PROUVOST A. Hydrogenylase, formicodéshydrogénase et hydrogénase chez Escherichia coli. C R Hebd Seances Acad Sci. 1951 Dec 19;233(25):1690–1692. [PubMed] [Google Scholar]
- GUNSALUS I. C. The chemistry and function of the pyruvate oxidation factor (lipoic acid). J Cell Physiol Suppl. 1953 Mar;41(Suppl 1):113–136. doi: 10.1002/jcp.1030410409. [DOI] [PubMed] [Google Scholar]
- JAKOBY W. B. Aldehyde oxidation. II. Evidence for closely juxtaposed sulfhydryl groups on dehydrogenases. J Biol Chem. 1958 May;232(1):89–97. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORTLOCK R. P., VALENTINE R. C., WOLFE R. S. Carbon dioxide activation in the pyruvate clastic system of Clostridium butyricum. J Biol Chem. 1959 Jul;234(7):1653–1656. [PubMed] [Google Scholar]
- McCormick N. G., Ordal E. J., Whiteley H. R. DEGRADATION OF PYRUVATE BY MICROCOCCUS LACTILYTICUS II. : Studies of Cofactors in the Formate-Exchange Reaction. J Bacteriol. 1962 Apr;83(4):899–906. doi: 10.1128/jb.83.4.899-906.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVELLI G. D. The exchange of H14COOH with the carboxyl group of pyruvate by Clostridium butylicum and Micrococcus lactilyticus. Biochim Biophys Acta. 1955 Dec;18(4):594–596. doi: 10.1016/0006-3002(55)90170-0. [DOI] [PubMed] [Google Scholar]
- Nirenberg M. W., Jakoby W. B. ON THE SITES OF ATTACHMENT AND REACTION OF ALDEHYDE DEHYDROGEN ASES. Proc Natl Acad Sci U S A. 1960 Feb;46(2):206–212. doi: 10.1073/pnas.46.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. Formic dehydrogenase and the hydrogenlyase enzyme complex in coli-aerogenes bacteria. J Bacteriol. 1957 Jun;73(6):706–721. doi: 10.1128/jb.73.6.706-721.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEEL J. L. The breakdown of pyruvate by cell-free extracts of the rumen micro-organism LC. Biochem J. 1960 Mar;74:525–541. doi: 10.1042/bj0740525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANADI D. R., LANGLEY M., WHITE F. alpha-Ketoglutaric dehydrogenase. VII. The role of thioctic acid. J Biol Chem. 1959 Jan;234(1):183–187. [PubMed] [Google Scholar]
- SAYRE F. W., ROBERTS E. Preparation and some properties of a phosphateactivated glutaminase from kidneys. J Biol Chem. 1958 Nov;233(5):1128–1134. [PubMed] [Google Scholar]
- STADTMAN E. R., BARKER H. A. Fatty acid synthesis by enzyme preparations of Clostridium kluyveri. VI. Reactions of acyl phosphates. J Biol Chem. 1950 Jun;184(2):769–793. [PubMed] [Google Scholar]
- STRECKER H. J. Formate fixation in pyruvate by Escherichia coli. J Biol Chem. 1951 Apr;189(2):815–830. [PubMed] [Google Scholar]
- WHITELEY H. R., DOUGLAS H. C. The fermentation of purines by Micrococcus lactilyticus. J Bacteriol. 1951 May;61(5):605–616. doi: 10.1128/jb.61.5.605-616.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITELEY H. R., ORDAL E. J. Fermentation of alpha keto acids by Micrococcus aerogenes and Micrococcus lactilyticus. J Bacteriol. 1957 Sep;74(3):331–336. doi: 10.1128/jb.74.3.331-336.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITELEY H. R., OSBORN M. J., HUENNEKENS F. M. Purification and properties of the formate-activating enzyme from Micrococcus aerogenes. J Biol Chem. 1959 Jun;234(6):1538–1543. [PubMed] [Google Scholar]
- WOLFE R. S., O'KANE D. J. Cofactors of the carbon dioxide exchange reaction of Clostridium butyricum. J Biol Chem. 1955 Aug;215(2):637–643. [PubMed] [Google Scholar]
- WOLFE R. S., O'KANE D. J. Cofactors of the phosphoroclastic reaction of Clostridium butyricum. J Biol Chem. 1953 Dec;205(2):755–765. [PubMed] [Google Scholar]
- Wilson J., Krampitz L. O., Werkman C. H. Reversibility of a phosphoroclastic reaction. Biochem J. 1948;42(4):598–600. doi: 10.1042/bj0420598. [DOI] [PMC free article] [PubMed] [Google Scholar]


