Context
ASCO assembled an Expert Panel to develop a new guideline on recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer.1
Thrombosis affects 4% to 20% of patients with cancer, and VTE is a leading cause of their deaths. The risk of VTE increases several-fold for people with cancer and is also associated with several forms of active cancer treatment. Methods of pharmacologic and mechanical prophylaxis are available, but underutilized.
Methodology
A comprehensive systematic review of the medical literature available through December 2006 on prevention and treatment of VTE for patients with cancer was conducted.
Discussion
Multiple randomized trials in a variety of patient populations have been conducted in the last 30 years demonstrating that primary prophylaxis can reduce deep vein thrombosis (DVT), pulmonary embolism (PE), and fatal PE. While a limited number of meta-analyses of the value of anticoagulation for patients with cancer have been conducted, few have reported prespecified outcomes for patients with cancer. Other guidelines provide only limited information on cancer-associated thrombosis. The American College of Chest Physicians (ACCP) guidelines on prevention of VTE recommend prophylaxis for acutely ill hospitalized patients with cancer receiving medical or surgical therapy. (Geerts WH et al: Prevention of venous thromboembolism: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest;126:338S-400S, 2004 [suppl 3]; the ASCO guideline specifically considers issues related to people with cancer and is, therefore, complementary to the ACCP recommendation). Surveys of oncologists, however, show low rates of compliance with thromboprophylaxis, which may be caused by multiple factors. Identifying patients most at risk for VTE and providing effective prophylaxis would significantly impact morbidity, delivery of cancer therapy, cancer-related outcomes, use of health care resources, and, above all, deaths of patients with cancer.
The primary forms of pharmacologic prophylaxis and treatment referred to in this guideline are unfractionated heparin (UFH) and the low-molecular weight heparins (LMWH). Other pharmacologic agents include vitamin K antagonists (warfarin, coumarins) and fondaparinux.
Figure 1 contains algorithms for the prevention and treatment of venous thromboembolism (VTE) for patients with cancer.
Figure 1.
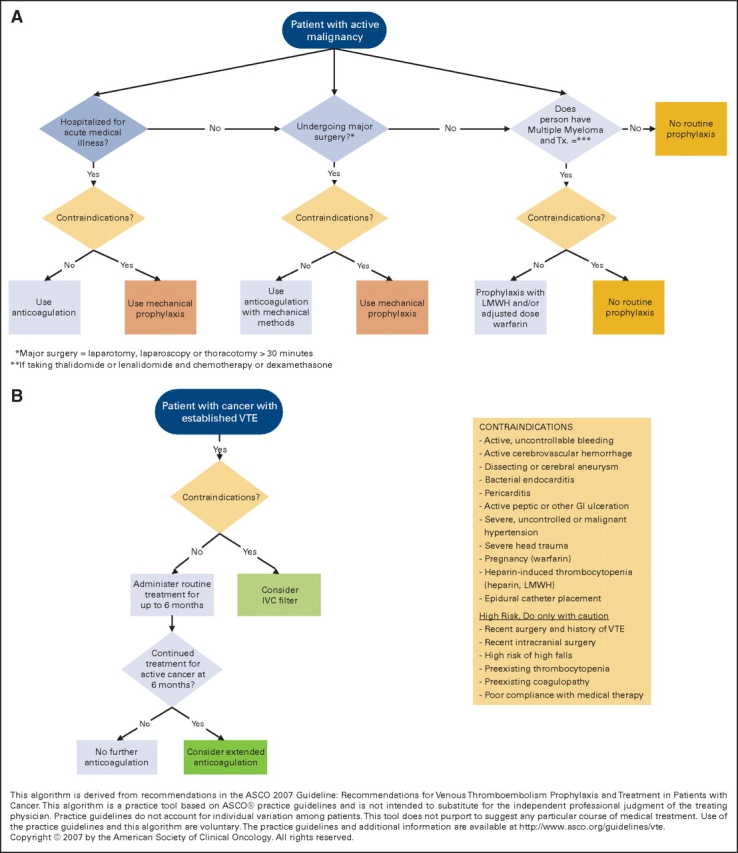
Algorithms for the prevention and treatment of venous thromboembolism for patients with cancer.
1. Should hospitalized patients with cancer receive anticoagulation for VTE prophylaxis?
The reported frequency of VTE in hospitalized patients with cancer has varied widely with reported incidences ranging from 0.6% to 18%, with the most recent studies reporting higher rates per hospitalization. Three double-blind placebo controlled multicenter studies of pharmacologic thromboprophylaxis with either LMWH or fondaparinux in acutely ill hospitalized medical patients, including patients with cancer, have been conducted and each reported a statistically significant reduction in VTE with pharmacologic prophylaxis. Few participants in these studies were patients with cancer, however, and data regarding bleeding complications in the cancer subgroup are not available. Even in the absence of clear treatment data for hospitalized patients with cancer, however, the high risk of VTE and low observed complication rates justify the use of pharmacologic prophylaxis.
2. Should ambulatory patients with cancer receive anticoagulation for VTE prophylaxis during systemic chemotherapy?
There are few data available on the prevention of VTE for patients with cancer who are ambulatory. The guideline does not recommend routine use of prophylaxis for ambulatory patients with cancer because of conflicting trial data, concern over bleeding, the need for laboratory monitoring and dose adjustment, and the relatively low incidence of VTE.
Patients with multiple myeloma taking thalidomide or lenalidomide in combination with chemotherapy or dexamethasone are clearly at higher risk of VTE than the general population of ambulatory patients with cancer but the risk may be decreased with pharmacologic prophylaxis based on data from nonrandomized studies. Therefore, the panel extrapolated from studies of prophylaxis in other groups and a trial of adjusted dose warfarin in breast cancer and recommends using LMWH or adjusted dose warfarin (INR∼1.5) for this ambulatory patient population.
3. Should patients with cancer undergoing surgery receive perioperative VTE prophylaxis?
VTE is a common complication of patients with cancer undergoing surgery both in the immediate postoperative period and after discharge. All patients with cancer undergoing surgery are at high risk, but the need for prophylaxis must be balanced against the risk and consequences of bleeding complications.
Pharmacologic Methods of Prophylaxis
Dosing. Initiate prophylaxis within 24 hours of surgery. Prophylaxis should be continued for at least 7 to 10 days postoperatively. Prolonged prophylaxis for up to 4 weeks may be considered in patients at high risk for VTE after discharge such as those undergoing major abdominal or pelvic surgery for cancer with residual malignant disease after operation, patients with obesity, and those with a previous history of VTE.
Agents. UFH and LMWH are equally effective in reducing DVT. The potential advantages of LMWHs over UFH in cancer surgery prophylaxis include once-daily versus thrice-daily injections and a lower risk of heparin-induced thrombocytopenia. Fondaparinux, a factor Xa inhibitor, was found to be at least as effective as one of the LMWHs. Recent randomized studies suggest that prolonging the duration of prophylaxis up to four weeks is even more effective in reducing postoperative VTE and does not increase bleeding complications.
Mechanical Methods of Prophylaxis
There are three methods of mechanical prophylaxis: intermittent pneumatic compression (IPC), graduated compression stockings, and mechanical foot pumps. They all decrease DVT when used as monotherapies; particularly in gynecological malignancies and intracranial surgery (including brain tumors). IPC has been found to decrease DVT and has other advantages including safety, ease of use, and lower cost than pharmacologic methods. However, data are lacking about its comparative effectiveness regarding PE and mortality.
Combined Methods
In patients at high risk, clinicians may consider combining mechanical and pharmacologic methods, but not using mechanical methods alone. For people with cancer at high risk for IPC failure with one of two additional factors—age older than 60 years and/or prior VTE—the combined approach is appropriate (also recommended by ACCP; Geerts WH et al). Combined methods benefit those receiving neurosurgery. Patients with gynecologic malignancies constitute a high-risk subgroup of patients with cancer receiving surgery and have been specifically considered in clinical trials of both pharmacologic and mechanical thromboprophylaxis.
4. What is the best treatment for patients with cancer with established VTE to prevent recurrence?
There is evidence that LMWH for up to six months is more effective than vitamin K antagonists. The risks of LMWH therapy include bleeding, thrombocytopenia, and osteoporosis. The bleeding risks are comparable to those of using UFH or LMWH followed by an oral vitamin K antagonist.
Anticoagulant therapy is absolutely contraindicated for patients with active bleeding. The insertion of a vena cava filter is only indicated for patients with contraindications to anticoagulant therapy or recurrence while on adequate anticoagulation. Thrombolysis is indicated for selected patients with life-threatening PE and for selected patients with massive or nonresolving ileo-femoral thrombosis.
Treatment with subcutaneous LMWH should be given for at least six months. Consider indefinite treatment for selected patients with active cancer, such as those with metastatic disease or receiving chemotherapy; cancer is a strong continuing risk factor for recurrent VTE. The relative benefits and risks of continuing LMWH beyond six months, versus switching to oral vitamin K antagonist therapy remains a clinical judgment for the individual patient in the absence of clinical trial data.
Treatment with LMWH alone resulted in better outcomes in most trials than combined treatment with UFH or LMWH followed by a vitamin K antagonist. The US Food and Drug Administration recently approved a LMWH, dalteparin sodium, for extended treatment of symptomatic VTE to reduce the risk of recurrence of VTE in patients with cancer (FDA 2007; www.fda.gov/cder/foi/appletter/2007/020287s035ltr.pdf).
For those with recurrent VTE despite adequate anticoagulant therapy or who have a contraindication to anticoagulation, clinicians should consider insertion of a vena cava filter. The vena cava filter may be effective for preventing clinically important PE, but data in a cancer-specific population are lacking. In addition, it carries risks of increasing recurrent leg DVT. The role of removable vena cava filters remainsuncertain due to a lack of randomized controlled trials evaluating their effectiveness and clinical outcomes.
Patients with intracranial tumors are at increased risk for thrombotic complications. Avoid anticoagulation for those with active intracranial bleeding, recent intracranial surgery, or preexisting bleeding diathesis (coagulopathy, thrombocytopenia). Use caution in those at high risk of falls and poor compliance with medical therapy. However, the presence of an intracranial tumor or brain metastases without evidence of active bleeding is not an absolute contraindication to anticoagulation.
Limited data are available regarding the safety and efficacy of antithrombotic therapy for patients with cancer with primary or metastatic tumors of the brain who develop concurrent thrombosis. Dose adjusted UFH and warfarin effectively reduce the risk of VTE without an increase in intracranial bleeding, deaths, or recurrent thromboses. There are very limited data regarding older persons with cancer who frequently have concurrent cancer and thrombosis, as both entities increase with age.
5. Should patients with cancer receive anticoagulants in the absence of established VTE to improve survival?
Some studies have investigated whether administering anticoagulants in the absence of established VTE could improve survival. Inhibiting the hemostatic system could change the biology of cancer and increase survival, independent of the effect on VTE. Two types of studies have explored this question: those with participants with cancer and VTE with survival as a secondary end point and those with participants with cancer without VTE with survival as a primary end point.
Studies in small-cell lung cancer combining warfarin and chemotherapy and LMWH with chemotherapy are encouraging but insufficient for drawing firm conclusions. In addition to small study sample size, these studies are limited by the use of posthoc and subgroup analyses, heterogeneous patient populations, and the multiple treatment strategies employed. These limited data are inadequate to prove or disprove an effect on survival with anticoagulant therapy for patients with cancer and upon which to base a recommendation at this time. Significant effects of vitamin K antagonists and UFH or LMWHs on survival are possible and warrant further study.
Relative Contraindications to Anticoagulation
Relative contraindications to anticoagulation include, among other conditions: active, uncontrollable bleeding; active cerebrovascular hemorrhage; dissecting or cerebral aneurysm; bacterial endocarditis; pericarditis, active peptic or other GI ulceration; severe, uncontrolled or malignant hypertension; severe head trauma, pregnancy (warfarin), heparin-induced thrombocytopenia (heparin, LMWH) and epidural catheter placement.
Additional Resources
ASCO has published the full-text of the guideline online at www.jco.org (doi:10.1200/JCO.2007.14.1283). Additional resources including algorithms, an Orders and Flow Sheet, and slides are available at www.asco.org/guidelines/vte. A Patient Guide on VTE is available at www.plwc.org/patientguides.
It is important to realize that many management questions have not been comprehensively addressed in randomized trials, and guidelines cannot always account for individual variation among patients. A guideline is not intended to supplant physician judgment with respect to particular patients or special clinical situations and cannot be considered inclusive of all proper methods of care or exclusive of other treatments reasonably directed at obtaining the same results.
Accordingly, ASCO considers adherence to this guideline to be voluntary, with ultimate determination regarding its application to be made by the physician in light of each patient's individual circumstances. In addition, the guideline describes administration of therapies in clinical practice; it cannot be assumed to apply to interventions performed in the context of clinical trials, given that clinical studies are designed to test innovative and novel therapies in a disease and setting for which better therapy is needed. Because guideline development involves a review and synthesis of the latest literature, a practice guideline also serves to identify important questions for further research and those settings in which investigational therapy should be considered.
Supplementary Material
Footnotes
The 2007 ASCO Guideline on Recommendations for Venous Thromboembolism Prophylaxis and Treatment in Patients with Cancer was developed and written by Gary H. Lyman, Alok A. Khorana, Anna Falanga, Daniel Clarke-Pearson, Christopher Flowers, Mohammad Jahanzeb, Ajay Kakkar, Nicole M. Kuderer, Mark N. Levine, Howard Liebman, David Mendelson, Gary Raskob, Mark R. Somerfield, Paul Thodiyil, David Trent, and Charles W. Francis.
Reference
- 1.Lyman GH, Khorana AA, Falanga A, et al: American Society of Clinical Oncology guideline: Recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. doi:10.1200/JCO.2007.14.1283 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


