Abstract
Purpose
This article examines the potential use of personal digital assistant (PDA) data capture systems for real-time linear monitoring of health-related quality of life (HRQOL) in prostate cancer research and clinical care.
Methods
We discuss the benefits and potential issues of using PDA data capture in the clinical health care setting. In addition, we describe the development and potential use of a PDA data capture system specific to managing HRQOL in prostate cancer treatment.
Conclusion
Follow-up health care clinics require a practical and systematic process of HRQOL data capture and analysis. Traditional paper questionnaire data capture is problematic. Data manipulation required for clinical decision-making is impractical for patient feedback on same-day clinic visits. Furthermore, the process of transforming paper questionnaire data to analysis-quality data can compromise data integrity. In contrast, research findings confirm the acceptability, ease of use, and reliability of PDAs in capturing data across health care settings, including the collection of serial HRQOL data. The main concern for PDA capture systems is the ability to compare respondent's answers between the paper and PDA questionnaire. Other challenges included patients reporting a lack of computer literacy and/or poor eyesight, as well as initial start-up costs. If issues are successfully addressed, the use of a PDA data capture system, such as the PDA HRQOL system at Princess Margaret Hospital's Prostate Centre, allows for valid and economical data collection with the possibility of linear real-time measurement of changes in HRQOL. Accordingly, there appears to be significant potential for PDA data collection of serial HRQOL in prostate cancer clinic settings.
Introduction
In clinical and research practice linked to prostate cancer treatment, it is essential to frequently monitor a patient's health-related quality of life (HRQOL). Prostate cancer treatment regularly results in physical and emotional morbidity. Common physical adverse effects of treatment include disruption in sexual,1,2 urinary,3 and bowel functions.4 Correspondingly, patients report severe distress associated with these adverse effects.5–7 Combined, the physical and emotional correlates of prostate cancer therapy have a significant impact on patient HRQOL.4,8 For this reason, HRQOL outcomes need to play an important role in determining prostate cancer treatment follow-up care. Likewise, comprehensive outcomes assessment of prostate cancer treatment requires analysis beyond tumor and survival measures to include patient-reported HRQOL.
To monitor patient HRQOL regularly and continually, prostate cancer treatment and follow-up health care clinics require a practical and systematic process of data capture and analysis. The traditional paper questionnaire format for data capture is problematic for both the clinician and researcher. Data manipulation required for clinical decision-making is time-consuming and impractical for patient feedback on same-day clinic visits.9 Furthermore, the process of transforming paper questionnaire data into analysis-quality data can compromise data integrity and the validity of research outcomes.10,11 The limitations of traditional data capture on paper raise the question of whether the use of personal digital assistant (PDA) electronic data collection systems would be a practical alternative. This article outlines the benefits and potential issues of PDA use in the clinical health care setting, and specifically discusses the potential use in prostate cancer research and clinical care.
Background
Clinicians and researchers have used PDA-administered questionnaires for patients across a number of clinical settings and disease types, including orthopedics,12,13 anaesthesiology,14 rheumatoid disease,15 smoking cessation,16 irritable bowel syndrome,17 and allergic rhinitis.18 PDA data collection in these studies has proven to be comparable, or superior to, paper survey methods. Agreement between paper questionnaires and PDA responses is high.14,16 Patients report feeling more comfortable completing a PDA survey and say they prefer it to the paper questionnaire.14,16 Likewise, research specific to HRQOL data collection also supports the use of PDA over paper surveys. Test-retest reliability is reported as similar for both modalities,17 with no significant differences in feasibility, including time needed to complete questionnaires and patient preference.15,17
PDA-administered questionnaires in an oncology setting have not yet been reported in the literature. However, related research involving touch-screen and desktop administration of questionnaires has illustrated acceptability in both oncology inpatient and outpatient environments.19–22 In essence, the research found that oncology patients report little difficulty using computer formats21 and expressed preference for this format.19
Benefits of PDA Data Capture Systems
The benefits of a PDA collection system to both the clinician and researcher are potentially lower costs,10,12 improved data quality through tailored data collection and capture,10,23 and more efficient and effective data manipulation,10,12 including immediate printout of data.24
In health care clinics serving a large volume of patients, the PDA system may be seen as clearly cost efficient when compared with paper questionnaire costs (paper and reproduction), and costs of data entry, coding and cleaning.
The PDA provides for improved data quality by tailoring data collection and capture to meet the requirements of both clinical and research tasks. PDA data collections allow for making complicated branching and skip patterns that remain invisible to interviewees.10,23 The device can be programmed to time stamp data collection,23 to automatically record time to complete questionnaires,10 and to secure data through password protection.25 The questionnaire format can be customized for different font sizes, number of questions per screen, and number of lines allotted to question and response text.10,23 Additionally, PDA use can reduce the frequency of unintentionally missed questions by recalling the missed question at the end of the survey, or by preventing continuation if a question has not been answered. The format of the PDA precludes multiple responses for a single question, and it ensures answers are clearly indicated.
Furthermore, with PDA administration, data can be downloaded directly, thereby eliminating the error-prone step of data entry and potential for bias. This results in faster data manipulation and analysis turnaround times. An explicit advantage of the effective and efficient data manipulation is that results can be immediately scored, displayed, and printed.16 This instant access to outcomes allows the researcher to retrieve and report on up-to-the-minute findings. Similarly, the immediate printout of data enables the clinician to review and interpret a patient profile in the company of the patient and discuss possible treatment decisions.24
Previous research has shown that real-time feedback results in an enhanced clinical interview for both the patient and physician.24,26 One recent study found that oncology patients receiving immediate feedback of HRQOL information reported that their physicians inquired about daily activity and emotional problems more often than without computerized results. The physicians of these patients indicated that HRQOL information improved communication and assisted in disease management decisions.24 This enrichment of the clinical interview appears to have a direct and positive impact on overall clinical outcomes. A recent study demonstrated that cancer patients exhibited clinically meaningful improvements in HRQOL after three sessions of using immediate feedback printouts.27 Furthermore, real-time review of HRQOL information helped physicians identify patients experiencing significant reductions in their quality of life, allowing for rapid assessment of patient health and the promotion of timely intervention.20
Potential Issues Confronting PDA Data Capture Systems
The main concern for PDA capture systems is the ability to compare respondents' answers across paper and PDA administration of various questionnaires. Responses may vary as a result of the direct transfer of an existing paper questionnaire to a PDA administration format.28 There are a number of potential moderators of mode of administration effects. First, the quality of paper and PDA surveys may differ in terms of completion pace and forced sequencing (eg, patients might experience a difference in how easily they can return and change previous responses).28 Second, social desirability may affect comparability. Using the Marlowe-Crowne Social Desirability Scale to compare paper and computerized surveys, studies found lower social desirability scores associated with computerized administration.29–31 Third, comparability of PDA and paper questionnaires may be affected by the nature of the subject matter being assessed. Research has shown that patients may be more comfortable disclosing sensitive and personal information on computerized versus paper surveys.31–33 Finally, mode of administration may also be affected by respondents' beliefs and attitudes. In a trial examining substance abuse, it was found that respondents with a low “general trust in others” had a tendency to report lower substance use on a computer-assisted survey compared with a paper survey.31 Overall, these findings warn that mode effects can exist and that the magnitude of them may vary due to a number of factors, including differences in functional responding patterns, area of study, and respondent characteristics.
Although studies examining the comparability of computerized and paper surveys typically find equivalent scores,15,17,22 it is essential that any response differences be identified. Mode of administration effects may lead to differential responses that do not correspond to the response profiles of the reliability and validity studies defining the psychometric properties of the original paper survey.28 Consequently, the generalizability of computerized survey responses may be compromised. To protect against this limitation, the psychometric properties of PDA administrations of surveys need to be confirmed or established on a measure-by-measure basis.
Other concerns regarding PDA data capture systems center on feasibility issues, including when patients report a lack of computer literacy as a reason for preferring and/or being more comfortable with paper forms over computerized versions.16 There also is concern that in older patient populations participants are more likely to have problems such as poor eyesight and, consequently, have difficulty with a PDA's relatively small display screen.13,22,23 Finally, the initial start-up costs associated with PDA data collections systems can be imposing. This cost may be offset by the practical/functional costs of paper survey collection and the benefits of PDA systems for research and patient care.
Overall, research findings support the several benefits of using PDA devices in collecting questionnaire data from patients, provided that additional validation of the PDA-administered surveys is performed and that feasibility issues are assessed. Accordingly, there appears to be considerable potential for PDA data collection of serial HRQOL in prostate cancer clinic settings.
PDA Data Collection in a Prostate Cancer Clinical and Research Setting
The Prostate Centre at Princess Margaret Hospital receives approximately 200 prostate cancer patient visits per week. The patient population includes high-risk, newly diagnosed, recently treated, and long-term follow-up patients. Currently, patients complete a HRQOL paper questionnaire package at each visit. The questionnaire packages are handed out and collected by the clinic clerk. Following the clinic, a research assistant enters the questionnaire responses into the Prostate Centre database. It takes approximately 3 minutes to enter each questionnaire package, which averages to 2 hours of data entry per clinic day. The data are analyzed aggregately on a semiannual basis to determine quality of care indices for the Prostate Centre. The data are not used for individual patient-physician feedback due to the time-consuming process of data collection, entry, and manipulation. Given that follow-up visits are generally a minimum of 3 months apart, patient-physician feedback and decision-making based on previous visit data collection may be inappropriate. Therefore, for immediate care, physicians include HRQOL questions as part of their clinic visit interview. This lengthens visits and does not allow for standardized procedures/guidelines of physician practice. Consequently, the Prostate Centre developed and is piloting a PDA-administered data collection system to increase efficiency of serial HRQOL data capture and to support a real-time mechanism for individual physician-patient feedback. The following describes the development of a PDA data collection system specific to HRQOL measures in the Prostate Centre.
Prostate Centre PDA HRQOL Data Capture System
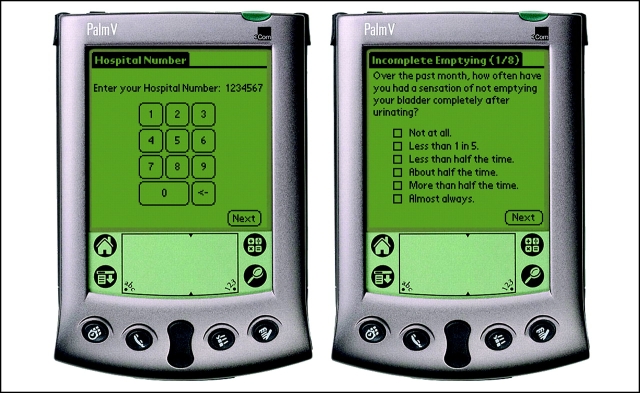
The Prostate Centre PDA HRQOL data capture system software was developed in C++ for the Palm Vx PDA series (Palm Inc, Sunnyvale, California). The software development process incorporated industry-standard quality-assurance activities, including the creation of automated unit test suites, design and code reviews, mockup and review of user interface models, system integration testing, and end-user testing. The software developed for the PDA allows a single participant to enter demographics and survey response information (Fig 1). Participants enter data into the PDA using a touch-screen stylus and are able to select only one response per question, but they have the option of changing answers if an error is made. If the participant fails to respond to a question, this question is repeated at the end of the survey. At this time, the participant has the option to provide a response, or confirm that he would like to skip the question.
Figure 1.
Sample questionnaire on a Prostate Centre PDA.
On completion of the survey, the participant returns the PDA to research staff for data synchronization with a personal computer (PC). During the synchronization, demographics information is checked against that of known patients stored in Microsoft Access (Microsoft Corp, Redmond, Washington), a relational database on the PC. If no demographics match is found, the participant is asked to correct his demographics information on the PDA and resynchronize (survey responses are preserved). Survey data from the PDA is transferred into the database on the PC, and a report is generated for the patient. Synchronization (Palm COM Conduit; Palm Inc) and report generation software are written in Visual Basic (Microsoft Corp).
Patients completing PDA-administered HRQOL questionnaires are identified by medical record number and date of birth. Patient data are protected through the use of multiple levels of encryption and a password access system. Once the PDA has been synchronized with the PC, the patient self-reported data are purged from the unit. These features ensure that sensitive information is only accessible to appropriate clinic staff. All other medical data are stored in the Prostate Centre's main database and are not directly accessible on either the PC or PDA. The Prostate Centre database is safeguarded through industry standard security and operational protections.
The initial development cost of the Prostate Centre PDA HRQOL platform, including Palm hardware and system research and design, was $3,000. The cost associated with implementation, real-time scoring, graphic output, and testing for three HRQOL measures was $1,400 per questionnaire, for a total of $4,200. Finally, the cost of integrating PDA data capture with the Prostate Centre database and troubleshooting was $5,300. Thus, the overall cost of the Prostate Centre HRQOL-PDA system was $12,500. These costs do not include the cost of the PC/laptop hardware and operating system.
Health-Related Quality of Life Measures
The HRQOL measures incorporated into the PDA introduced in the Prostate Centre include the International Prostate Symptom Score (IPSS),34 the Patient Oriented-Prostate Cancer Utility Survey (PORPUS),35 and the International Index of Erectile Function-5 (IIEF-5).36 The IPSS is an eight-item measure of patient urinary voiding function and includes a quality of life score. The PORPUS is a prostate cancer–specific comprehensive instrument for measuring HRQOL. The 10-item psychometric instrument assesses 10 quality of life domains: pain, energy, social support, communication with physician, emotional well-being, urinary frequency, bladder control, sexual function, sexual interest, and bowel problems. The IIEF-5 is an abbreviated version of the International Index of Erectile Function37 and was developed for use as a screening tool in clinical settings to discern men with erectile dysfunction. All measures are reliable and valid.34,38,39
Immediate Printout: Physician-Patient Feedback
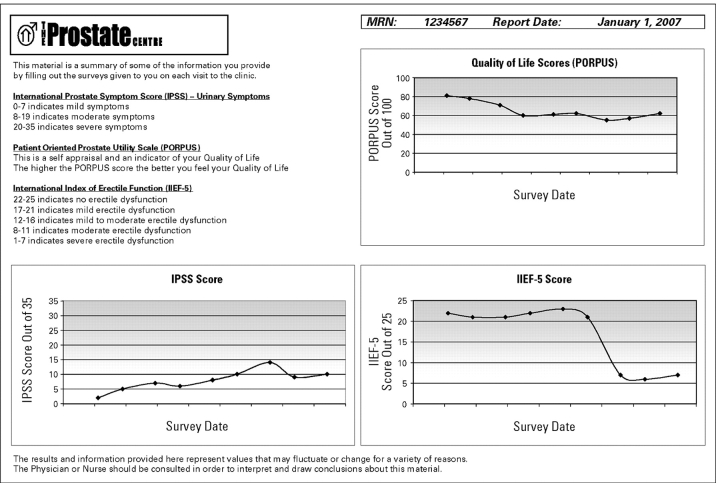
The Prostate Centre PDA HRQOL data capture system was designed to produce an immediate feedback printout directly following the PDA data synchronization with a PC (Fig 2). The printout consists of a brief lay explanation of the IPSS, PORPUS, and IIEF-5, with a summary graph depicting outcome scores over time for each questionnaire.
Figure 2.
Feedback report from the Prostate Centre PDA HRQOL survey.
The Next Step
Before PDA data collection techniques can be fully established for clinical or research purposes in health care, the mode needs to be adequately validated for use with specific measures and patient populations. As part of our piloting of the Prostate Centre PDA HRQOL, we are comparing the use of our Prostate Centre PDA HRQOL data capture system to paper/pencil questionnaires in a randomized control trial. Evaluation will include an assessment of feasibility (participation rates, time to completion, and preference), data quality and validity (completeness of data, response correlation, and internal consistency), and patient satisfaction. The unique relevance of this research is its focus on prostate cancer patients' responses to the PDA data collection system, as well as on the validity and reliability of the IPSS, PORPUS, and the IIEF-5 using the PDA to administer surveys. If this study and others support the use of PDA collection and feedback systems in prostate cancer settings, the potential for a beneficial impact on prostate cancer research and clinical care will be significant.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following authors or their immediate family members indicated a financial interest. No conflict existed for drugs or devices used in a study if they are not being evaluated as part of the investigation.
| Authors | Employment | Leadership | Consultant | Stock | Honoraria | Research Funds | Testimony | Other |
|---|---|---|---|---|---|---|---|---|
| Robin W. Kalnin | Meridian Software Development | Meridian Software Development | The Prostate Centre, Princess Margaret Hospital |
References
- 1.Matthew AG, Goldman A, Trachtenberg J, et al. Sexual dysfunction after radical prostatectomy: Prevalence, treatments, restricted use of treatments and distress. J Urol. 2005;174:2105–2110. doi: 10.1097/01.ju.0000181206.16447.e2. [DOI] [PubMed] [Google Scholar]
- 2.Litwin MS, Flanders SC, Pasta DJ, et al. Sexual function and bother after radical prostatectomy or radiation for prostate cancer: Multivariate quality-of-life analysis from CaPSURE. Urology. 1999;54:503–508. doi: 10.1016/s0090-4295(99)00172-7. [DOI] [PubMed] [Google Scholar]
- 3.Hassouna MM, Heaton JPW. Prostate cancer: 8. Urinary incontinence and erectile dysfunction. CMAJ. 1999;160:78–86. [PMC free article] [PubMed] [Google Scholar]
- 4.Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA. 1995;273:129–135. doi: 10.1001/jama.273.2.129. [DOI] [PubMed] [Google Scholar]
- 5.Cooperberg MR, Koppie TM, Lubeck DP, et al. How potent is potent? Evaluation of sexual function and bother in men who report potency after treatment for prostate cancer: Data from CaPSURE. Urology. 2003;61:190–196. doi: 10.1016/s0090-4295(02)02118-0. [DOI] [PubMed] [Google Scholar]
- 6.Fosså SD, Woehre H, Kurth K-H, et al. Influence of urological morbidity of quality of life in patients with prostate cancer. Eur Urol. 1997;31:3–8. doi: 10.1159/000474553. [DOI] [PubMed] [Google Scholar]
- 7.Helgason ÁR, Adolfsson J, Dickman P, et al. Distress due to unwanted side-effects of prostate cancer treatment is related to impaired well-being (quality of life) Prostate Cancer Prostatic Dis. 1998;1:128–133. doi: 10.1038/sj.pcan.4500226. [DOI] [PubMed] [Google Scholar]
- 8.Clark JA, Rieker P, Propert KJ, et al. Changes in quality of life following treatment for early prostate cancer. Urology. 1999;53:161–168. doi: 10.1016/s0090-4295(98)00457-9. [DOI] [PubMed] [Google Scholar]
- 9.Wilson AS, Kitas GD, Carruthers DM, et al. Computerized information-gathering in specialist rheumatology clinics: An initial evaluation of an electronic version of the Short Form 36. Rheumatology. 2002;41:268–273. doi: 10.1093/rheumatology/41.3.268. [DOI] [PubMed] [Google Scholar]
- 10.Gravlee CC. Mobile computer-assisted personal interviewing with handheld computers: The Entryware System 3.0. Field Methods. 2002;14:322–336. [Google Scholar]
- 11.Cella DF. Methods and problems in measuring quality of life. Supportive Care in Cancer. 1995;3:11–22. doi: 10.1007/BF00343916. [DOI] [PubMed] [Google Scholar]
- 12.Giammattei FP. Implementing a total joint registry using personal digital assistants. Orthop Nurs. 2003;22:284–288. doi: 10.1097/00006416-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Saleh KJ, Radosevich DM, Kassim RA, et al. Comparison of commonly used orthopaedic outcome measures using Palm-top computers and paper surveys. J Orthop Res. 2002;20:1146–1151. doi: 10.1016/S0736-0266(02)00059-1. [DOI] [PubMed] [Google Scholar]
- 14.VanDenKerkhof EG, Goldstein DH, Blaine WC, et al. A Comparison of paper with electronic patient-completed questionnaires in a preoperative clinic. Anesth Anal. 2005;101:1075–1080. doi: 10.1213/01.ane.0000168449.32159.7b. [DOI] [PubMed] [Google Scholar]
- 15.Kvien TK, Mowinckel P, Heiberg T, et al. Performance of health status measures with a pen based personal digital assistant. Ann Rheum Dis. 2005;64:1480–1484. doi: 10.1136/ard.2004.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernhardt JM, Strecher VJ, Bishop KR, et al. Handheld computer-assisted self-interviews: User comfort level and preferences. Am J Health Behav. 2001;25:557–563. doi: 10.5993/ajhb.25.6.5. [DOI] [PubMed] [Google Scholar]
- 17.Bushnell DM, Reilly MC, Galani C, et al. Validation of electronic data capture of the irritable bowel syndrome: Quality of life measure, the work productivity and activity impairment questionnaire for irritable bowel syndrome and the EuroQol. Value Health. 2006;9:98–105. doi: 10.1111/j.1524-4733.2006.00087.x. [DOI] [PubMed] [Google Scholar]
- 18.Koop A, Mösges R. The use of handheld computers in clinical trials. Control Clin Trials. 2002;23:469–480. doi: 10.1016/s0197-2456(02)00224-6. [DOI] [PubMed] [Google Scholar]
- 19.Aiello EJ, Taplin S, Reid R, et al. In a randomized controlled trial, patients preferred electronic data collection of breast and cancer risk-factor information in a mammography setting. J Clin Epidemiol. 2006;59:77–81. doi: 10.1016/j.jclinepi.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Boyes A, Newell S, Girgis A. Rapid assessment of psychosocial well-being: Are computers the way forward in a clinical setting? Qual Life Res. 2002;11:27–35. doi: 10.1023/a:1014407819645. [DOI] [PubMed] [Google Scholar]
- 21.Buxton J, White M, Osoba D. Patients' experiences using a computerized program with a touch-sensitive video monitor for the assessment of health-related quality of life. Qual Life Res. 1998;7:513–519. doi: 10.1023/a:1008826408328. [DOI] [PubMed] [Google Scholar]
- 22.Velikova G, Wright EP, Smith AB, et al. Automated collection of quality-of-life data: A comparison of paper and computer touch-screen questionnaires. J Clin Oncol. 1999;17:998–1007. doi: 10.1200/JCO.1999.17.3.998. [DOI] [PubMed] [Google Scholar]
- 23.Palmblad M, Tiplady B. Electronic diaries and questionnaires: Designing user interfaces that are easy for all patients to use. Qual Life Res. 2004;13:1199–1207. doi: 10.1023/B:QURE.0000037501.92374.e1. [DOI] [PubMed] [Google Scholar]
- 24.Velikova G, Brown JM, Smith AB, et al. Computer-based quality of life questionnaires may contribute to doctor-patient interactions in oncology. Br J Cancer. 2002;86:51–59. doi: 10.1038/sj.bjc.6600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Ubaydli M. Handheld computers. BMJ. 2004;328:1181–1184. doi: 10.1136/bmj.328.7449.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang CH, Cella D, Masters GA, et al. Real-time clinical application of quality-of-life assessment in advanced lung cancer. Clinical Lung Cancer. 2002;4:104–109. doi: 10.3816/clc.2002.n.020. [DOI] [PubMed] [Google Scholar]
- 27.Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: A randomized controlled trial. J Clin Oncol. 2004;22:714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 28.Streiner DL, Norman GR. Toronto, Canada: Oxford University Press; 1995. Health measurement scales: A Practical Guide to Their Development and Use; p. 231. [Google Scholar]
- 29.Martin CL, Nagao DH. Some effects of computerized interviewing on job applicant responses. J Appl Psychol. 1989;74:72–80. [Google Scholar]
- 30.Kiesler S, Sproull LS. Response effects in the electronic eurvey. Public Opin Q. 1986;50:402–413. [Google Scholar]
- 31.Wright DL, Aquilino WS, Supple AJ. A comparison of computer-assisted and paper-and-pencil self-administered questionnaires in a survey on smoking, alcohol, and drug use. Public Opin Q. 1998;62:331–353. [Google Scholar]
- 32.Turner CF, Ku L, Rogers SM, et al. Adolescent sexual behavior, drug use, and violence: Increased reporting with computer survey technology. Science. 1998;280:867–873. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- 33.Tourangeau R, Smith TW. Asking sensitive questions: The impact of data collection mode, question format, and question context. Public Opin Q. 1996;60:275–304. [Google Scholar]
- 34.Barry MJ, Fowler FJJ, O'Leary MP, et al. The American Urological Association Symptom Index for Benign Prostatic Hyperplasia. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 35.Krahn M, Ritvo P, Irvine J, et al. Construction of the patient-oriented prostate utility scale (PORPUS): A multiattribute health state classification system for prostate cancer. J Clin Epidemiol. 2000;53:920–930. doi: 10.1016/s0895-4356(00)00211-0. [DOI] [PubMed] [Google Scholar]
- 36.Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 37.Rosen RC, Riley A, Wagner G, et al. The International Index of Erectile Function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 38.Cappelleri JC, Siegel RL, Glasser DB, et al. Relationship between patient self-assessment of erectile dysfunction and the sexual health inventory for men. Clin Ther. 2001;23:1707–1719. doi: 10.1016/s0149-2918(01)80138-7. [DOI] [PubMed] [Google Scholar]
- 39.Ritvo P, Irvine J, Naglie G, et al. Reliability and validity of the PORPUS, a combined psychometric and utility-based quality-of-life instrument for prostate cancer. J Clin Epidemiol. 2005;58:466–474. doi: 10.1016/j.jclinepi.2004.08.019. [DOI] [PubMed] [Google Scholar]