Abstract
Purpose
To investigate whether recurrence score (RS) as determined using a commercial reference laboratory test influences clinicians' treatment recommendations and eventual treatment in patients with early-stage breast cancer.
Methods
A retrospective analysis was performed on 74 patients from a community-based oncology practice with estrogen receptor (ER) –positive, lymph node (LN) –negative stage I or II breast cancer for which RS was obtained. Demographic and pathology information was extracted from medical records. Ten-year relapse-free survival was calculated using Adjuvant! Online. Treatment recommendations before the RS knowledge were compared with treatment recommendations after RS knowledge and to the treatment eventually administered.
Results and Conclusion
A weak correlation was found between RS and both patient age and tumor size, modest correlation between RS and tumor grade, and modest correlation between RS and 10-year recurrence as determined by Adjuvant! Online. For 21% and 25% of patients, knowledge of the RS changed the clinicians' treatment recommendations and eventual treatment, respectively. The decision to change from hormone therapy to chemotherapy (with or without hormone therapy) was generally associated with high RS (high distant recurrence risk as determined by the commercial reference laboratory test), whereas the decision to change from chemotherapy to hormone therapy was generally associated with low RS (low distant recurrence risk as determined by the commercial reference laboratory test). Knowledge of the RS changed treatment recommendations and eventual treatment in patients with ER-positive/LN-negative early-stage breast cancer. Use of genomic-based prognosis may result in more accurate estimates of true recurrence risk than currently possible with commonly used prognostic factors (such as patient age, tumor size, and tumor grade) alone and thus lead to an increase in appropriate adjuvant therapy decision making.
Introduction
One of the most complex decisions faced by an oncologist involves the use of adjuvant treatment in early-stage breast cancer. In these instances, the oncologist must consider the patient's risk of recurrence, the benefits and toxicity of any proposed therapy, and the patient's input in the decision-making process.
Traditionally, oncologists have used certain risk factors to help gauge the risk of recurrence of early-stage breast cancer. These factors include tumor size, hormone receptor status, and tumor grade.1 The Oncotype DX breast cancer assay (Genomic Health Inc, Redwood City, CA) has been validated as an independent prognostic measure of the risk of recurrence for women with estrogen receptor–positive and lymph node–negative breast cancer.2 By calculating a recurrence score (RS) based on the expression of 21 genes, the Oncotype DX assay gives a quantitative risk of distant recurrence of breast cancer at 10 years when patients are treated with systemic tamoxifen alone. This risk estimate has been shown to be independent of and superior to patient age, tumor size, and tumor grade as a prognostic factor for breast cancer recurrence in this subgroup of women.2
This study was designed to see how the RS influences the adjuvant treatment recommendations in a busy oncology practice. The primary purpose of this study was to investigate whether the availability of the RS changed practice patterns and specifically to explore whether the availability of the RS was associated with a change in adjuvant treatment recommendations originally made on the basis of commonly-used prognostic factors and whether the availability of the RS was associated with a difference in actual treatment received compared with the original recommendations.
The secondary purposes of the study were to assess the correlation of the RS with the patient's age and with tumor size and tumor grade and to assess the correlation of the risk of recurrence predicted by the RS with that predicted by Adjuvant! Online (www.adjuvantonline.com), a popular tool based on the standard measures that are commonly used by oncologists to assess recurrence risk.
Methods
Study Design
Rocky Mountain Cancer Centers is headquartered in Denver, Colorado. It comprises a large, private practice oncology group, part of the US Oncology Network. All four oncologists from that group participated in this study. Seventy-four consecutive breast cancer patients with estrogen receptor–positive, lymph node–negative disease for whom the Oncotype DX assay was ordered were selected for the study. These patients spanned a time period from January 2004 through April 2005. There were no set criteria for ordering the Oncotype DX assay; each oncologist ordered the test when he/she felt it was clinically appropriate. All patients for whom the assay was ordered during that time period were included in this analysis. The assays were ordered through the normal commercial process from the Genomic Health reference laboratory in Redwood City, California. RS was determined by using this assay on tumor tissue from 72 of these patients (two patients [3%] had insufficient tumor to allow RS determination). Demographic information and pathology data were obtained from the medical records for all of these patients. In particular, patient age, tumor size, and tumor grade were noted.
The medical records were also examined to determine the original adjuvant treatment recommendation made before knowledge of the RS and the adjuvant treatment actually received by the patient. The original treatment recommendation had not been recorded for four patients, which left 68 patients for this study.
Relevant clinical information extracted from the medical record was also entered into the Adjuvant! Online program for these 68 patients. This Internet program generates a 10-year risk of disease recurrence (distant and local) based on large databases of information.
Statistical Analysis
Statistical analyses were performed according to a predefined statistical analysis plan. A descriptive analysis was performed to stratify patients according to initial treatment recommendation (before knowledge of the RS), after RS treatment recommendation, and actual treatment administered (hormone therapy alone [HT] or chemotherapy ± HT [CT]). These groups were further analyzed according to predefined RS risk categories (low, < 18; intermediate, 18 to 30; high, ≥ 31).
Analysis of variance was used to evaluate the association between RS and tumor grade. The Tukey-Kramer method for pairwise comparisons was used to test the statistical significance of differences between the mean RS for each tumor grade while maintaining the overall type I error rate at .05.3 Correlation analyses were utilized to determine the association between the RS and patient age, tumor size, and the risk of 10-year distant recurrence as determined by Adjuvant! Online.
The influence of the RS on the final treatment recommendation was investigated by comparing the treatment recommendation made in the absence of the RS to the treatment recommendation after knowledge of the RS. The odds ratio was used to compare the likelihood of a change in the treatment recommendation given a low versus a high RS. The same approach was used to evaluate the influence of the RS on the treatment actually administered. Exact inference methods were used for significance testing of the odds ratio.4
Results
Demographics
The demographic information for 68 patients can be found in Table 1. It is interesting to note that 32 (47%) of 68 patients had tumors ≤ 1 cm in size. Because these patients were drawn from a consecutive series of breast cancer patients, this may reflect the large number of small tumors seen in clinical practice.
Table 1.
Patient Demographics and Baseline Characteristics
| Characteristic | No. | % |
|---|---|---|
| Sex | ||
| Male | 1 | |
| Female | 67 | |
| Age, years | ||
| Median | 54 | |
| Range | 35–77 | |
| Tumor type | ||
| Infiltrating ductal carcinoma | 55 | 81 |
| Infiltrating lobular carcinoma | 4 | 6 |
| Mucinous carcinoma | 4 | 6 |
| Tubular carcinoma | 1 | 1.5 |
| Mixed | 3 | 4 |
| Missing | 1 | 1.5 |
| Tumor size, cm | ||
| Mean | 1.2 | |
| SD | 0.6 | |
| Tumor size distribution, cm | ||
| 0–1 | 32 | 47 |
| 1.1–2.0 | 28 | 41 |
| 2.1–3.0 | 8 | 12 |
| Tumor grade distribution | ||
| I | 30 | 44 |
| II | 24 | 35 |
| III | 14 | 21 |
Table 2 lists the distribution of RS obtained for the 68 patients in this study. This distribution is similar to that obtained in the validation study of Oncotype DX involving 668 patients, where a distribution RS showed that 51% of patients were low risk (RS, < 18), 22% were intermediate risk (RS, 18 to 30), and 27% were high risk (RS, ≥ 31). 2
Table 2.
Recurrence Score Distribution
| Risk Group | No. | % |
|---|---|---|
| Total | ||
| Mean | 22 | |
| Range | 5–68 | |
| Low risk | 32 | 47 |
| Intermediate risk | 22 | 32 |
| High risk | 14 | 21 |
Correlation Between RS and Tumor Grade, Tumor Size, Patient Age, and Risk As Determined by Adjuvant! Online
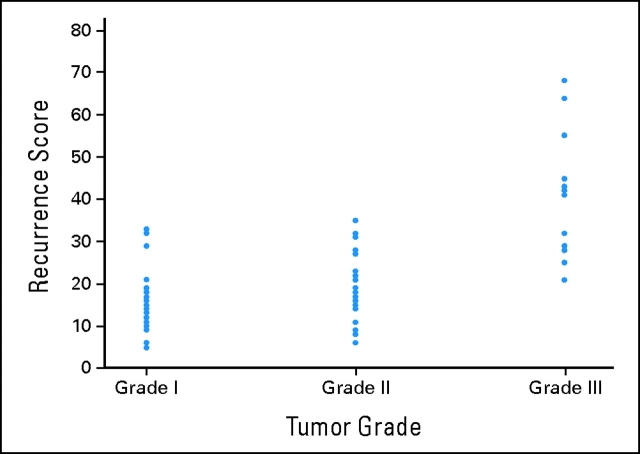
The mean (standard deviation) RSs for grades 1, 2, and 3 tumors were 15.5 (6.8), 19.0 (8.0), and 40.4 (14.3), respectively. A statistically significant difference (95% CI) was found between grade 1 and 3 means (−24.8; −32.0 to −17.9) and between grade 2 and 3 means (−1.4; −28.8 to −14.0) but not between grade 1 and 2 means (−3.4; −9.44 to 2.64). Figure 1 presents the scatterplot of RS by tumor grade.
Figure 1.
Correlation between recurrence score and tumor grade.
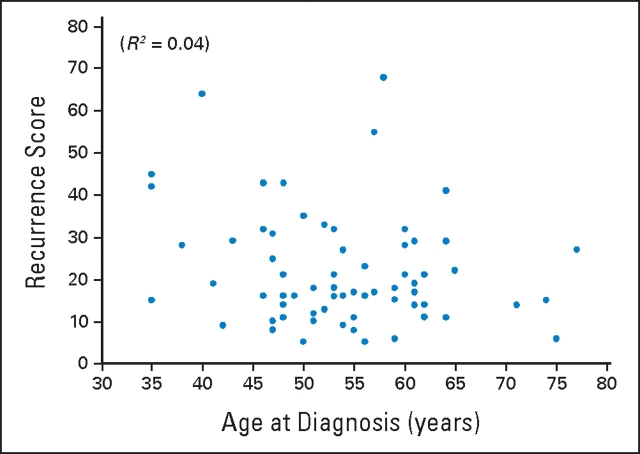
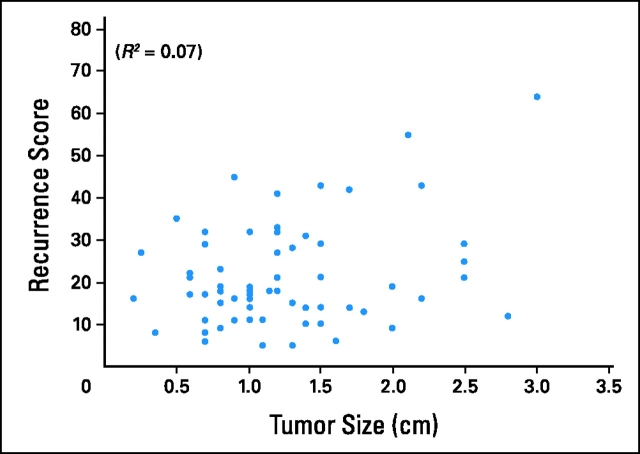
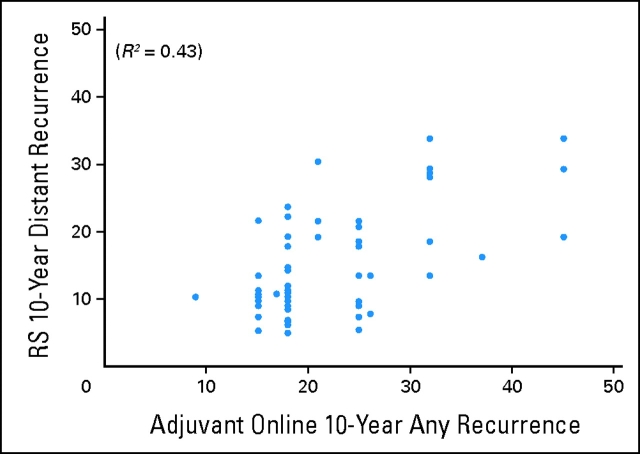
A series of analyses was performed to examine the correlation of the RS generated for these 68 patients with patient age, tumor size, and the risk of 10-year recurrence as determined by Adjuvant! Online. A weak correlation was found between RS and both patient age (Fig 2; R2 = 0.04; 95% CI, 0.00 to 0.17; P < .01) and tumor size (Fig 3; R2 = 0.07; 95% CI, 0.00 to 0.22; P = .03). The correlation between the risk of distant recurrence at 10 years as estimated from the RS and the risk of overall recurrence (distant or local) at 10 years as estimated from Adjuvant! Online was modest (Fig 4; R2 = 0.43; 95% CI, 0.24 to 0.59; P < .01).
Figure 2.
Correlation between recurrence score and patient age.
Figure 3.
Correlation between recurrence score and tumor size.
Figure 4.
Correlation between 10-year distant recurrence as determined by the recurrence score (RS) and 10-year recurrence as determined by Adjuvant! Online.
RS and Treatment
The effect of the RS on adjuvant treatment recommendations is presented in Table 3. Among the 68 patients, the treating oncologist changed the adjuvant treatment recommendation in 14 (20%) after knowledge of the RS became available. In seven (50%) of these 14 patients, the recommendation was changed from HT to CT; in the remaining seven (50%), it was changed from CT to HT. In patients for whom the recommendation was changed from HT to CT, six (83%) of seven had an intermediate or high RS. The odds of the physician treatment recommendation changing from HT to CT were significantly higher (P = .0011) if the RS was high (odds ratio, 4:0) versus low (odds ratio, 1:18). There were no patients for whom an initial recommendation for HT was maintained after knowledge that the RS was high. In patients for whom the recommendation was changed from CT to HT, six (83%) of seven had a low RS. The odds of the physician treatment recommendation changing from CT to HT were significantly higher (P = .0340) if the RS was low (odds ratio, 6:7) versus high (odds ratio, 0:10). For patients with a high RS, there were no patients for whom an initial recommendation for CT changed to a recommendation for HT.
Table 3.
Physician Treatment Recommendations Before and After RS Knowledge
| Treatment Recommendation Before RS Knowledge | Treatment Recommendation After RS Knowledge | RS (No.) | Total | |||
|---|---|---|---|---|---|---|
| Low | Intermediate | High | No. | % | ||
| HT | HT | 18 | 10 | 0 | 28 | 41 |
| HT | CT | 1 | 2 | 4 | 7 | 10 |
| CT | HT | 6 | 1 | 0 | 7 | 10 |
| CT | CT | 7 | 9 | 10 | 26 | 38 |
Abbreviations: RS, recurrence score; HT, hormone therapy; CT, chemotherapy with or without hormone therapy.
Comparison of before RS adjuvant recommendations with actual treatment received are presented in Table 4. Of 68 patients, 17 (25%) received a different adjuvant treatment (HT v CT) from that recommended before knowledge of the RS. For three patients who eventually received CT, the original recommendation was HT. Two (67%) of these three patients had a high RS. The odds of CT treatment, given an initial physician treatment recommendation for HT, were significantly higher (P = .0474) if the RS was high (odds ratio, 2:2) versus low (odds ratio, 0:19). In 14 patients who eventually received HT, the original recommendation was CT. Nine (64%) of these 14 patients had a low RS. The odds of HT treatment, given an initial physician treatment recommendation for CT, were 20.3 times higher (P = .0130) if the RS was low (odds ratio, 9:4) versus high (odds ratio, 1:9).
Table 4.
Physician Treatment Recommendations Before RS Knowledge and Actual Adjuvant Treatment Administered
| Treatment Recommendation Before RS Knowledge | Treatment Administered After RS Knowledge | RS (No.) | Total | |||
|---|---|---|---|---|---|---|
| Low | Intermediate | High | No. | % | ||
| HT | HT | 19 | 11 | 2 | 32 | 47 |
| HT | CT | 0 | 1 | 2 | 3 | 4 |
| CT | HT | 9 | 4 | 1 | 14 | 21 |
| CT | CT | 4 | 6 | 9 | 19 | 28 |
Abbreviations: RS, recurrence score; HT, hormone therapy; CT, chemotherapy with or without hormone therapy.
Discussion
The Oncotype DX assay is the first commercially available genomic test to help clinicians and patients determine the risk of distant recurrence in early-stage breast cancer. The assay is a validated prognostic test that assesses the risk of recurrence of a particular breast cancer based on a genomic expression profile.2,5 Physicians have tried to estimate recurrence risk, which is essential for an adequate and appropriate adjuvant treatment decision, through the use of commonly used factors, such as patient age, hormone receptor status, tumor size, and tumor grade. This approach is problematic, however, given the lack of a uniform weighting of these elements and the poor correlation of tumor grading among various pathologists.6 Adjuvant! Online represents an attempt to objectify a recurrence risk using these variables.
The demographic data regarding these consecutive patients is worthy of comment. These results are similar to those found in the major validation study of Oncotype DX.2 Specifically, almost one half (47%) of the RS values were in the low risk group, compared with slightly more than one half (51%) in the major validation study. The major difference was tumor size distribution. In the main validation study (in which tumor samples were derived from the original National Surgical Adjuvant Breast and Bowel Project B-14 study and were therefore collected more than 15 years ago), 414 (62%) of 668 patients had a tumor ≤ 2.0 cm, whereas in this study, 60 (88%) of 68 patients had a tumor ≤ 2.0 cm. This higher percentage of smaller tumors in this study could reflect a nationwide trend toward smaller tumors at diagnosis, or reflect practice patterns for selecting patients for the test. In addition, the fact that the Oncotype DX assay was ordered in a patient population with tumors ≤ 1 cm is consistent with the fact that the 10-year distant recurrence rate in T1a and T1b tumors is not negligible; not all of these patients do well.7
This retrospective view from a large community practice demonstrates that knowledge of the RS changed both the recommended adjuvant treatment and the actual treatment received compared with the initial adjuvant treatment recommendation made before the RS was available. In 21% of patients (14 of 68), knowledge of the RS changed the physician's treatment recommendation. An important finding is that the direction of the change was consistent with information provided by the RS. The odds of the physician's treatment recommendation changing from HT to CT were significantly higher (estimated as infinitely higher) if the RS was high versus low risk. The odds of the physician's treatment recommendation changing from CT to HT were significantly higher (estimated as infinitely higher) if the RS was low versus high risk. Thus, knowledge of the RS was associated with a statistically significant change in the adjuvant treatment recommendation consistent with the recurrence risk suggested by the RS.
In this study, recommendation changes were equally divided between CT to HT and HT to CT. This could have been due to the small sample size in this study. In larger data sets, approximately 50% of patients had a low RS and 25% of patients had a high RS.2 Hornberger et al8 demonstrated that, given current treatment recommendations and the expected distribution of RS, the change in treatment recommendations after RS knowledge is expected to be more from CT to HT than from HT to CT. It should also be mentioned that each oncologist ordered the Oncotype DX assay per his/her clinical ordering patterns as opposed to a rigid criteria. Thus, the choice of patients for whom the test was ordered could have influenced the distribution of recommendations.
In 25% of patients (17 of 68), knowledge of the RS changed the actual treatment received compared with the pre-RS recommendation. Even though the direction of treatment recommendation change was evenly divided between CT to HT and HT to CT, the great majority of changes in actual treatment (14 of 17 patients; 82%) were from CT to HT and only a few (three of 17 patients; 18%) were from HT to CT. This discrepancy can be at least partially explained by the role of the patient in the decision-making process, which was not specifically examined in this study. For example, it is possible that a physician recommendation to change treatment from CT to HT would be more often accepted by a patient than a recommendation to change treatment from HT to CT. The role of the patient in the decision-making process warrants further study.
In situations where the recommendation or eventual treatment was not changed after RS knowledge was obtained, physicians and patients reported anecdotally that the RS provided increased confidence and reassurance that the treatment plan was appropriate. This result could potentially translate to increased patient compliance with the treatment plan. Future studies looking specifically at the patient component of decision making utilizing the RS are planned.
This study demonstrated only a poor correlation between the RS and both patient age and tumor size and a modest correlation between RS and tumor grade. These results are consistent with the main validation study of the Oncotype DX assay where patient age and tumor size, but not poor tumor grade lost their predictive power of distant recurrence when the multivariate analysis included the RS.2
The value of the Oncotype DX assay is, in part, that it provides an objectively measured risk of recurrence that is independent from the traditional factors. Adjuvant! Online is a practical tool that incorporates these traditional factors to yield a risk of recurrence. The risk of 10-year distant recurrence generated from the RS and the risk of recurrence generated from Adjuvant! Online were only modestly correlated, suggesting that the RS provides information not contained in Adjuvant! Online. Part of this difference may be explained by noting that Adjuvant! Online generates a risk that includes both distant and local recurrence, whereas the risk generated from the RS is only for distant recurrence. However, Bryant et al9 demonstrated that the RS information regarding the risk of distant recurrence is independent from and superior to that provided by Adjuvant! Online.
Recently, Paik et al10 demonstrated that the RS correlates not just with risk of distant recurrence at 10 years but also with chemotherapy benefit. The great majority of benefit from adjuvant chemotherapy in the National Surgical Adjuvant Breast and Bowel Project B-20 study was found in patients with a high RS, whereas those with a low RS had minimal if any benefit from adjuvant chemotherapy.9 Thus, by identifying patients with a high (or low) risk of recurrence, the clinician is also identifying patients with a significant (or minimal) chemotherapy benefit.
In summary, this study demonstrates that knowledge of RS does indeed result in a change in clinical practice, both in treatment recommendations and actual adjuvant therapy received. Compared with the commonly used prognostic factors alone, knowledge of the RS can facilitate better alignment of patient recurrence risk with adjuvant treatment, which is the oncologist's goal in making adjuvant treatment decisions.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following authors or their immediate family members indicated a financial interest. No conflict existed for drugs or devices used in a study if they are not being evaluated as part of investigation.
Table 5.
| Authors | Employment | Leadership | Consultant | Stock | Honoraria | Research Funds | Testimony | Other |
|---|---|---|---|---|---|---|---|---|
| Ruth Oratz | Genomic Health Inc |
References
- 1.Mincey BA, Palmieri FM, Perez EA: Adjuvant therapy for breast cancer: Recommendations for management based on consensus review and recent clinical trials. Oncologist 7:246-250, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Paik S, Shak S, Tang G, et al: A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817-2826, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Kramer CY: Extension of multiple range tests to group means with unequal numbers of replications. Biometrics 12:307-310, 1956 [Google Scholar]
- 4.Mehta CR, Nitin R, Gray R: Computing an exact confidence interval for the common odds ratio in several 2×2 contingency tables. J Am Stat Assoc 80:969-973, 1985 [Google Scholar]
- 5.Habel LA, Shak S, Jacobs MK, et al: A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients: Breast Cancer Res, 2006. http://breast-cancer-research.com/content/8/3/R25 [DOI] [PMC free article] [PubMed]
- 6.Robbins P, Pinder S, de Klerk N, et al: Histological grading of breast carcinomas: A study of interobserver agreement. Hum Pathol 26:873-879, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Hanrahan EO, Valero V, Gonzalez-Angulo A, et al: Prognosis and management of patients with node-negative invasive breast carcinoma that is 1 cm or smaller in size (stage 1; T1a, bN0M0): A review of the literature. J Clin Oncol 224:2113-2122, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Hornberger J, Cosler LE, Lyman GH: Economic analysis of targeting chemotherapy using a 21-Gene RT-PCR assay in lymph-node-negative, estrogen receptor-positive early-stage breast cancer. Am J Manag Care 11:313-324, 2005 [PubMed] [Google Scholar]
- 9.Bryant J: Is the “standard adjuvant regimen” good for all? the 9th International Conference of Primary Therapy of Early Breast Cancer, St Gallen, Switzerland, January 26–29, 2005. Presented at
- 10.Paik S, Tang G, Shak S, et al: Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 224:3726-3734, 2006 [DOI] [PubMed] [Google Scholar]