Fifty years ago, Hubel & Wiesel (1962) proposed their hierarchical model to explain how the intricate response properties of Complex cells in cat visual cortex arose from multiple inputs from less complicated Simple cells. Although this model was perhaps an oversimplification already at the outset, the general concept of the repeated neocortical circuit motif has stood the test of time well.
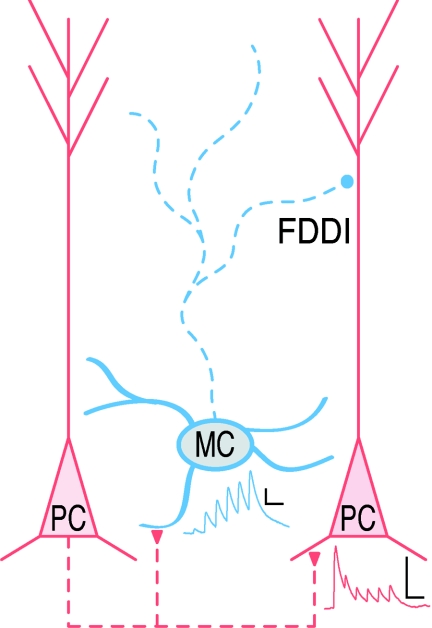
While inhibition has featured prominently in experiments, the interneuronal cell type has been conspicuously absent from many ideas about the repeated cortical circuit module. Maybe the sheer diversity of inhibitory interneuron classes in the neocortex has been too overwhelming. Amidst the disarray of neocortical interneuronal types, however, the Martinotti cell (MC) stands out as one that is relatively well demarcated. This ubiquitous inhibitory cell type, which is found across several neocortical layers, possesses a striking ascending axon that mainly arborizes in the top three layers, where it makes synaptic contacts onto the apical dendrites of pyramidal cells (PCs; Fig. 1). The MC also exhibits a characteristic slowly accommodating firing pattern with spikes triggered at low threshold and it also expresses the peptide somatostatin (Wang et al. 2004).
Figure 1. A ubiquitous circuit motif in neocortex.
In several neocortical regions, around 1 in 7 of nearby PC pairs are connected, but the disynaptic PC–MC–PC motif that mediates FDDI is more than twice as prevalent (Berger et al. 2009). Whereas PC–PC connections are depressing (red trace), the PC–MC pathway is facilitating (blue trace), so PCs must fire at high frequency to drive MCs and generate FDDI (Silberberg & Markram, 2007). Dashed lines: axons; continuous lines: dendrites; scalebars: 1 mV, 50 ms.
In an elegant study, Silberberg & Markram (2007) reported that high-frequency firing in PCs of the somatosensory cortex could readily trigger inhibitory responses in neighbouring PCs by activating intermediate MCs via a strongly facilitating synapse (Fig. 1), a concept here termed frequency-dependent disynaptic inhibition (FDDI). Interestingly, the prevalence of this disynaptic motif was more than twice that of the direct monosynaptic excitatory connection (Fig. 1, Silberberg & Markram, 2007). The omnipresence of this disynaptic motif seems to imply that it is important. But is it omnipresent across brain regions?
In this issue of The Journal of Physiology, Berger et al. (2009) report that this disynaptic motif is indeed widespread. Using multiple simultaneous whole-cell recordings of layer-5 PCs, they examined the prevalence of this disynaptic circuit motif across a wide variety of neocortical areas: somatosensory, motor, auditory, secondary visual, and medial prefrontal cortices. They characterised the connectivity and properties of both monosynaptic and disynaptic connections, and found that the incidence of FDDI among PCs was consistently twice that of monosynaptic excitatory connections. The connectivity rate of FDDI scaled with the monosynaptic connectivity in different areas, with the highest connectivity in auditory cortex, and the lowest in medial prefrontal cortex. The authors also observed a higher than expected level of reciprocal FDDI and monosynaptic connections, especially in somatosensory cortex. Finally, FDDI was use dependent, and rapidly disappeared if PCs were stimulated too often, probably due to fatigue of the PC–MC synapse.
Although FDDI remained stereotyped across regions, Berger et al. (2009) also observed some interesting area-specific differences. The auditory cortex stood out by having the highest FDDI connectivity, the largest amplitude, the fastest rise, and the most rapid decay of FDDI responses. The authors suggest that this may reflect the high temporal specificity requirements of auditory processing.
To examine FDDI, Berger et al. (2009) used a prolonged 70 Hz spike train. Although this activity pattern is a good experimental probe, it does not necessarily reflect the in vivo activity of individual PCs. A recent in vivo study, however, indicates that bursts in PCs activate MCs and thereby hyperpolarize PC apical dendrites via FDDI (Murayama et al. 2009). Burst firing is therefore self-limiting, as it initiates in the distal PC dendrite, but more importantly, MCs can also dynamically reshape the stimulus–response curve of PC dendrites (Murayama et al. 2009). Another study shows that recruitment of MCs, or cells similar to them, increases supralinearly with the number of sparsely active PCs, thus efficiently limiting cortical excitability during more widespread synchronous PC activity (Kapfer et al. 2007). Both reports thus hint that one possible function of FDDI is to keep activity within bounds via negative feedback. But if this is true, then the finding of Berger et al. (2009) that FDDI disappears with increased use seems puzzling: during periods of increased activity, a mechanism that promotes stability should not fatigue but persist or up-regulate.
An additional possible functional role for FDDI is that it serves to gate synaptic plasticity. It has been shown that excitatory inputs onto the distal apical PC dendrite undergo Hebbian long-term potentiation only when the dendrite is in a depolarized state (Sjöström & Häusser, 2006). PCs triggering FDDI would clamp apical dendrites of nearby PCs at hyperpolarized potentials, thus preventing potentiation.
The functionality of FDDI thus remains uncertain and its possible role in plasticity needs to be investigated. It is also unclear if this disynaptic motif becomes more or less prevalent with development and maturity. Regardless, it is clear that FDDI is a new principle and that the disynaptic PC–MC–PC motif is generic and ubiquitous in neocortical microcircuits.
References
- Berger TK, Perin R, Silberberg G, Markram H. J Physiol. 2009;587:5411–5425. doi: 10.1113/jphysiol.2009.176552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Nat Neurosci. 2007;10:743–753. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama M, Perez-Garci E, Nevian T, Bock T, Senn W, Larkum ME. Nature. 2009;457:1137–1141. doi: 10.1038/nature07663. [DOI] [PubMed] [Google Scholar]
- Silberberg G, Markram H. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Häusser M. Neuron. 2006;51:227–238. doi: 10.1016/j.neuron.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. J Physiol. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]