Abstract
In the wake of the obesity pandemic, increased research efforts are under way to define how peripheral hormones and metabolites regulate energy homeostasis. The melanocortin system, comprising anorexigenic proopiomelanocortin (POMC) expressing neurons and orexigenic agouti-related protein (AgRP)/neuropeptide Y (NPY) coexpressing neurons in the arcuate nucleus of the hypothalamus are crucial for normal energy homeostasis both in rodents and humans. They are regulated by peripheral hormones such as leptin and insulin, as well as nutrients such as glucose, amino acids and fatty acids. Although much progress has been made, recent reports continue to underline how restricted our understanding of POMC and AgRP/NPY neuron regulation by these signals is. Importantly, ATP-dependent potassium (KATP) channels are regulated both by ATP (from glucose metabolism) and by leptin and insulin, and directly control electrical excitability of both POMC and AgRP neurons. Thus, this review attempts to offer an integrative overview about how peripheral signals, particularly leptin, insulin and glucose, converge on a molecular level in POMC and AgRP neurons of the arcuate nucleus of the hypothalamus to control energy homeostasis.
POMC and AgRP neurons as regulators of energy homeostasis
Hypothalamic neurons have been implicated in the control of diverse body functions such as stress response, sexual behaviour and energy homeostasis. The arcuate nucleus (Arc), located next to the third ventricle, is privileged among these nuclei due to its close contact with the median eminence, a site characterized by an incomplete blood–brain barrier (BBB). Thus, this location allows neurons to sense acute fluctuations of hormones or other signals in the blood. Accordingly, peripheral injection of hormones induces rapid (<10 min) activation of their signalling cascades in the Arc.
Possibly the most extensively studied neuronal populations in the Arc are the propiomelanocortin (POMC) and agouti-related protein (AgRP)/neuropeptide Y (NPY) expressing neurons. In POMC neurons, the neuropeptide precursor POMC is cleaved to α-melanocyte stimulating hormone (α-MSH), which after secretion activates melanocortin 4 receptors expressed on secondary neuron populations, located in the paraventricular nucleus (PVN) of the hypothalamus, among other nuclei. Thus, membrane depolarization of POMC neurons leads to α-MSH release, MC4R activation and ultimately decreases food intake and increases energy expenditure. However, both the downstream pathways and the nature of the MC4R expressing neurons are only incompletely understood.
On the other hand, neuropeptide Y (NPY) release by AgRP/NPY neurons has an orexigenic effect, mediated by different subtypes of NPY receptors on downstream neurons. Agouti related protein (AgRP) directly blocks α-MSH mediated activation of the MC4R, thus inhibiting α-MSH action. Supporting the critical, antagonizing role of POMC-derived peptides and AgRP, POMC knockout mice and human patients with POMC mutations are obese, and acute ablation of AgRP/NPY neurons in adult mice leads to starvation (Yaswen et al. 1999; Gropp et al. 2005; Luquet et al. 2005).
Many hormones implicated in control of energy homeostasis have been shown to affect either POMC/AgRP mRNA expression or POMC/AgRP neuron excitability, including insulin and leptin (see below). More recently, it has been demonstrated that nutrients, such as fatty acids and glucose, may also play an important role in regulation of POMC and AgRP neuron activity. Importantly, all of these signals share the ability to regulate ATP-dependent potassium (KATP) channels.
Role of KATP channels and AMPK in glucose sensing
During the day, blood glucose concentrations fluctuate depending on physical activity or meal intake. More than 40 years ago it was shown that specific neuronal populations have the ability to sense the dynamics of blood glucose concentrations (Oomura et al. 1964), although the mechanism underlying this effect was not well defined at that time. Glucose is actively transported across the blood–brain barrier and depending on the brain area, differential tightness of the local blood–brain barrier can either enhance or decrease the ability of neurons to sense acute changes in glycaemia. Partially due to their location close to the median eminence, Arc neurons including POMC and AgRP/NPY neurons are well known to respond to changes in ambient glucose concentrations, either as being glucose excited (increase in glucose leads to increase in firing) or glucose inhibited (increase in glucose leads to decrease in firing) (Burdakov et al. 2005; Claret et al. 2007; Parton et al. 2007).
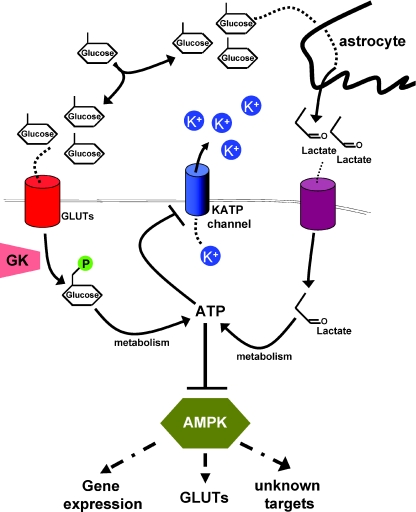
According to the ‘neuronal sensor’ model, glucose is taken up by the neuron via glucose transporters (GLUT), such as GLUT2. Then, glucose is phosphorylated by glucose kinase (GK), and subsequently metabolized to generate ATP. In line with this model, mice with GLUT2 insufficiency show disturbed refeeding behaviour (Bady et al. 2006) and mice heterozygous for GK show hyperphagia and hyperglycaemia (Yang et al. 2007).
On the other hand, the ‘Magistretti’ hypothesis suggests that glucose itself is taken up by astrocytes, metabolized to lactate, which is taken up by neurons and converted to pyruvate, which is then oxidized with production of ATP (Magistretti et al. 1999) (Fig. 1). This idea is backed by the finding that in mice lacking central GLUT2, which suffer from deregulation of glucagon levels, re-expressing GLUT2 in astrocytes restores normal control of glucagon levels (Marty et al. 2005). However, it is conceivable that depending on substrate availability, one or both glucose sensing pathways are used by different neuronal populations.
Figure 1. Direct and indirect models of glucose sensing.
Glucose reaches neurons via cerebrospinal fluid or leaky blood–brain barrier. It is taken up directly by the neuron via a glucose transporter (GLUT), phosphorylated by glucose kinase (GK) and then metabolized to produce ATP, or it is taken up by astrocytes, converted to lactate, and then transported to neurons, where it is again used for ATP generation. ATP binds to and closes KATP channels, which leads to depolarization and an increase in firing. On the other hand, ATP generation decreases the AMP/ATP ratio, which leads to a decrease in AMPK activity. AMPK itself seems to be necessary for glucose sensing (at least in POMC and AgRP neurons), but it is unclear if gene expression, regulation of glucose transporters or other mechanisms contribute to that.
Nonetheless, regardless of substrate use, neuronal metabolism generates ATP which binds to and closes the ATP-dependent potassium (KATP) channels, which leads to reduced potassium outflow, depolarization of the neuron membrane and ultimately to an increase in electrical activity. KATP channels, which consist of a channel pore subunit (KIR 6.1 or 6.2) and a regulatory sulphonylurea (SUR1 or SUR2) subunit, are widely expressed in the brain including POMC and AgRP neurons (Ibrahim et al. 2003; Plum et al. 2006b; Konner et al. 2007). Therefore, if this mechanism is important for normal POMC neuron function, inhibition of ATP sensitivity of KATP channels should impair POMC glucose sensing. Recently, Parton et al. (2007) tested this hypothesis by expressing a mutated KATP channel subunit with strongly reduced ATP sensitivity specifically in POMC neurons. Indeed, glucose sensing of mutant POMC neurons was abolished, as increasing ambient glucose concentrations did not change electrical activity. While glucose tolerance was impaired in these mice, indicating that sensing of high glucose levels of POMC neurons is necessary for maintenance of normal glucose homeostasis, body weight was unchanged between mutant and control mice. Of note, POMC neurons from wild-type mice fed a high fat diet lost the ability to sense glucose, likely due to increased proton leak across the mitochondrial membrane, which leads to reduced ATP generation and thus less KATP channel closure (Parton et al. 2007). Thus, KATP channel regulation provides a relevant mechanism controlling glucose homeostasis, which is altered upon development of obesity.
In the same year, Claret et al. (2007) reported another mutant with glucose insensitive POMC neurons, but here body weight was increased, whereas glucose homeostasis remained unchanged. These mice lacked the AMP-activated protein kinase (AMPK) specifically in POMC neurons. AMPK serves as a sensor of the intracellular AMP/ATP ratio, which is increased when intracellular energy stores are depleted. Thus, AMPK activation occurs, when intracellular energy supply is low (Fig. 1). Mice lacking AMPK in POMC neurons developed obesity caused by decreased energy expenditure, but showed unchanged glucose tolerance (Claret et al. 2007). While a proportion of wild-type POMC cells were hyperpolarized by low glucose levels, this was not observed in POMC neurons lacking AMPK, indicating that at least in POMC neurons, AMPK function is necessary for glucose sensing (Claret et al. 2007). It is currently unknown at the molecular level how AMPK allows POMC neurons to sense glucose and thus alters POMC neuronal excitation. In POMC neurons lacking AMPK, basal KATP function was unchanged, indicating that loss of AMPK did not lead to channel degradation. On the other hand, AMPK signalling has been shown to regulate expression and/or intracellular localization of several GLUT family members in other cell types. Therefore, differential GLUT regulation provides a potential mechanism for AMPK-dependent POMC-neuron regulation, but it is unknown if additionally AMPK signalling can affect KATP channel opening directly or other neuronal AMPK targets involved in ion channel activation. It will be informative whether gene expression changes or direct phosphorylation of target proteins underlies this function of AMPK (Fig. 1). Taken together, both KATP-channel and AMPK regulation provide important mechanisms allowing neurons of critical importance in energy homeostasis to adapt their activity to fuel availability and it will be crucial to understand if/how they interact.
Leptin and insulin as regulators of energy homeostasis
Besides glucose, the Arc neurons are able to sense and integrate information from blood borne hormones such as insulin and leptin. The peptide hormone insulin is acutely secreted by pancreatic β cells depending on blood glucose concentrations, whereas over the long term, it circulates in proportion to body-wide adipose storage. Leptin on the other hand, is secreted directly by adipocytes, and therefore circulating leptin concentrations increase with accumulation of fat. Mice lacking leptin (ob/ob mice) are massively obese and hyperglycaemic, and treatment of ob/ob mice or patients with mutations in the leptin gene with recombinant leptin rapidly induces weight loss and normoglycaemia (Halaas et al. 1995; Farooqi et al. 1999). Nonetheless, most obese patients suffer from a state of so-called leptin resistance, that is, high circulating leptin concentrations cannot decrease energy intake (Considine et al. 1995; Maffei et al. 1995). The cause of this leptin resistance and likely one of the major causes of obesity in human populations is still incompletely understood; multiple mechanisms including reduced transport of leptin across the blood–brain barrier and increased expression of the leptin signalling inhibitor suppressor of cytokine signalling (SOCS) 3 as well as increased circulating concentrations of c-reactive protein (CRP, capable of binding leptin) have been proposed (Bjorbaek et al. 1998; Banks et al. 2004; Chen et al. 2006).
Similar to leptin receptors, also insulin receptors are widely expressed in the CNS (Havrankova et al. 1978). While mice lacking the insulin receptor specifically in the CNS display only mild and sex-specific obesity (Bruning et al. 2000), interestingly obese men are resistant to insulin's anorexigenic effects, suggesting, that both central and peripheral insulin resistance occur in human obesity (Hallschmid et al. 2008). Thus, deciphering the molecular basis of leptin and insulin resistance is a prerequisite for successful development of efficient novel anti-obesity treatments.
Function of STAT3 and PI3K in POMC and AgRP Neurons
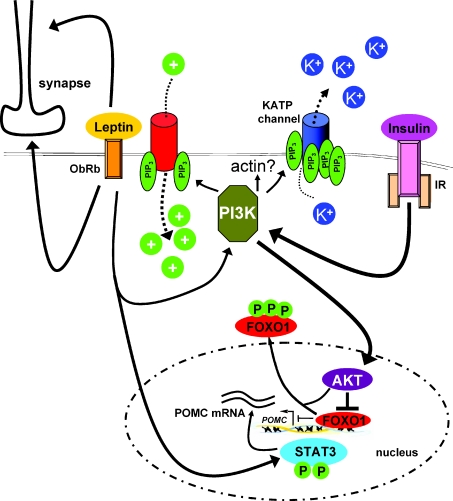
To understand the molecular basis of leptin and insulin resistance, one first has to define the signalling cascades mediating insulin's and leptin's effects in POMC and AgRP neurons. Leptin mediates phosphorylation, activation (homodimerization) and nuclear translocation of the transcription factor STAT3. STAT3 binding to the POMC promoter leads to an increase in POMC mRNA expression by recruitment of histone acetylases, whereas STAT3 in AgRP neurons decreases AgRP (and possibly NPY) expression by recruiting histone deacetylases (Kim et al. 2006; Kitamura et al. 2006). However, mice with ablation of STAT3 specifically in AgRP neurons show increased NPY but normal AgRP expression, whereas mice with overexpression of a constitutively active version of STAT3 in AgRP neurons show increased locomoter activity and leanness, but unchanged neuropeptide expression (Gong et al. 2008; Mesaros et al. 2008). On the other hand, consistent with a role for STAT3 in control of POMC expression, STAT3 deletion in POMC neurons causes a slight decrease in POMC expression and increase in body weight as well as compensatory hyperleptinaemia (Xu et al. 2007). This increase in leptin might mask the full phenotype resulting from the lack of Stat3 signalling in POMC neurons by acting on neuron populations different from POMC neurons, as well as by activating other leptin sensitive signalling pathways, such as phosphatidylinositol 3-kinase (PI3K) signalling within POMC cells. PI3K, when activated, phosphorylates the membrane lipid phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3), whereas several lipid phosphatases, among them the tumour suppressor PTEN (phosphatase and tensin homologue), act to reverse this process. Accumulation of PIP3 leads to recruitment of several kinases to the plasma membrane, which carry on the signalling cascade (Plum et al. 2006a). Interestingly, it had been demonstrated that both insulin's and leptin's acute effects to reduce food intake and body weight could be inhibited by central preadministration of a PI3K inhibitor (Niswender et al. 2001, 2003).
PI3K mediated phosphorylation of FOXO1 and thus nuclear exclusion of FOXO1 has been shown to cooperatively increase POMC mRNA expression (Kitamura et al. 2006; Belgardt et al. 2008) (Fig. 2). Indeed, leptin induces PI3K activation in both POMC and NPY/AgRP neurons, and leptin stimulated PI3K signalling seems to decrease NPY and AgRP mRNA expression (Morrison et al. 2005), although it is possible that leptin activates PI3K signalling not in AgRP/NPY neurons itself, but through synaptic transmission (Xu et al. 2005). Moreover, the acute effects of insulin and leptin on AgRP neurons differ. Insulin either hyperpolarizes (Konner et al. 2007) or depolarizes (Claret et al. 2007) AgRP neurons. While insulin activates PI3K signalling in AgRP neurons, leptin has the opposite effect (Xu et al. 2005). Leptin either hyperpolarizes AgRP neurons (van den Top et al. 2004) or has no effect (Claret et al. 2007). To further clarify the role of PI3K signalling in POMC neurons, the Elmquist group has recently generated mice with POMC specific deletion of the regulatory PI3K subunits. Interestingly, the acute effect of centrally applied leptin on food intake was severely blunted in these mice (Hill et al. 2008). Unexpectedly, POMC specific PI3K inhibition did not alter body weight, glucose homeostasis and neuropeptide expression compared to control mice (Hill et al. 2008). However, in electrophysiology studies leptin could not decrease the membrane potential nor induce firing of POMC neurons anymore, indicating that leptin engages PI3K signalling to control neuronal firing, at least in POMC neurons (Hill et al. 2008). On the other hand, it had been previously shown that leptin activates the PI3K pathway to hyperpolarize unidentified hypothalamic neurons by opening ATP-dependent potassium (KATP) channels in the same manner as insulin (Spanswick et al. 1997; Spanswick et al. 2000; Mirshamsi et al. 2004; Irani et al. 2008). Moreover, we and others have shown that insulin stimulates PI3K signalling in POMC and AgRP neurons, resulting in a KATP channel-dependent hyperpolarization and electrical silencing (Plum et al. 2006b; Konner et al. 2007; Belgardt et al. 2008; Hill et al. 2008). Insulin's ability to hyperpolarize POMC neurons has been shown to be dependent on PI3K signalling and KATP channel activation (Plum et al. 2006b). Thus, insulin and leptin activate the same signalling cascade (PI3K) either leading to membrane depolarization and an increase in firing rate (leptin) or membrane hyperpolarization and electrical silencing (insulin). At this time, it is unknown how activation of the same cascade leads to these contrasting functional outcomes. One possibility is that differences in activation magnitude and/or kinetics underlie this phenomenon, that is, that a certain threshold of PIP3 accumulation in the plasma membrane opens cation channels leading to depolarization, whereas more PIP3 or possibly longer exposure of KATP channels to PIP3 leads to opening of KATP channels and subsequent hyperpolarization (Fig. 2).
Figure 2. The role of PI3K and KATP channels in leptin and insulin signalling.
Leptin and insulin both activate PI3K to induce either depolarization (leptin) or hyperpolarization (insulin). It is unclear how activation of the same signalling cascade leads to the two contrary outcomes, but subtle differences in activation strength and/or duration may contribute. Moreover, regulation of actin filaments may be necessary for KATP channel activation. PI3K activation also induces nuclear export of FOXO1, and thus abrogates FOXO1's inhibition of POMC expression. STAT3 activation induced by leptin is the driving force behind POMC expression, and competes with FOXO1 to bind the POMC promoter. At the same time, leptin, either by cell autonomous or presynaptic mechanisms, controls synaptic input onto POMC neurons. AKT, protein kinase B; PI3K, phosphatidylinositol 3-kinase; IR, insulin receptor; ObRb, long (signalling) isoform of the leptin receptor; PIP3, phosphatidylinositol-3,4,5-trisphosphate.
Still, differences in the signalling cascades of insulin and leptin, such as reorganization of actin filaments, might also explain the different outcomes of PI3K activation. It has been demonstrated that actin filament stabilization prevents KATP channel opening (Mirshamsi et al. 2004). Moreover, in hypothalamic cell lines, leptin can raise PIP3 levels by phosphorylation and subsequent inhibition of PTEN, leading to PIP3 accumulation without classical PI3K activation (Ning et al. 2006), although in other cell types the ability of leptin to influence actin filaments has been shown to depend on classical PI3K activation (Zeidan et al. 2007). Similarly, insulin-dependent KATP channel opening can also be blocked by actin filament stabilization, yet PDK1 and thus AKT activation/downstream signalling is not necessary for insulin mediated KATP channel opening at least in POMC neurons (Mirshamsi et al. 2004; Belgardt et al. 2008). Thus, identifying the PI3K-dependent regulators of actin reorganization is expected to further define PI3K mediated KATP channel regulation.
Further expanding this model, leptin and insulin may modulate POMC neuron firing by presynaptic action on AgRP neurons, which produce the inhibitory neurotransmitter GABA. GABAergic synapses on POMC neurons had been noted previously, which opened up the possibility that AgRP neurons inhibit POMC neuron firing by direct synaptic inhibition. Indeed, mice whose AgRP neurons are unable to release GABA show decreased inhibitory input on POMC neurons (Tong et al. 2008). Thus, hormonal regulation of POMC neuron activity critically involves both cell autonomous and presynaptic effects.
It is likely, that some of these discordant findings are due to different conditions of electrophysiological studies. In patch clamps studies, investigators use glucose concentrations between 2 and 11 mm in their bath solutions nurturing the brain slices. Problematically, the ‘real’ glucose concentration seen by POMC or AgRP neurons in vivo is unknown, because they may not only sense glucose fluctuations in the intracerebrospinal fluid (via the 3rd ventricle), but also by extending fibres into the median eminence (Kiss et al. 1985). Also, most groups use the so-called whole cell patch clamp technique, in which the recording pipette (which is prefilled with solution supposed to emulate actual neuron ATP/GTP concentrations) punctures the target neuron. Use of this technique can lead to shutdown of neuronal functions in a relatively short time likely to be due to wash out of the existing intracellular ions. More advanced, the perforated patch technique uses a small pore opener such as nystatin in the patch pipette to create smaller perforations, reducing dilution of neuronal cytoplasm. Indeed, in ventromedial neurons, whole cell recordings induce neuron hyperpolarization in a matter of minutes, whereas perforated patch recordings show normal membrane potential and firing for several hours (our unpublished observations). It has also been suggested that a cytosolic regulator of KATP channels exists, which modulates KATP channel sensitivity (Teramoto et al. 2006). Dilution of this unknown factor would affect KATP channel function and thus distort the recording data. Lastly, brain slices of young mice (<3 weeks old) are usually used for recordings because denseness of hypothalamic tissue increases over time, which complicates efficient recording. There are major changes in the melanocortin circuits already in the first three postnatal weeks, leading to the question how the melanocortin system changes afterwards (Melnick et al. 2007). Further improvements in patching techniques would thus help to carefully investigate neuronal networks in adult mice.
Rapid synaptic rewiring of arcuate nucleus neurons
Results obtained by electron microscopy have shed light on the time course of how fast hormones such as leptin regulate neuronal circuits. Mice lacking leptin have an increase in excitatory synapses and a decrease in inhibitory synapse on NPY neurons, which is rapidly, that is in hours, normalized by leptin administration. Conversely, mice lacking leptin show a decrease in excitatory synapses on POMC neurons and leptin treatment increases excitatory input (Pinto et al. 2004). The notion that hormones can rapidly rearrange synapses on neurons important for energy expenditure was further worked out by the same laboratory, when it was shown that oestrogen, known to be anorexigenic, increases the number of excitatory inputs on POMC neurons in a STAT3-dependent pathway, whereas ghrelin, known to be orexigenic, decreases excitatory input onto POMC neurons (Pinto et al. 2004; Gao et al. 2007). It is unknown if all hormones implicated in energy homeostasis and, quite possibly, fuel/metabolites such as fatty acids or glucose can influence synaptic wiring. Furthermore, it would be highly interesting to know which signalling pathways, and especially which signalling molecules in the end attract or deflect synapses. It is also unknown if pre- or postsynaptic hormonal action leads to synaptic rewiring, that is if neurons actually attract the synapses, or synapses are ‘sent’ to get in contact with POMC neurons, for example. Moreover, chronic hyperactivation of the PI3K signalling pathway in POMC neurons leads to an increase of inhibitory synaptic input onto them (Plum et al. 2006b). Additionally, insulin stimulation increases the amount of GABAA receptor in neuron membranes in vitro, still, at this time, it is unknown if acute insulin stimulation has an effect on rewiring in the same way as leptin on POMC or AgRP neurons (Wan et al. 1997). Moreover, to our knowledge, it has not been tested if nutrients, such as glucose or fatty acids, can induce synaptic change in POMC or AgRP neurons.
Central PI3K regulates adipose tissue function
Analysis of central PI3K signalling in energy homeostasis is hampered by the fact that deletion of PTEN, the main negative regulator of PI3K signalling, leads to embryonic death, whereas the generation of mice with central deletion of all PI3K subunits is tedious at best. Thus, several groups have tried to further define the effects of central activation of PI3K compared to investigating PI3K activation in discrete neuron populations using chemical PI3K inhibitors such as wortmannin. It seems that central PI3K signalling plays a role in regulation of adipose tissue mass and energy expenditure. Increasing PI3K activity in all cells expressing the long form of the leptin receptor (only the long form will upon leptin binding activate the signal cascade intracellularly) induces changes in white adipose tissue (WAT) energy expenditure and leanness in mice (Plum et al. 2007), whereas in rats, central leptin action depends on intact PI3K signalling to inhibit lipogenesis in WAT (Buettner et al. 2008). Interestingly, chronic low dose intracerebroventricular insulin (with no effect on food intake) increases lipogenesis and WAT mass in mice (Koch et al. 2008). Although the findings in rats and mice appear dissimilar, which might be due to species differences, sympathetic nervous system activation is thought to underlie this brain → WAT pathway.
Outlook
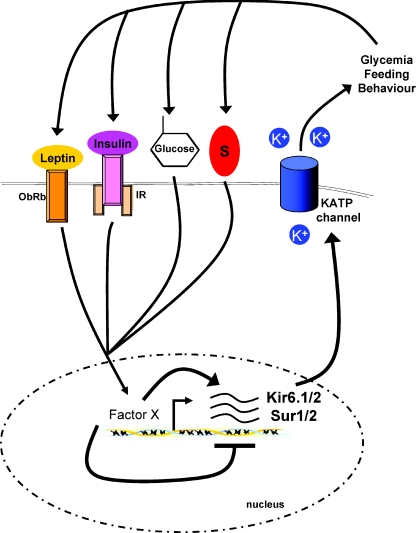
It is now clear that it is likely that all metabolites such as glucose, amino acids and fatty acids as well as a plethora of peripheral hormones act as messengers to regulate energy homeostasis, and importantly, it is also clear that these mechanisms meant to decrease energy intake in times of energy surplus, ultimately fail in obese patients. i.c.v. infusion of oleic acid (a fatty acid) fails to inhibit feeding in rats fed a high-fat diet (HFD) for only 3 days (Morgan et al. 2004). Glucose sensing is diminished in HFD fed animals, and leptin resistance develops in POMC neurons after 6 days of HFD (Munzberg et al. 2004). Analysis of these pathomechanisms is hampered by the fact that our understanding of even basal functions of those neuron populations is fragmentary. A subtle change in ambient glucose concentration or temperature for example can potently affect findings in electrophysiological studies. Thus, it seems necessary to repeat experiments at multiple glucose concentrations, to gain insight into the way the neuron reacts to the spectrum of conditions. Moreover, it is clear now that there are subpopulations in these neuron populations, and by lack of genetic markers for these sub-groups, we cannot distinguish between them. For example, only ∼50% of POMC neurons react to insulin with hyperpolarization. It is absolutely unknown why insulin sensitivity (at least at the electrophysiological level) is unnecessary for the rest. Most interestingly, it appears that mRNA expression of KATP channel subunits can be regulated by glucose and hormones such as sex steroids (Acosta-Martinez & Levine, 2007; Huang et al. 2008). Contemplating this, glucose (and hormone) levels might regulate KATP channel expression in key hypothalamic neurons, which sense glucose levels, and thus by affecting peripheral glucose production regulate peripheral glucose levels, which then regulate hormone release (Fig. 3). Taken together, cell specific analysis of gene expression, ion channel regulation and synaptic plasticity along with standardized experimental methods are necessary to understand not only POMC and AgRP neurons, but overall central regulation of energy homeostasis.
Figure 3. Hypothetical regulation of KATP channel subunit expression by hormones and nutrients.
KATP channel subunits KIR6.1/6.2 and SUR1/2 have been shown to be regulated by glycaemia and hormones such as oestradiol and progesterone. Changes in subunit expression may lead to differences in neuronal response towards glucose, which ultimately affects peripheral glucose production, which again either reinforces or diminishes KATP channel subunit expression. Besides neuropeptide expression, acute adjustment of ion channel activity and synaptic plasticity, regulation of subunit expression might be essential for central homeostatic control. The factors crucially involved in the KIR/SUR expression are not understood and thus stated as ‘Factor X’. S, steroids.
Acknowledgments
We apologize to all colleagues whose important contribution could not be cited due to space limitations. We thank G. Schmall for excellent secretarial assistance and all members of the Brüning and the Kloppenburg lab for helpful discussion of the manuscript. This work was supported by grants from the CMMC (TV2) and the EU (LSHM-CT-2003-503041) to J.C.B., the Fritz Thyssen Stiftung (Az.10.04.1.153/Az. 10.06.2.175) to J.C.B., the EFSD/Lilly European Diabetes Research Programme to J.C.B., and the DFG (Br. 1492/7-1) to J.C.B..
References
- Acosta-Martinez M, Levine JE. Regulation of KATP channel subunit gene expression by hyperglycemia in the mediobasal hypothalamus of female rats. Am J Physiol Endocrinol Metab. 2007;292:E1801–1807. doi: 10.1152/ajpendo.00700.2006. [DOI] [PubMed] [Google Scholar]
- Bady I, Marty N, Dallaporta M, Emery M, Gyger J, Tarussio D, Foretz M, Thorens B. Evidence from glut2-null mice that glucose is a critical physiological regulator of feeding. Diabetes. 2006;55:988–995. doi: 10.2337/diabetes.55.04.06.db05-1386. [DOI] [PubMed] [Google Scholar]
- Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B, Klockener T, Alessi D, Kloppenburg P, Bruning JC. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, Schwartz GJ, Kunos G, Rossetti L, Buettner C. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc Lond B Biol Sci. 2005;360:2227–2235. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Li F, Li J, Cai H, Strom S, Bisello A, Kelley DE, Friedman-Einat M, Skibinski GA, McCrory MA, Szalai AJ, Zhao AZ. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat Med. 2006;12:425–432. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine RV, Considine EL, Williams CJ, Nyce MR, Magosin SA, Bauer TL, Rosato EL, Colberg J, Caro JF. Evidence against either a premature stop codon or the absence of obese gene mRNA in human obesity. J Clin Invest. 1995;95:2986–2988. doi: 10.1172/JCI118007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signalling in obese animals. Nat Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- Gong L, Yao F, Hockman K, Heng HH, Morton GJ, Takeda K, Akira S, Low MJ, Rubinstein M, MacKenzie RG. Signal transducer and activator of transcription-3 is required in hypothalamic agouti-related protein/neuropeptide Y neurons for normal energy homeostasis. Endocrinology. 2008;149:3346–3354. doi: 10.1210/en.2007-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Bruning JC. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Hallschmid M, Benedict C, Schultes B, Born J, Kern W. Obese men respond to cognitive but not to catabolic brain insulin signalling. Int J Obes (Lond) 2008;32:275–282. doi: 10.1038/sj.ijo.0803722. [DOI] [PubMed] [Google Scholar]
- Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signalling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Acosta-Martinez M, Levine JE. Ovarian steroids stimulate adenosine triphosphate-sensitive potassium (KATP) channel subunit gene expression and confer responsiveness of the gonadotropin-releasing hormone pulse generator to KATP channel modulation. Endocrinology. 2008;149:2423–2432. doi: 10.1210/en.2007-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express KATP channels. Endocrinology. 2003;144:1331–1340. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- Irani BG, Le Foll C, Dunn-Meynell A, Levin BE. Effects of leptin on rat ventromedial hypothalamic neurons. Endocrinology. 2008;149:5146–5154. doi: 10.1210/en.2008-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, Kim YB, Lee KU. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9:901–906. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Mezey E, Cassell MD, Williams TH, Mueller GP, O’Donohue TL, Palkovits M. Topographical distribution of pro-opiomelanocortin-derived peptides (ACTH/β-END/α-MSH) in the rat median eminence. Brain Res. 1985;329:169–176. doi: 10.1016/0006-8993(85)90522-0. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- Koch L, Wunderlich FT, Seibler J, Konner AC, Hampel B, Irlenbusch S, Brabant G, Kahn CR, Schwenk F, Bruning JC. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest. 2008;118:2132–2147. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Marty N, Dallaporta M, Foretz M, Emery M, Tarussio D, Bady I, Binnert C, Beermann F, Thorens B. Regulation of glucagon secretion by glucose transporter type 2 (glut2) and astrocyte-dependent glucose sensors. J Clin Invest. 2005;115:3545–3553. doi: 10.1172/JCI26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick I, Pronchuk N, Cowley MA, Grove KL, Colmers WF. Developmental switch in neuropeptide Y and melanocortin effects in the paraventricular nucleus of the hypothalamus. Neuron. 2007;56:1103–1115. doi: 10.1016/j.neuron.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Mesaros A, Koralov SB, Rother E, Wunderlich FT, Ernst MB, Barsh GS, Rajewsky K, Bruning JC. Activation of Stat3 signalling in AgRP neurons promotes locomotor activity. Cell Metab. 2008;7:236–248. doi: 10.1016/j.cmet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Mirshamsi S, Laidlaw HA, Ning K, Anderson E, Burgess LA, Gray A, Sutherland C, Ashford ML. Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATP channel activation. BMC Neurosci. 2004;5:54. doi: 10.1186/1471-2202-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K, Obici S, Rossetti L. Hypothalamic responses to long-chain fatty acids are nutritionally regulated. J Biol Chem. 2004;279:31139–31148. doi: 10.1074/jbc.M400458200. [DOI] [PubMed] [Google Scholar]
- Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signalling. Am J Physiol Endocrinol Metab. 2005;289:E1051–1057. doi: 10.1152/ajpendo.00094.2005. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- Ning K, Miller LC, Laidlaw HA, Burgess LA, Perera NM, Downes CP, Leslie NR, Ashford ML. A novel leptin signalling pathway via PTEN inhibition in hypothalamic cell lines and pancreatic beta-cells. EMBO J. 2006;25:2377–2387. doi: 10.1038/sj.emboj.7601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Kimura K, Ooyama H, Maeno T, Iki M, Kuniyoshi M. Reciprocal activities of the ventromedial and lateral hypothalamic areas of cats. Science. 1964;143:484–485. doi: 10.1126/science.143.3605.484. [DOI] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Plum L, Belgardt BF, Bruning JC. Central insulin action in energy and glucose homeostasis. J Clin Invest. 2006a;116:1761–1766. doi: 10.1172/JCI29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Alber J, Belgardt BF, Koch L, Seibler J, Schwenk F, Fekete C, Suzuki A, Mak TW, Krone W, Horvath TL, Ashcroft FM, Bruning JC. Enhanced PIP3 signalling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006b;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Rother E, Munzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Konner AC, Alber J, Suzuki A, Krone W, Horvath TL, Rahmouni K, Bruning JC. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 2007;6:431–445. doi: 10.1016/j.cmet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci. 2000;3:757–758. doi: 10.1038/77660. [DOI] [PubMed] [Google Scholar]
- Teramoto N, Tomoda T, Yunoki T, Ito Y. Different glibenclamide-sensitivity of ATP-sensitive K+ currents using different patch-clamp recording methods. Eur J Pharmacol. 2006;531:34–40. doi: 10.1016/j.ejphar.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABAA receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest. 2005;115:951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu AW, Ste-Marie L, Kaelin CB, Barsh GS. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology. 2007;148:72–80. doi: 10.1210/en.2006-1119. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Mastaitis J, Mizuno T, Mobbs CV. Glucokinase regulates reproductive function, glucocorticoid secretion, food intake, and hypothalamic gene expression. Endocrinology. 2007;148:1928–1932. doi: 10.1210/en.2006-1312. [DOI] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- Zeidan A, Paylor B, Steinhoff KJ, Javadov S, Rajapurohitam V, Chakrabarti S, Karmazyn M. Actin cytoskeleton dynamics promotes leptin-induced vascular smooth muscle hypertrophy via RhoA/ROCK- and phosphatidylinositol 3-kinase/protein kinase B-dependent pathways. J Pharmacol Exp Ther. 2007;322:1110–1116. doi: 10.1124/jpet.107.122440. [DOI] [PubMed] [Google Scholar]