Abstract
The P2X2 channel is a ligand-gated channel activated by ATP. Functional features that reflect the dynamic flexibility of the channel include time-dependent pore dilatation following ATP application and direct inhibitory interaction with activated nicotinic acetylcholine receptors on the membrane. We have been studying the mechanisms by which P2X2 channel functionality is dynamically regulated. Using a Xenopus oocyte expression system, we observed that the pore properties, including ion selectivity and rectification, depend on the open channel density on the membrane. Pore dilatation was apparent when the open channel density was high and inward rectification was modest. We also observed that P2X2 channels show voltage dependence, despite the absence of a canonical voltage sensor. At a semi-steady state after ATP application, P2X2 channels were activated upon membrane hyperpolarization. This voltage-dependent activation was also [ATP] dependent. With increases in [ATP], the speed of hyperpolarization-induced activation was increased and the conductance–voltage relationship was shifted towards depolarized potentials. Based on analyses of experimental data and various simulations, we propose that these phenomena can be explained by assuming a fast ATP binding step and a rate-limiting voltage-dependent gating step. Complete elucidation of these regulatory mechanisms awaits dynamic imaging of functioning P2X2 channels.
The P2X2 channel is a member of the P2X family of ligand-gated ion channels activated by ATP. It is expressed in dorsal ganglia neurons, mesenteric ganglia neurons, taste buds and astrocytes, and plays a key role in synaptic transmission in various systems (Edwards et al. 1992; Evans et al. 1992; Khakh, 2001; North, 2002; Koizumi et al. 2005; Burnstock, 2007; Surprenant & North, 2008). Complementary DNA (cDNA) encoding P2X2 was first isolated through expression cloning, and the encoded protein was shown to have two transmembrane (TM) segments (Brake et al. 1994; Valera et al. 1994). Various lines of evidence suggest that the functional unit is a trimer (Surprenant & North, 2008). Indeed, atomic force microscopic analysis (Barrera et al. 2005), single particle structure analyses (Mio et al. 2005, 2009) and crystal structure analysis (Kawate et al. 2009) have now confirmed that P2X2 is trimeric.
The mechanism by which P2X2 channel activity is regulated exhibits several unique features. One is the time-dependent dilatation of the pore following ATP application (Khakh & Lester, 1999; Virginio et al. 1999; Eickhorst et al. 2002). This was made manifest by the time-dependent increase in P2X2 channel permeability to N-methyl-d-glucamine (NMDG), a large cation known to be generally impermeable to cation channels, following application of ATP. Another unique feature is the functional interaction of P2X2 channels with nicotinic acetylcholine receptor channels on the membrane. When P2X2 and the nicotinic acetylcholine receptor were co-expressed, simultaneous activation of both receptors under voltage clamp evoked a membrane current that was clearly smaller than the sum of the currents carried by the two channels individually, indicating direct mutual interaction among the activated channels (Nakazawa, 1994; Khakh et al. 2000). These phenomena are reflections of dynamic and flexible features of the P2X2 channel. We have also been engaged in investigating some novel and dynamic regulation mechanisms of P2X2 channels (Fujiwara & Kubo, 2004, 2006; Fujiwara et al. 2009). Our findings to date are summarized in this article.
Density-dependent changes in pore properties
While carrying out experiments in Xenopus oocytes, we noticed that the magnitude of the inward rectification of rat P2X2 channel currents varied such that it appeared to be dependent on the current amplitude or the channel density on the membrane, i.e. inward rectification declined with increases in the channel density (Fujiwara & Kubo, 2004). We also observed that the aforementioned time-dependent pore dilatation seen after ATP application was also dependent on the channel density. In other words, pore dilatation, as reflected by the depolarizing shift in the reversal potential with NMG in the bath solution, was less apparent when the channel density was low than when it was high (Fujiwara & Kubo, 2004). Moreover, even when the expression level of the channel was high, if only a fraction of the channels was activated due to application of only a low concentration of ATP, the phenotype was similar to that seen when the expression level of the channel was low (Fujiwara & Kubo, 2004). Based on these results, we proposed that there is an interaction between open P2X2 channels, whereby the channel pore becomes a weak rectifier with high NMG permeability (Fig. 1). We eventually identified a point mutant (I328C) whose phenotype was less affected by open channel density than wild-type P2X2 (Fujiwara & Kubo, 2004).
Figure 1. Schematic drawings explaining the dynamic variation of inward rectification intensity depending on the open channel density.
The equilibrium is inclined toward the O2 state, depending on the open channel density. Definitions: O1, a state showing a strong inward rectification and a low permeability to NMDG; O2, a state showing no inward rectification; and O3, a state showing a high permeability to NMDG (from Fig. 7 of Fujiwara & Kubo, 2004).
The crystal structure of the zebrafish P2X4 (zP2X4) channel in the closed state was solved recently (Kawate et al. 2009). Although the subtype and the species are different from the rat P2X2 (rP2X2) which we used in the experiments, the overall structure is expected to be conserved between them. The residue Ile336 of zP2X4 (Ile328 of rP2X2) is located in the second transmembrane region and just above the residues which are reported to form the narrowest part of the pore (Leu340, Gly343 and Ala344 of zP2X4; Fig. 2A). In the closed state, the side-chain of Ile336 does not point to the outside of the trimer and it does not appear to serve as antennae for the inter-trimer interaction. It therefore remains to be elucidated how I328C mutation decreases the expression density-dependent changes.
Figure 2. The crystal structure of zebrafish P2X4 (zP2X4) in the closed state and the location of the amino acid residues identified by the mutagenesis study.
A, the alignment of the amino acid residues in the second transmembrane region of rat P2X2 (rP2X2) and zP2X4. B, the crystal structure deposited in Protein Data Bank (accession number 3H9V) by Kawate et al. (2009) was used as a template, and the graphic presentation was made by PyMol software. Only one subunit of the trimer observed from the pore centre side is shown. The residue Ile336 (I328 of rP2X2) is coloured in orange, while V352 (corresponding position to Gly344 of rP2X2) is in red. The amino acid residues reported to form the gate (Leu340, Gly343 and Ala344) are shown in yellow, pink and green, respectively. The key amino acid residues of the ATP binding site, Lys70 and Lys72 (Lys69 and Lys71 of rP2X2), are shown in blue, and Arg298 and Lys316 (Arg290 and Lys308 of rP2X2) are shown in purple.
Density-dependent changes in the pore properties have potential implications for physiological regulation, e.g. a change of the local density of the channel at a synapse due to the presence/absence or binding/unbinding of an anchoring molecule(s) could change the properties of the channel pore and, in turn, the properties of the synapse.
Regulation of desensitization and pore dilatation
The P2X channels show varying degrees of desensitization, and it is known that the C-terminal cytoplasmic region is the critical mediator of that desensitization (Koshimizu et al. 1999; Smith et al. 1999). Using wild-type and mutant P2X2 channels, we observed that the binding of phosphoinositides to the proximal C-terminal cytoplasmic region, a region in which positively charged amino acids are clustered, is critical for preventing desensitization and thus maintaining P2X2 channel activity (Fujiwara & Kubo, 2006). We also observed that channels enter the desensitized state not from the standard open state, but from the pore-dilated open state, suggesting that the channel–phosphoinositide interaction is weakened in the pore-dilated state (Fujiwara & Kubo, 2006).
Gating of the P2X2 channel is voltage and [ATP] dependent, despite the absence of a canonical voltage sensor domain
The P2X2 channel has only two TM regions, and it does not have a canonical voltage sensor. Nonetheless, P2X2 channels exhibit voltage-dependent activation upon hyperpolarization (Nakazawa et al. 1997; Zhou & Hume, 1998; Nakazawa & Ohno, 2005). Similar voltage-dependent gating in the absence of a canonical voltage sensor has also been reported for the nicotinic acetylcholine receptor (Charnet et al. 1992; Figl et al. 1996). Prompted by our interest in the origins of voltage dependence, we used a Xenopus oocyte expression system to analyse the voltage dependence of the rat P2X2 channel in detail (Fujiwara et al. 2009).
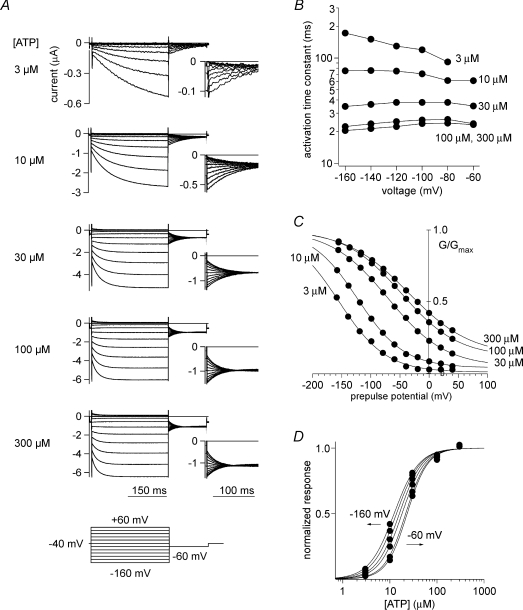
We applied voltage step pluses under two-electrode voltage clamp during the semi-steady state after ATP application and analysed the speed of the hyperpolarization-induced activation. We also analysed the conductance–voltage (G–V) relationship by measuring tail current amplitudes (Fig. 3A). With this approach, we made the novel and unexpected observation that the τ (activation time constant)–V relationship (Fig. 3B) and the normalized G–V relationship (Fig. 3C) were both clearly dependent on the applied [ATP]. When [ATP] was low, hyperpolarization-induced activation was slow, and the G–V relationship was shifted to hyperpolarized potentials. With increases in [ATP], the activation speed increased, and G–V was shifted to more depolarized potentials. The z value in the Boltzmann equation fitted to the G–V curve was approximately 0.5, which is much smaller than in typical voltage-dependent channels, and it did not indicate clear [ATP] dependence. The EC50 was also slightly voltage dependent, so that the value was reduced at more depolarized potentials (Fig. 3D). An obvious possibility is that the voltage-dependent activation is due to the block/unblock by a blocker extrinsic to the channel. However, when we carried out excised patch experiments, which enabled us to control both the internal and the external solutions, we still observed obvious voltage-dependent gating, confirming that the gating is truly intrinsic.
Figure 3. Macroscopic current recordings through P2X2 evoked by step pulses during the steady state after application of various ATP concentrations, and analyses of the voltage-dependent gating.
A, macroscopic currents through wild-type P2X2 evoked by step pulses in the presence of various concentrations of ATP. The pulse protocol is indicated at the bottom. These current traces were recorded from a single oocyte and are shown after subtracting data obtained in the absence of ATP. B, dependence of the activation kinetics on voltage and [ATP]. The activation phases of the currents shown in A were fitted with a single exponential function, and the time constants of the fittings at each membrane potential are plotted. C, normalized G–V relationships derived from the recording in A. Tail current amplitudes at −60 mV were measured. Data were fitted with the two-state Boltzmann equation. D, normalized [ATP]–response relationships. From Figs 2 and 3A, C and E of ©Fujiwara et al. (2009), originally published in J Gen Physiol doi:10.1085/jgp.200810002.
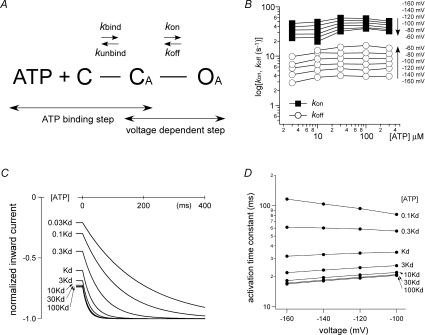
What then is the origin of voltage-dependent gating that also depends on [ATP]? It is generally accepted that, during activation, ATP-gated channels proceed through three states: C, closed and ATP unbound; CA, closed with ATP bound; and OA, open with ATP bound. The transition between C and CA is the ATP binding step, while the transition between CA and OA is the gating step. Using this three-state, two-transition model, we analysed the gating properties of the P2X2 channel (Fig. 4A). Given that the voltage-dependent gating is clearly dependent on [ATP], one simple and straightforward possibility is that the ATP binding step is the origin of the voltage dependence. The ATP binding could be voltage dependent if the binding site was located within an effective electric field, even if it was not embedded in the lipid bilayer. Another possibility is that the gating step following ATP binding is voltage dependent. In that case, the question is why the voltage-dependent gating also shows dependence on [ATP].
Figure 4. Three-state, two-transition model of voltage- and [ATP]-dependent gating, and simulation analyses of the activation phase evoked by a voltage step.
A, simple three-state model consisting of an ATP binding step and a gating step. Definitions: C represents the closed state with no bound ATP, CA represents the closed state after ATP is bound, and OA represents the open state. B, kon and koff were calculated as described by Fujiwara et al. (2009). C, reproduction of the activation phase by simulation. The activation phases evoked by step pulses from −60 to −160 mV in the presence of various ATP concentrations were simulated. The applied [ATP] relative to Kd is indicated in the figure. D, summary of the simulation of the activation kinetics at various voltages and ATP concentrations. The activation phases of the simulated currents could be fitted satisfactorily with a single exponential function, and the time constants of the fittings at various concentrations of ATP relative to Kd were plotted versus membrane potential. From Figs 10A and B and 11A and C of ©Fujiwara et al. (2009), originally published in J Gen Physiol doi:10.1085/jgp.200810002.
The rate constants β and α in the simple C–O model can be readily obtained, as τ= 1/(β+α) and normalized G=β/(β+α). Using the calculated α and β values, we next calculated the on-rate (kon) and the off-rate (koff) values of the gating step in the C–CA–OA model (Fig. 4A). Two assumptions used were that the ATP binding step is fast and the gating step is rate limiting. We also used the reported Kd values (Ding & Sachs, 1999) to obtain the ratio of the unbinding-rate (kunbind) and the binding-rate (kbind) values. Although we had no concrete values for kbind and kunbind, we were able to calculate the kon and koff values as described in detail by Fujiwara et al. (2009). The calculated kon and koff values showed clear voltage dependence with no [ATP] dependence; kon was larger at hyperpolarized potentials, while koff was larger at depolarized potentials (Fig. 4B). In addition, by using the calculated kon and koff values with the reported kbind and kunbind values (Ding & Sachs, 1999), we were able to reproduce the voltage-dependent activation as well as the ATP dependence (Fig. 4C and D). We also tried simulations using arbitrary kbind parameters with voltage dependence. Although we managed to reproduce the ATP dependence of the rate of voltage-dependent gating, the EC50 value was steeply voltage dependent and clearly deviated from the experimental results. Thus, it was not possible to explain and reproduce the experimental results by simply assuming the ATP binding step is voltage dependent. Instead, our findings suggest that the gating step after the ATP binding step accounts for the voltage dependence. Notably, the fact that the gating step is rate limiting and much slower than the ATP binding step can also explain and reproduce the ATP dependence.
In the case of voltage-gated K+ channels, a gating hinge is known to be present in the middle of the last (sixth) TM segment (Jiang et al. 2002; Magidovich & Yifrach, 2004; Ding et al. 2005). We identified Gly344 situated in the middle of the last (second) TM segment as the gating hinge in the rat P2X2 channel (Fujiwara et al. 2009). By substituting an Ala for Gly344 (G344A), we were able to largely eliminate the voltage dependence of P2X2 activation (a G344P mutant showed a slower voltage-dependent activation). Furthermore, by reintroducing a Gly at position 344 of the G344A mutant, voltage-dependent gating could be restored. Thus, in a manner analogous to voltage-gated K+ channels, Gly344 serves as is the gating hinge involved in the voltage-gated activation of the P2X2 channel (Fujiwara et al. 2009).
The amino acid residues of the second transmembrane region of rP2X2 and zP2X4 are aligned in Fig. 2A. As shown, Gly344 of rP2X2 is not conserved in zP2X4. The residues Leu340, Gly343 and Ala344 in zP2X4 were shown to form the narrowest part of the pore (Kawate et al. 2009). The position in the crystal structure of these amino acid residues as well as Ile340 (Ile328 of rP2X2) in the previous section is marked in Fig. 2B. The structure shown is that of only one subunit observed from the pore centre side. Based on these data, we speculate that the structure, especially the position of the gating hinge, differs significantly between the two clones. The difference might partly explain the difference in the extent of pore dilatation.
Future aspects
Expression density-dependent changes
Channel density-dependent modulation of pore properties is a novel mechanism for regulating P2X2 channel activity, but a similar mechanism could account for the functional regulation of other membrane proteins. For example, the outward rectification of Transient Receptor Potential (TRP) channel currents in Xenopus oocytes shows similar amplitude-dependent changes (Nagatomo & Kubo, 2008).
Figure 1 illustrates a proposed mechanism whereby mutual interaction among activated channels determines density-dependent pore properties (Fujiwara & Kubo, 2004). In addition, there may be cases in which density-dependent changes in pore properties reflect the availability of one or more auxiliary molecules, such as a specific lipid. It would therefore be of interest to carry out analyses in other expression systems, including mammalian cultured cell lines.
We carried out single particle structure analyses of negatively stained electron microscopic images (Mio et al. 2005) and cryo-electron microscopic images (Mio et al. 2009), which enabled us to determine that the P2X2 channel has a vase-like structure with lateral tunnels above the membrane. Although the resolution is much lower than that obtained with X-ray crystallography (Kawate et al. 2009), this approach may be better suited for analysis of density-dependent changes to the channel structure. To reproduce various densities, it might be effective to carry out single particle structure analyses using liposomes with embedded recombinant proteins (Wang & Sigworth, 2009). Since the density of recombinant proteins on liposome membranes can be manipulated, one would be able to analyse density-dependent structural changes, which are hard to analyse within a tightly packed crystal.
From a physiological point of view, it would be interesting to see the behavioural changes elicited in knock-in mice carrying the I328C mutation or a different P2X2 mutant, whose density-dependent pore properties are altered with no obvious changes to other basic functions.
Voltage-dependent activation
We reported that the fast ATP binding step and the rate-limiting voltage-dependent gating step could reproduce the experimental results (Fujiwara et al. 2009). The most important and interesting questions that arise from those observations have to do with the structural background of the voltage dependence of the gating step. What happens after ATP binds? How is information about ATP binding transmitted to the main body of the channel to open the gate? Why is this step voltage dependent?
Through systematic mutagenic analysis, the ATP binding region in the extracellular loop was identified and shown to be rich in positive charges (Roberts et al. 2006). The contributions of amino acid residues in TM1 and TM2 to ATP-evoked gating and/or permeation have been studied extensively (Surprenant & North, 2008). It may be that a complex comprised of the negatively charged ATP and the positively charged binding site interacts directly or indirectly with the extracellular end of the TM segment involved in the gating, and that the interaction with the TM segment is under the influence of the electric field. The mutagenesis studies cited above were carried out from the point of view of ATP-induced activation, but so far no studies have been done from the point of view of voltage dependence. It would therefore seem worthwhile to characterize the voltage dependence of P2X2 channels with mutations in the ATP binding region, linker region and/or the upper TM regions, and to examine the effect of ATP analogues carrying four negative charges.
Voltage dependence has also been reported for metabotropic receptors such as muscarinic acetylcholine receptors (Ben-Chaim et al. 2003, 2006) and metabotropic glutamate receptors (Ohana et al. 2006). Although the biochemical analyses performed by these groups indicate that the ligand binding step itself is voltage dependent, our findings suggest that the voltage dependence of the binding step cannot explain the results (Fujiwara et al. 2009), though we cannot exclude the possibility that the binding step also has voltage dependence. Biochemical analysis of the binding would clarify this point.
The ATP binding site was clearly identified in the crystal structure of zP2X4 (Kawate et al. 2009). It was shown to locate on the surface of the intersubunit boundary at a position distant from the transmembrane regions (Fig. 2B). Judging from the location of the binding site, it seems unlikely that the ATP binding site is in the electric field and that the ATP binding step is voltage dependent. Also, the interaction of the ATP–ATP binding site complex with the transmebrane regions mentioned above would be indirect and possibly mediated by the linker region.
Ben-Chaim et al. (2006) successfully recorded gating currents from muscarinic receptors, although the z value is relatively small. It would also be worth trying to record gating currents from cells expressing P2X2 receptors. Of particular interest would be whether the gating current was observed only when ATP was bound or whether it could also be observed in the absence of ATP. If the origin of voltage dependence is the formation of a complex comprised of ATP and its binding site, movement of the gating charge would be recorded only when ATP is bound. Another powerful approach to characterizing the dynamic aspects of channel activity is real-time measurement of conformational changes using an optical technique. For instance, Fluorescent Resonance Energy Transfer (FRET)-based analyses have revealed conformational changes in the cytoplasmic region of P2X2 channels during ATP-evoked activation and desensitization (Fisher et al. 2004). It would be interesting to apply this technique to analyse the conformational changes during the voltage-dependent gating.
Author's present address
Y. Fujiwara: Department of Integrative Physiology, Graduate School and Faculty of Medicine, Osaka University, Osaka 565-0871, Japan.
References
- Barrera NP, Ormond SJ, Henderson RM, Murrell-Lagnado RD, Edwardson JM. Atomic force microscopy imaging demonstrates that P2X2 receptors are trimers but that P2X6 receptor subunits do not oligomerize. J Biol Chem. 2005;280:10759–10765. doi: 10.1074/jbc.M412265200. [DOI] [PubMed] [Google Scholar]
- Ben-Chaim Y, Chanda B, Dascal N, Bezanilla F, Parnas I, Parnas H. Movement of ‘gating charge’ is coupled to ligand binding in a G-protein-coupled receptor. Nature. 2006;444:106–109. doi: 10.1038/nature05259. [DOI] [PubMed] [Google Scholar]
- Ben-Chaim Y, Tour O, Dascal N, Parnas I, Parnas H. The M2 muscarinic G-protein-coupled receptor is voltage-sensitive. J Biol Chem. 2003;278:22482–22491. doi: 10.1074/jbc.M301146200. [DOI] [PubMed] [Google Scholar]
- Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Charnet P, Labarca C, Cohen BN, Davidson N, Lester HA, Pilar G. Pharmacological and kinetic properties of α4β2 neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Physiol. 1992;450:375–394. doi: 10.1113/jphysiol.1992.sp019132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Ingleby L, Ahern CA, Horn R. Investigating the putative glycine hinge in Shaker potassium channel. J Gen Physiol. 2005;126:213–226. doi: 10.1085/jgp.200509287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Sachs F. Single channel properties of P2X2 purinoceptors. J Gen Physiol. 1999;113:695–720. doi: 10.1085/jgp.113.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Eickhorst AN, Berson A, Cockayne D, Lester HA, Khakh BS. Control of P2X2 channel permeability by the cytosolic domain. J Gen Physiol. 2002;120:119–131. doi: 10.1085/jgp.20028535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RJ, Derkach V, Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Figl A, Labarca C, Davidson N, Lester HA, Cohen BN. Voltage-jump relaxation kinetics for wild-type and chimeric beta subunits of neuronal nicotinic receptors. J Gen Physiol. 1996;107:369–379. doi: 10.1085/jgp.107.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JA, Girdler G, Khakh BS. Time-resolved measurement of state-specific P2X2 ion channel cytosolic gating motions. J Neurosci. 2004;24:10475–10487. doi: 10.1523/JNEUROSCI.3250-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Keceli B, Nakajo K, Kubo Y. Voltage- and [ATP]-dependent gating of the P2X2 ATP receptor channel. J Gen Physiol. 2009;133:93–109. doi: 10.1085/jgp.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Kubo Y. Density-dependent changes of the pore properties of the P2X2 receptor channel. J Physiol. 2004;558:31–43. doi: 10.1113/jphysiol.2004.064568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Kubo Y. Regulation of the desensitization and ion selectivity of ATP-gated P2X2 channels by phosphoinositides. J Physiol. 2006;576:135–149. doi: 10.1113/jphysiol.2006.115246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–522. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci. 2001;2:165–174. doi: 10.1038/35058521. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Lester HA. Dynamic selectivity filters in ion channels. Neuron. 1999;23:653–658. doi: 10.1016/s0896-6273(01)80025-8. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Zhou X, Sydes J, Galligan JJ, Lester HA. State-dependent cross-inhibition between transmitter-gated cation channels. Nature. 2000;406:405–410. doi: 10.1038/35019066. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Fujishita K, Inoue K. Regulation of cell-to-cell communication mediated by astrocytic ATP in the CNS. Purinergic Signal. 2005;1:211–217. doi: 10.1007/s11302-005-6321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu T, Koshimizu M, Stojilkovic SS. Contributions of the C-terminal domain to the control of P2X receptor desensitization. J Biol Chem. 1999;274:37651–37657. doi: 10.1074/jbc.274.53.37651. [DOI] [PubMed] [Google Scholar]
- Magidovich E, Yifrach O. Conserved gating hinge in ligand- and voltage-dependent K+ channels. Biochemistry. 2004;43:13242–13247. doi: 10.1021/bi048377v. [DOI] [PubMed] [Google Scholar]
- Mio K, Kubo Y, Ogura T, Yamamoto T, Sato C. Visualization of the trimeric P2X2 receptor with a crown-capped extracellular domain. Biochem Biophys Res Commun. 2005;337:998–1005. doi: 10.1016/j.bbrc.2005.09.141. [DOI] [PubMed] [Google Scholar]
- Mio K, Ogura T, Yamamoto T, Hiroaki Y, Fujiyoshi Y, Kubo Y, Sato C. Reconstruction of the P2X2 receptor reveals a vase-shaped structure with lateral tunnels above the membrane. Structure. 2009;17:266–275. doi: 10.1016/j.str.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Nagatomo K, Kubo Y. Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc Natl Acad Sci U S A. 2008;105:17373–17378. doi: 10.1073/pnas.0809769105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K. ATP-activated current and its interaction with acetylcholine-activated current in rat sympathetic neurons. J Neurosci. 1994;14:740–750. doi: 10.1523/JNEUROSCI.14-02-00740.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Liu M, Inoue K, Ohno Y. Voltage-dependent gating of ATP-activated channels in PC12 cells. J Neurophysiol. 1997;78:884–890. doi: 10.1152/jn.1997.78.2.884. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Ohno Y. Characterization of voltage-dependent gating of P2X2 receptor/channel. Eur J Pharmacol. 2005;508:23–30. doi: 10.1016/j.ejphar.2004.12.005. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Ohana L, Barchad O, Parnas I, Parnas H. The metabotropic glutamate G-protein-coupled receptors mGluR3 and mGluR1a are voltage-sensitive. J Biol Chem. 2006;281:24204–24215. doi: 10.1074/jbc.M513447200. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Vial C, Digby HR, Agboh KC, Wen H, Atterbury-Thomas A, Evans RJ. Molecular properties of P2X receptors. Pflugers Arch. 2006;452:486–500. doi: 10.1007/s00424-006-0073-6. [DOI] [PubMed] [Google Scholar]
- Smith FM, Humphrey PP, Murrell-Lagnado RD. Identification of amino acids within the P2X2 receptor C-terminus that regulate desensitization. J Physiol. 1999;520:91–99. doi: 10.1111/j.1469-7793.1999.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, North RA. Signalling at purinergic P2X receptors. Annu Rev Physiol. 2008;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- Wang L, Sigworth FJ. Structure of the BK potassium channel in a lipid membrane from electron cryomicroscopy. Nature. 2009;461:292–295. doi: 10.1038/nature08291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hume RI. Two mechanisms for inward rectification of current flow through the purinoceptor P2X2 class of ATP-gated channels. J Physiol. 1998;507:353–364. doi: 10.1111/j.1469-7793.1998.353bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]