Abstract
Voltage-gated proton channels have been described in different cells and organisms since the early ’80s, but the first member of the family, Hv1, was cloned only recently. The Hv1 channel was found to contain a voltage-sensing domain (VSD), similar to those of voltage-gated sodium, potassium and calcium channels. All these other channels also contain a pore domain, which forms a central pore at the interface of the four subunits. The pore domain is missing in Hv1. This raised several questions on the location of the proton pore and on the mechanism of gating. Here, we briefly review our effort to understand the structural organization of Hv1 channels and discuss the relationship between the gating of Hv1 and the gating of ion-conducting pores recently discovered in the VSDs of mutant voltage-gated potassium and sodium channels.
Voltage-gated potassium, sodium and calcium channels are made of four voltage-sensing domains (VSDs) that control one permeation pathway located at the centre of a distinct pore domain (Yu & Catterall, 2004; Long et al. 2005; Tombola et al. 2006). Recently, a new addition to the family of VSD-containing channels has been made with the cloning of the first voltage-gated proton channel, Hv1, also known as VSOP (Ramsey et al. 2006; Sasaki et al. 2006). Voltage-gated proton channels were first identified in snail neurons more than a quarter of a century ago (Thomas & Meech, 1982). Since then, their biophysical properties and biological role have been elucidated in detail (Decoursey, 2003; DeCoursey et al. 2003), but the lack of candidate genes for these channels has prevented molecular studies on channel architecture and gating. This kind of study is now possible, and several groups have begun tackling different aspects of channel structure and function (Alabi et al. 2007; Koch et al. 2008; Lee et al. 2008, 2009; Musset et al. 2008; Li et al. 2009; Okochi et al. 2009; Ramsey et al. 2009; Tombola et al. 2008).
When Hv1 was cloned, its sequence revealed that the predicted membrane-spanning region consists solely of the VSD, lacking a homologue to the pore domain of other voltage-gated channels (Ramsey et al. 2006; Sasaki et al. 2006). This raised several questions about how voltage-gated proton channels work, as follows. (1) If Hv1 lacks a pore domain then where is the permeation pathway located? (2) Is Hv1 made of four VSDs, like other voltage-gated channels? (3) How is the voltage sensor movement linked to channel opening in Hv1? Here, we briefly describe our attempts to answer these questions.
Most ion channels are protein complexes made of multiple subunits. A single ion-conducting pore is normally located in the centre of the complex, at the interface between subunits (Hille, 2001). Voltage-gated chloride channels (ClCs) and aquaporins are important exceptions. They are also made of multiple subunits, but each subunit contains its own pore and thus there are as many pores as there are subunits (Ludewig et al. 1996; Middleton et al. 1996; Fu et al. 2000; Sui et al. 2001; Dutzler et al. 2002; King et al. 2004). We first set out to determine the number of subunits in Hv1 and then to determine whether the permeation pathway is located within a single subunit or at the interface between multiple subunits.
The Hv1 channel is a dimer, with dimerization driven by the cytoplasmic domain
We used a single molecule technique (Ulbrich & Isacoff, 2007) to visualize Green Fluorescent Protein (GFP)-tagged Hv1 channels on the cell surface with Total Internal Reflection Fluorescent Microscopy (TIRFM). The advantages of this method are that it focuses exclusively on the plasma membrane, where channels reach only after they have undergone the quality control processes of membrane targeting and the site of channel function, and that subunit stoichiometry is assessed for individual proteins, rather than via bulk methods, which may not detect heterogeneity from average behaviours.
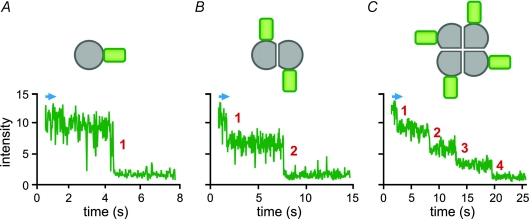
We determined the number of subunits per channel by counting the number of photo-bleaching events from channels that were expressed at a sufficiently low density to ensure that practically all of the fluorescent spots on the cell surface corresponded to individual proteins (Fig. 1). We tested both wild-type (WT) Hv1 channels, tagged with GFP, and ones whose mobility was reduced by an additional protein interaction domain from scaffolding proteins PSD95, Dlg1 and Zoe-1 (PDZ)-interaction domain and co-expression of the PDZ protein PSD95. In both cases, the fraction of fluorescent spots that bleached in two steps was very similar to what was seen in a known reference (NMDA receptors tagged with GFP on only two of the four subunits) and clearly differed from two other references, one that carries a single GFP per channel and one that carries four GFPs per channel (Ulbrich & Isacoff, 2007).
Figure 1. Determination of the number of subunits in individual protein complexes by counting photobleaching steps.
Each labelled complex appears as a fluorescent spot on the movie acquired under total internal reflection microscopy. When the bleaching light is turned on (blue arrow) the fluorescence intensity of the spot decays in a stepwise manner until, one by one, all the fluorophores are bleached. A monomer, such as Ci-VSP, produces spots with only one bleaching step (A). A dimer, such as Hv1, gives spots with two bleaching steps (B). A tetramer, such as a cyclic nucleotide-gated channel, gives spots with four bleaching steps (C).
Chimeras between Hv1 and the voltage-dependent phosphatase Ci-VSP, which was recently shown to be monomeric (Kohout et al. 2008), or the C-terminal of the Shaker Kv1 channel, showed that dimerization depends on the cytoplasmic domain, and not the membrane domain, of Hv1. The evidence for this was that substitution of the N-terminal of Hv1 was found to compromise dimerization, and substitution of the C-terminus was found to disrupt it completely, while transplantation of the two terminals from Hv1 onto the membrane domain of Ci-VSP was sufficient to dimerize the normally monomeric Ci-VSP.
Each Hv1 subunit contains a pore
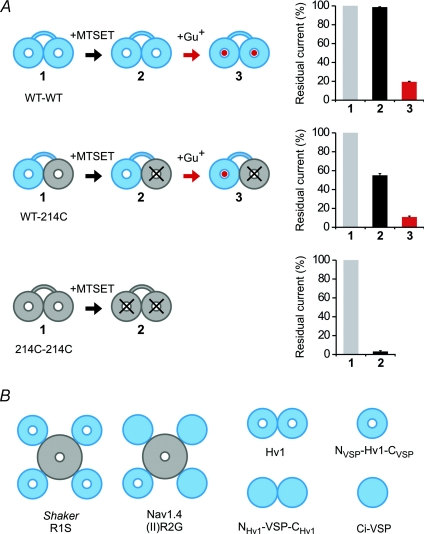
Having found that the Hv1 channel is made of two subunits, we asked whether there are one or two pores per channel. We identified a site in Hv1, N214, that when mutated to cysteine makes the channel susceptible to block by the thiol-reactive methanethiosulfonate (MTS) reagents, enabling us to modify the conduction pathway. We then constructed tandem dimers of Hv1 that would allow us to introduce the N214C mutation independently into the two subunits. Channels formed by the linked homodimers, WT–WT or 214C–214C, were the same as those formed by the free co-assembly of unlinked WT or 214C subunits, respectively, enabling the analysis. We also found that the WT channel is blocked by free guanidinium, providing a second blocking probe of the pore. We then tested the expectation that only if the channel has two pores, a separate one in each of its two subunits, would manipulation in one subunit leave unaffected the flow through the unmutated pore (Fig. 2A).
Figure 2. Each of two Hv1 subunits contains its own pore and gate.
A, block of the proton current of Hv1 linked dimers by MTSET and guanidinium (Gu+). Only the pore of the 214C subunit is sensitive to MTSET. The block by guanidinium of the WT pore after MTSET treatment is the same as the block without MTSET treatment. B, examples of VSDs (in blue) permeable or impermeable to protons or solution ions. The VSDs of Shaker and the VSD of domain II of Nav1.4 become ion conducting when one of the S4 arginines is mutated to a smaller uncharged residue. In wild-type Hv1, an asparagine replaces the forth S4 arginine (at position 214). The Hv1 pore is still able to conduct protons when N214 is mutated to cysteine. The mutant N214R, in contrast, is non-conducting, like the VSDs of wild-type Shaker and Nav1.4, or the VSD of Ci-VSP. The chimera in which the N- and C-termini of Hv1 are replaced by the corresponding parts of Ci-VSP (NVSP–Hv1–CVSP) is monomeric and still works as a voltage-gated proton channel. The reverse chimera, in which the N- and C-termini of Ci-VSP are replaced by the corresponding parts of Hv1 (NHv1–VSP–CHv1), forms non-conducting dimers.
The following three lines of evidence pointed to a two-pore construction of the Hv1 dimer, with one pore in each subunit. (1) The fractional block of the heterodimeric WT–214C and 214C–WT constructs was similar to that of the 214C–214C homodimer using different MTS reagents with distinct steric and electrostatic properties. (2) When 2-(trimethylammonium)ethyl methanethiosulfonate (MTSET) completely blocks 214C, then further block by guanidinium of WT–214C and 214C–WT follows exactly what would be expected for block of a separate WT pore (Fig. 2A). (3) When 2-(Aminocarbonyl)ethyl]methanethiosulfonate (MTSACE) partly blocks 214C then guanidinium block of WT–214C and 214C–WT follows the predicted combination of normal block of the WT pore and reduced block of a separate 214C–MTSACE pore. A fourth line of evidence also showed that each subunit has its own pore, when we found that the monomerized Hv1 chimera, NVSP–Hv–CVSP, functions as a voltage-gated proton channel (Fig. 2B). These findings argue strongly that the Hv1 dimer contains two separate pores.
Model of the permeation pathway and the mechanism of voltage-dependent gating
Our evidence that Hv1 is a dimer containing two separate pores raised questions about where the permeation pathway lies within each subunit and how the voltage sensor controls the gate of each pore. We recently described a metal-cation-selective pore, the omega pore, that opens in the VSD of the Shaker voltage-gated K+ channel when the first S4 arginine (R1) is mutated to a smaller uncharged amino acid and the channel is in the resting conformation at negative voltage (Tombola et al. 2005, 2007). A similar omega pore has been described in mutant voltage-gated Na+ channels (Sokolov et al. 2005, 2007; Struyk et al. 2008). Proton pores have also been described in the Shaker VSD with histidine substitutions R1H or R4H (Starace & Bezanilla, 2004; Starace et al. 1997). What is the relationship between these omega/proton pores in K+ and Na+ channels (Fig. 2B) and the proton pore of the Hv1 channel? Our study of Hv1 reveals intriguing similarities between these VSD pores.
Asparagine 214 (N214) of the WT Hv1 channel aligns with the fourth S4 arginine (R4) of the Shaker channel. While replacement of N214 with cysteine yields conducting channels, we found that replacement of N214 with arginine abolishes the proton current. In Shaker, the nature of the side-chains at the R1 position determines the size of the omega current (Tombola et al. 2005), and when R1 is substituted by a histidine the omega pore becomes proton selective (Starace & Bezanilla, 2004). In Hv1, N214C can react with thiol-modifying agents in the intracellular solution, consistent with the internal exposure of R4 and positions around it in the Shaker K+ and in the Na+ channel (Larsson et al. 1996; Yang et al. 1996). The omega pathway opens when the membrane potential is negative and the VSD reaches its resting conformation (S4 ‘down’; Durell et al. 2004; Campos et al. 2007; Tombola et al. 2007; Yarov-Yarovoy et al. 2006). This places the R1 position in the middle of the membrane electric field (Larsson et al. 1996; Yang et al. 1996; Gandhi & Isacoff, 2002), corresponding to the narrowest portion of the omega pore (Tombola et al. 2007). Alternatively, depolarization of Shaker moves the R4 position to the middle of the membrane electric field (S4 ‘up’) to replace R1 (Larsson et al. 1996; Gandhi & Isacoff, 2002), and in these conditions the R4H mutant of Shaker opens and conducts protons (Starace et al. 1997). In Hv1, the proton pore opens at positive voltages (S4 ‘up’), consistent with the residue at position R4, i.e. asparagine 214, entering a location in the narrowest part of the VSD pathway and enabling protons to pass. In support of this model, both substitution of N214 with arginine and modification of N214C with MTS reagents block the Hv1 pore.
Based on these similarities between voltage-gated currents of the Hv1 VSD and the voltage-gated omega/proton pores in the VSDs of the Shaker K+ channel and Na+ channels, we propose that the mechanism of gating of the Hv1 channel is similar to that of the omega/proton pores in other voltage-gated channels, where gating in Hv1 occurs via S4 movement into a conformation that lets protons pass through the VSD only in the ‘up’ state by placing a small polar residue into the pathway otherwise occupied, and blocked, by large positively charged arginine residues.
To explain the high energy barrier that protons have to overcome to permeate voltage-gated proton channels, DeCoursey & Cherny (1998) proposed that the rate-limiting step for proton permeation is not diffusion to the mouth of the channel but proton transfer in a narrow region of the permeation pathway. The existence of a constriction in the VSD permeation pathway can provide a simple explanation for the finding that guanidinium ions added intracellularly block the proton channel. The constriction that prevents guanidinium permeation in Hv1 may be the selectivity filter for protons. Further studies will be needed to pinpoint the selectivity filter and to determine the contribution of the side-chain at the ‘R4’ position to the proton permeation pathway.
Conclusion
Using a single molecule optical method that we recently developed, we found that, in contrast to the classical tetrameric voltage-gated channels and to the monomeric Ci-VSP, the Hv1 proton channel is a dimer. Each of the subunits of Hv1 has its own permeation pathway, which is likely to be situated in the heart of the VSD. Similar results were obtained by two other groups using a Fluorescence Resonance Energy Transfer (FRET) approach and cross-linking techniques on the purified Hv1 protein (Koch et al. 2008; Lee et al. 2008). In particular, Koch and colleagues showed that the deletion of the C-terminal coiled-coil domain of Hv1 produces monomeric channels, which are still functional. Lee and colleagues found evidence that, while the two Hv1 subunits are held together in the cytosol by the coiled-coil domains, in the membrane the interface between subunits is primarily made of S1 transmembrane segments.
In conclusion, it appears that each of the Hv1 permeation pathways has its own gate controlled by one voltage sensor, similar to the omega pathway of the Shaker voltage-gated K+ channel VSD. The dimerization in Hv1 depends on the cytosolic domain of the channel, with the coiled-coil C-terminal domain playing a key role. These findings are consistent with a single ion channel domain combining two functions that are separate in most other channels, those of input and output, by serving as both a sensor and a gate. This represents a unique solution to the coupling problem. We have now shown that the pair of Hv1 channels interact to sculpt their gating properties (Tombola et al. 2009).
Acknowledgments
The research was supported by the National Institutes of Health (R01NS035549 to E.Y.I.), by the American Heart Association WSA (09BGIA2160044 to F.T.) and by postdoctoral fellowships from the American Heart Association and Deutsche Forschungsgesellschaft (to M.H.U.).
References
- Alabi AA, Bahamonde MI, Jung HJ, Kim JI, Swartz KJ. Portability of paddle motif function and pharmacology in voltage sensors. Nature. 2007;450:370–375. doi: 10.1038/nature06266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos FV, Chanda B, Roux B, Bezanilla F. Two atomic constraints unambiguously position the S4 segment relative to S1 and S2 segments in the closed state of Shaker K channel. Proc Natl Acad Sci U S A. 2007;104:7904–7909. doi: 10.1073/pnas.0702638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Cherny VV. Temperature dependence of voltage-gated H+ currents in human neutrophils, rat alveolar epithelial cells, and mammalian phagocytes. J Gen Physiol. 1998;112:503–522. doi: 10.1085/jgp.112.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE, Morgan D, Cherny VV. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–534. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]
- Durell SR, Shrivastava IH, Guy HR. Models of the structure and voltage-gating mechanism of the shaker K+ channel. Biophys J. 2004;87:2116–2130. doi: 10.1529/biophysj.104.040618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- Fu D, Libson A, Miercke LJ, Weitzman C, Nollert P, Krucinski J, Stroud RM. Structure of a glycerol-conducting channel and the basis for its selectivity. Science. 2000;290:481–486. doi: 10.1126/science.290.5491.481. [DOI] [PubMed] [Google Scholar]
- Gandhi CS, Isacoff EY. Molecular models of voltage sensing. J Gen Physiol. 2002;120:455–463. doi: 10.1085/jgp.20028678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3rd edn. Sunderland: Sinauer Associates Inc.; 2001. [Google Scholar]
- King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- Koch HP, Kurokawa T, Okochi Y, Sasaki M, Okamura Y, Larsson HP. Multimeric nature of voltage-gated proton channels. Proc Natl Acad Sci U S A. 2008;105:9111–9116. doi: 10.1073/pnas.0801553105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout SC, Ulbrich MH, Bell SC, Isacoff EY. Subunit organization and functional transitions in Ci-VSP. Nat Struct Mol Biol. 2008;15:106–108. doi: 10.1038/nsmb1320. [DOI] [PubMed] [Google Scholar]
- Larsson HP, Baker OS, Dhillon DS, Isacoff EY. Transmembrane movement of the Shaker K+ channel S4. Neuron. 1996;16:387–397. doi: 10.1016/s0896-6273(00)80056-2. [DOI] [PubMed] [Google Scholar]
- Lee SY, Letts JA, Mackinnon R. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc Natl Acad Sci U S A. 2008;105:7692–7695. doi: 10.1073/pnas.0803277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Letts JA, MacKinnon R. Functional reconstitution of purified human Hv1 H+ channels. J Mol Biol. 2009;387:1055–1060. doi: 10.1016/j.jmb.2009.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Zhao Q, Zhou Q, Zhai Y. Expression, purification, crystallization and preliminary crystallographic study of the carboxyl-terminal domain of the human voltage-gated proton channel Hv1. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009;65:279–281. doi: 10.1107/S1744309109003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- Ludewig U, Pusch M, Jentsch TJ. Two physically distinct pores in the dimeric ClC-0 chloride channel. Nature. 1996;383:340–343. doi: 10.1038/383340a0. [DOI] [PubMed] [Google Scholar]
- Middleton RE, Pheasant DJ, Miller C. Homodimeric architecture of a ClC-type chloride ion channel. Nature. 1996;383:337–340. doi: 10.1038/383337a0. [DOI] [PubMed] [Google Scholar]
- Musset B, Cherny VV, Morgan D, Okamura Y, Ramsey IS, Clapham DE, DeCoursey TE. Detailed comparison of expressed and native voltage-gated proton channel currents. J Physiol. 2008;586:2477–2486. doi: 10.1113/jphysiol.2007.149427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi Y, Sasaki M, Iwasaki H, Okamura Y. Voltage-gated proton channel is expressed on phagosomes. Biochem Biophys Res Commun. 2009;382:274–279. doi: 10.1016/j.bbrc.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Ruchti E, Kaczmarek JS, Clapham DE. Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc Natl Acad Sci U S A. 2009;106:7642–7647. doi: 10.1073/pnas.0902761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- Sokolov S, Scheuer T, Catterall WA. Ion permeation through a voltage-sensitive gating pore in brain sodium channels having voltage sensor mutations. Neuron. 2005;47:183–189. doi: 10.1016/j.neuron.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Sokolov S, Scheuer T, Catterall WA. Gating pore current in an inherited ion channelopathy. Nature. 2007;446:76–78. doi: 10.1038/nature05598. [DOI] [PubMed] [Google Scholar]
- Starace DM, Bezanilla F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature. 2004;427:548–553. doi: 10.1038/nature02270. [DOI] [PubMed] [Google Scholar]
- Starace DM, Stefani E, Bezanilla F. Voltage-dependent proton transport by the voltage sensor of the Shaker K+ channel. Neuron. 1997;19:1319–1327. doi: 10.1016/s0896-6273(00)80422-5. [DOI] [PubMed] [Google Scholar]
- Struyk AF, Markin VS, Francis D, Cannon SC. Gating pore currents in DIIS4 mutations of NaV1.4 associated with periodic paralysis: saturation of ion flux and implications for disease pathogenesis. J Gen Physiol. 2008;132:447–464. doi: 10.1085/jgp.200809967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui H, Han BG, Lee JK, Walian P, Jap BK. Structural basis of water-specific transport through the AQP1 water channel. Nature. 2001;414:872–878. doi: 10.1038/414872a. [DOI] [PubMed] [Google Scholar]
- Thomas RC, Meech RW. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature. 1982;299:826–828. doi: 10.1038/299826a0. [DOI] [PubMed] [Google Scholar]
- Tombola F, Pathak MM, Gorostiza P, Isacoff EY. The twisted ion-permeation pathway of a resting voltage-sensing domain. Nature. 2007;445:546–549. doi: 10.1038/nature05396. [DOI] [PubMed] [Google Scholar]
- Tombola F, Pathak MM, Isacoff EY. Voltage-sensing arginines in a potassium channel permeate and occlude cation-selective pores. Neuron. 2005;45:379–388. doi: 10.1016/j.neuron.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Tombola F, Pathak MM, Isacoff EY. How does voltage open an ion channel? Annu Rev Cell Dev Biol. 2006;22:23–52. doi: 10.1146/annurev.cellbio.21.020404.145837. [DOI] [PubMed] [Google Scholar]
- Tombola F, Ulbrich MH, Isacoff EY. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron. 2008;58:546–556. doi: 10.1016/j.neuron.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombola F, Ulbrich M, Kohout SC, Isacoff EY. The opening of the two pores of the Hv1 voltage-gated proton channel is tuned by cooperativity. Nat. Struct. Molec. Bio. 2009 doi: 10.1038/nsmb.1738. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrich MH, Isacoff EY. Subunit counting in membrane-bound proteins. Nat Methods. 2007;4:319–321. doi: 10.1038/NMETH1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, George AL, Jr, Horn R. Molecular basis of charge movement in voltage-gated sodium channels. Neuron. 1996;16:113–122. doi: 10.1016/s0896-6273(00)80028-8. [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, Baker D, Catterall WA. Voltage sensor conformations in the open and closed states in ROSETTA structural models of K+ channels. Proc Natl Acad Sci U S A. 2006;103:7292–7297. doi: 10.1073/pnas.0602350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Catterall WA. The VGL-chanome: a protein superfamily specialized for electrical signalling and ionic homeostasis. Sci STKE. 2004;2004:re15. doi: 10.1126/stke.2532004re15. [DOI] [PubMed] [Google Scholar]