Abstract
G-protein-coupled receptors (GPCRs) are key players in the precise tuning of intercellullar communication. In the brain, both major neurotransmitters, glutamate and GABA, act on specific GPCRs [the metabotropic glutamate (mGlu) and GABAB receptors] to modulate synaptic transmission. These receptors are encoded by the largest gene family, and have been found to associate into both homo- and hetero-oligomers, which increases the complexity of this cell communication system. Here we show that dimerization is required for mGlu and GABAB receptors to function, since the activation process requires a relative movement between the subunits to occur. We will also show that, in contrast to the mGlu receptors, which form strict dimers, the GABAB receptors assemble into larger complexes, both in transfected cells and in the brain, resulting in a decreased G-protein coupling efficacy. We propose that GABAB receptor oligomerization offers a way to increase the possibility of modulating receptor signalling and activity, allowing the same receptor protein to have specific properties in neurons at different locations.
G-protein-coupled receptors (GPCRs) play a pivotal role in cell communication by activating intracellular events through both G-protein-dependent and -independent processes (Bockaert & Pin, 1999). These receptors are encoded by the largest gene family in mammals and offer multiple ways to modulate activity of specific cells. It is then not surprising that these receptors constitute the main target of drugs on the market and still represent the most promising targets for drug development (Hopkins & Groom, 2002; Overington et al. 2006). Even though these receptors represent a very complex receptor-signalling system, this complexity is likely to be higher considering that these receptors can organize into oligomeric complexes (Bouvier, 2001; Milligan, 2006). However, the existence of such large receptor assemblies in native systems and their functional consequences remain largely unsolved (Ferre et al. 2009).
Both major neurotransmitters, GABA and glutamate, activate not only ionotropic receptors, but also metabotropic receptors coupled to G-proteins, the GABAB receptor and the eight subtypes of metobotropic glutamate (mGlu) receptors (Conn & Pin, 1997; Bettler & Tiao, 2006). These receptors are part of a specific class of GPCRs, called class C, which represent promising targets for drug development in the area of addiction, anxiety and schizophrenia as well as for the treatment of neurodegenerative diseases such as Parkinson's disease.
Class C GPCRs represent an interesting model for study of the role of oligomerization in receptor function. Indeed, these receptors are well-recognized dimers, both in transfected cells and in neurons (Pin et al. 2003). While mGlu receptors form homodimers stabilized by an intersubunit disulphide bridge, the GABAB receptor is an obligatory heteromer composed of two subunits, GABAB1, where GABA binds, and GABAB2, which is responsible for G-protein activation (Kaupmann et al. 1998; Pin et al. 2004; White et al. 2007). Such a heteromeric assembly of the GABAB receptor at the cell surface is controlled by a quality control mechanism that involves the C-terminal domain of both subunits. Indeed, a binding site for the coat protein I complex (COP1, a protein complex involved in the trafficking of proteins from the cis-Golgi back to the endoplasmic reticulum) is located in the C-terminal tail of the GABAB1 subunit, preventing the export of this subunit through the Golgi and then leading to its retention in the endoplasmic reticulum (Margeta-Mitrovic et al. 2000; Brock et al. 2005). Through a coiled coil interaction with the GABAB2 C-terminal tail, the COP1 binding site is made inaccessible to COP1, allowing the heteromer to proceed through the Golgi and to reach the cell surface (Margeta-Mitrovic et al. 2000; Pagano et al. 2001).
Here we will summarize our recent work aiming to clarify the role of class C GPCR oligomerization. We will show that association of two subunits is required for function. We will then show that while mGlu receptors form strict dimers, the GABAB receptor can form larger complexes, offering new ways to modulate its activity.
Metabotropic glutamate and GABAB receptor dimerization is mandatory for G-protein activation
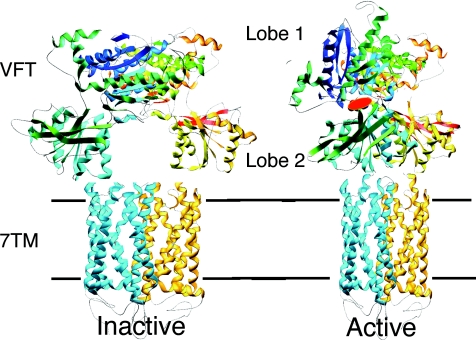
All GPCRs are integral membrane proteins with seven transmembrane helical segments (the so-called 7TM domain). In rhodopsin-like receptors, the ligand binds in a central cavity located in the extracellular side of the 7TM protein. Agonist binding results in a change in conformation, i.e. the opening of the intracellular surface, allowing G-protein binding and activation (Schwartz & Hubbell, 2008). Class C GPCRs also have a 7TM domain, but the agonist binding site is located in a specific large extracellular domain structurally similar to the bacterial amino acid binding proteins such as leucine–isoleucine–valine binding protein (Fig. 1). This domain, called the Venus Flytrap domain (VFT), is composed of two lobes separated by a clef where the agonist binding site is located (Kunishima et al. 2000). The VFT is in an open conformation, and reaches a closed state upon agonist binding, as is well illustrated by the crystal structure of the mGlu1 VFT solved with and without bound glutamate (Kunishima et al. 2000). We confirmed that competitive antagonists act by preventing VFT closure (Bessis et al. 2002), while locking the VFT in a closed conformation with a disulphide bridge results in a fully active receptor (Kniazeff et al. 2004b).
Figure 1. A model for the activation mechanism of the heterodimeric GABAB receptor based on the solved three-dimensional structure of the dimeric extracellular domain of mGlu1.
GABAB1 is in blue, GABAB2 in yellow. Binding of GABA in the cleft of GABAB1 Venus Flytrap domain (VFT) leads to domain closure and a reorientation of both VFTs in the dimer. This is expected to induce a relative movement of the seven transmembrane (7TM) domains, allowing activation of GABAB2 7TM. A similar activation mechanism is proposed for the mGlu receptors even though these have an additional cystein-rich domain that links their VFT to their 7TM.
But how can the closed VFT activate the 7TM domain? The first crystal structures of the mGlu1 VFTs revealed that these domains spontaneously assemble into dimers through a large hydrophobic surface located in lobe 1, leaving apart both lobes 2 in the inactive state (Kunishima et al. 2000; Tsuchiya et al. 2002). The closure of at least one VFT in the dimer allows a new stable association of both VFTs, with a direct contact between lobes 2 (Fig. 1). It was therefore proposed that this relative movement between the VFTs could lead to a relative movement of the 7TMs, resulting in their activation (Tateyama et al. 2004). However, the crystal structure of the mGlu3 VFT with bound agonists did not reveal such a movement of the VFTs (Muto et al. 2007), leaving open any other possibilities for the activation process.
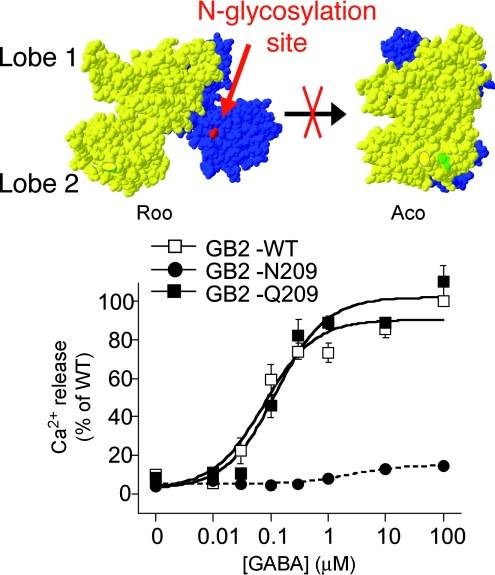
Using the GABAB receptor as a model, we aimed to prevent any possible association between lobes 2 by inserting an N-glycan [through the introduction of an N-glycosylation site, Nx(S/T)] at the lobe 2 interface. By doing so in either GABAB1 VFT or GABAB2 VFT, we did not impair the correct assembly of both subunits and reaching the cell surface, nor the binding of GABA in the GABAB1 VFT (Rondard et al. 2008). However, we completely prevented receptor activation, in agreement with the lobe 2 interface being exposed to the solvent in the inactive state of the receptor, but being in close proximity in the active state (Rondard et al. 2008; Fig. 2). To further demonstrate that such relative VFT movement was indeed sufficient for class C GPCR activation, we introduced additional cystein residues in the cystein-rich domain of the mGlu2 receptor, at a position suspected to form an additional disulphide bridge between both subunits in the active orientation only. Indeed, we succeeded in producing mutants that were constitutively active, and not further activated by agonists, nor inhibited by competitive antagonists (S. Huang, P. Rondard, J.F. Liu and J.P. Pin, unpublished data).
Figure 2. Insertion of an N-glycan at the lobe 2 interface of GABAB1 prevents GABAB receptor activation.
The illustrated models depict the resting conformation with both VFTs open (Roo) and the active conformation with one VFT closed and the other open (Aco), as based on the solved structure of the mGlu1 VFT dimer without (Roo) and with bound agonist (Aco). Intracellular Ca signals geberated by different concentrations of GABA were measured in cells expressing the wild-type GABAB receptor (GB2-WT), or that containg the GB2 subunit carrying an additional N-glycosylation site at position 209 (GB2-N209), or a variant not glycosylated (GB2-Q209). Note that replacement of the Asn residue in the glycosylation site by a Gln residue that is not glycosylated restores function. Adapted from Rondard et al. (2008).
Taken together, these data further validated the model of class C GPCR activation involving a relative movement of the VFTs within a dimeric entity to activate the 7TM domains. Accordingly, class C GPCR dimerization is mandatory for agonist activation of the 7TM domain, even though a single 7TM domain is activated at a time both in mGlu receptor homodimers (Goudet et al. 2005; Hlavackova et al. 2005) and in the GABAB receptors, in which GABAB2 7TM only is responsible for G-protein activation (Galvez et al. 2001; Duthey et al. 2002).
Analysis of mGlu and GABAB receptor oligomers at the cell surface using time-resolved fluorescence energy transfer (FRET)
Atomic force microscopy (AFM) images of rhodopsin in native retinal disk membranes revealed that this receptor probably organizes into large complexes best described as associated rows of dimers (Fotiadis et al. 2003). Owing to the expected high density of the mGlu and GABAB receptors at the synapse, we wondered whether these receptors could form larger complexes than dimers.
Energy transfer technologies, such as fluorescence energy transfer (FRET) or bioluminescence energy transfer (BRET) are now commonly used to validate the close proximity of proteins in living cells (Selvin, 2000; Bouvier, 2001; Vogel et al. 2006). These technologies are based on the non-radiative transfer of energy between a donor and an acceptor, leading to light emission of the acceptor. Since the distance between the fluorophore needed to obtain transfer is lower than 100 Å, FRET is commonly accepted as an indication that both partners are in very close proximity, contacting each other either directly or via a unique protein. For example, FRET between cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) fused to the different partners is used to validate the protein association in transfected cells. Alternatively, the CFP donor is replaced by a luciferase in the BRET approach. However, these approaches suffer from a number of limitations, firstly because the energy transfer is probably facilitated in the intracellular compartments (endoplasmic reticulum, Golgi and intracellular transport vesicles), where the expressed fusion proteins accumulate and, secondly, because the spectral properties of the fluorophores are not optimal, since the acceptor is excited at the wavelength used to excite the donor (in FRET) and the donor still emits at the peak emission wavelength of the acceptor (in both FRET and BRET).
We therefore decided to use fluorophores compatible with time-resolved FRET [TR-FRET; based on the use of long life-time fluorophores (caged europium or terbium) as donors; Mathis, 1995; Selvin, 2000; Bazin et al. 2002]. Using these fluorophore pairs, the FRET signal to noise ratio is increased by one thousand, firstly because of an optimal spectral compatibility and, secondly, because the acceptor emission resulting from FRET can be specifically measured 50 μs after donor excitation, when the emission of common fluorophores has returned to zero. This decay between excitation and measurement of FRET then leads to a dramatic decrease of the background noise owing to fluorescence of the cell components.
We combined the use of these fluorophores with two different labelling approaches of cell surface proteins, thus allowing the analysis of protein proximity at the cell surface exclusively. The first labelling approach is based on the use of monoclonal antibodies conjugated with the indicated fluorophores and targeting either an extracellular heamagglutinin (HA), flag or myc epitope inserted in the coding sequence, or a native epitope of the protein (Maurel et al. 2004; Fig. 3). The second approach is based on the use of snap-tag technology. The snap-tag is a 20 kDa protein derived from the 06-alkylguanine-DNA alkyltransferase (AGT) that can be covalently labelled with benzylguanine derivatives carrying the fluorophore of interest (Keppler et al. 2003, 2004). By inserting a snap-tag at the extracellular N-terminal end of the receptor, and addition of non-permeant benzylguanine derivatives, we could specifically label cell surface proteins with TR-FRET-compatible fluorophores (Maurel et al. 2008; Fig. 4).
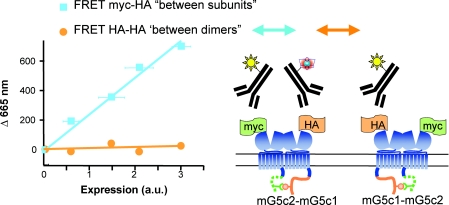
Figure 3. Metabotropic glutamate receptors form strict dimers at the cell surface.
mGlu5 subunits carrying the C-tail of GABAB1 (mG5c1 in orange, not reaching the cell surface alone) or GABAB2 (mG5c2, in green, allowing targeting to the cell surface of the C1-C2 combination), and taged with a myc and HA epitope, respectively, were labelled with FRET-compatible anti-HA antibodies only, or with a combination of anti-HA and anti-myc antibodies. Experiments were performed with cells expressing different amounts of receptors at the cells surface as quantified by ELISA. Note that a large TR-FRET emission, proportional to the amount of cell surface receptors, is observed when both subunits of the dimer are labelled. In contrast, no TR-FRET can be measured if only one subunit per dimer is labelled. Adapted from Kniazeff et al. (2004a).
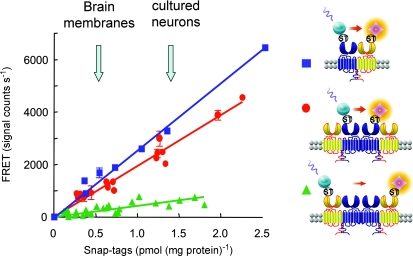
Figure 4. GABAB receptors organize into larger complexes.
GABAB1 (blue), GABAB2 (yellow) or both subunits were fused to a snap-tag (ST) at their N-termini, expressed at various densities in transfected cells, and labelled with benzylguanine derivatives carrying either Eu-crytate (TR-FRET donor) or d2-crytate (TR-FRET acceptor). The TR-FRET measurements revealed a close proximity between GABAB1 and GABAB2 subunits, but also a similar FRET between two GABAB1 subunits. In contrast, very low FRET was measured between two GABAB2 subunits. Note that the FRET emission is proportional to the amount of receptors at the cell surface (represented here as the amount of ST that can be labelled on intact cells) and is clearly detectable for densities similar to those reported in brain membranes or in cultured cortical neurons (arrows in the graph). In the graph, TR-FRET efficacy is represented by the slope of the curve and is then constant over the receptor densities analysed. Adapted from Maurel et al. (2008).
Using these approaches, we confirmed that a large TR-FRET signal could be detected at the cell surface between both subunits of the mGlu (Fig. 3) and GABAB receptors (Fig. 4; Maurel et al. 2004, 2008; Rives et al. 2009), in agreement with their known dimeric nature as indicated above.
We next aimed to analyse whether these dimers could assemble into larger complexes by inserting a single fluorophore (either donor or acceptor) per dimer. With that aim, we used the natural quality control system of the GABAB receptor, such that the subunit carrying the C-terminal tail of GABAB1 only reaches the cell surface when associated with a subunit carrying the C-terminal tail of GABAB2. Using this approach, and labelling the dimers with either antibodies or snap-tags, we have not been able to detect any significant FRET between mGlu receptor dimers (Kniazeff et al. 2004a; Brock et al. 2007; Maurel et al. 2008), indicating that these receptors did not spontaneously form larger complexes in transfected cells (Fig. 3). However, one cannot exclude the possibility that scaffolding proteins may help such larger complexes to form in neurons; however, at least this revealed that such oligomerization may not be necessary for proper receptor function.
In contrast, when similar experiments were conducted with the GABAB receptor, a large signal was measured when only the GABAB1 subunits were labelled, but not when GABAB2 carried the fluorophores (Maurel et al. 2008; Fig. 4). Such data cannot be interpreted as the consequence of a random clustering of GABAB dimers, since this would generate equivalent FRET signals whether only GABAB1 or GABAB2 was labelled. In contrast, these data are best explained by the association between GABAB dimers through GABAB1, leaving apart GABAB2 such that very low FRET can be measured between these subunits (Maurel et al. 2008). Further demonstration of GABAB receptor oligomers was obtained through cell surface co-immunoprecipitation experiments, demonstrating that HA-GABAB1 immunoprecipitation can pull down another GABAB1 subunit not carrying the HA epitope (Rives et al. 2009; L. Comps-Agrar, J. Kniazeff, J.P. Pin, unpublished data). The specificity of this association was further demonstrated by showing that the mGlu1 receptor did not associate with the GABAB receptor at the cell surface, as measured either by cell surface TR-FRET using antibodies or snap-tag technologies, or by co-immunoprecipitation experiments (Rives et al. 2009).
It is noteworthy that the efficacy of the TR-FRET signal measured between GABAB1 subunits was constant over a range of cell surface density and, most importantly, was observed for GABAB receptor densities reported in brain membranes and in cultured neurons (Fig. 4), further illustrating that this phenomenon was not a consequence of the overexpression of the receptor in transfected cells.
This led us to examine whether such GABAB large complexes could also be detected in the brain. With that aim, we used monoclonal antibodies specifically directed against the GABAB1a subunit (a variant of GABAB1 carrying an additional set of two sushi domains at the N-terminus) labelled with TR-FRET-compatible fluorophores. These antibodies were first used to label cell surface GABAB1a subunits in transfected cells, and revealed a clear TR-FRET signal, as observed with the other approaches using fusion proteins (L. Comps-Agrar, J. Kniazeff, M. Gassmann, B. Bettler, E. Trinquet, J.P. Pin, unpublished data). It is noteworthy that a large and significant TR-FRET signal was also measured from brain membranes (but not from mammary gland membranes known not to express the GABAB receptor), indicating the presence of GABAB oligomers in native tissue (L. Comps-Agrar, J. Kniazeff, M. Gassmann, B. Bettler, E. Trinquet, J.P. Pin, unpublished data).
Taken together, these data indicate that, in contrast to the mGlu receptors, which form strict dimers, the GABAB receptor can assemble into larger complexes through interactions involving the GABAB1 subunit (Fig. 5).
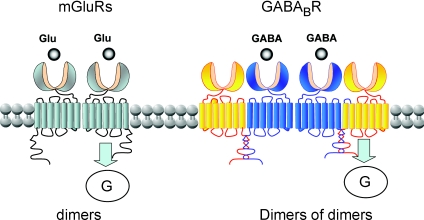
Figure 5. Schematic view of the oligomeric assembly of mGlu and GABAB receptors.
While the mGlu receptors are strict dimers, with a single subunit at a time activating G-proteins (Goudet et al. 2005; Hlavackova et al. 2005), the GABAB receptors assemble into dimers of dimers via interaction of the GABAB1 subunits (blue), with possibly a single receptor entity activating a G-protein at a time.
Oligomerization of GABAB receptors affects G-protein coupling efficacy
To start analysing the functional consequences of GABAB receptor association into large complexes, we aimed to prevent such association using a minimal domain of GABAB1 corresponding to the 7TM region, and then deleted of the GABA binding site. We assumed that such a small protein, by interacting with the GABAB1 subunit of the GABAB heterodimer, will prevent the formation of larger complexes. Overexpression of such a truncated GABAB1 subunit largely decreased the FRET measured between GABAB1 subunits (Fig. 6), but left constant the FRET between GABAB1 and GABAB2, consistent with the expected competition (Maurel et al. 2008). Is is interesting that disruption of the GABAB oligomers into heterodimers largely increased the coupling efficacy of the receptor to the G-protein (Maurel et al. 2008).
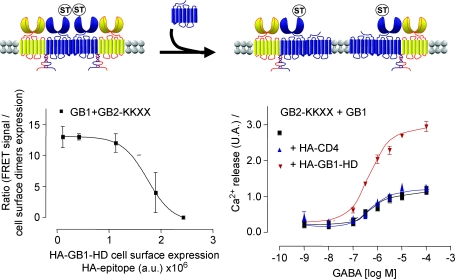
Figure 6. Disruption of GABAB oligomers increases G-protein coupling efficacy.
Left graph: FRET signals between the ST-labelled GB1 subunits co-expressed with a GB2 subunits carrying an ER retention signal (GB2-KKXX), as a function of the cell surface expression of the truncated form of GB1 (HA-GB1-HD). Right panel, Ca-mediated responses generated by increasing concentration of GABA in cells expresing GB1+GB2-KKXX alone (Back squares), or in the presence of a excess HA-CD4 (blue triangles), or the truncated form of GB1 (HA-GB1-HD) (inverted red triangles). By overexpressing the 7TM domain of GABAB1, deleted of both the VFT and the coiled coil domain, a disruption of the large GABAB receptor complexes is observed, as revealed by the large decrease in the TR-FRET signal between snap-tag (ST) GABAB1. In parallel, a twofold increase in the G-protein activation is observed, even though the amount of wild-type GABAB receptor heterodimers at the cell surface remained constant. Adapted from Maurel et al. (2008).
Although more work is necessary to confirm this interpretation, we propose that in a dimer of GABAB heterodimers, a single receptor unit (the association between a GABAB1 and a GABAB2 subunit) is able to activate a G-protein, such that separation of both units allows each hetorodimer to activate G-proteins, leading to a twofold increase in G-protein coupling efficacy (Fig. 6).
Conclusion
Taken together, these data confirm that class C GPCRs must assemble into dimers to allow agonist binding in their large extracellular domain to activate the 7TM domain, leading to G-protein activation. The data also revealed the surprising observation that GABAB receptors assemble into larger complexes. Even though a strict GABAB heterodimer is sufficient for proper G-protein activation, such larger assembly impairs G-protein coupling as if a single receptor unit per dimer of dimers could be active at a time. This can be the case if steric hindrance prevents the simultaneous association of two G-proteins per dimer of dimers. Alternatively, it is also possible that allosteric transition between the 7TMs prevents both receptors from reaching a fully active state at the same time. However, we cannot exclude the possibility that both receptor entities are functional, but display a lower coupling efficacy.
These findings raise the question of why such assemblies form in neurons if they are associated with a decreased signalling per receptor unit. So far, only the ability of the GABAB receptor to activate G-proteins has been analysed, such that it remains possible that this favours other signalling cascades by allowing more proteins to associate with the complex simultaneously. Such assembly may also influence the trafficking or cell surface dynamics of the receptor. More work will be necessary to solve these issues, not only in transfected cells, but most importantly in neurons.
Acknowledgments
This work was supported by the ‘Centre National de la Recherche Scientifique’ (CNRS), the ‘Institut National de la Santé et de la Recherche Médicale’ (INSERM), CisBio International, and by grants from the French Ministry of Research, Action Concertée Incitative ‘Biologie Cellulaire Moléculaire et Structurale’ (ACI-BCMS 328), the Agence Nationale de la Recherche (ANR-05-PRIB-02502, ANR-BLAN06-3_135092, ANR-05-NEUR-035 and ANR-NT09-481664), and by an unrestricted grant from Senomyx (La Jolla, CA, USA). D.M. was supported by the ADER Languedoc Roussillon, and C.M. by the Fondation pour la Recherche Médicale.
References
- Bazin H, Trinquet E, Mathis G. Time resolved amplification of cryptate emission: a versatile technology to trace biomolecular interactions. J Biotechnol. 2002;82:233–250. doi: 10.1016/s1389-0352(01)00040-x. [DOI] [PubMed] [Google Scholar]
- Bessis A-S, Rondard P, Gaven F, Brabet I, Triballeau N, Prézeau L, Acher F, Pin J-P. Closure of the Venus Flytrap module of mGlu8 receptor and the activation process: insights from mutations converting antagonists into agonists. Proc Natl Acad Sci USA. 2002;99:11097–11102. doi: 10.1073/pnas.162138699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettler B, Tiao JY. Molecular diversity, trafficking and subcellular localization of GABAB receptors. Pharmacol Ther. 2006;110:533–543. doi: 10.1016/j.pharmthera.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Pin J-P. Molecular tinkering of G-protein coupled receptors: an evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M. Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosci. 2001;2:274–286. doi: 10.1038/35067575. [DOI] [PubMed] [Google Scholar]
- Brock C, Boudier L, Maurel D, Blahos J, Pin J-P. Assembly-dependent surface targeting of the heterodimeric GABAB receptor is controlled by COPI, but not 14-3-3. Mol Biol Cell. 2005;16:5572–5578. doi: 10.1091/mbc.E05-05-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock C, Oueslati N, Soler S, Boudier L, Rondard P, Pin J-P. Activation of a dimeric metabotropic glutamate receptor by inter-subunit rearrangement. J Biol Chem. 2007;282:33000–33008. doi: 10.1074/jbc.M702542200. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin J-P. Pharmacology and functions of metabotropic glutamate receptors. Ann Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Duthey B, Caudron S, Perroy J, Bettler B, Fagni L, Pin J-P, Prézeau L. A single subunit (GB2) is required for G-protein activation by the heterodimeric GABAB receptor. J Biol Chem. 2002;277:3236–3241. doi: 10.1074/jbc.M108900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, Fuxe K, George SR, Javitch JA, Lohse MJ, Mackie K, Milligan G, Pfleger KD, Pin JP, Volkow ND, Waldhoer M, Woods AS, Franco R. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. 2009;5:131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotiadis D, Liang Y, Filipek S, Saperstein DA, Engel A, Palczewski K. Atomic-force microscopy: rhodopsin dimers in native disc membranes. Nature. 2003;421:127–128. doi: 10.1038/421127a. [DOI] [PubMed] [Google Scholar]
- Galvez T, Duthey B, Kniazeff J, Blahos J, Rovelli G, Bettler B, Prézeau L, Pin J-P. Allosteric interactions between GB1 and GB2 subunits are required for optimal GABAB receptor function. EMBO J. 2001;20:2152–2159. doi: 10.1093/emboj/20.9.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudet C, Kniazeff J, Hlavackova V, Malhaire F, Maurel D, Acher F, Blahos J, Prézeau L, Pin J-P. Asymmetric functioning of dimeric metabotropic glutamate receptors disclosed by positive allosteric modulators. J Biol Chem. 2005;280:24380–24385. doi: 10.1074/jbc.M502642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlavackova V, Goudet C, Kniazeff J, Zikova A, Maurel D, Vol C, Trojanova J, Prézeau L, Pin J-P, Blahos J. Evidence for a single heptahelical domain being turned on upon activation of a dimeric GPCR. EMBO J. 2005;24:499–509. doi: 10.1038/sj.emboj.7600557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K. A general method for the covalent labelling of fusion proteins with small molecules in vivo. Nat Biotechnol. 2003;21:86–89. doi: 10.1038/nbt765. [DOI] [PubMed] [Google Scholar]
- Keppler A, Pick H, Arrivoli C, Vogel H, Johnsson K. Labelling of fusion proteins with synthetic fluorophores in live cells. Proc Natl Acad Sci USA. 2004;101:9955–9959. doi: 10.1073/pnas.0401923101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeff J, Bessis A-S, Maurel D, Ansanay H, Prezeau L, Pin J-P. Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat Str Mol Biol. 2004a;11:706–713. doi: 10.1038/nsmb794. [DOI] [PubMed] [Google Scholar]
- Kniazeff J, Saintot P-P, Goudet C, Liu J, Charnet A, Guillon G, Pin J-P. Locking the dimeric GABAB G-protein coupled receptor in its active state. J Neurosci. 2004b;24:370–377. doi: 10.1523/JNEUROSCI.3141-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407:971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABAB receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- Mathis G. Probing molecular interaction with homogeneous techniques based on rare earth cryptates and fluorescence energy transfer. Clin Chem. 1995;41:1391–1397. [PubMed] [Google Scholar]
- Maurel D, Comps-Agrar L, Brock C, Rives M-L, Bourrier E, Ayoub MA, Bazin H, Tinel N, Durroux T, Prézeau L, Trinquet E, Pin J-P. Cell surface protein-protein interaction analysis with combined time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nat Methods. 2008;5:561–567. doi: 10.1038/nmeth.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel D, Kniazeff J, Mathis G, Trinquet E, Pin J-P, Ansanay H. Cell surface detection of membrane protein interaction with homogeneous time-resolved fluorescence resonance energy transfer technology. Anal Biochem. 2004;329:253–262. doi: 10.1016/j.ab.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Milligan G. G-protein-coupled receptor heterodimers: pharmacology, function and relevance to drug discovery. Drug Discov Today. 2006;11:541–549. doi: 10.1016/j.drudis.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Muto T, Tsuchiya D, Morikawa K, Jingami H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc Natl Acad Sci USA. 2007;104:3759–3764. doi: 10.1073/pnas.0611577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Pagano A, Rovelli G, Mosbacher J, Lohmann T, Duthey B, Stauffer D, Ristig D, Schuler V, Meigel I, Lampert C, Stein T, Prézeau L, Blahos J, Pin J-P, Froestl W, Kuhn R, Heid J, Kaupmann K, Bettler B. C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABAB receptors. J Neurosci. 2001;21:1189–1202. doi: 10.1523/JNEUROSCI.21-04-01189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin J-P, Galvez T, Prézeau L. Evolution, structure and activation mechanism of family 3/C G-protein coupled receptors. Pharmacol Ther. 2003;98:325–354. doi: 10.1016/s0163-7258(03)00038-x. [DOI] [PubMed] [Google Scholar]
- Pin J-P, Kniazeff J, Binet V, Liu J, Maurel D, Galvez T, Duthey B, Havlickova M, Blahos J, Prézeau L, Rondard P. Activation mechanism of the heterodimeric GABAB receptor. Biochem Pharmacol. 2004;68:1565–1572. doi: 10.1016/j.bcp.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rives M-L, Vol C, Tinel N, Trinquet E, Ayoub MA, Pin J-P, Prézeau L. Crosstalk between GABAB and mGlu1a receptors reveals new insights into GPCR signal integration. EMBO J. 2009;28:2195–2208. doi: 10.1038/emboj.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondard P, Huang S, Monnier C, Tu H, Blanchard B, Oueslati N, Malhaire F, Li Y, Maurel D, Trinquet E, Labesse G, Pin J-P, Liu J. Functioning of the dimeric GABAB receptor extracellular domain revealed by glycan wedge scanning. EMBO J. 2008;27:1321–1332. doi: 10.1038/emboj.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz TW, Hubbell WL. Structural biology: a moving story of receptors. Nature. 2008;455:473–474. doi: 10.1038/455473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin PR. The renaissance of fluorescence resonance energy transfer. Nat Struct Biol. 2000;7:730–734. doi: 10.1038/78948. [DOI] [PubMed] [Google Scholar]
- Tateyama M, Abe H, Nakata H, Saito O, Kubo Y. Ligand-induced rearrangement of the dimeric metabotropic glutamate receptor 1α. Nat Struct Mol Biol. 2004;11:637–642. doi: 10.1038/nsmb770. [DOI] [PubMed] [Google Scholar]
- Tsuchiya D, Kunishima N, Kamiya N, Jingami H, Morikawa K. Structural views of the ligand-binding cores of a metabotropic glutamate receptor complexed with an antagonist and both glutamate and Gd3+ Proc Natl Acad Sci USA. 2002;99:2660–2665. doi: 10.1073/pnas.052708599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel SS, Thaler C, Koushik SV. Fanciful FRET. Sci STKE. 2006;2006:re2. doi: 10.1126/stke.3312006re2. [DOI] [PubMed] [Google Scholar]
- White JF, Grodnitzky J, Louis JM, Trinh LB, Shiloach J, Gutierrez J, Northup JK, Grisshammer R. Dimerization of the class A G protein-coupled neurotensin receptor NTS1 alters G protein interaction. Proc Natl Acad Sci USA. 2007;104:12199–12204. doi: 10.1073/pnas.0705312104. [DOI] [PMC free article] [PubMed] [Google Scholar]