Abstract
Calcium microdomains generated by tight clusters of calcium channels regulate fusion of small vesicles at the synaptic terminal and have also been suggested to trigger exocytosis of large dense-core vesicles from neuroendocrine cells. To test this idea, we have compared sites of exocytosis and the spatial distribution of calcium channels in chromaffin cells. Fusion of individual vesicles was visualized using interference reflection microscopy and the submembranous calcium signal was assessed using total internal reflection fluorescence microscopy. Depolarization triggered a burst of exocytosis from up to seven sites in a membrane area of 11 μm2, but these sites did not colocalize with calcium microdomains. Instead, calcium influx occurred in large patches (averaging 34 μm2) containing a mixture of P/Q- and N-type channels. About 20% of fusion events occurred outside calcium channel patches. Further, the delay between the onset of stimulation and a burst of exocytosis was prolonged for several seconds by increasing the concentration of the slow calcium chelator EGTA from 1.5 to 5 mm. These results demonstrate that while calcium channels and release sites tend to congregate in specialized regions of the surface membrane, these have dimensions of several micrometres. The dominant calcium signal regulating release in chromaffin cells is generated by the cooperative action of many channels operating over distances of many micrometres rather than discrete clusters of calcium channels generating localized microdomains.
Introduction
It is unclear how secretion in neuroendocrine cells is coupled to the opening of Ca2+ channels. One view is that a docked vesicle is triggered to fuse by a nearby ‘Ca2+ microdomain’ generated around a tight cluster of open Ca2+ channels (Olivos Ore & Artalejo, 2004), leading to the suggestion that excitation–secretion coupling in neuroendocrine cells is similar to synapses, where vesicles and Ca2+ channels cluster tightly at active zones (Cohen et al. 1991; Zenisek et al. 2003; Beaumont et al. 2005). However, this view is difficult to square with the functional and morphological differences in excitation–secretion coupling at synapses and endocrine cells. At synapses, vesicles begin fusing < 1 ms after Ca2+ channels open in response to an action potential (Robitaille et al. 1990; Cohen et al. 1991), while secretion in neuroendocrine cells is at least 100-fold slower (Almers, 1990; Martin, 1994; Zhou & Misler, 1995). Further, amperometry and capacitance experiments testing the effects of Ca2+ buffers indicate that delayed secretion is due to Ca2+ diffusion over distances of micrometres between Ca2+ channels and release sites (Chow et al. 1994, 1996).
The spatial organization of the secretory machinery in synapses is also very different to that in neuroendocrine cells. Fast release of neurotransmitter occurs at specialized active zones a few hundred nanometres in diameter where synaptic vesicles are tightly docked to the surface membrane prior to fusion. In contrast, docked granules in chromaffin cells display mobility within a 220 nm cage when unprimed and only become immobile after priming has taken place (Nofal et al. 2007). The structure organizing calcium channels in a synaptic terminal is the active zone (Khimich et al. 2005; Schoch & Gundelfinger, 2006; Long et al. 2008), but these are not observed in neuroendocrine cells. It is not clear, therefore, whether one might expect the same spatial organization of calcium signal and fusion site in neuroendocrine cells and synapses.
To understand excitation–secretion coupling in neuroendocrine cells, the submembranous Ca2+ signal should be compared with the spatial distribution of release sites. While Ca2+ microdomains have been visualized at synapses (Cohen et al. 1991; Tucker & Fettiplace, 1996; Zenisek et al. 2003; Beaumont et al. 2005), they have not been clearly demonstrated in neuroendocrine cells. Pulsed-laser imaging and amperometry were used to monitor Ca2+ influx and exocytosis in chromaffin cells and the results indicated that these processes tend to occur in similar regions of the membrane, although the spatial resolution did not allow detection of Ca2+ microdomains or ‘active zones’ of exocytosis (Robinson et al. 1995). A technique that provides better spatial resolution for detecting calcium influx at the membrane is total internal reflection fluorescence microscopy (TIRFM) (Zenisek et al. 2003; Beaumont et al. 2005; Demuro & Parker, 2006; Serulle et al. 2007). Becherer et al. (2003) applied TIRFM to chromaffin cells and inferred the existence of microdomains from local spikes in the noisy Ca2+ signal, but the stimulus they used – depolarization for several seconds by application of high K+– did not allow spatial calcium microdomains to be observed. We therefore set out to visualize calcium influx in chromaffin cells by using TIRFM to monitor calcium signals generated by very brief (2 ms) stimuli. Sites of granule fusion in the same region of membrane were then mapped using interference reflection microscopy (IRM; Llobet et al. 2008). The results we present demonstrate that while Ca2+ channels and release sites tend to occupy the same region of membrane, they are not tightly coupled in space. Excitation–secretion coupling in chromaffin cells therefore stands in contrast to synapses, where Ca2+ channels form tight clusters at the active zone (Cohen et al. 1991; Tucker & Fettiplace, 1996; Zenisek et al. 2003; Beaumont et al. 2005; Neher & Sakaba, 2008).
Methods
Cell preparation and solutions
Bovine adrenal chromaffin cells were prepared as described by Seward & Nowycky (1996). Adrenal glands were obtained from a local slaughterhouse and chromaffin cells isolated by collagenase digestion followed by purification using a Percoll gradient. Cells were plated onto poly-l-lysine-coated coverslips and used 1–3 days after preparation. Ringer solution contained (in mm): 140 NaCl, 2.5 KCl, 1 MgCl2, 10 Hepes, 10 glucose, 2.5 CaCl2, pH 7.4, 300 mosmol kg−1. For TIRFM experiments 1 nm tetrodotoxin (Tocris, Ellisville, MO, USA) was added to Ringer solution. Nifedipine was from Sigma (St Louis, MO, USA), nimodipine from EMD Biosciences (San Diego, CA, USA); ω-agatoxin IVA and ω-conotoxin GVIA were from Alomone Labs (Jerusalem, Israel). Solutions were perfused onto cells using an array of quartz pipes (World Precision Instruments, Sarasota, FL, USA) controlled by a system of valves (Warner Instrument Corp., Hamden, CT, USA). The solution in the patch pipette contained (in mm): 120 caesium methanesulfonate, 10 TEA-Cl, 5 MgCl2, 20 Hepes, 3 Na2ATP, and 1 NaGTP, pH 7.2, 290 mosmol kg−1. For IRM experiments, the pipette solution contained variable amounts of Ca2+ buffer, either (in mm) 0.1 EGTA, 1.5 EGTA, 5 EGTA or 0.4 BAPTA. For TIRFM experiments, pipette solution contained either 0.1 mm OregonGreen488-BAPTA-2 (Kd= 0.6 μm; OGBAPTA-2) or 0.2 mm Fluo-5N (Kd= 90 μm; Molecular Probes, Eugene, OR, USA).
Electrophysiology and imaging
IRM imaging was performed as described previously (Llobet et al. 2003; Beaumont et al. 2005). Cells were voltage clamped in the whole-cell configuration using an Axopatch 200A amplifier (Axon Instruments, Union City, CA, USA) and acquired with a G4 Macintosh equipped with an ITC-16 interface (Instrutech Corp., Port Washington, NY, USA) controlled by the Pulse Control extension (Herrington & Bookman, 1994) of IGOR Pro software (Wavemetrics, Lake Oswego, OR, USA). Exocytosis was elicited by depolarizing from −80 mV to +10 mV, either as a single 500 ms step or as a train of 24 × 105 ms or 4 × 90 ms pulses. We used 105 ms pulses to allow each to be delivered at the beginning of a frame acquired by the camera. Image acquisition was performed using IPLab software and triggered by a TTL pulse delivered by the ITC-16 at the beginning of the electrophysiological recording. Calcium currents were leak subtracted using macros written in IGOR Pro. All IRM images and movies were ‘background subtracted’ using a macro written in IPLab (Scanalytics, Fairfax, VA, USA) to better visualize changes.
‘Through the objective’ TIRFM was combined with IRM as described (Llobet et al. 2003; Beaumont et al. 2005). A 488 nm beam from an argon laser provided the TIRFM excitation light. The light for IRM originated from a 100 W xenon lamp (Newport-Oriel, Irvine, CA, USA) and was transmitted through a 510WB40 filter (Omega Optical, Brattleboro, VT, USA) before passing through a combining cube (Oriel). The filter block contained a 505DRLP dichroic (Omega), which reflected >95% at 488 nm and about 35% at 510 nm, while transmitting > 80% at wavelengths > 515 nm. About 35% reflection was sufficient for IRM if the xenon lamp was at maximum power. When only TIRFM was used, emitted light was filtered through a HQ510LP filter (Chroma Technology, Rockingham, VT, USA) and 488 nm notch filter (Coherent, Santa Clara, CA, USA). When TIRFM was combined with IRM, emitted light was filtered through a 545AF75 filter (Omega). Images were magnified × 2.2 before being acquired by a Princeton Instruments Pentamax CCD camera and Winview32 acquisition software (Roper Scientific, Trenton, NJ, USA). Image acquisition was synchronized with the electrophysiological recording using a Master-8 pulse generator (AMPI, Jerusalem, Israel). The membrane current and camera exposure signal time were digitized (20 kHz) and filtered (5 kHz) by a Digidata 1322A interface (Axon).
Images of Ca2+ influx in Figs 4–9 are shown as ΔF/F0, where ΔF=F–F0, F is the individual frame and F0 is the average image at rest (obtained from between 4 and 15 consecutive frames acquired before the stimulus). The protocol for identifying sites of calcium influx by TIRFM is described further in Supplemental Fig. 2. To quantify changes in Ca2+ influx between different stimulus trains (Figs 8 and 9), we compared ΔF values measured in the frame in which the 2 ms stimulus was applied. Each pixel from the ΔF image obtained after adding a blocker of Ca2+ channels was divided by the corresponding pixel in the ΔF image obtained before addition of the blocker. The ratio values for each pixel within the Ca2+ channel patch were then averaged to give the mean intensity ratio (±s.d.) over all pixels in the patch. The ratio image of Stim 2/Stim 1 therefore provided a measure of the relative change in the amount of Ca2+ influx in the footprint. Measurements of the free [Ca2+] under the membrane using Fluo-5N were calibrated (Fig. 3) by measuring Fmin in pipette solution containing 10 mm EGTA and Fmax in pipette solution containing 1 mm CaCl2. The Kd for Fluo-5N was assumed to be 90 μm, measured by the manufacturers. Results are expressed as mean ±s.e.m. unless otherwise indicated.
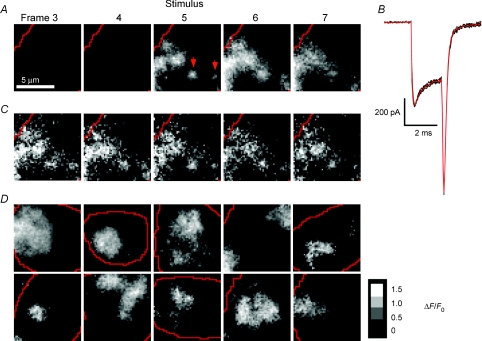
Figure 4. Visualizing sites of calcium influx by TIRFM.
A, images showing the relative change in fluorescence (ΔF/F) of OGBAPTA-2 (0.1 mm) in the footprint of a chromaffin cell. Averaged from six movies of 7 × 11 ms frames acquired at 10 s intervals, all from the same cell. Calcium influx was triggered by a 2 ms depolarization (−80 to +10mV) delivered at the beginning of frame 5; a 1 ms prepulse from −80 mV to +150 mV was delivered immediately preceding the 2 ms depolarization. Red lines indicate the edges of the footprint. B, whole-cell Ca2+ currents recorded for each of the 6 stimuli delivered to the cell in A (black traces) and the average of the 6 traces (red). C, five individual examples of the stimulus frames used to make the averaged frame 5 in A. D, a gallery of averaged stimulus frames showing the relative change in intensity (ΔI/I0) from 10 different cells on a grey scale. Each image is the average of 6 or 8 stimulus frames. Scale bar in arbitrary units shows ΔI/I0.
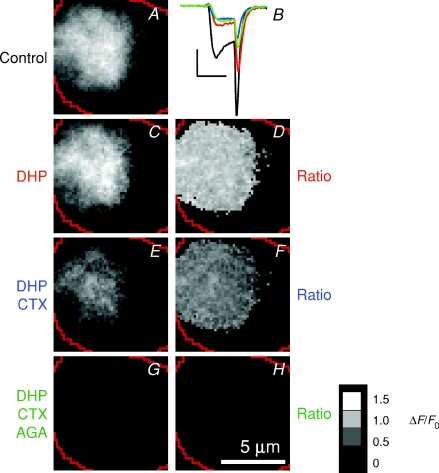
Figure 9. Absence of L-type calcium channels in the footprint.
A, images of Ca2+ influx (ΔF/F) obtained at rest, then after successively adding 20 μm nifedipine and 20 μm nimodipine (C), 1 μmω-conotoxin GVIA (E) and 200–400 nmω-agatoxin IVA (G), all to the same cell. The corresponding ratio images are shown in D, F and H; the Ca2+ currents are shown in B. Dihydropyridines blocked the majority of Ca2+ channels in the surface membrane of this cell as a whole (B), but none in the footprint (D). Calibration bar in B: 200 pA × 2 ms; scale bar, 5 μm; ratio calibration bar = 1.5 (white), 1.0, 0.5, 0 (black).
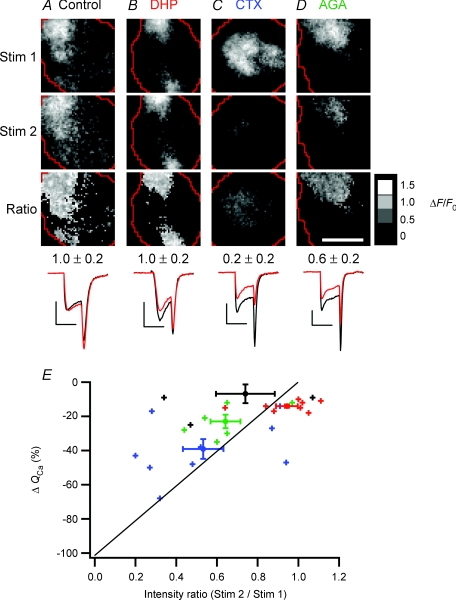
Figure 8. Calcium channel patches in footprints consist primarily of N- and P/Q-type channels.
A–D, images of Ca2+ influx (ΔF/F) obtained at rest (Stim 1, top row) and in a test condition (Stim 2, second row), as well as the ratio of Stim 2/Stim 1 (Ratio, third row). Each frame is averaged from 6 responses to depolarizations lasting 2 ms. Before and during the second stimulus set (Stim 2), cells were perfused either with normal Ringer solution (A), 20 μm nifedipine and 20 μm nimodipine (B), 1 μmω-conotoxin GVIA (C) or 200–400 nmω-agatoxin IVA (D). Mean ratio values (±s.d.) over the patch of Ca2+ channels are shown below the ratio images. The averaged whole-cell Ca2+ currents for Stim 1 (black) and Stim 2 (red) are at bottom; all calibration bars: 200 pA × 2 ms. Scale bar: 5 μm; ratio calibration bar = 1.5 (white), 1.0, 0.5, 0 (black). E, collected results (n= 28 cells), showing the percentage change in Ca2+ influx over the whole cell (ΔQCa) versus the intensity ratio over the patch of Ca2+ channels. Small crosses represent data from individual cells perfused with either control Ringer solution (black), dihydropyridines (red), conotoxin (blue), or agatoxin (green) before collecting the second set of stimulus images. Large markers with error bars represent means. The diagonal line marks the relationship expected if ΔF/F were directly proportional to QCa and all types of Ca2+ channels were randomly distributed over the whole surface of the cell, including the footprint.
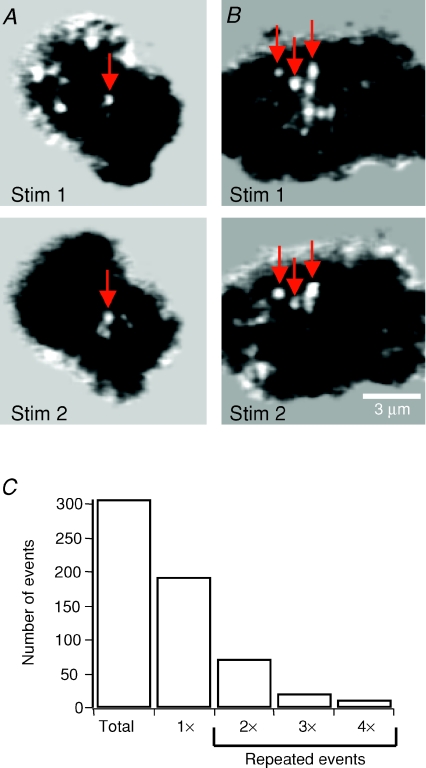
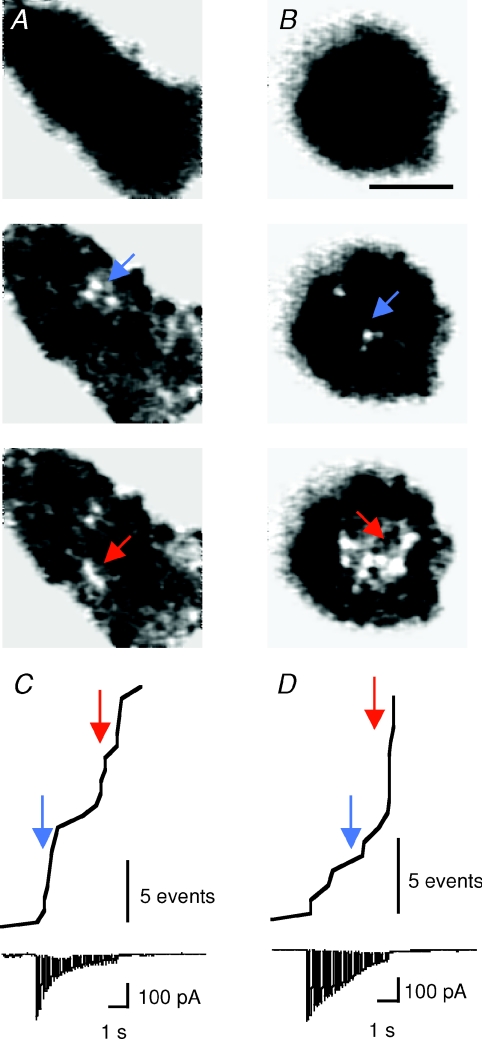
Figure 3. Specialized sites of fusion.
A and B, background-subtracted images from the IRM movies of two cells, each stimulated twice (Stim 1 and Stim 2). Arrows are located in the same position during the first and second stimulations, indicating fusion events occurring in the same spot. Stim 1 and Stim 2 images in B are from Supplemental Movie 2 (available online only). C, histogram showing the number of fusion events that occurred within the same spots two, three, or four times, from a total of 307 events. The bar marked ‘1×’ is the number of events that did not overlap with any others.
Results
Using IRM to detect fusion of large dense-core vesicles
To investigate excitation–secretion coupling in chromaffin cells we visualized sites of fusion using IRM, a technique that detects local invaginations of the surface membrane generated by fusion of a vesicle. Examples of such events are shown in Fig. 1A and B, where they appear as localized ‘spots’ within the footprint of the cell (see Movie 1 in Supplemental material, available online only). A large number of experimental observations indicate that these spots are generated by the Ω-shapes formed when a vesicle fuses with the surface membrane (Llobet et al. 2003, 2008). First, spots in the footprint only occur during and after the application of a depolarizing stimulus. Second, they are synchronized in time and space with the release of FM4-64, acridine orange or pro-atrial natriuretic peptide (ANP)-GFP within granules, as visualized by simultaneous TIRFM and IRM imaging. Third, the number of events in a footprint is directly proportional to the total increase in surface area measured by the capacitance technique and follows the same time course. Fourth, the average capacitance increase per event is 1.43 fF, which is very similar to the average capacitance of a granule estimated by Ales et al. (1999). Fifth, the kinetics of IRM signals are altered by a number of experimental manipulations known to affect the fusion or retrieval of granules. For instance, the speed of IRM signals are calcium dependent: recovery of the signal is blocked by strontium, which also blocks vesicle retrieval assayed by capacitance (Llobet et al. 2008). The full procedure by which IRM was used to identify the timing and location of fused granules is described in the online ‘Supplementary Information’ and Supplemental Fig. 1.
Figure 1. Bursts of fusion events occurring in clusters on the membrane.
A and B, background-subtracted IRM images from 2 different cells stimulated with a train of 24 × 105 ms depolarizations at 5 Hz. Before stimulation, IRM footprints were uniformly dark (top row), but discrete spots appear on depolarization. A was recorded with 1.5 mm EGTA in the patch pipette and B with 5 mm EGTA. Image in B comes from Supplemental Movie 1, available online only. Scale bar, 5 μm. C and D, the cumulative number of fusion events and the corresponding whole-cell Ca2+ currents plotted for cells in A and B. Coloured arrows correspond to time points at which the frames shown in A and B were extracted. Note the slower inactivation of Ca2+ currents in D compared to C. Scale bar, 5 fusion events.
Bursts of exocytosis were delayed by EGTA
When a chromaffin cell was depolarized, fusion events within the footprint often occurred in bursts, and the timing of these depended on the concentration of the Ca2+ chelator inside the whole-cell pipette. The cell in Fig. 1A was dialysed with 1.5 mm EGTA, which binds Ca2+ ions with a Kd of ∼150 nm. While Ca2+ channels were closed, there was no activity (top), but a stimulus train lasting 5 s triggered the appearance of discrete bright spots. A cumulative plot of fusion events shows that exocytosis in this footprint occurred in two distinct bursts, the first complete within 1 s of the beginning of the stimulus train and the second occurring more slowly over a period of several seconds (Fig. 1C). Two phases of exocytosis are also observed in chromaffin cells using the capacitance technique and release of caged Ca2+: the initial exocytic burst is complete within a few hundred milliseconds at 10–20 μm Ca2+ and the second occurs at a rate of 0.1 s−1 (Xu et al. 1998). In comparison, we estimated that the [Ca2+] within ∼100 nm of the membrane reached ∼20–30 μm during depolarization under conditions of low Ca2+ buffering (Fig. 7).
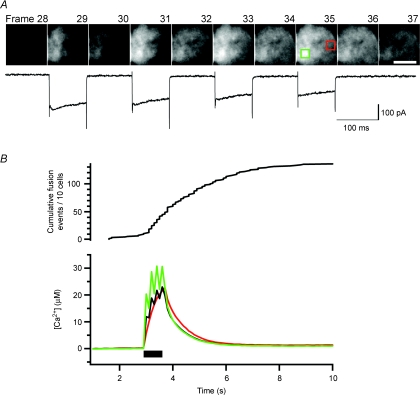
Figure 7. Visualizing calcium influx with a low affinity calcium indicator.
A, ten consecutive frames showing ΔF/F from a cell loaded with 0.2 mm Fluo-5N through the patch pipette. The whole-cell Ca2+ current is shown below. The four 90 ms depolarization steps occurred within frames 29, 31, 33 and 35 (100 ms per frame). Scale bar: 5 μm. B, the submembranous [Ca2+] averaged over the whole footprint (black) for the same cell shown in A was compared with the [Ca2+] of two ROIs (each 10 pixels × 10 pixels), one ROI centred over a patch of Ca2+ channels (green box in A) and one remote from Ca2+ channels (red box). The black bar indicates the time of the depolarization. Similar results were seen for seven other cells. The cumulative exocytosis events measured by IRM in response to the same stimulus is shown above (n= 10 cells).
When the concentration of EGTA inside the pipette was increased from 1.5 mm to 5 mm, the initial rate of exocytosis was slowed (Fig. 1D). Averaged time courses of exocytosis in 0.1 mm, 1.5 mm and 5 mm EGTA are shown by the cumulative histograms in Fig. 2A–C. Each plot is averaged from 4–5 footprints, and was obtained by making cumulative histograms of all events under each condition, then dividing by the number of footprints from which these measurements were collected. The initial rates of granule fusion were then compared by making linear fits to the cumulative histograms (bold lines in Fig. 2D). Under these stimulation conditions, the frequency of fusion events was sensitive to EGTA, as would be expected from capacitance measurements (Moser & Neher, 1997). The initial rate of fusion in 0.1 mm EGTA was sevenfold higher than in 5 mm EGTA (Fig. 2D), but there was still an obvious burst of exocytosis in 5 mm EGTA; the crucial difference was that the most rapid phase of fusion in 5 mm EGTA was delayed by 3–4 s. In other words, the slowing of exocytosis by EGTA could be overcome by more prolonged Ca2+ influx. This observation argues strongly against the idea that the signal triggering granule fusion is a localized microdomain, for two reasons. First, both theory (Naraghi & Neher, 1997) and experimental measurements (Zenisek et al. 2003; Beaumont et al. 2005) demonstrate that Ca2+ microdomains persist in EGTA concentrations up to 5 mm, because this chelator binds Ca2+ ions relatively slowly. The major effect of EGTA is to suppress the ‘global’ increase in Ca2+ generated by diffusion of Ca2+ away from sites of influx (Zenisek et al. 2003; Beaumont et al. 2005). Second, Ca2+ microdomains are generated within milliseconds when Ca2+ channels open, even in the presence of high concentrations of EGTA (Zenisek et al. 2003). The delayed burst of exocytosis in 5 mm EGTA must therefore reflect the gradual accumulation of free Ca2+ ions some distance from calcium channels.
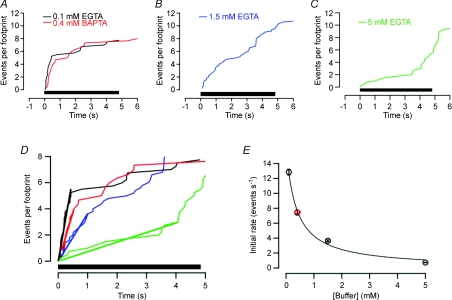
Figure 2. EGTA increased the delay between the beginning of stimulation and the first burst of exocytosis.
A–C, cumulative plot of number of fusion events per footprint observed during trains of 24 × 105 ms depolarizations delivered at 5 Hz. Cells were dialysed with 0.1 mm EGTA (A; n= 4), 1.5 mm EGTA (B; n= 5), 5 mm EGTA (C; n= 4) or 0.4 mm BAPTA (A; n= 3). D, the traces in A–C superimposed on an expanded time scale. The initial phases of release have been fitted with straight lines through the origin (bold). E, the initial rate of fusion events (per second per footprint) as a function of the concentration of EGTA or BAPTA in the patch pipette. The line through the points is the least-squares fit of the relation R=Rmax[1 − (E/(E+E1/2))], where E is the concentration of buffer in mm, E1/2 is the concentration where inhibition is half-maximal (0.36 mm) and Rmax is the maximum rate of release in the absence of any calcium buffer in the patch pipette (16 events per second per footprint). Both Rmax and E1/2 were free parameters in the fitting process. The red point is a measurement in BAPTA and the black points are measurements in EGTA. Error bars show the standard deviation of the linear fits in D (some of these are narrower than the points).
Secretion triggered by calcium microdomains at the synapse can be inhibited much more efficiently by the Ca2+ chelator BAPTA compared to EGTA (Adler et al. 1991; Burrone et al. 2002). BAPTA binds Ca2+ ions with a similar affinity to EGTA but about 60-fold faster, and therefore has a stronger effect on calcium signals close to calcium channels (Neher, 1998). To compare the effects of these buffers on granule fusion in chromaffin cells, we first looked at the relation between the initial rate of fusion and the EGTA concentration, shown by the black circles in Fig. 2E. These measurements could be described empirically by a function assuming that EGTA inhibits granule fusion according to the relation R=Rmax[1 − (E/(E+E1/2))], where E is the concentration of EGTA in mm, E1/2 is the concentration where inhibition is half-maximal (0.36 mm) and Rmax is 16 events per second per footprint. To test whether BAPTA was any more effective than EGTA at slowing granule fusion, we introduced 0.4 mm BAPTA, which would be expected to have an effect that was approximately half-maximal if the two chelators were equally effective at antagonizing granule fusion (Fig. 2E). The red point in Fig. 2E shows that BAPTA was not significantly more, or less, effective than EGTA. This observation indicates that the equilibrium dissociation constant of these chelators is more important than the association constant for Ca2+ binding in inhibiting granule fusion, and that this process is therefore triggered by the global increase in [Ca2+] rather than Ca2+ microdomains.
Repeated fusion events at specialized sites
At some sites in the membrane, fusion events occurred repeatedly during a train of stimuli lasting 5 s. Figure 3A and B shows examples of two cells which were stimulated twice, and the arrows identify fusion events which occurred in the same spot in the separate stimuli (see also online Supplemental Movie 2). Of 307 fusion events in 12 cells stimulated 3–6 times, 115 occurred within one pixel of each other (the centre of the event being defined as the pixel in which the IRM signal was at its maximum and each pixel being 73 nm square). In some cases, different fusion events occurred centred over the same pixel three or more times (Fig. 3C). Thus at least 37% of fusion events occurred at sites that were fixed in the membrane over the tens of minutes time scale of these experiments. This figure will be a lower limit because the relatively low average release probability at a single site would have required many repetitive stimuli to identify all the preferred sites in this way. Nonetheless, these results indicate that the membrane of chromaffin cells contains areas at which vesicles can fuse repeatedly. Clustering of multiple fusions in a single site could be due to the sequential arrival of different granules to preferential release zones of ∼0.4 μm2 (Rickman et al. 2004; Kishimoto et al. 2006) and/or to a small granule population undergoing repeated cycles of fusion and re-sealing in a circumscribed portion of the membrane (Perrais et al. 2004).
Calcium influx occurred in large patches
We next sought to investigate how sites of fusion were organized spatially relative to Ca2+ channels in the membrane, and in particular whether depolarization generated discrete calcium microdomains or more diffuse signals. Submembranous Ca2+ was imaged by TIRFM using the indicator OGBAPTA-2 (0.1 mm). At the synapse of goldfish retinal bipolar cells, Ca2+ microdomains generated by tight clusters of Ca2+ channels can be clearly visualized after depolarizations lasting 20 ms (Zenisek et al. 2003; Beaumont et al. 2005). We sought to capture an even briefer ‘snap-shot’ of Ca2+ entry in chromaffin cells by depolarizing for just 2 ms and integrating the signal over a single frame lasting 11 ms. This stimulus moved a Ca2+ charge of 0.9 ± 0.1 pC into the cell, similar to the ∼1 pC delivered in response to a single action potential (Giovannucci et al. 1999). The resulting calcium signal is shown in Fig. 4A, which is a series of 5 out of 7 consecutive frames, with the depolarization delivered at the beginning of frame 5. Calcium influx was not uniform across the footprint, but occurred in distinct patches. Then, after Ca2+ channels closed, the fluorescence gradually dissipated as Ca2+ diffused away from entry sites (Fig. 4A, frames 6 and 7). The source of Ca2+ influx was a relatively fixed array of channels because when the depolarization was repeated (Fig. 4B), the submembranous Ca2+ signal was similar for each of five stimuli (Fig. 4C). Calcium influx therefore occurred diffusely rather than in discrete puncta. Calcium influx visualized by TIRFM is also shown in Supplemental Movies 3 and 4.
Might these ‘patchy’ Ca2+ signals reflect non-uniform attachment of the footprint to the glass coverslip? Three observations argue against this possibility. First, the IRM experiments showed that footprints presented a homogeneous pattern of interference before stimulation (Fig. 1A and B). Moreover, signals arising exclusively from the edges of the footprint were not considered because these were the membrane regions that tended to display a looser attachment to the coverslip. Second, the fluorescence intensity of OGBAPTA-2 viewed by TIRF was relatively uniform across the footprint at resting levels of Ca2+ (Fig. 5 below). And, third, the submembranous Ca2+ signal measured by TIRFM was also relatively uniform when a large Ca2+ load was introduced and allowed to dissipate after closure of Ca2+ channels. An example of this behaviour can be seen in Fig. 7A, where there is a patchy Ca2+ signal evident during the first depolarization of a train (frame 29), but a much more uniform signal after closure of Ca2+ channels at the end of the train (frame 36). We conclude that the patchy signals reported by OGBAPTA-2 reflected the distribution of calcium channels in the surface membrane.
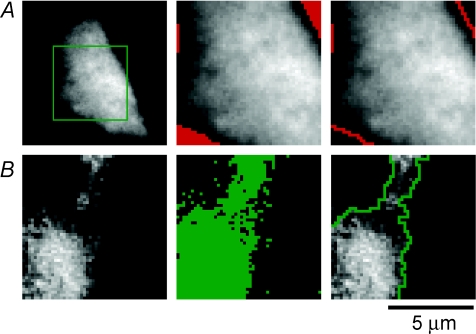
Figure 5. Defining the area of the footprint and calcium patches.
A, defining the area of the footprint. TIRFM image of a voltage-clamped chromaffin cell, at rest, loaded with 0.1 mm OGBAPTA-2. To acquire TIRFM images at 11 ms per frame, data collection was restricted to a 50 pixels × 50 pixels ROI (green box, left). To define the outline of the footprint, we determined the minimum (MINframe1) and maximum (MAXframe1) pixel intensities for the first frame in a movie. A threshold was then set at the minimum pixel intensity (MINframe1) + 20% of the total intensity range (MAXframe1) – (MINframe1). All pixels below the threshold are marked in red (middle), and the margins of these regions are shown by the lines in image at right. B, defining the area of calcium influx. An image of the relative change in the fluorescence of OGBAPTA-2 (ΔF/F) in the frame in which Ca2+ influx was activated for 2 ms is shown at left. This image was thresholded by first determining the maximum pixel intensity over all the prestimulus frames (MAXframe1-4) and the maximum pixel intensity of the stimulus frame (MAXframe5). The minimum threshold was set as (MAXframe1-4) + 20% of the intensity range (MAXframe5) – (MAXframe1-4). The pixels above this threshold are marked in green in the middle panel and the margins of this area are shown by the green line in the right panel.
Measuring areas of calcium influx
The area covered by arrays of Ca2+ channels varied widely. Two relatively localized Ca2+ signals, indicating a small cluster of Ca2+ channels, are shown by the red arrows in Fig. 4A. These signals might be termed ‘microdomains’ because their spatial scale is 1–2 μm (Neher & Sakaba, 2008), but the predominant signal was much more widely distributed. The areas of Ca2+ influx in the footprints of ten other cells are shown in Fig. 4D. Surveying these examples, it is possible to see ‘hotspots’ within the larger regions of Ca2+ influx, indicating that the density of Ca2+ channels per unit area was not necessarily uniform across these patches. The basic observation was that independent microdomains (such as arrowed in Fig. 4A) were rare, and the predominant Ca2+ signal was distributed over much wider regions. These patches of Ca2+ influx were relatively well circumscribed and their boundaries could be recognized by eye (Fig. 4D).
To measure the areas of the footprint and patches of Ca2+ influx we applied a thresholding technique. Figure 5A shows the resting fluorescence of a chromaffin cell loaded with 0.1 mm OGBAPTA-2 (left). To allow imaging at 11 ms per frame, we sampled a 50 pixels × 50 pixels region of interest (ROI). The minimum and maximum pixel intensities for the first frame in a movie were measured (MINframe1 and MAXframe1), and a threshold set at 20% of the total intensity range (i.e. MINframe1+ 0.2(MAXframe1− MINframe1)). This thresholding criterion was chosen as one which effectively separated the footprint from the background signal over the neighbouring coverslip. An example is shown in Fig. 5A, where all pixels below the threshold are marked in red (middle), and the margins of these regions, marking the outline of the footprint, are shown by the lines in the image on the right. Similar criteria were applied to define patches of Ca2+ influx. Figure 5B shows the relative change in the fluorescence of OGBAPTA-2 in the frame in which Ca2+ influx was activated for 2 ms (left). First we determined the maximum pixel intensity over all the prestimulus frames (MAXframe1-4), then the maximum pixel intensity of the stimulus frame (MAXframe5). The minimum threshold was set at 20% of this range (i.e. MAXframe1-4+ 0.2(MAXframe5− MAXframe1-4)). The pixels above this threshold are marked in green in the middle panel in Fig. 5B, and the margins of the contiguous area are shown by the green line in the right panel. The size of the Ca2+ channel arrays ranged from 10 to 78 μm2, with a mean size of ∼34 μm2. On average there were two distinct patches per footprint.
Exocytosis within patches of calcium channels
The results presented in Figs 1, 3 and 5 indicated that clusters of fusion events occurred on spatial scales similar to patches of Ca2+ channels. To determine more precisely where exocytosis occurred relative to Ca2+ channels, we applied IRM and TIRFM in the same cells. Three examples are shown in Fig. 6A–C. First, the IRM images at the top were obtained by integrating over a period of 12 s from the onset of the stimulus, which was delivered in the perforated patch configuration. The longer integration time compared to Fig. 1 provided a view of all the sites where granules fused over this period, and the brighter white spots reflect sites where two or more fusion events occurred (as assessed by observing the movie of difference images frame by frame, as shown in Fig. 1). The corresponding TIRFM images at the bottom show Ca2+ channel patches visualized in the same footprint by going whole-cell with the pipette containing 0.1 mm OGBAPTA-2. Overlaid onto the Ca2+ channel patches are yellow and blue spots marking the sites of fusion events occurring during the stimulus (synchronous) and after the stimulus (asynchronous), identified from the IRM movies. Most sites of fusion were within the large regions of the surface membrane containing Ca2+ channels.
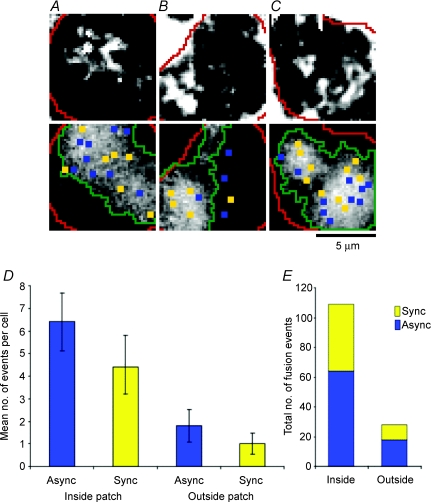
Figure 6. Fusion events occurred preferentially close to calcium channels.
IRM images averaged over a 12 s period from the start of the stimulus are shown in the top row and areas of Ca2+ influx visualized by TIRFM are shown below for three different cells (A–C). To trigger the fusion events (A–C, top), cells were voltage clamped in the perforated-patch configuration and stimulated with 4 × 90 ms depolarizations at 5 Hz. We then broke-through to whole-cell configuration and loaded cells with 0.1 mm OGBAPTA-2. The TIRFM images of Ca2+ influx (A–C, bottom) show the average stimulus frames (ΔF/F), as in Fig. 4D. Overlaid onto the TIRFM images are the sites of synchronous (yellow) and asynchronous (blue) fusion events visualized by IRM, the outline of the footprints (red lines), and the perimeter of the Ca2+ channel patches (green lines). D, average number, per cell, of asynchronous and synchronous fusion events occurring inside vs. outside of calcium channel patches. The differences between the means for asynchronous events inside vs. outside were significantly different (t test, P= 0.006), as were the differences between the means for synchronous events inside vs. outside (P= 0.03). Error bars are s.e.m.; n= 10 cells for each. E, total number of fusion events recorded for 10 cells (137 total events) is broken down to reveal the ratio of synchronous to asynchronous, inside and outside of the calcium channel patches.
To determine whether fusion events occurred preferentially within the Ca2+ channel patches, we measured the area of the footprint (Afoot) and the area covered by patches of Ca2+ channels (Apatch) for each cell (n= 10). If all of the fusion events visualized under IRM (Ntotal= 137 events) were randomly distributed across the footprint, then the predicted number of fusion events within Apatch would be: Npredict= (Apatch/Afoot) ×Ntotal. The actual number of fusion events occurring within the Ca2+ channel patch (Nactual) was compared to Npredict for each cell. The total Nactual was 109 events, significantly larger (P= 0.01, Student's paired, two-tailed t test) than the total Npredict of 92 events. In 70% of the cells, Nactual > Npredict, while in the remaining 30%Nactual=Npredict. These results confirmed that fusion events in the footprints of chromaffin cells were not randomly distributed but occurred preferentially within patches of Ca2+ channels.
Might the clustering of fusion events within Ca2+ channel patches simply reflect the higher local Ca2+ concentration? Two observations argued against this possibility. First, while most fusion sites occurred in regions of the surface membrane containing Ca2+ channels, several were towards the margins of these patches (e.g. Fig. 6A) and 20% outside (e.g. Fig. 6B). Second, when we analysed all the fusion events in 10 cells, we found that asynchronous events also occurred preferentially within patches of Ca2+ channels (Fig. 6D). These results indicate that the local Ca2+ concentration was not the only factor causing fusion to occur preferentially in regions of the surface membrane containing Ca2+ channels. It seems most likely that greater numbers of vesicles were docked in these areas or that the vesicles were better primed for release.
The calcium signal triggering synchronous and asynchronous fusion events
The stimulus we used to visualize the distribution of Ca2+ channels – a depolarization lasting 2 ms – was too brief to trigger exocytosis. To better understand excitation–secretion coupling we therefore also imaged the submembranous Ca2+ signal during more prolonged Ca2+ influx sufficient to trigger exocytosis, using trains of 4 × 90 ms depolarizations. Fluo-5N was used as the Ca2+ indicator because its relatively low affinity (Kd= 90 μm) prevented saturation in the face of these much larger Ca2+ loads. Figure 7A shows a series of consecutive frames lasting 100 ms, with 90 ms depolarizations delivered during frames 29, 31, 33 and 35. Patches of Ca2+ channels could be recognized in response to the first stimulus in the train (frame 29), but diffusion of Ca2+ from these regions caused a gradual build-up under the whole membrane (compare frames 30 and 36). Figure 7B shows the submembranous Ca2+ concentration over the whole footprint (black) compared with two 10 pixels × 10 pixels ROIs, one within (green) and one outside (red) a patch of Ca2+ channels. Average Ca2+ levels reached a maximum of about 30 μm within the Ca2+ patch during the stimulus, but rapidly fell after closure of Ca2+ channels. Of a total of 137 fusion events, 43% occurred 1 s or more after Ca2+ influx had stopped, at Ca2+ concentrations below 6 μm (Fig. 7B). Clearly, exocytosis was not tightly coupled to the opening of Ca2+ channels. These results provide further evidence against the idea that fusion of secretory vesicles in chromaffin cells is driven by localized Ca2+ microdomains.
Calcium channel patches consisted primarily of N- and P/Q-type channels
Bovine adrenal chromaffin cells express three types of voltage-activated Ca2+ channels, N-, L- and P/Q-type (García et al. 2006). Several studies have examined the efficiency with which the different channel subtypes trigger exocytosis in chromaffin cells but with conflicting results (Artalejo et al. 1994; Lara et al. 1998; Gil et al. 2001). N- and P/Q-type channels contain a synprint region that allows interaction with syntaxin, SNAP-25, and synaptotagmin (Catterall, 1999); thus, these channels may localize to regions of the surface membrane where the SNARE complex has formed in preparation for fusion. We therefore compared how different subtypes of Ca2+ channels were distributed within and between patches.
After imaging sites of Ca2+ influx under normal conditions, we applied dihydropyridines (DHP) to block L-type channels, ω-conotoxin GVIA (CTX) to block N-type channels, or ω-agatoxin IVA (AGA) to block P/Q-type channels. The averaged responses to the first set of 2 ms stimuli under control conditions are shown in the top row in Fig. 8A–D (‘Stim 1’); the response to the second set of stimuli under test conditions is shown in the middle row (‘Stim 2’), and the ratio images of Stim 2/Stim 1 are at the bottom. When the second set of stimuli was also delivered in the absence of any pharmacological agents, the average ratio of (Stim 2/Stim 1) was one (Fig. 8A), indicating there was little change in Ca2+ influx between the two sets of stimuli (Fig. 8A, bottom). The (Stim 2/Stim 1) ratio was also equal to one after treatment with dihydropyridines, indicating that blocking L-type Ca2+ channels did not significantly decrease Ca2+ influx in the footprint, even though the whole-cell Ca2+ current was inhibited by 20% (Fig. 8B). In contrast, treatment with CTX resulted in an 80% decrease in Ca2+ influx between Stim 1 and Stim 2 (Fig. 8C), while application of AGA led to a 40% decrease (Fig. 8D). These results indicate that patches of Ca2+ channels within the footprint were made up primarily of N- and P/Q-types, with very few active L-type channels.
The ratio images (Stim 2/Stim 1) in Fig. 8C and D were relatively uniform, as they were in 13 other cells tested, indicating that the distributions of N- and P/Q-type channels were similar across a patch. Figure 8E compares how these blockers of Ca2+ channels affected the total amount of Ca2+ influx into the cell (measured as the integral of the Ca2+ current, Q) and the influx into the footprint (measured as the mean pixel intensity in the ratio image Stim 2/Stim 1). In cells treated with CTX and AGA, inhibition of the Ca2+ current was correlated with a decrease in the amount of Ca2+ entering through the footprint. In contrast, in cells treated with DHP, Ca2+ influx into the footprint was not significantly reduced. Thus, L-type channels were segregated away from N- and P/Q-type channels in the footprint. This conclusion was reinforced by successively blocking each Ca2+ channel subtype in the same cell. In the example in Fig. 9, applying DHP reduced the total Ca2+ current by about 70% (Fig. 9B), without any effect on the Ca2+ influx through the footprint (mean pixel intensity ratio of 0.99 ± 0.13 in Fig. 9D). When CTX was added in addition to DHP, the mean intensity ratio was 0.59 ± 0.12 (Fig. 9F), while further addition of AGA completely blocked Ca2+ influx (Fig. 9H). Thus the footprint contained only N- and P/Q-type channels, and the ratio images show that these were distributed relatively uniformly.
Discussion
The results of this study demonstrate that the time and place of vesicle fusion in chromaffin cells is not tightly coupled to the opening of Ca2+ channels in the surface membrane. Direct visualization of the submembranous Ca2+ signal associated with brief openings of Ca2+ channels demonstrated that these were not discrete microdomains, but large areas, often covering tens of square micrometres (Fig. 4).
Three aspects of the results obtained with Ca2+ buffers also argued against tight coupling between sites of granule fusion and Ca2+ channels within these large regions of the membrane. First, vesicle fusion in chromaffin cells was very sensitive to EGTA (Fig. 2), a chelator that binds Ca2+ ions relatively slowly and which is therefore only effective at reducing the Ca2+ signal at micrometre distances from Ca2+ channels (Neher, 1998). Second, the rate of vesicle fusion accelerated as Ca2+ accumulated in the cytoplasm over periods of seconds (Fig. 2C). The time scale over which calcium microdomains are generated is three orders of magnitude shorter. Third, BAPTA, a fast chelator of Ca2+ ions, was no more effective at slowing the rate of exocytosis than EGTA, which binds Ca2+ ions about 60-fold more slowly (Fig. 2E). Although the majority of fusion sites were located within areas of the surface membrane containing patches of Ca2+ channels, exocytosis also occurred at sites remote from channels (Fig. 6). In addition, asynchronous release after closure of Ca2+ channels was an obvious feature of exocytic responses (Fig. 7), providing direct evidence that release did not require Ca2+ microdomains generated by closely coupled Ca2+ channels. All these observations run counter to the suggestion that fusion of large vesicles in chromaffin cells is driven by localized Ca2+ signals similar to those observed at the synapse (Becherer et al. 2003; Olivos Ore & Artalejo, 2004).
The results we have presented stand in contrast to those of Becherer et al. (2003), who reported that brief (< 100 ms) Ca2+ transients could be generated in the footprint of chromaffin cells during 3 s applications of high K+, localized to submicrometre areas. It is unclear how such localized calcium gradients might be suddenly generated during a maintained depolarization that will lead to widespread diffusion of submembranous Ca2+ and channel inactivation (Fig. 7). The approach we have used to detect regions of calcium influx, a depolarization of 2 ms followed by the immediate imaging of the calcium signal over a period of 11 ms, is likely to be a more reliable method for identifying sites of calcium entry, and has been used to detect calcium microdomains at synapses (Tucker & Fettiplace, 1996; Zenisek et al. 2003; Beaumont et al. 2005). This approach failed to uncover discrete calcium microdomains in chromaffin cells.
Calcium channels distributed over large areas of membrane
On depolarization, calcium influx occurred through areas of the surface membrane covering many tens of squared micrometres, and ‘microdomains’ could not be detected. TIRFM has reliably detected calcium microdomains at the active zones of retinal bipolar cells and hair cells (Zenisek et al. 2003; Beaumont et al. 2005), so the lack of such signals in chromaffin cells is unlikely to be a limitation of the technique. Although imaging indicated that the density of channels within a patch was not uniform (Figs 6 and 7), it was clear that the Ca2+ signal over most areas of the membrane was affected by Ca2+ influx over many channels distributed widely.
How many channels formed a patch in a chromaffin cell? The average surface area of a chromaffin cell is about 710 μm2 (assuming a specific membrane capacitance of 8 fF μm−2), and Ca2+ channel patches covered an average of 60% of the footprint in 11 cells. If the estimated 10 000 to 20 000 Ca2+ channels (Fenwick et al. 1982; Artalejo et al. 1992) in a chromaffin cell were distributed homogeneously throughout these patches, the average patch would contain 800–1700 channels at a density of about 25–50 μm−2. These results stand in contrast to the way Ca2+ channels are distributed in the membrane of large synaptic terminals, such as ribbon synapses of hair cells, bipolar cells or the calyx of Held, where all channels occur in a few very tight clusters, a fraction of a micrometre in diameter, covering less than 5% of the surface membrane (Roberts et al. 1990; Tucker & Fettiplace, 1996; Meinrenken et al. 2002; Zenisek et al. 2003; Beaumont et al. 2005). Electron microscopy and electrophysiology studies in frog hair cells indicate that an average active zone contains about 90 Ca2+ channels covering an area of 0.06 μm2, equivalent to a density of 1500 channels μm−2 (Roberts et al. 1990). Calcium influx through these tight clusters of channels can be readily observed by imaging (Cohen et al. 1991; Tucker & Fettiplace, 1996; Zenisek et al. 2003; Beaumont et al. 2005).
Excitation–secretion coupling in neuroendocrine cells and synapses
The results of this study allow us to compare the membrane organization of Ca2+ channels and fusion sites in chromaffin cells and presynaptic terminals. Synapses provide specific sites of communication between neurons, and release of neurotransmitter occurs at active zones on the spatial scale of hundreds of nanometres directed towards postsynaptic receptors that are similarly localized. In contrast, chromaffin cells in the adrenal gland release catecholamines into the vasculature that travels along the entire apical surface of the cell, and it is across this large region of surface membrane that granules are docked (Chan et al. 2005). These distinctive differences in the spatial organization of exocytic sites in neurons and neuroendocrine cells might be correlated with differences in the spatial organization of Ca2+ channels. Larger domains of calcium entry in chromaffin cells compared to synapses may reflect the lack of spatial precision in the release of hormones compared to neurotransmitters.
Glossary
Abbreviations
- IRM
interference reflection microscopy
- TIRFM
total internal reflection fluorescence microscopy
Author contributions
All authors contributed to the conception, analysis and interpretation of experiments as well as writing and revising the article.
Authors’ present addresses
M. M. Wu: Department of Molecular and Cellular Physiology, Stanford University School of Medicine, Stanford, CA 94305, USA.
A. Llobet: Laboratori de Neurobiologia-CIBERNED, IDIBELL-Universitat de Barcelona 08907, L’Hospitalet de Llobregat, Spain.
Supplemental material
Supplementary Figure 1. Fusion of individual granules detected by IRM
Supplementary Figure 2. Experimental protocol for imaging calcium influx sites by TIRFM.
Movie 1. Clustered fusion events visualized by IRM.
Movie 2. Repeated fusion events at specialized sites.
Movies 3 & 4. Calcium macrodomains visualized by TIRFM.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albillos A, Neher E, Moser T. R-type Ca2+ channels are coupled to the rapid component of secretion in mouse adrenal slice chromaffin cells. J Neurosci. 2000;20:8323–8330. doi: 10.1523/JNEUROSCI.20-22-08323.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ales E, Tabares L, Poyato JM, Valero V, Lindau M, Alvarez de Toledo G. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat Cell Biol. 1999;1:40–44. doi: 10.1038/9012. [DOI] [PubMed] [Google Scholar]
- Allersma MW, Bittner MA, Axelrod D, Holz RW. Motion matters: secretory granule motion adjacent to the plasma membrane and exocytosis. Mol Biol Cell. 2006;17:2424–2438. doi: 10.1091/mbc.E05-10-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Exocytosis. Annu Rev Physiol. 1990;52:607–624. doi: 10.1146/annurev.ph.52.030190.003135. [DOI] [PubMed] [Google Scholar]
- Artalejo CR, Adams ME, Fox AP. Three types of Ca2+ channel trigger secretion with different efficacies in chromaffin cells. Nature. 1994;367:72–76. doi: 10.1038/367072a0. [DOI] [PubMed] [Google Scholar]
- Artalejo CR, Rossie S, Perlman RL, Fox AP. Voltage-dependent phosphorylation may recruit Ca2+ current facilitation in chromaffin cells. Nature. 1992;358:63–66. doi: 10.1038/358063a0. [DOI] [PubMed] [Google Scholar]
- Beaumont V, Llobet A, Lagnado L. Expansion of calcium microdomains regulates fast exocytosis at a ribbon synapse. Proc Natl Acad Sci U S A. 2005;102:10700–10705. doi: 10.1073/pnas.0501961102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becherer U, Moser T, Stuhmer W, Oheim M. Calcium regulates exocytosis at the level of single vesicles. Nat Neurosci. 2003;6:846–853. doi: 10.1038/nn1087. [DOI] [PubMed] [Google Scholar]
- Burrone J, Neves G, Gomis A, Cooke A, Lagnado L. Endogenous calcium buffers regulate fast exocytosis in the synaptic terminal of retinal bipolar cells. Neuron. 2002;33:101–112. doi: 10.1016/s0896-6273(01)00565-7. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Interactions of presynaptic Ca2+ channels and snare proteins in neurotransmitter release. Ann N Y Acad Sci. 1999;868:144–159. doi: 10.1111/j.1749-6632.1999.tb11284.x. [DOI] [PubMed] [Google Scholar]
- Chan SA, Polo-Parada L, Smith C. Action potential stimulation reveals an increased role for P/Q-calcium channel-dependent exocytosis in mouse adrenal tissue slices. Arch Biochem Biophys. 2005;435:65–73. doi: 10.1016/j.abb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Chan SA, Smith C. Physiological stimuli evoke two forms of endocytosis in bovine chromaffin cells. J Physiol. 2001;537:871–885. doi: 10.1111/j.1469-7793.2001.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SA, Smith C. Low frequency stimulation of mouse adrenal slices reveals a clathrin-independent, protein kinase C-mediated endocytic mechanism. J Physiol. 2003;553:707–717. doi: 10.1113/jphysiol.2003.053918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RH, Klingauf J, Heinemann C, Zucker RS, Neher E. Mechanisms determining the time course of secretion in neuroendocrine cells. Neuron. 1996;16:369–376. doi: 10.1016/s0896-6273(00)80054-9. [DOI] [PubMed] [Google Scholar]
- Chow RH, Klingauf J, Neher E. Time course of Ca2+ concentration triggering exocytosis in neuroendocrine cells. Proc Natl Acad Sci U S A. 1994;91:12765–12769. doi: 10.1073/pnas.91.26.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MW, Jones OT, Angelides KJ. Distribution of Ca2+ channels on frog motor nerve terminals revealed by fluorescent omega-conotoxin. J Neurosci. 1991;11:1032–1039. doi: 10.1523/JNEUROSCI.11-04-01032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie KP, Fox AP. Comparison of N- and P/Q-type voltage-gated calcium channel current inhibition. J Neurosci. 1997;17:4570–4579. doi: 10.1523/JNEUROSCI.17-12-04570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyar VE, Allersma MW, Axelrod D, Holz RW. Increased motion and travel, rather than stable docking, characterize the last moments before secretory granule fusion. Proc Natl Acad Sci U S A. 2007;104:15929–15934. doi: 10.1073/pnas.0705406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuro A, Parker I. Imaging single-channel calcium microdomains. Cell Calcium. 2006;40:413–422. doi: 10.1016/j.ceca.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engisch KL, Nowycky MC. Calcium dependence of large dense-cored vesicle exocytosis evoked by calcium influx in bovine adrenal chromaffin cells. J Neurosci. 1996;16:1359–1369. doi: 10.1523/JNEUROSCI.16-04-01359.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick EM, Marty A, Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García AG, García-De-Diego AM, Gandía L, Borges R, García-Sancho J. Calcium signalling and exocytosis in adrenal chromaffin cells. Physiol Rev. 2006;86:1093–1131. doi: 10.1152/physrev.00039.2005. [DOI] [PubMed] [Google Scholar]
- Gil A, Viniegra S, Neco P, Gutierrez LM. Co-localization of vesicles and P/Q Ca2+-channels explains the preferential distribution of exocytotic active zones in neurites emitted by bovine chromaffin cells. Eur J Cell Biol. 2001;80:358–365. doi: 10.1078/0171-9335-00168. [DOI] [PubMed] [Google Scholar]
- Giovannucci DR, Hlubek MD, Stuenkel EL. Mitochondria regulate the Ca2+-exocytosis relationship of bovine adrenal chromaffin cells. J Neurosci. 1999;19:9261–9270. doi: 10.1523/JNEUROSCI.19-21-09261.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington J, Bookman RJ. PULSE CONTROL v4.0: IGOR XOPS for Patch Clamp Data Acquisition and Capacitance Measurements. Miami, FL, USA: University of Miami Press; 1994. [Google Scholar]
- Khimich D, Nouvian R, Pujol R, Tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–894. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Kimura R, Liu T, Nemoto T, Takahashi N, Kasai H. Vacuolar sequential exocytosis of large dense-core vesicles in adrenal medulla. EMBO J. 2006;25:673–682. doi: 10.1038/sj.emboj.7600983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara B, Gandía L, Martinez-Sierra R, Torres A, García AG. Q-type Ca2+ channels are located closer to secretory sites than L-type channels: functional evidence in chromaffin cells. Pflugers Arch. 1998;435:472–478. doi: 10.1007/s004240050541. [DOI] [PubMed] [Google Scholar]
- Llobet A, Beaumont V, Lagnado L. Real-time measurement of exocytosis and endocytosis using interference of light. Neuron. 2003;40:1075–1086. doi: 10.1016/s0896-6273(03)00765-7. [DOI] [PubMed] [Google Scholar]
- Llobet A, Wu M, Lagnado L. The mouth of a dense-core vesicle opens and closes in a concerted action regulated by calcium and amphiphysin. J Cell Biol. 2008;182:1017–1028. doi: 10.1083/jcb.200807034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long AA, Kim E, Leung HT, Woodruff E, 3rd, An L, Doerge RW, Pak WL, Broadie K. Presynaptic calcium channel localization and calcium-dependent synaptic vesicle exocytosis regulated by the Fuseless protein. J Neurosci. 2008;28:3668–3682. doi: 10.1523/JNEUROSCI.5553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TF. The molecular machinery for fast and slow neurosecretion. Curr Opin Neurobiol. 1994;4:626–632. doi: 10.1016/0959-4388(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Meinrenken CJ, Borst JG, Sakmann B. Calcium secretion coupling at calyx of Held governed by nonuniform channel-vesicle topography. J Neurosci. 2002;22:1648–1667. doi: 10.1523/JNEUROSCI.22-05-01648.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Neher E. Rapid exocytosis in single chromaffin cells recorded from mouse adrenal slices. J Neurosci. 1997;17:2314–2323. doi: 10.1523/JNEUROSCI.17-07-02314.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naraghi M, Neher E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J Neurosci. 1997;17:6961–6973. doi: 10.1523/JNEUROSCI.17-18-06961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Neher E. A comparison between exocytic control mechanisms in adrenal chromaffin cells and a glutamatergic synapse. Pflugers Arch. 2006;453:261–268. doi: 10.1007/s00424-006-0143-9. [DOI] [PubMed] [Google Scholar]
- Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Nofal S, Becherer U, Hof D, Matti U, Rettig J. Primed vesicles can be distinguished from docked vesicles by analyzing their mobility. J Neurosci. 2007;27:1386–1395. doi: 10.1523/JNEUROSCI.4714-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivos Ore L, Artalejo AR. Intracellular Ca2+ microdomain-triggered exocytosis in neuroendocrine cells. Trends Neurosci. 2004;27:113–115. doi: 10.1016/j.tins.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Perrais D, Kleppe I, Taraska J, Almers W. Recapture after exocytosis causes differential retention of protein in granules of bovine chromaffin cells. J Physiol. 2004;560:413–428. doi: 10.1113/jphysiol.2004.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman C, Meunier FA, Binz T, Davletov B. High affinity interaction of syntaxin and SNAP-25 on the plasma membrane is abolished by botulinum toxin E. J Biol Chem. 2004;279:644–651. doi: 10.1074/jbc.M310879200. [DOI] [PubMed] [Google Scholar]
- Roberts WM, Jacobs RA, Hudspeth AJ. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990;10:3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson IM, Finnegan JM, Monck JR, Wightman RM, Fernandez JM. Colocalization of calcium entry and exocytotic release sites in adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1995;92:2474–2478. doi: 10.1073/pnas.92.7.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R, Adler EM, Charlton MP. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990;5:773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- Schoch S, Gundelfinger ED. Molecular organization of the presynaptic active zone. Cell Tissue Res. 2006;326:379–391. doi: 10.1007/s00441-006-0244-y. [DOI] [PubMed] [Google Scholar]
- Serulle Y, Sugimori M, Llinás RR. Imaging synaptosomal calcium concentration microdomains and vesicle fusion by using total internal reflection fluorescent microscopy. Proc Natl Acad Sci U S A. 2007;104:1697–1702. doi: 10.1073/pnas.0610741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seward EP, Nowycky MC. Kinetics of stimulus-coupled secretion in dialyzed bovine chromaffin cells in response to trains of depolarizing pulses. J Neurosci. 1996;16:553–562. doi: 10.1523/JNEUROSCI.16-02-00553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JY, Wu XS, Wu W, Jin SX, Dondzillo A, Wu LG. Capacitance measurements at the calyx of Held in the medial nucleus of the trapezoid body. J Neurosci Methods. 2004;134:121–131. doi: 10.1016/j.jneumeth.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Tucker TR, Fettiplace R. Monitoring calcium in turtle hair cells with a calcium activated potassium channel. J Physiol. 1996;494:613–626. doi: 10.1113/jphysiol.1996.sp021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Binz T, Niemann H, Neher E. Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nat Neurosci. 1998;1:192–200. doi: 10.1038/642. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Davila V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J Neurosci. 2003;23:2538–2548. doi: 10.1523/JNEUROSCI.23-07-02538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Misler S. Action potential-induced quantal secretion of catecholamines from rat adrenal chromaffin cells. J Biol Chem. 1995;270:3498–3505. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.