Abstract
Brief interruption of voluntary EMG in a hand muscle by focal transcranial magnetic stimulation (TMS) of the ipsilateral primary motor cortex (M1), the so-called ipsilateral silent period (ISP), is a measure of interhemispheric motor inhibition. However, little is known about how volitional motor activity would modulate the ISP. Here we tested in 30 healthy adults to what extent and under what conditions voluntary activation of the stimulated right M1 by moving the left hand strengthens interhemispheric inhibition as indexed by an enhancement of the ISP area in the maximally contracting right first dorsal interosseous (FDI). Left index finger abduction, already at low levels of contraction, significantly enhanced the ISP compared to left hand at rest. Even imagination of left index finger movement enhanced the ISP compared to rest or mental calculation. This enhancement occurred in the absence of motor-evoked potential amplitude modulation in the left FDI, thus excluding a non-specific contribution from an increase in right M1 corticospinal excitability. Contraction of the left extensor indicis, but not contraction of more proximal left upper limb or left or right lower limb muscles also enhanced the ISP. A reaction time experiment showed that the ISP enhancement developed at a late stage of movement preparation just before or at movement onset. Interhemispheric inhibition of the motor-evoked potential as tested by a bifocal paired-pulse TMS protocol and thought to be mediated via a neuronal circuit different to the ISP was not enhanced when tested under identical motor task conditions. Finally, ISP enhancement by contraction of the left FDI correlated inversely with EMG mirror activity in the right FDI during phasic abductions of the left index finger. Our findings strongly suggest that voluntary M1 activation by real or imagined movement of the contralateral hand increases interhemispheric motor inhibition of the opposite M1. This phenomenon shows substantial topographical, temporal and neuronal circuit specificity, and has functional significance as it probably plays a pivotal role in suppressing mirror activity.
Introduction
In humans, intricate and independent finger movements are enabled by a largely crossed system of fast-conducting axons that provides mono-synaptic connections between primary motor cortex (M1) and contralateral spinal motoneurones (Porter & Lemon, 1993). Execution of unimanual or asymmetric bilateral movements relies on a neural network that is capable of lateralising motor cortical output (Carson, 2005; Cincotta & Ziemann, 2008). While a full characterisation of this distributed network is still lacking, data from lesioned monkeys (Brinkman, 1984) and human patients (Chan & Ross, 1988) are in keeping with the view that it probably includes the supplementary motor area and the cingulate gyrus. Positron emission tomography (PET) findings (Sadato et al. 1997) and transcranial magnetic stimulation (TMS) data in healthy human subjects (Meyer-Lindenberg et al. 2002; Cincotta et al. 2004; Giovannelli et al. 2006) suggest that the dorsal premotor cortex is also involved.
This notion of a neuronal network for movement lateralisation upstream of M1 by no means rules out the possibility that movement lateralisation is supported, in addition, by active inhibition from the voluntarily active M1 to the opposite M1. TMS studies that examined interhemispheric inhibition (IHI) by a paired-pulse protocol with the conditioning stimulus delivered to one M1 and the test stimulus delivered to the other M1 support this hypothesis (Ferbert et al. 1992; Mochizuki et al. 2004; Duque et al. 2007; Hübers et al. 2008). In particular, volitional activity in the M1 receiving the conditioning pulse, e.g. slight unilateral contraction of the contralateral hand, facilitates inhibition of the motor-evoked potential (MEP) elicited by a test stimulus delivered 10 ms later to the opposite M1 (interhemispheric inhibition at short-interstimulus interval, S-IHI) when compared to the rest condition (Ferbert et al. 1992; Mochizuki et al. 2004; Talelli et al. 2008).
Besides S-IHI of the MEP, interhemispheric inhibition can also be studied by a short attenuation or interruption of ongoing voluntary electromyographic (EMG) activity in hand muscles induced by focal TMS of the ipsilateral M1 (Wassermann et al. 1991; Ferbert et al. 1992; Meyer et al. 1995; Trompetto et al. 2004; Cincotta et al. 2006). This ipsilateral silent period (ISP) begins 30–40 ms after a single magnetic pulse and lasts, on average, 25 ms (Meyer et al. 1995). Studies in patients with callosal lesions indicate that the ISP reflects interhemispheric cortico-cortical inhibitory mechanisms mediated by fibres passing through the posterior half of the trunk of the corpus callosum (Meyer et al. 1995, 1998). By examining the effects of different stimulus intensities and current directions, Chen et al. (2003) suggested that the neural mechanisms underlying the ISP differ from those responsible for the S-IHI of the MEP. The ISP is currently considered as a measure that provides complementary but not identical information on interhemispheric inhibition compared to S-IHI measurements (Perez & Cohen, 2009). As the ISP measures inhibition of volitional motor activity rather than inhibition of MEPs, the ISP appears to be an original and particularly suited tool to investigate interhemispheric control of voluntary cortical motor output.
The aim of the present study was to investigate to what extent and under what conditions the ISP is modulated when the stimulated M1 is engaged in a voluntary motor task. Hence, we examined the effects of different real or imagined movements of the contralateral hand and of other body segments on the ISP in healthy humans. Furthermore, we tested whether motor task-related modulation of the ISP could be dissociated from modulation of the S-IHI. Finally, the functional significance of motor task-related modulation of the ISP was examined by testing its relation to the occurrence of EMG mirror activity, i.e. the failure to lateralise intended unilateral hand movements.
Methods
Subjects
Thirty right-handed healthy volunteers (sixteen women, mean age 32.1 years, range 19–56 years) were included in the study. Handedness was confirmed by the Edinburgh inventory (Oldfield, 1971) (mean laterality index 86.7, range 60–100). The study was performed according to the latest version of the Declaration of Helsinki and approved by the local ethics committee in Florence. Participants gave their written informed consent. During the experiments, subjects were seated in a comfortable chair with their arms fully supported.
EMG recording
Surface EMG activity was recorded simultaneously from the right and left first dorsal interosseous (FDI) muscles (‘target’ and ‘task’ muscle, respectively). Moreover, in control experiments aiming to explore the topographical specificity of motor task-related modulation of the ISP, EMG recordings were also made from the left extensor indicis proprius (EIP), the left extensor carpi radialis (ECR), the left biceps brachii (BB), and the right or left tibialis anterior (TA) muscles, which acted as task muscles in specific experimental conditions (see below). Signals were amplified, analog filtered (100–2000 Hz), digitised (A/D rate, 5 kHz) by a micro 1401 unit and Signal 2 software (Cambridge Electronic Design, UK) and stored on a personal computer for off-line analysis. Analysis time was 1.5 s with 1 s preceding the TMS pulse. Except for the experiment specifically aiming to test the effects of unintended, slight contraction (‘physiological’ mirroring) of the left FDI on the ISP in the right FDI (see below), appropriate relaxation or activation of the task muscles in different experimental conditions was monitored throughout the experiments by an acoustic feedback from high gain EMG and was a precondition for delivery of TMS. In this way, in all the other experiments, off-line analysis revealed very slight unwanted EMG activity in the left FDI in less than 5% of the trials. These traces were discarded from further analysis. Moreover, in all conditions where full relaxation of the task muscle (left FDI) was requested, the root mean squares (RMS) of the pre-stimulus EMG activity were calculated off-line to confirm full relaxation. Group RMS values (mean ±s.d.) ranged from 0.008 ± 0.007 mV to 0.011 ± 0.006 mV throughout experiments. These very low values were well within the range of previously reported RMS data in resting muscles (Möller et al. 2009).
TMS
Single-pulse TMS was delivered using a Magstim 200 stimulator (Magstim Co., UK) with a monophasic current waveform, connected to a figure-of-eight-shaped coil (external diameter of each loop, 9 cm) held tangentially to the scalp. The centre of the junction of the coil was placed over the hand area of the right or left M1 at the optimal position (hot spot) to elicit MEPs in the contralateral FDI, with the handle pointing backwards and 45 deg away from the midline. With this coil orientation, the induced current flowed in an anterior–medial direction, approximately perpendicular to the central sulcus. In most experiments, the right M1 was stimulated. In one of the two experiments addressing the circuit specificity of task-related modulation of the ISP, focal TMS was delivered to the left M1 to study the cortical silent period (CSP) in the right FDI (see below). In the other experiment, S-IHI of the MEP was tested using two Magstim 200 stimulators, each connected to a figure-of-eight-shaped coil. The coils were placed over the FDI hot spot of the M1 of either hemisphere (Ferbert et al. 1992).
General experimental design
We performed a series of experiments designed to thoroughly examine to what extent the ISP in the right FDI is modulated by tonic or phasic voluntary contractions of the left FDI, by different levels of voluntary contraction of the left FDI, by unintended, slight contraction (‘physiological’ mirroring) of the left FDI, by imagined movements involving the left index finger, by voluntary contraction of muscles other than the left FDI (addressing topographical specificity of ISP modulation) or during preparation of left FDI contraction (addressing temporal specificity). Further experiments explored the effects of left FDI contraction on other measures of cortical inhibition in the right FDI (circuit specificity) and the relationship between the motor task-related modulation of the ISP and the ability to lateralise voluntary movements of the left hand (addressing behavioural significance of ISP modulation). Single experiments were carried out on separate days. For all ISP experiments, subjects were always asked to perform an isometric contraction of the right FDI at the maximum strength level that they could steadily maintain (Trompetto et al. 2004; Cincotta et al. 2006), whereas the task of the left hand (or other body parts) differed across experimental conditions. A large background EMG level in the right FDI and relatively low TMS intensity (see below) minimised the possibility of ceiling or floor effects in task-related ISP modulation. Within each experimental session, the order of task conditions was varied pseudorandomly in a counterbalanced manner across subjects. The interval between consecutive TMS trials varied between 5 and 10 s to minimise anticipation of the next trial. Pauses were inserted whenever necessary to avoid muscle fatigue. These precautions resulted in a close match of background EMG levels across conditions (see Results).
ISP modulation by different tonic and phasic contractions of the left FDI (‘main experiment’)
It is important to note that in this and all other ISP experiments the basic requirement throughout was maximum isometric contraction of the right FDI. Therefore, the term ‘full relaxation’ means that all other body parts were relaxed except the right FDI. Twelve subjects participated in the main experiment. The ISP in the right FDI was recorded whilst the left FDI was engaged in four different tasks: (i) full relaxation; (ii) maximal isometric contraction; (iii) repetitive thumb-to-index tapping with the left hand; and (iv) sequential left index tapping movements on a flat surface with the palm immobile and facing downward, with subjects instructed to touch with the tip of the left index finger three target points placed about 1.3 cm apart corresponding to three different levels of index abduction. Prior to the ISP measurements, the resting motor threshold (RMT) was determined in the left FDI according to the recommendations of the IFCN Committee (Rossini et al. 1994). RMT was measured in 1% increments of maximum stimulator output (MSO), and defined as the minimum stimulus intensity, which produced MEPs of >50 μV in at least half of 10 consecutive trials in the relaxed FDI. RMT (mean ±s.d.) was 39.4 ± 7.0% MSO. For the ISP measurements, TMS of the right M1 was delivered at an intensity of 120% RMT. Twenty stimuli were delivered in each experimental condition. When the tasks consisted of phasic movements of the left index, TMS was delivered as soon as the operator recognised by visual inspection the index abduction in the repetitive task and the maximal of the three levels of index abduction in the sequential task. Off-line evaluation of recordings confirmed that TMS pulses were delivered during the voluntary EMG bursts in the left FDI.
ISP modulation by isometric left FDI contraction at different strength levels
Seven subjects participated in this experiment. TMS procedures were identical to the main experiment and were performed whilst the left FDI was engaged in three different tasks: (i) full relaxation; (ii) isometric contraction at the minimum strength level that they could steadily maintain against resistance (approximately 5% of maximum voluntary contraction); and (iii) maximum isometric contraction.
ISP modulation by unintended, slight contraction (‘physiological’ mirroring) of the left FDI
In eight subjects, TMS pulses identical to the main experiment were delivered during maximal voluntary isometric contraction of the right FDI alone, irrespective of whether or not high gain EMG showed unintended activation of the left FDI (‘physiological’ mirroring). This experiment addresses the question as to whether voluntary suppression of mirror EMG activity in the left FDI has affected the ISP in the right FDI. Traces were recorded until at least 20 trials without and 20 trials with unwanted EMG activity in the left FDI were collected. As the percentage of traces with mirroring ranged between 16% and 41% of the trials, the number of recordings ranged between 49 and 128 across subjects. The first 20 ‘fully relaxed’ trials and the 20 trials with unintended EMG activity in the left FDI were used for analysis. In the ‘fully relaxed’ and ‘physiological mirroring’ conditions, the group RMS values (mean ±s.d.) of the pre-stimulus EMG activity in the left FDI were 0.008 ± 0.002 mV and 0.016 ± 0.009 mV, respectively. Although the unintended EMG activity in the left FDI was slight and non-persistent, this difference was significant (paired t test: P= 0.02).
ISP modulation by imagined movements involving the left FDI
Eleven subjects participated in this experiment. TMS procedures were identical to the main experiment and were performed in three different experimental conditions of the left hand: (i) full relaxation; (ii) imagination of tonic thumb-to-index opposition; and (iii) full relaxation whilst performing a mental calculation task. Mental calculation consisted of counting backwards by 7 beginning with 100. In each condition, TMS to the right M1 was delivered only when the acoustic feedback from high gain EMG indicated complete absence of muscle activity in the left FDI.
ISP modulation by isometric contraction of muscles other than the left FDI (topographical specificity)
A set of three sub-experiments was performed to explore the topographical specificity of the ISP enhancement observed in the main experiment when contracting the left FDI (see Results). The sub-experiments were performed on separate days and designed to obtain a progressive refinement of the topographical specificity of the ISP modulation. Sub-experiment 1 (10 subjects) consisted of four experimental conditions: (i) full relaxation; (ii) maximum isometric voluntary contraction of the left FDI (to confirm data in the main experiment); (iii) maximum isometric voluntary contraction of the right TA; and (iv) maximum isometric voluntary contraction of the left EIP. In sub-experiment 2 (eight subjects), two different experimental conditions were compared: (i) full relaxation; and (ii) maximum isometric voluntary contraction of the left TA. Finally, sub-experiment 3 (eight subjects) consisted of three different conditions: (i) full relaxation; (ii) maximum isometric voluntary contraction of the left ECR; and (iii) maximum isometric voluntary contraction of the left BB.
ISP modulation by preparation of left FDI contraction (temporal specificity)
A simple visual reaction time (RT) task was used to investigate whether the ISP enhancement in the right FDI observed during phasic movements of the left index (see Results of the main experiment), was expressed already before the onset of left FDI contraction. Seven subjects participated in this experiment. The visual go-signal (duration, 100 ms) consisted of a 5 cm yellow circle displayed at eye level on a blank computer screen 1 m in front of the subject. Subjects were instructed to perform a single, rapid thumb-to-index tap with the left hand as soon as possible after the go-signal whilst they maintained maximum isometric voluntary contraction of the right FDI. TMS pulses were delivered in three different experimental conditions: (i) without the go-signal (baseline); (ii) 100 ms after the go-signal; and (iii) 200 ms after the go-signal. These intervals were chosen to deliver TMS before and during the EMG burst in the left FDI, respectively (Ziemann et al. 1997). When this was not the case, the trial was discarded. TMS trials of the three experimental conditions were randomly intermixed until at least 20 valid trials were obtained in each condition. To test whether the visual go-signal per se could modulate the ISP in the right FDI, the same protocol was applied whilst the subjects were asked to observe the computer screen but not to respond with the left hand.
Circuit specificity of task-related ISP modulation
In order to test directly whether ISP modulation was related to modification of S-IHI, both measures were tested in the right FDI during identical motor tasks: (i) unilateral isometric contraction of the right FDI; and (ii) bilateral isometric FDI contraction at the maximum strength level (i.e. conditions (i) and (ii) of the main experiment). Ten subjects participated in this experiment. During each task, three different TMS conditions were randomly performed: stimulation of the right M1 alone (20 trials) for ISP recordings, stimulation of the left M1 alone (10 trials) for unconditioned test MEP recordings, and conditioning stimulation of the right M1 followed by test stimulation of the left M1 at a 10 ms interstimulus interval (10 trials) to evaluate S-IHI. TMS of the right M1 was delivered at an intensity of 120% RMT. For left M1 stimulation, TMS intensity was adjusted to elicit an unconditioned MEP of approximately 2 mV peak-to-peak amplitude in the maximally contracting right FDI. Stimulus intensity ranged from 20% to 32% MSO.
In order to explore if the ISP modulation involved inhibitory circuits in the left M1, we tested to what extent contraction of the left FDI modulated the CSP elicited in the right FDI by TMS of the left M1. Eight subjects participated in this experiment. Prior to the CSP measurements, the active motor threshold (AMT) of the right FDI was measured in 1% increments of MSO and was defined as the minimum stimulus intensity that produced MEPs of >50 μV in at least half of 10 consecutive trials during a minimal isometric contraction (approximately 5% of maximum voluntary strength) of the right FDI. AMT (mean ±s.d.) was 31.8 ± 7.9% MSO. For the CSP measurements, TMS of the left M1 was delivered at an intensity equal to the AMT. In this way, the duration and depth of the CSP were comparable to those of the ISP in the right FDI in the ISP experiments. Twenty stimuli were delivered in either one of two different experimental conditions: (i) unilateral maximum isometric voluntary contraction of the right FDI; and (ii) bilateral maximum isometric voluntary contraction of the right and left FDI (i.e. same conditions (i) and (ii) as in the main experiment).
Relationship between motor task-related ISP modulation and voluntary hand movement lateralisation (behavioural significance)
In ten subjects, the ISP was tested in two experimental conditions: (i) unilateral maximum isometric voluntary contraction of the right FDI; and (ii) bilateral maximum isometric voluntary contraction of the right and left FDI (i.e. same conditions (i) and (ii) as in the main experiment). TMS procedures were also identical to the main experiment. Voluntary movement lateralisation was measured by employing a well-established experimental protocol (Mayston et al. 1999; Giovannelli et al. 2006; Hübers et al. 2008): Subjects were instructed to perform a voluntary phasic (‘brief and brisk’) abduction of the left index finger in response to a visual go-signal, while maintaining a tonic contraction of the right FDI at the minimum strength level that they could steadily maintain against resistance, under the guidance of auditory feedback of the EMG. The go-signal was identical to the one used in the simple RT protocol (see above, ‘ISP modulation by preparation of left FDI contraction). Twenty trials were performed at intertrial intervals ≥ 10 s.
Data analysis
For each experimental condition, single trials were rectified and averaged off-line and the resulting traces were exported in ASCII format to a Microsoft Excel spreadsheet for analysis. The ISP was quantified in the right FDI by the suppression of the voluntary EMG activity between the onset and the offset of the ISP (ISP area, in mV ms). ISP onset and offset were established using a method of statistical process control (Garvey et al. 2001, 2005). Briefly, the variation limits of background EMG activity were calculated by the average level of the 1 s EMG epochs immediately preceding the TMS pulse and by the mean consecutive difference between data points. ISP onset was defined as the first of five consecutive data points to fall below the lower 95% variation limit (equivalent to 2 s.d.) in the appropriate time window. ISP offset was the first data point above the lower 95% limit if 4 of the 9 subsequent points were also above the limit. In addition, traces were inspected to see whether ipsilateral excitatory responses (Ziemann et al. 1999) preceded the ISP. An ipsilateral MEP (iMEP) was considered to be present if the averaged rectified EMG signal exceeded 120% of the mean background EMG level for at least 5 ms. The method used to quantify the area of the CSP was the same as for ISP quantification. The amplitudes of the contralateral MEPs were measured in the experiments examining the effects of movement imagination (left FDI) and in the CSP experiment (right FDI) by taking the difference from baseline (0 mV) to the peak of the major deflection in the rectified averaged EMG. In the experiment comparing task-related ISP and S-IHI modulation, the raw EMG recordings of unconditioned and conditioned test MEPs were also separately averaged off-line. For each motor task, S-IHI was calculated as the percentage of peak-to-peak amplitude of the mean conditioned test MEP over the mean unconditioned test MEP. For ISP area and S-IHI, the difference between the measures obtained during bilateral FDI contraction and the measures obtained during unilateral contraction of the right FDI was expressed as a percentage of the mean value of the two measurements (percentage difference). These percentage differences were used to test whether motor task-induced changes in ISP area and S-IHI were correlated. In the experiment addressing the ‘Relationship between motor task-related ISP modulation and voluntary hand movement lateralisation’, EMG mirror activity in the right FDI was measured as the average of the rectified EMG traces and expressed as the percentage difference between the mean EMG level during the 50 ms after voluntary EMG burst onset in the left FDI and the mean baseline EMG amplitude in the 800 ms before burst onset. EMG mirror activity and the percentage difference in ISP area between bilateral FDI contraction and unilateral contraction of the right FDI were correlated in a linear regression analysis to test for any relationship between motor task-induced ISP modulation and mirror activity.
Statistics
In experiments consisting of more than two experimental conditions, EMG measures were entered in one-way repeated-measures analyses of variance (rmANOVA) with ‘Experimental condition’ as a within-subject factor (Sigmastat 3.1 software, Jandel Scientific, Erkrath, Germany). Post hoc tests were performed using Newman–Keuls multiple comparisons. In experiments consisting of two different experimental conditions, comparisons were made by using two-tailed paired t tests. In the experiment comparing ISP and S-IHI, ISP area, unconditioned test MEP amplitude, and S-IHI were compared between the two motor tasks by two-tailed paired t tests. Correlations between motor task-induced changes in ISP area and S-IHI, and between task-induced ISP modulation and EMG mirror activity were analysed by linear regression. In all tests, the level of significance was set at P < 0.05.
Results
None of the participants reported adverse effects during or after the experimental procedures. According to the relatively low stimulus intensity used in the present study, no iMEP preceded the ISP in any of the experimental conditions.
ISP modulation by different tonic and phasic contractions of the left FDI
The rmANOVA revealed a significant effect of ‘Experimental condition’ on the ISP area (F3,33= 5.80, P= 0.003). Post hoc tests showed that each of the three tasks where the left FDI was involved in voluntary motor activity (maximal isometric contraction, repetitive thumb-to-index tapping of the left hand, and sequential tapping movements of the left index) resulted in an increase in ISP area in the right FDI when compared with the left FDI in the rest condition (Table 1, Fig. 1). The trend towards a stronger increase of ISP area with sequential movements compared to repetitive thumb-to-index tapping or tonic contraction was not significant (Table 1). In addition, there was a significant effect of ‘Experimental condition’ on the background EMG in the right FDI (F3,33= 3.32, P= 0.032). Post hoc testing revealed that this effect was caused by slightly but consistently lower background EMG with repetitive thumb-to-index tapping and sequential movements of the left index than maximum isometric contraction of the left FDI (Table 1). It is unlikely that so small a difference in background EMG level could exert a relevant influence on ISP measurements. In that case, however, the ISP enhancement in the phasic movement conditions of the left index finger would anyway have been slightly underestimated when compared to the maximum isometric contraction condition. Importantly, post hoc tests showed no significant difference in the background EMG of the right FDI between left FDI relaxation and the three experimental conditions where the left FDI was involved in voluntary motor activity (Table 1).
Table 1.
ISP area and background EMG level in the right FDI (mean ±s.d.) in the different experimental conditions
| ISP area (mV ms) | Background EMG level (mV) | |
|---|---|---|
| Different tonic and phasic contractions of the left FDI | ||
| Unilateral isometric contraction of the right FDI | 1.83 ± 0.85* | 0.399 ± 0.120 |
| Bilateral isometric contraction of the FDI | 3.37 ± 1.61 | 0.423 ± 0.121 |
| Isometric contraction of the right FDI and repetitive movements of the left index | 3.27 ± 1.76 | 0.377 ± 0.117*a |
| Isometric contraction of the right FDI and sequential movements of the left index | 4.04 ± 2.60 | 0.377 ± 0.099*a |
| Isometric left FDI contraction at different strength levels | ||
| Unilateral isometric contraction of the right FDI | 2.72 ± 1.77* | 0.424 ± 0.095 |
| Isometric contraction of the right FDI and minimal contraction of the left FDI | 4.27 ± 2.50 | 0.448 ± 0.077 |
| Isometric contraction of the right FDI and maximal contraction of the left FDI | 4.72 ± 2.33 | 0.427 ± 0.064 |
| Imagined movements involving the left FDI | ||
| Unilateral isometric contraction of the right FDI | 2.88 ± 1.92 | 0.435 ± 0.133 |
| Isometric contraction of the right FDI and imagined left thumb-to-index opposition | 3.81 ± 2.59* | 0.425 ± 0.126 |
| Isometric contraction of the right FDI and mental calculation | 2.95 ± 2.35 | 0.418 ± 0.131 |
| Isometric contraction of other muscles (topographical specificity) | ||
| Sub-experiment 1 | ||
| Unilateral isometric contraction of the right FDI | 2.66 ± 1.90 | 0.423 ± 0.063 |
| Bilateral isometric FDI contraction | 4.07 ± 3.00*b | 0.415 ± 0.072 |
| Isometric contraction of the right FDI and the right TA | 2.24 ± 1.16 | 0.420 ± 0.080 |
| Isometric contraction of the right FDI and the left EIP | 4.72 ± 2.54*b | 0.422 ± 0.074 |
| Sub-experiment 2 | ||
| Unilateral isometric contraction of the right FDI | 3.26 ± 2.23 | 0.434 ± 0.127 |
| Isometric contraction of the right FDI and the left TA | 3.52 ± 2.14 | 0.435 ± 0.129 |
| Sub-experiment 3 | ||
| Unilateral isometric contraction of the right FDI | 3.21 ± 1.72 | 0.433 ± 0.146 |
| Isometric contraction of the right FDI and the left ECR | 3.98 ± 1.79 | 0.427 ± 0.106 |
| Isometric contraction of the right FDI and the left BB | 2.57 ± 0.90 | 0.425 ± 0.090 |
| Preparation of left FDI contraction (temporal specificity) | ||
| Unilateral isometric contraction of the right FDI and no RT (baseline) | 2.47 ± 2.07 | 0.376 ± 0.173 |
| Isometric contraction of the right FDI during RT (TMS before the EMG onset in the left FDI) | 2.95 ± 2.42 | 0.380 ± 0.147 |
| Isometric contraction of the right FDI during RT (TMS during the EMG burst in the left FDI) | 4.48 ± 2.45*c | 0.372 ± 0.140 |
ISP, ipsilateral silent period; EMG, electromyographic; FDI, first dorsal interosseous muscle; TA, tibialis anterior muscle; EIP, extensor indicis proprius muscle; ECR, extensor carpi radialis; BB, biceps brachii. *P < 0.05, repeated-measures ANOVA, and post hoc Newman–Keuls multiple comparison tests. aWith respect to bilateral isometric contraction of the FDI. bWith respect to contraction of the right FDI alone or simultaneous isometric contraction of the right FDI and right TA. cWith respect to unilateral isometric contraction of the right FDI and no RT (baseline).
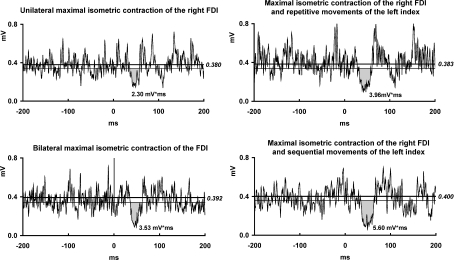
Figure 1. ISP modulation by different tonic and phasic contractions of the left FDI.
EMG recordings from the maximally contracting right first dorsal interosseous (FDI) muscle of one representative subject in four experimental conditions: (i) left FDI at rest; (ii) left FDI maximally contracting; (iii) repetitive left thumb-to-index tapping; (iv) sequential movements of the left index finger. The waveforms are averages of 20 rectified trials. Grey regions represent ipsilateral silent period (ISP) areas (in mV ms). ISP onset and offset were determined by using statistical process control (see Methods). The upper horizontal line represents the mean background EMG level and the lower line represents the lower 95% variation limit of the mean background EMG. All motor tasks of the left hand enhanced the ISP area in the right FDI when compared to the left hand at rest.
ISP modulation by isometric left FDI contraction at different strength levels
The rmANOVA showed a significant effect of ‘Experimental condition’ on the ISP area (F2,12= 4.73, P= 0.032). Post hoc testing revealed that, during minimal as well as maximal isometric contraction of the left FDI, the ISP area in the right FDI was significantly larger than in the left FDI resting condition (Table 1, Fig. 2). In addition, there was a non-significant trend for a larger ISP area with maximal than with minimal isometric contraction of the left FDI (Table 1). The level of background voluntary EMG activity in the left FDI was significantly different between maximal and minimal isometric contraction of the task muscle (mean ±s.d.: 0.410 ± 0.168 vs. 0.088 ± 0.053 mV, P < 0.001). ‘Experimental condition’ had no significant effect on the background EMG in the right FDI (F3,12= 0.83, P= 0.46; Table 1).
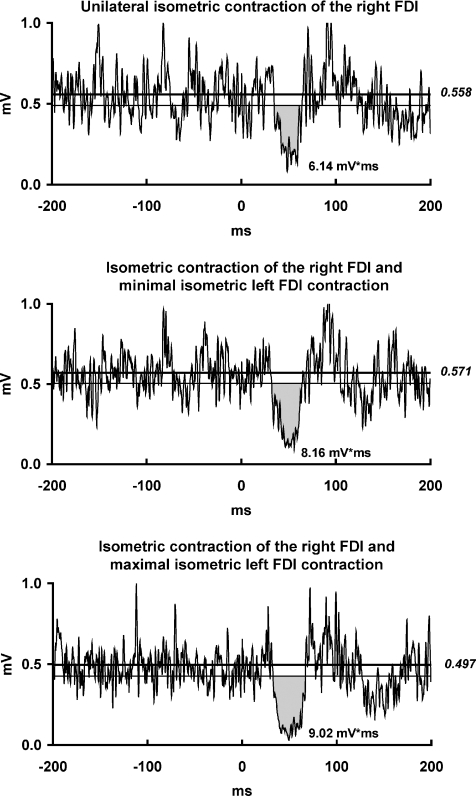
Figure 2. ISP modulation by isometric left FDI contraction at different strength levels.
EMG recordings from the maximally contracting right FDI muscle of one representative subject in three experimental conditions: (i) left FDI at rest; (ii) minimal isometric contraction of the left FDI; (iii) maximal isometric contraction of the left FDI. For further arrangements and conventions, see Fig. 1. Isometric contraction of the left FDI at either minimal or maximal strength level enhanced the ISP area in the right FDI when compared to the left FDI at rest.
ISP modulation by unintended, slight contraction (‘physiological’ mirroring) of the left FDI
Paired t tests showed that neither the ISP area (P= 0.12) nor the background EMG in the right FDI (P= 0.09) differed significantly between the conditions of ‘physiological mirroring’ and ‘full relaxation’ of the left FDI (ISP area: mean ±s.d.: 2.13 ± 1.28 vs. 2.43 ± 1.43 mV ms; background EMG: 0.336 ± 0.118 vs. 0.383 ± 0.131 mV, respectively).
ISP modulation by imagined movements involving the left FDI
rmANOVA demonstrated a significant effect of ‘Experimental condition’ on the ISP area (F2,20= 4.11, P= 0.032). Post hoc testing showed that, during imagined tonic thumb-to-index opposition of the left hand, the ISP area in the right FDI was significantly greater than during isometric contraction of the right FDI alone or during mental calculation (Table 1, Fig. 3). There was no effect of ‘Experimental condition’ on the background EMG in the right FDI (F2,20= 1.98, P= 0.16). In addition, ‘Experimental condition’ had no significant effect on the MEP amplitude in left FDI (F2,20= 0.61, P= 0.56). MEP amplitude (mean ±s.d.) was slightly larger during motor imagery (3.6 ± 1.6 mV) than during left FDI rest (3.3 ± 1.7 mV) or mental calculation (3.4 ± 1.7 mV), but these differences were not significant. RMS (mean ±s.d.) of the pre-stimulus EMG level in the left FDI was 0.008 ± 0.007 mV in the resting condition, 0.009 ± 0.005 mV during motor imagery, and 0.011 ± 0.008 mV during mental calculation. The rmANOVA did not reveal an effect of ‘Experimental condition’ on these very low RMS values (F2,20= 1.08, P= 0.36). These findings confirm full relaxation of the left FDI in the motor imagination condition. Therefore, unwanted activation of the left FDI cannot be attributed as a possible cause of the observed enhancement of the ISP area by motor imagery.
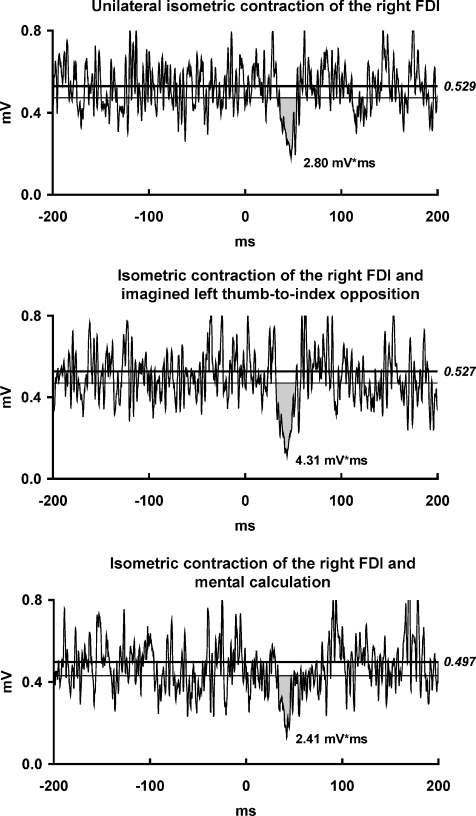
Figure 3. ISP modulation by imagined movements involving the left FDI.
EMG recordings from the maximally contracting right FDI muscle of one representative subject in three experimental conditions: (i) left hand at rest, no mental calculation; (ii) imagined tonic left thumb-to-index opposition; (iii) left hand at rest and mental calculation. For further arrangements and conventions, see Fig. 1. Motor imagery of tonic thumb-to-index opposition of the left hand enhanced the ISP area in the right FDI when compared to relaxation or mental calculation.
ISP modulation by isometric contraction of other muscles (topographical specificity)
In sub-experiment 1, rmANOVA showed a significant effect of ‘Experimental condition’ on the ISP area (F3,27= 8.35, P < 0.001). Post hoc testing indicated that maximum isometric voluntary contraction of the left FDI or the left EIP led to an increase in ISP area in the right FDI when compared to the conditions of left FDI at rest and maximum voluntary contraction of the right TA (Table 1, Fig. 4). ‘Experimental condition’ had no significant effect on the background EMG in the right FDI (F3,27= 0.08, P= 0.97; Table 1).
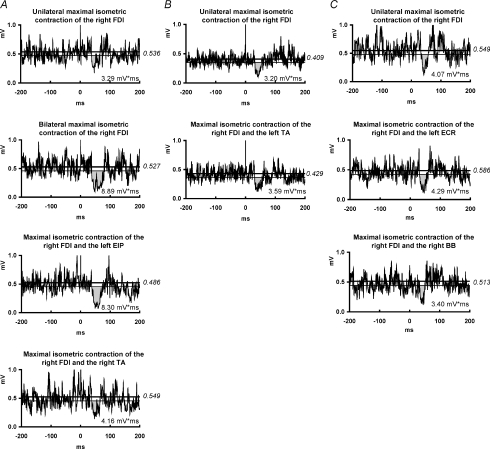
Figure 4. ISP modulation by isometric contraction of other muscles (topographical specificity).
A, sub-experiment 1: EMG recordings from the maximally contracting right FDI muscle of one representative subject in four experimental conditions: (i) no other muscle contracting; (ii) maximal isometric contraction of the left FDI; (iii) maximal isometric contraction of the left extensor indicis proprius (EIP); (iv) maximal isometric contraction of the right tibialis anterior (TA). B, sub-experiment 2: EMG recordings from the maximally contracting right FDI of one representative subject in two experimental conditions: (i) no other muscle contracting; (ii) maximal isometric contraction of the left TA. C, sub-experiment 3: EMG recordings from the maximally contracting right FDI of one representative subject in three experimental conditions: (i) no other muscle contracting; (ii) maximal isometric contraction of the left extensor carpi radialis (ECR); (iii) maximal isometric contraction of the left biceps brachii (BB). For further arrangements and conventions, see Fig. 1. Maximal isometric contraction of the left FDI or left EIP increased the ISP area in the right FDI, whereas this was not the case for maximal isometric contraction of any of the other tested muscles, suggesting topographical specificity of motor task-related ISP enhancement.
In sub-experiment 2, paired t tests showed that neither the ISP area (P= 0.36) nor the background EMG in the right FDI (P= 0.87), differed significantly between conditions of relaxation and maximum isometric voluntary contraction of the left TA (Table 1, Fig. 4).
In sub-experiment 3, rmANOVA showed that ‘Experimental condition’ had no significant effect on the ISP area (F2,14= 2.88, P= 0.09) or the background EMG in the right FDI (F2,14= 0.04, P= 0.96). The trend toward a larger ISP area in the condition of maximal isometric voluntary contraction of the left ECR compared to the other two experimental conditions was not significant (Table 1, Fig. 4).
ISP modulation by preparation of left FDI contraction (temporal specificity)
When the stimulation protocol used in the RT protocol was applied whilst the subjects were requested to observe the visual go-signal but not to react with the left hand, the rmANOVA showed no significant effect of ‘Experimental condition’ on the ISP area (F2,12= 0.76, P= 0.49). This indicates that the visual go-signal per se did not modulate the ISP in the right FDI.
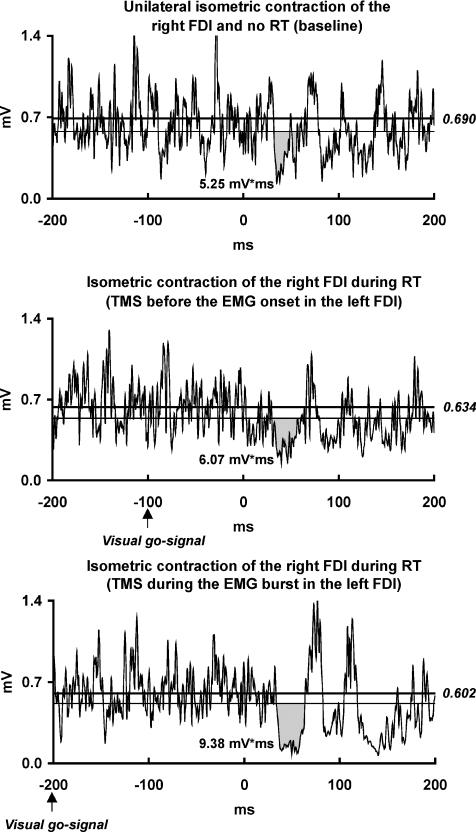
During the RT protocol, rmANOVA revealed a significant effect of ‘Experimental condition’ on the ISP area (F2,12= 4.67, P= 0.032). Post hoc tests showed that the ISP area in the right FDI was significantly larger when the TMS pulse was delivered 200 ms after the go-signal (i.e. during the EMG burst in the left FDI) than when the left FDI was not engaged in the RT (baseline) or when TMS was delivered 100 ms after the go-signal (i.e. before the EMG bursts in the left FDI, Table 1, Fig. 5). There was a non-significant trend in the 100 ms condition for a slightly larger ISP area than in the baseline condition (Table 1, Fig. 5). There was no effect of ‘Experimental condition’ on the background EMG in the right FDI (F2,12= 0.19, P= 0.83; Table 1). The RT values (mean ±s.d.) were 122 ± 18 ms (range 88–147 ms) when the TMS pulse was delivered during the EMG burst and 209 ± 30 ms (range 181–273) when TMS was delivered prior to the EMG bursts in the left FDI. The average EMG level in the left FDI during the phasic movement of the left index (mean ±s.d.) was 0.313 ± 0.119 mV. These values corresponded to 85 ± 27% of the individual EMG level during maximal isometric contraction of the left FDI.
Figure 5. ISP modulation by preparation of left FDI contraction (temporal specificity).
EMG recordings from the maximally contracting right FDI muscle of one representative subject. The subject was instructed to perform a rapid thumb-to-index tap with the left hand as soon as possible after a visual go-signal whilst maintaining maximal isometric contraction of the right FDI. Recordings were made in three experimental conditions: (i) without go-signal (baseline); (ii) TMS 100 ms after the go-signal (before onset of the voluntary EMG burst in the left FDI); (iii) TMS 200 ms after the go-signal (during the EMG burst in the left FDI). For further arrangements and conventions, see Fig. 1. ISP area in the right FDI was enhanced after onset of the voluntary EMG burst in the left FDI but not during its preparation when compared with the baseline condition.
Circuit specificity of task-related ISP modulation
The comparison of task-related ISP vs. S-IHI modulation showed that the ISP area in the right FDI was significantly larger during bilateral maximum isometric FDI contraction than during unilateral right FDI contraction (P= 0.025, Table 2). In contrast, no significant task-related differences in unconditioned test MEP amplitude (P= 0.84) or S-IHI of the MEP in the right FDI (P= 0.94) were seen when evaluated under identical motor task conditions as used for ISP assessment (Table 2). MEP amplitude in the left FDI during unilateral right FDI contraction and bilateral FDI contraction was 3.4 ± 2.7 and 6.8 ± 2.1 mV, respectively. rmANOVA revealed no difference in the background EMG in the right FDI across motor tasks and TMS conditions (Table 2). The percentage differences of ISP area vs. S-IHI in the right FDI as calculated from the comparison between bilateral FDI contraction and unilateral right FDI contraction did not correlate with each other (r= 0.15, P= 0.68).
Table 2.
ISP vs. S-IHI modulation: ISP area, test MEP amplitude, S-IHI of the MEP, and background EMG level in the right FDI (mean ± s.d.) in the different motor tasks
| TMS of the right M1 alone |
TMS of the left M1 alone |
Paired pulse TMS at an ISI of 10 ms |
||||
|---|---|---|---|---|---|---|
| ISP area (mV ms) | Background EMG level (mV) | Unconditioned test MEP amplitude (mV) | Background EMG level (mV) | IHI of the MEP (%)a | Background EMG level (mV) | |
| Unilateral contraction of the right FDI | 2.79 ± 2.20 | 0.382 ± 0.074 | 2.05 ± 1.23 | 0.373 ± 0.078 | 65 ± 33 | 0.365 ± 0.107 |
| Bilateral contraction of the FDI | 3.96 ± 2.65* | 0.372 ± 0.093 | 2.10 ± 1.21 | 0.363 ± 0.068 | 66 ± 28 | 0.351 ± 0.114 |
P < 0.05, two-tailed paired t test.
Unconditioned/conditioned test MEP amplitude (%). ISP, ipsilateral silent period; S-IHI, interhemispheric inhibition at short interstimulus interval; MEP, motor-evoked potential; EMG, electromyographic; FDI, first dorsal interosseous muscle.
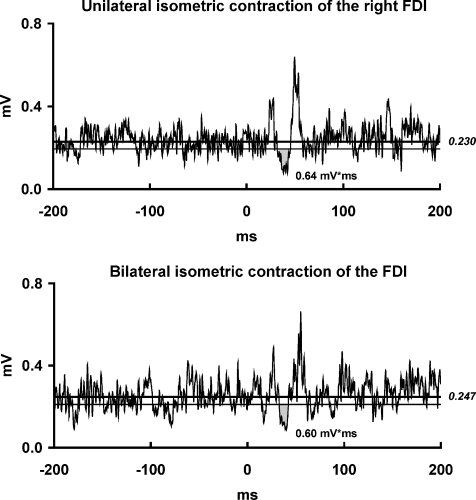
The CSP area was not different when comparing unilateral (mean ±s.d.: 0.65 ± 0.68 mV ms) and bilateral maximal isometric contraction of the FDI (0.79 ± 0.80 mV ms, P= 0.41) (Fig. 6). Likewise, neither the amplitude of the contralateral MEP preceding the CSP (P= 0.40) nor the background EMG in the right FDI (P= 0.07) differed significantly between unilateral and bilateral FDI contraction (MEP amplitude: mean ±s.d.: 2.7 ± 1.6 vs. 2.6 ± 1.7 mV; background EMG: 0.365 ± 0.100 vs. 0.391 ± 0.116 mV, respectively). Note that, in this subset of eight subjects, the ISP area obtained in the main experiment was larger during bilateral (mean ±s.d.: 3.43 ± 1.89 mV ms) than during unilateral FDI contraction (mean ±s.d.: 1.56 ± 0.81 mV ms, P= 0.02).
Figure 6. Circuit specificity of motor task-related ISP modulation.
EMG recordings from the maximally contracting right FDI muscle of one representative subject in two experimental conditions: (i) left FDI at rest; (ii) maximal isometric contraction of the left FDI. The waveforms are averages of 20 rectified trials. Grey region represents the cortical silent period (CSP) area (in mV ms). CSP onset and offset were determined by using statistical process control (see Methods). The upper line represents the mean background EMG amplitude and lower line represents the lower 95% variation limit of the mean background EMG amplitude. Note that there was no difference in the CSP area between the two experimental conditions.
Relationship between motor task-related ISP modulation and voluntary hand movement lateralisation
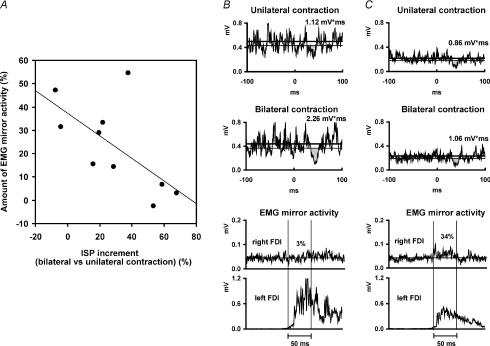
A paired t test confirmed that the ISP area in the right FDI was larger during bilateral FDI contraction (mean ±s.d.: 4.08 ± 2.48 mV ms) than during unilateral right FDI contraction (3.13 ± 2.26 mV ms, P= 0.019) with non-differing levels of background EMG (0.429 ± 0.111 and 0.445 ± 0.122 mV, respectively, P= 0.13). The percentage increment in ISP area in the right FDI with bilateral FDI contraction vs. unilateral right FDI contraction was inversely correlated (r=−0.64, P= 0.046) to the amount of EMG mirror activity observed in the right FDI during phasic left index finger abduction (Fig. 7A). In contrast, no significant correlation was seen between the absolute ISP area during unilateral (r=−0.17, P= 0.63) or bilateral (r=−0.44, P= 0.20) FDI contraction and the amount of EMG mirror activity.
Figure 7. Relation between motor task-related ISP modulation and EMG mirror activity.
A, data from all 10 subjects. The increment of ISP area in the right FDI observed when comparing bilateral maximal isometric FDI contraction with unilateral maximal isometric contraction of the right FDI is expressed as the percentage difference between the two measurements (x-axis). Positive and negative values indicate increases vs. decreases in ISP area, respectively. The amount of EMG mirror activity in the right FDI is given on the y-axis (for details, see Methods). ISP area increment and amount of EMG mirror activity correlated inversely (r=−0.64, P= 0.046). B, recordings from one representative subject with large motor task-related ISP area increment and low EMG mirror activity. C, recordings from one other representative subject with low motor task-related ISP area increment but a large amount of EMG mirror activity.
Discussion
The main novel result of the present study is that, in healthy adults, volitional motor activity of the stimulated M1 by movement of the contralateral hand produces a significant enhancement of the ISP area in the ipsilateral hand. Additional experiments characterised this phenomenon by showing its early recruitment with low levels of contraction, topographical specificity, temporal specificity, neuronal circuit specificity and functional significance. The following paragraphs provide a detailed discussion of each of these features of motor task-related ISP modulation.
Motor task-related ISP modulation
Motor task-related ISP enhancement in the right FDI is a robust finding, because it was observed with either tonic or phasic contractions of the left FDI, at different levels of isometric contraction (minimal vs. maximal), and even with just imagination of a thumb-to-index opposition of the left hand. Furthermore, maximum voluntary contraction of the left FDI does not increase the ISP enhancement observed with minimal contraction any further. This indicates early recruitment of this motor task-related enhancement of interhemispheric inhibition, for left-hand movement, from the voluntarily active right M1 to the opposite left M1. We showed in another experiment that the ISP modulation correlated inversely with the amount of EMG mirror activity in the target FDI (see Fig. 7). Therefore, it can be concluded that, in terms of functional significance, early recruitment of ISP enhancement is probably particularly effective in warding off unwanted mirror activity in the case of low contraction levels. This prediction is consistent with several observations that show occurrence of mirror activity mainly with high forces or demanding motor tasks (Cernacek, 1961; Zijdewind & Kernell, 2001; Bodwell et al. 2003; Post et al. 2008, 2009). In addition, we showed that the presence of slight, unintended EMG activity in the left FDI (physiological mirroring) during intended maximal unilateral isometric contraction of the right FDI did not modify the ISP in the right FDI when compared with full voluntary relaxation of the left FDI. Hence, it can be safely concluded that any intention to suppress physiological mirroring in the task FDI did not influence the ISP in the ipsilateral target FDI. Therefore, it was appropriate to use the condition ‘full left FDI relaxation’ as a control condition in all the other experiments.
These results could not be explained by changes in background EMG activity in the ISP target muscle (right FDI), as the background EMG did not change across experimental conditions within a given experimental session (cf. Tables 1 and 2). In addition, the observed ISP enhancement was not accounted for by a non-specific increase in motor cortical excitability in the stimulated right M1 for the following reasons. It is well known that imagery of upper limb movements enhances the excitability of the corticomotoneuronal system in the contralateral M1, as revealed by facilitation of the size of the TMS-induced MEP (Izumi et al. 1995; Rossi et al. 1998; Fadiga et al. 1999; Rossini et al. 1999). On the other hand, the slight increase of MEP amplitude we observed in the left FDI involved in the imagined motor task was not significant. However, it should be noted that the strong isometric contraction of the right FDI necessary for the ISP measurements goes along with an increase in the excitability of the crossed corticospinal system in the right M1 (Rossini et al. 1994; Muellbacher et al. 2000; Cincotta et al. 2004). It is possible that an interaction of these two different mechanisms produced a ceiling effect that made any MEP facilitation due to motor imagery in the right M1 unappreciable. An alternative explanation is that, in right-handed subjects, increased excitability of the corticospinal neurones during motor imagery may predominate in the left M1, and occurs to a lesser extent or not at all in the right M1 (Stinear et al. 2006). Whatever the explanation, it can be concluded that the ISP enhancement during motor imagery of the contralateral hand cannot be accounted for by a non-specific increase in excitability of the stimulated right M1. This proposition is supported further by the lack of task-related S-IHI enhancement from the right to left M1 in the presence of ISP enhancement (see below). Other experiments showed that short-interval intracortical inhibition and intracortical facilitation are reduced rather than increased when the stimulated M1 is engaged in volitional motor activity, further indicating that different neuronal circuits in M1 can show enhancement, depression or no change under the conditions of an identical motor task (Ridding et al. 1995; Zoghi & Nordstrom, 2007). Finally, somatosensory re-afferent feedback may cause changes in interhemispheric inhibition. In patients after a cerebral stroke, transient ischaemic anaesthesia of the intact hand resulted in a decrease of S-IHI from the lesional to the contralesional M1 (Floel et al. 2008). Modulation of the ISP area by somatosensory re-afferent feedback has never been investigated, but the present experiments suggest that it does not play a major role, as the ISP enhancement can occur during motor imagery, i.e. independent of changes in the proprioceptive feedback as it would be produced by real movements of the contralateral hand.
Topographical and temporal specificity of motor task-related ISP modulation
Enhancement of the ISP does not necessarily require voluntary activation of the left hand muscle that is homologous to the ISP target muscle (right FDI), as it also occurred with contraction of the left EIP, another distal upper limb muscle. However, lacking ISP enhancement with more proximal left upper limb muscles (ECR, BB), or a left or right lower limb muscle (TA) clearly indicates that the motor task-related ISP enhancement is substantially topographically specific. This is in good accord with anatomical data in monkeys (Rouiller et al. 1994) and magnetic resonance imaging (MRI) data in humans (Wahl et al. 2007), which supported the notion that callosal fibres connect largely homologous M1 body representations in the two hemispheres.
Furthermore, the RT experiment suggests that enhancement of the ISP occurs relatively late in the neuronal processes related to contralateral hand movements, as only a non-significant trend toward ISP facilitation was seen during preparation of a finger movement of the opposite hand 100 ms after the go-signal. A higher temporal resolution was excluded by the experimental design. However, the time course of ISP modulation seems to differ from the time course of S-IHI modulation from the active to non-active M1 in preparation for a unimanual movement, where a maximal inhibition was found ∼100 ms after the go-signal, compared to less inhibition in a control resting condition and very shortly before movement onset (Duque et al. 2007). This supports the notion of dissociable processes underlying the ISP and the S-IHI (see further below). Another bifocal paired-pulse TMS study investigated the interhemispheric interactions between the left dorsal premotor cortex, an area important for movement selection and preparation, and the right M1, and showed an early inhibition 100 ms after the go-signal, if the right hand had to be moved (Koch et al. 2006). This is another example pointing to the rather late-stage operation of the motor task-related ISP modulation in the present study.
Circuit specificity of motor task-related ISP modulation
The ISP induced by focal single-pulse TMS of the M1 ipsilateral to the target hand muscle measures transcallosal inhibition of voluntary motor output from the non-stimulated contralateral M1 (Wassermann et al. 1991; Ferbert et al. 1992; Meyer et al. 1995; Trompetto et al. 2004; Cincotta et al. 2006). The ISP is absent or delayed in patients with agenesis or surgical lesions of the corpus callosum (Meyer et al. 1995, 1998). A transcallosal route of the ISP is further supported by ISP abnormalities in several neurological and psychiatric disorders with frequent structural alteration of the callosal interhemispheric connections, such as cerebral stroke (Boroojerdi et al. 1996), multiple sclerosis (Boroojerdi et al. 1998; Höppner et al. 1999; Schmierer et al. 2000; Lenzi et al. 2007), movement disorders (Niehaus et al. 2001; Trompetto et al. 2003; Wolters et al. 2004), schizophrenia (Höppner et al. 2001; Fitzgerald et al. 2002; Bajbouj et al. 2004), and attention deficit hyperactivity disorder (Buchmann et al. 2006).
S-IHI reflects the suppressive effect of a conditioning TMS pulse applied to one M1 on the MEP elicited by a test TMS pulse given some 10 ms later to the M1 of the other hemisphere (Ferbert et al. 1992). Epidural cervical spinal cord recordings of the corticospinal volley induced by the test stimulus (Di Lazzaro et al. 1999) and a significant correlation between magnitude of S-IHI and fractional anisotropy, an MRI-based measure of microstructural integrity, of callosal motor fibres (Wahl et al. 2007) strongly suggest that S-IHI is also mediated through the corpus callosum. A number of paired-pulse studies demonstrated motor task-related S-IHI modulation. Weak unilateral isometric contraction of the FDI slightly enhances S-IHI from the voluntarily active to the non-active M1 (Ferbert et al. 1992; Mochizuki et al. 2004; Talelli et al. 2008).
It can be argued that the ISP enhancement observed in the present study by imagined or real motor activity of the hand contralateral to the stimulated M1 does not merely confirm those findings of the previous S-IHI studies. First, the increase of S-IHI observed during slight unilateral contraction of the FDI contralateral to the conditioning stimulus disappeared when the ipsilateral FDI was contracted in addition (Ferbert et al. 1992). In contrast, ISP enhancement produced by imagined or real movements of the contralateral hand is observed during isometric contraction of the ISP target muscle (right FDI ipsilateral to the stimulated M1), a mandatory prerequisite for ISP recordings. In addition, by matching conditioned and test MEP amplitudes across different force levels, Perez & Cohen (2008) recently found that S-IHI decreased rather than increased when the hand contralateral to the conditioning pulse was engaged in generating high levels of force when compared to rest and slight muscle contraction. Finally, Chen et al. (2003) did not find any correlation between S-IHI and ISP duration or ISP area across different stimulus intensities (45, 60, 75 and 90% of MSO) and different current directions induced in the brain. Taken together, these results provide substantial evidence that the neuronal circuits responsible for the ISP differ from those underlying the S-IHI. Here we put this notion a step further by demonstrating that motor task-induced modulations of the ISP and the S-IHI were not correlated with each other. On the other hand, Chen et al. (2003) and Avanzino et al. (2007) found similarities between ISP and IHI tested at a long interstimulus interval of 40 ms (L-IHI). Several other recent studies support the notion that S-IHI and L-IHI constitute physiologically distinct forms of interhemispheric inhibition (Kukaswadia et al. 2005; Irlbacher et al. 2007; Ni et al. 2008). Whether or not motor task-related modulation of the ISP and L-IHI is similar as well as the question of whether ISP and S-IHI modulation can also be dissociated using lower levels of target FDI contraction is beyond the scope of this study and may be tested in further experiments.
Hence, the present ISP findings expand the current knowledge on the interhemispheric control of hand movements because ISP measurements deal more directly than S-IHI with interhemispheric inhibition of voluntary motor cortical output.
At present, however, it is not entirely clear in which of the two hemispheres the motor task-related ISP enhancement originates. The CSP elicited in the right FDI by stimulation of the left M1, which was carefully matched to the duration of the ISP, was not modulated by left FDI contraction. This dissociation between ISP and CSP suggests that the motor task-related ISP enhancement occurs separately from the inhibitory circuits responsible for the CSP in the left M1, and this may be taken as supportive although not conclusive evidence for an origin of the ISP modulation in the stimulated right M1.
Finally, a possible hemispheric asymmetry of the task-related ISP modulation was not assessed in this study, as only the modulation from the non-dominant right M1 (voluntarily active for left hand movements) to the dominant left M1 (non-active for left hand movements) was tested. This may constitute a slight limitation of the present study. However, one previous study by Duque et al. (2007) showed that task modulation of S-IHI in right-handed subjects did not show hemispheric asymmetry if tested from the voluntarily active to the non-active M1. Whether this holds true for the motor task-related ISP modulation needs to be tested in further experiments.
Functional significance of motor task-related ISP modulation
The enhancement of the ISP seen in our experiments reflects a task-specific facilitation of interhemispheric inhibitory influences from the stimulated M1 to the opposite M1. Numerous studies showed that bimanual coordination is more stable in motor tasks requiring mirror-symmetrical rather than non-symmetrical contraction of homologous muscles (for review, see Swinnen, 2002; Swinnen & Wenderoth, 2004). Spatial and temporal constraints limiting the execution of non-symmetrical bimanual movements have been identified (Swinnen, 2002; Swinnen & Wenderoth, 2004). In addition, although healthy adults are usually able to perform unilateral movements in daily life (Schott & Wyke, 1981), a subtle, involuntary mirror activation is often present (Cernacek, 1961; Armatas et al. 1994; Zijdewind & Kernell, 2001; Bodwell et al. 2003; Baliz et al. 2005; Ottaviani et al. 2008; Post et al. 2008, 2009). This physiological mirroring, which increases with more demanding motor tasks, fatigue, cognitive distraction, decrease in attentional capacity, and age, depends on unintended motor output along the crossed corticospinal projection from the M1 ipsilateral to the voluntary task (‘mirror’ M1) and suggests a tendency for movements of the upper extremities to be drawn towards one another (for review, see Cincotta & Ziemann, 2008). Therefore, performance of symmetrical bimanual voluntary movements is considered a basic coordinative behaviour of the nervous system, whereas asymmetrical bimanual motor tasks as well as unimanual movements require complex mechanisms of motor control (Cincotta & Ziemann, 2008) in order to set into action the so-called ‘non-mirror transformation’ of default ‘symmetrical’ motor programmes (Chan & Ross, 1988). In the present study, we demonstrated that the ISP enhancement observed when the stimulated M1 is generating motor output to the contralateral hand inversely correlated to the amount of EMG mirror activity in the tonically contracted target hand muscle by intended unilateral phasic contraction of the homologous contralateral muscle. These data provide novel evidence as to the functional significance of the ISP by directly linking its motor task-related modulation to neural mechanisms involved in lateralisation of voluntary movements. This strongly support the hypothesis that the M1 contralateral to a given voluntary movement contributes to this process by a late-stage inhibition of unwanted ‘mirror’ motor output in the opposite M1. This view is in keeping with the absence of the ISP in childhood (Heinen et al. 1998; Koerte et al. 2009), when physiological mirroring is far greater than in adults.
Acknowledgments
This project was supported by a grant from ‘Ente Cassa di Risparmio di Firenze’, Florence, Italy.
Glossary
Abbreviations
- BB
biceps brachii
- CSP
cortical silent period
- ECR
extensor carpi radialis
- EIP
extensor indicis proprius
- EMG
electromyographic
- FDI
first dorsal interosseous
- IHI
interhemispheric inhibition
- ISP
ipsilateral silent period
- L-IHI
long-interval interhemispheric inhibition
- M1
primary motor cortex
- MEP
motor-evoked potential
- MSO
maximum stimulator output
- RMS
root mean squares
- RMT
resting motor threshold
- RT
reaction time
- S-IHI
short-interval interhemispheric inhibition
- TA
tibialis anterior
- TMS
transcranial magnetic stimulation
Author contributions
F.G., A.B., MC, and U.Z. conceived and designed the experiments; F.G., A.B., F.B., and M.C. carried out the experiments; F.G. and M.P.V. performed data analysis; F.G., A.B., and F.B. drafted the paper; G.Z., M.P.V., M.C., and U.Z. revised the paper. All authors approved the final version of the article. The experiments were done in the TMS laboratory at the Unit of Neurology, Florence Health Authority, Florence, Italy.
References
- Armatas CA, Summers JJ, Bradshaw JL. Mirror movements in normal adult subjects. J Clin Exp Neuropsychol. 1994;16:405–413. doi: 10.1080/01688639408402651. [DOI] [PubMed] [Google Scholar]
- Avanzino L, Teo JTH, Rothwell JC. Intracortical circuits modulate transcallosal inhibition in humans. J Physiol. 2007;583:99–114. doi: 10.1113/jphysiol.2007.134510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajbouj M, Gallinat J, Niehaus L, Lang UE, Roricht S, Meyer BU. Abnormalities of inhibitory neuronal mechanisms in the motor cortex of patients with schizophrenia. Pharmacopsychiatry. 2004;37:74–80. doi: 10.1055/s-2004-815529. [DOI] [PubMed] [Google Scholar]
- Baliz Y, Armatas C, Farrow M, Hoy KE, Fitzgerald PB, Bradshaw JL, Georgiou-Karistianis N. The influence of attention and age on the occurrence of mirror movements. J Int Neuropsychol Soc. 2005;11:855–862. doi: 10.1017/s1355617705051003. [DOI] [PubMed] [Google Scholar]
- Bodwell JA, Mahurin RK, Waddle S, Price R, Cramer SC. Age and features of movement influence motor overflow. J Am Geriatr Soc. 2003;51:1735–1739. doi: 10.1046/j.1532-5415.2003.51557.x. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996;144:160–170. doi: 10.1016/s0022-510x(96)00222-5. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Hungs M, Mull M, Topper R, Noth J. Interhemispheric inhibition in patients with multiple sclerosis. Electroencephalogr Clin Neurophysiol. 1998;109:230–237. doi: 10.1016/s0924-980x(98)00013-7. [DOI] [PubMed] [Google Scholar]
- Brinkman C. Supplementary motor area of the monkey's cerebral cortex: short- and long-term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci. 1984;4:918–929. doi: 10.1523/JNEUROSCI.04-04-00918.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann J, Gierow W, Weber S, Hoeppner J, Klauer T, Wittstock M, Benecke R, Haessler F, Wolters A. Modulation of transcallosally mediated motor inhibition in children with attention deficit hyperactivity disorder (ADHD) by medication with methylphenidate (MPH) Neurosci Lett. 2006;405:14–18. doi: 10.1016/j.neulet.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Carson RG. Neural pathways mediating bilateral interactions between the upper limbs. Brain Res Brain Res Rev. 2005;49:641–662. doi: 10.1016/j.brainresrev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Cernacek J. Contralateral motor irridation-cerebral dominance. Arch Neurol. 1961;4:61–68. doi: 10.1001/archneur.1961.00450080047005. [DOI] [PubMed] [Google Scholar]
- Chan JL, Ross ED. Left-handed mirror writing following right anterior cerebral artery infarction: evidence for nonmirror transformation of motor programs by right supplementary motor area. Neurology. 1988;38:59–63. doi: 10.1212/wnl.38.1.59. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Jie-Yuan L. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Borgheresi A, Balestrieri F, Giovannelli F, Rossi S, Ragazzoni A, Zaccara G, Ziemann U. Involvement of the human dorsal premotor cortex in unimanual motor control: an interference approach using transcranial magnetic stimulation. Neurosci Lett. 2004;367:189–193. doi: 10.1016/j.neulet.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Giovannelli F, Borgheresi A, Balestrieri F, Zaccara G, Inghilleri M, Berardelli A. Modulatory effects of high-frequency repetitive transcranial magnetic stimulation on the ipsilateral silent period. Exp Brain Res. 2006;171:490–496. doi: 10.1007/s00221-005-0296-3. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Ziemann U. Neurophysiology of unimanual motor control and mirror movements. Clin Neurophysiol. 2008;119:744–762. doi: 10.1016/j.clinph.2007.11.047. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res. 1999;124:520–524. doi: 10.1007/s002210050648. [DOI] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, Olivier E, Cohen LG. Intermanual differences in movement-related interhemispheric inhibition. J Cogn Neurosci. 2007;19:204–213. doi: 10.1162/jocn.2007.19.2.204. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Buccino G, Craighero L, Fogassi L, Gallese V, Pavesi G. Corticospinal excitability is specifically modulated by motor imagery: a magnetic stimulation study. Neuropsychologia. 1999;37:147–158. doi: 10.1016/s0028-3932(98)00089-x. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Daskalakis ZJ, deCastella A, Kulkarni J. A study of transcallosal inhibition in schizophrenia using transcranial magnetic stimulation. Schizophr Res. 2002;56:199–209. doi: 10.1016/s0920-9964(01)00222-5. [DOI] [PubMed] [Google Scholar]
- Floel A, Hummel F, Duque J, Knecht S, Cohen LG. Influence of somatosensory input on interhemispheric interactions in patients with chronic stroke. Neurorehabil Neural Repair. 2008;22:477–485. doi: 10.1177/1545968308316388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey MA, Barker CA, Bartko JJ, Denckla MB, Wassermann EM, Castellanos FX, Dell ML, Ziemann U. The ipsilateral silent period in boys with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2005;116:1889–1896. doi: 10.1016/j.clinph.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Garvey MA, Ziemann U, Becker DA, Barker CA, Bartko JJ. New graphical method to measure silent periods evoked by transcranial magnetic stimulation. Clin Neurophysiol. 2001;112:1451–1460. doi: 10.1016/s1388-2457(01)00581-8. [DOI] [PubMed] [Google Scholar]
- Giovannelli F, Borgheresi A, Balestrieri F, Ragazzoni A, Zaccara G, Cincotta M, Ziemann U. Role of the right dorsal premotor cortex in ‘physiological’ mirror EMG activity. Exp Brain Res. 2006;175:633–640. doi: 10.1007/s00221-006-0581-9. [DOI] [PubMed] [Google Scholar]
- Heinen F, Glocker FX, Fietzek U, Meyer BU, Lücking CH, Korinthenberg R. Absence of transcallosal inhibition following focal magnetic stimulation in preschool children. Ann Neurol. 1998;43:608–612. doi: 10.1002/ana.410430508. [DOI] [PubMed] [Google Scholar]
- Höppner J, Kunescha E, Buchmann J, Hess A, Grossmann A, Benecke R. Demyelination and axonal degeneration in corpus callosum assessed by analysis of transcallosally mediated inhibition in multiple sclerosis. Clin Neurophysiol. 1999;110:748–756. doi: 10.1016/s1388-2457(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Höppner J, Kunesch E, Grossmann A, Tolzin CJ, Schulz M, Schlafke D, Ernst K. Dysfunction of transcallosally mediated motor inhibition and callosal morphology in patients with schizophrenia. Acta Psychiatr Scand. 2001;104:227–235. doi: 10.1034/j.1600-0447.2001.00247.x. [DOI] [PubMed] [Google Scholar]
- Hübers A, Orekhov Y, Ziemann U. Interhemispheric motor inhibition: its role in controlling electromyographic mirror activity. Eur J Neurosci. 2008;28:364–371. doi: 10.1111/j.1460-9568.2008.06335.x. [DOI] [PubMed] [Google Scholar]
- Irlbacher K, Brocke J, Mechow JV, Brandt SA. Effects of GABAA and GABAB agonists on interhemispheric inhibition in man. Clin Neurophysiol. 2007;118:308–316. doi: 10.1016/j.clinph.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Izumi S, Findley TW, Ikai T, Andrews S, Daum M, Chino N. Facilitatory effects of thinking about movement on motor-evoked potential transcranial magnetic stimulation of the brain. Am J Phys Med Rehab. 1995;74:207–213. doi: 10.1097/00002060-199505000-00005. [DOI] [PubMed] [Google Scholar]
- Koch G, Franca M, Del Olmo MF, Cheeran B, Milton R, Alvarez Sauco M, Rothwell JC. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci. 2006;26:7452–7459. doi: 10.1523/JNEUROSCI.1158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte I, Heinen F, Fuchs T, Laubender RP, Pomschar A, Stahl R, Berweck S, Winkler P, Hufschmidt A, Reiser MF, Ertl-Wagner B. Anisotropy of callosal motor fibres in combination with transcranial magnetic stimulation in the course of motor development. Invest Radiol. 2009;44:279–284. doi: 10.1097/RLI.0b013e31819e9362. [DOI] [PubMed] [Google Scholar]
- Kukaswadia S, Wagle-Shukla A, Morgante F, Gunraj C, Chen R. Interactions between long latency afferent inhibition and interhemispheric inhibitions in the human motor cortex. J Physiol. 2005;563:915–924. doi: 10.1113/jphysiol.2004.080010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Conte A, Mainero C, Frasca V, Fubelli F, Totaro P, Caramia F, Inghilleri M, Pozzilli C, Pantano P. Effect of corpus callosum damage on ipsilateral motor activation in patients with multiple sclerosis: a functional and anatomical study. Hum Brain Mapp. 2007;28:636–644. doi: 10.1002/hbm.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayston MJ, Harrison LM, Stephens JA. A neurophysiological study of mirror movements in adults and children. Ann Neurol. 1999;45:583–594. doi: 10.1002/1531-8249(199905)45:5<583::aid-ana6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Röricht S, Gräfin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118:429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, Woiciechowsky C. Topography of fibres in the human corpus callosum mediating interhemispheric inhibition between the motor cortices. Ann Neurol. 1998;43:360–389. doi: 10.1002/ana.410430314. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Ziemann U, Hajak G, Cohen L, Berman KF. Transitions between dynamical states of differing stability in the human brain. Proc Natl Acad Sci U S A. 2002;99:10948–10953. doi: 10.1073/pnas.162114799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H, Huang Y-Z, Rothwell JC. Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J Physiol. 2004;561:331–338. doi: 10.1113/jphysiol.2004.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller C, Arai N, Lücke J, Ziemann U. Hysteresis effects on the input-output curve of motor evoked potentials. Clin Neurophysiol. 2009;120:1003–1008. doi: 10.1016/j.clinph.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Facchini S, Boroojerdi B, Hallett M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol. 2000;111:344–349. doi: 10.1016/s1388-2457(99)00243-6. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Nelson AJ, Yeh IJ, Castillo G, Hoque T, Chen R. Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cereb Cortex. 2008;19:1654–1665. doi: 10.1093/cercor/bhn201. [DOI] [PubMed] [Google Scholar]
- Niehaus L, Alt-Stutterheim K, Roricht S, Meyer BU. Abnormal postexcitatory and interhemispheric motor cortex inhibition in writer's cramp. J Neurol. 2001;248:51–56. doi: 10.1007/s004150170269. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ottaviani D, Tiple D, Suppa A, Colosimo C, Fabbrini G, Cincotta M, De Fazio G, Berardelli A. Mirror movements in patients with Parkinson's disease. Mov Disord. 2008;23:253–258. doi: 10.1002/mds.21825. [DOI] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci. 2008;28:5631–5640. doi: 10.1523/JNEUROSCI.0093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Interhemispheric inhibition between primary motor cortices: what have we learned? J Physiol. 2009;587:725–726. doi: 10.1113/jphysiol.2008.166926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R, Lemon R. Corticospinal Function and Voluntary Movement. Oxford: Clarendon Press; 1993. [Google Scholar]
- Post M, Bayrak S, Kernell D, Zijdewind I. Contralateral muscle activity and fatigue in the human first dorsal interosseous muscle. J Appl Physiol. 2008;105:70–82. doi: 10.1152/japplphysiol.01298.2007. [DOI] [PubMed] [Google Scholar]
- Post M, Bakels R, Zijdewind I. Inadvertent contralateral activity during a sustained unilateral contraction reflects the direction of target movement. J Neurosci. 2009;29:6353–6357. doi: 10.1523/JNEUROSCI.0631-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Pasqualetti P, Tecchio F, Pauri F, Rossini PM. Corticospinal excitability modulation during mental simulation of wrist movements in human subjects. Neurosci Lett. 1998;243:147–151. doi: 10.1016/s0304-3940(98)00088-3. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH, Maertens de Noordhout AL, Marsden CD, Murray NMF, Rothwell JC, Swash M, Tomberg C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroenceph Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, Pasqualetti P, Tecchio F. Corticospinal excitability modulation to hand muscles during movement imagery. Cereb Cortex. 1999;9:161–167. doi: 10.1093/cercor/9.2.161. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M. Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Exp Brain Res. 1994;102:227–243. doi: 10.1007/BF00227511. [DOI] [PubMed] [Google Scholar]
- Sadato N, Yonekura Y, Waki A, Yamada H, Ishii Y. Role of the supplementary motor area and the right premotor cortex in the coordination of bimanual finger movements. J Neurosci. 1997;17:9667–9674. doi: 10.1523/JNEUROSCI.17-24-09667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer K, Niehaus L, Roricht S, Meyer BU. Conduction deficits of callosal fibres in early multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;68:633–638. doi: 10.1136/jnnp.68.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott GD, Wyke MA. Congenital mirror movements. J Neurol Neurosurg Psychiatry. 1981;44:586–599. doi: 10.1136/jnnp.44.7.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Fleming MK, Byblow WD. Lateralization of unimanual and bimanual motor imagery. Brain Res. 2006;1095:139–147. doi: 10.1016/j.brainres.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Swinnen SP. Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci. 2002;3:350–361. doi: 10.1038/nrn807. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Wenderoth N. Two hands, one brain: cognitive neuroscience of bimanual skill. Trends Cogn Sci. 2004;8:18–25. doi: 10.1016/j.tics.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Talelli P, Waddingham W, Ewas A, Rothwell JC, Ward NS. The effect of age on task-related modulation of interhemispheric balance. Exp Brain Res. 2008;186:59–66. doi: 10.1007/s00221-007-1205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompetto C, Bove M, Marinelli L, Avanzino L, Buccolieri A, Abbruzzese G. Suppression of the transcallosal motor output: a transcranial magnetic stimulation study in healthy subjects. Exp Brain Res. 2004;158:133–140. doi: 10.1007/s00221-004-1881-6. [DOI] [PubMed] [Google Scholar]
- Trompetto C, Buccolieri A, Marchese R, Marinelli L, Michelozzi G, Abbruzzese G. Impairment of transcallosal inhibition in patients with corticobasal degeneration. Clin Neurophysiol. 2003;114:2181–2187. doi: 10.1016/s1388-2457(03)00213-x. [DOI] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;45:12132–12128. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, Fuhr P, Cohen LG, Hallett M. Effects of transcranial magnetic stimulation on ipsilateral muscles. Neurology. 1991;41:1795–1799. doi: 10.1212/wnl.41.11.1795. [DOI] [PubMed] [Google Scholar]
- Wolters A, Classen J, Kunesch E, Grossmann A, Benecke R. Measurements of transcallosally mediated cortical inhibition for differentiating parkinsonian syndromes. Mov Disord. 2004;19:518–528. doi: 10.1002/mds.20064. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Ishii K, Borgheresi A, Yaseen Z, Battaglia F, Hallett M, Cincotta M, Wassermann EM. Dissociation of the pathways mediating ipsilateral and contralateral motor evoked potentials in human hand and arm muscles. J Physiol. 1999;518:895–906. doi: 10.1111/j.1469-7793.1999.0895p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Netz J, Hömberg V. Delay in simple reaction time after focal transcranial magnetic stimulation of the human brain occurs at the final motor output stage. Brain Res. 1997;744:32–40. doi: 10.1016/s0006-8993(96)01062-1. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Kernell D. Bilateral interactions during contractions of intrinsic hand muscles. J Neurophysiol. 2001;85:1907–1913. doi: 10.1152/jn.2001.85.5.1907. [DOI] [PubMed] [Google Scholar]
- Zoghi M, Nordstrom MA. Progressive suppression of intracortical inhibition during graded isometric contraction of a hand muscle is not influenced by hand preference. Exp Brain Res. 2007;177:266–274. doi: 10.1007/s00221-006-0669-2. [DOI] [PubMed] [Google Scholar]