Abstract
The stability of a physiological control system, such as the arterial baroreflex, depends critically upon both the magnitude (i.e. gain or sensitivity) and timing (i.e. latency) of the effector response. Although studies have examined resting arterial baroreflex sensitivity in older subjects, little attention has been given to the influence of ageing on the latency of peak baroreflex responses. First, we compared the temporal pattern of heart rate (HR) and mean arterial blood pressure (BP) responses to selective carotid baroreceptor (CBR) unloading and loading in 14 young (22 ± 1 years) and older (61 ± 1 years) subjects, using 5 s pulses of neck pressure (NP, +35 Torr) and neck suction (NS, −80 Torr). Second, CBR latency was assessed following pharmacological blockade of cardiac parasympathetic nerve activity in eight young subjects, to better understand how known age-related reductions in parasympathetic nerve activity influence CBR response latency. In response to NP, the time to the peak increase in HR and mean BP were similar in young and older groups. In contrast, in response to NS the time to peak decrease in HR (2.1 ± 0.2 vs. 3.8 ± 0.2 s) and mean BP (6.7 ± 0.4 vs. 8.3 ± 0.2 s) were delayed in older individuals (young vs. older, P < 0.05). The time to peak HR and mean BP were delayed in young subjects following cardiac parasympathetic blockade (glycopyrrolate). Collectively, these data suggest that ageing is associated with delayed peak cardiovascular responses to acute carotid baroreceptor loading that may be, in part, due to age-related reductions in cardiac parasympathetic tone.
Introduction
The arterial baroreflex buffers moment-to-moment fluctuations in arterial blood pressure (BP) via modulation of autonomic neural activity to the heart and vasculature. Due to its association with the genesis of arrhythmias (Chen et al. 2006), orthostatic intolerance (Robertson et al. 1999) and increased cardiovascular risk (La Rovere et al. 1998; La Rovere et al. 2001), alterations in the sensitivity (i.e. gain) of the arterial baroreflex has received much attention. However, it has been reported that the stability of a physiological control system, such as the arterial baroreflex, depends not only upon the magnitude of the effector response (i.e. sensitivity or gain), but also critically upon the timing of this response (i.e. latency) (Mackey & Glass, 1977; Madwed et al. 1989; Cavalcanti & Belardinelli, 1996; Keyl et al. 2001). Although studies have examined resting arterial baroreflex sensitivity for the control of heart rate (HR) and BP in older subjects (Gribbin et al. 1971; Ebert et al. 1992; Matsukawa et al. 1996; Davy et al. 1998; Fisher et al. 2007; Monahan, 2007), little attention has been given to the influence of ageing on the latency of peak baroreflex responses. This may be of particular importance for the baroreflex control of BP in which impairments in reflex sensitivity with ageing have not always been identified (Shi et al. 1996), even though older subjects tend to have inadequate responses to abrupt changes in BP (e.g. standing) (Shi et al. 2000). Furthermore, alterations in timing of the baroreflex-mediated effector response are quite relevant from a clinical standpoint, as prolonged baroreflex latency, in the absence of changes in reflex sensitivity, have been associated with poor orthostatic tolerance (Gulli et al. 2005), while instabilities in cardiac baroreflex function may precipitate the occurrence of ventricular arrhythmias (Schwartz & Stone, 1982; Vanoli et al. 1991).
In young healthy individuals, a delay in the latency of the peak cardiac baroreflex response has been reported during both heat stress (Yamazaki & Sone, 2005) and dynamic exercise (Strange et al. 1990; Linnarsson et al. 1996; Sundblad & Linnarsson, 1996). Although not directly tested in these studies, it was suggested that the mechanism for this prolonged response is the increase in cardiac sympathetic tone (Yang et al. 1994; Sundblad & Linnarsson, 1996) and/or reduction in cardiac parasympathetic tone (Keyl et al. 2001) that are elicited by these stimuli (Robinson et al. 1966; Mitchell, 1990; Kenney et al. 1995; Niimi et al. 1997). Given that ageing is characterised by increased basal sympathetic nerve activity (SNA) (Seals & Esler, 2000), and diminished cardiac parasympathetic nerve activity (Davies, 1975; Lipsitz et al. 1990; Taylor et al. 1995; Poller et al. 1997; Oberhauser et al. 2001; Stratton et al. 2003), it may be reasonable to expect that the cardiac baroreflex response latency is altered in older individuals, but this remains to be determined. Furthermore, although a previous study in animals has reported age-related prolongations in the peak BP response to bilateral carotid artery occlusion (Ferrari et al. 1991), the influence of ageing on the time course of baroreflex-mediated BP responses in normotensive humans has not been established.
Given this background, the present study was designed to determine how ageing influences the temporal pattern of beat-to-beat HR and mean BP responses elicited by acute carotid baroreceptor (CBR) unloading and loading (Fadel et al. 2003). To achieve this, the application of neck pressure and neck suction was utilized to determine the peak latency of the carotid–cardiac and carotid-vasomotor baroreflex responses in young and older subjects. Additionally, studies using parasympathetic blockade in young subjects were performed to better understand how known age-related reductions in parasympathetic nerve activity influence CBR response latency (Davies, 1975; Lipsitz et al. 1990; Taylor et al. 1995; Poller et al. 1997; Oberhauser et al. 2001; Stratton et al. 2003). We tested the following hypotheses: (1) older individuals would exhibit a prolonged HR and mean BP response latency to CBR perturbation compared to younger individuals, and (2) in young subjects, peak cardiovascular response latency to CBR loading and unloading would be delayed following blockade of cardiac parasympathetic nerve activity.
Methods
Subjects
For Protocol 1 (ageing and CBR latency) 14 young (age 22 ± 1 years) and 14 older (age 61 ± 1 years) healthy subjects participated in this study. Subject characteristics are shown in Table 1. For Protocol 2 (parasympathetic blockade and CBR latency) eight healthy young subjects (age 25 ± 1 years, weight 71 ± 2 kg, height 181 ± 2 cm) participated. All experimental procedures and protocols conformed to the Declaration of Helsinki and were approved by the University of Missouri–Columbia Health Sciences Institutional Review Board (Protocol 1) and The Ethics Committee of Copenhagen (Protocol 2). Prior to participation subjects provided written informed consent and completed a medical health history questionnaire. Older subjects also underwent a physical examination by a physician investigator. No subject had a history or symptoms of cardiovascular, pulmonary, metabolic, or neurological disease. Both young and older subjects were recreationally active typically engaging in low (e.g. walking) and moderate (e.g. jogging, stationary bike) intensity aerobic activities 2–4 days a week. No subject was using prescribed or over the counter medications. Fasting blood chemistry screening indicated that triglycerides, cholesterol, lipoproteins and glucose concentrations in the older subjects were within the normal range for healthy adults (Table 1) (Grundy, 1999).
Table 1.
Subject characteristics
| Young | Older | |
|---|---|---|
| Men/Women | 9/5 | 8/6 |
| Age (years) | 22 ± 1 | 61 ± 1* |
| Weight (kg) | 69 ± 3 | 77 ± 4 |
| Height (cm) | 170 ± 3 | 173 ± 3 |
| BMI (kg m2) | 24 ± 1 | 26 ± 1 |
| Systolic BP (mmHg) | 116 ± 2 | 117 ± 3 |
| Diastolic BP (mmHg) | 66 ± 2 | 75 ± 2* |
| Mean BP (mmHg) | 83 ± 1 | 89 ± 3* |
| Pulse Pressure (mmHg) | 50 ± 4 | 42 ± 2 |
| Heart Rate (beats min−1) | 60 ± 2 | 61 ± 2 |
| Triglycerides (mg dl−1) | — | 101 ± 11 |
| Cholesterol (mg dl−1) | — | 199 ± 8 |
| HDL (mg dl−1) | — | 54 ± 4 |
| LDL (mg dl−1) | — | 125 ± 7 |
| BUN (mg dl−1) | — | 16 ± 1 |
| Plasma Na+ (meq 1−1) | — | 140 ± 0.5 |
| Plasma K+ (meq 1−1) | — | 4.1 ± 0.1 |
| Glucose (mg dl−1) | — | 100 ± 3 |
Values are means ±s.e.m. BMI, body mass index; BP, blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; BUN, blood urea nitrogen.
Significantly different from young (P < 0.05).
CBR perturbation
Five-second pulses of +35 Torr neck pressure (NP) and −80 Torr neck suction (NS) were used to selectively unload and load the carotid baroreceptors, respectively. These stimuli were chosen to produce near maximal physiological amounts of carotid sinus hypotension and hypertension and to examine possible asymmetry in baroreflex latency to NP and NS (Potts & Raven, 1995; Fadel et al. 2001). The application of NP and NS was performed using a malleable lead collar fitted around the anterior two-thirds of the neck. Each stimulus was delivered 50 ms after the second consecutive R-R interval that did not vary by >50 ms using a customized computer controlled system (Potts & Raven, 1995; Fisher et al. 2007). Changes in neck collar pressure were generated by a variable pressure source and delivered to the neck collar through large-bore two-way solenoid valves (model 8215B, Asco, Florham Park, NJ, USA). A pressure transducer (model DP45, Validyne Engineering, Northridge, CA, USA) was connected to a port on the collar to accurately quantify the stimulus applied. To minimize respiratory-related modulation of HR, the 5 s pulses of NP and NS were delivered to the carotid sinus during a 12–15 s breath hold at end-expiration (Eckberg et al. 1980). Four to five trials of both NP and NS were performed with a minimum of 45 s of recovery allotted between trials to allow all physiological variables to return to pre-stimulus values (Potts & Raven, 1995; Ogoh et al. 2002, 2003; Fisher et al. 2007). Prior to the experimental session, appropriate neck chamber placement was ensured by first fitting the subjects based on observed neck size, and then performing resting trials of NP and NS to determine directionally appropriate and consistent HR and BP responses. In addition, all older subjects in Protocol 1 underwent Duplex ultrasound imaging to screen for significant carotid artery plaques and to identify the location of the carotid sinus bifurcation prior to performing NP and NS. The latter becomes particularly important to ensure correct collar placement in older subjects given the often blunted baroreflex responses observed with ageing (Brown et al. 2003; Fisher et al. 2007; Monahan, 2007).
Ageing and CBR latency (protocol 1)
To determine how ageing influences CBR latency, the temporal pattern of beat-to-beat HR and mean BP responses were assessed during the application of NP and NS in young and older subjects.
Instrumentation
HR was continuously monitored using a lead II ECG. Beat-to-beat arterial BP was measured using photoplethysmography from the middle finger of the left hand positioned at the level of the right atrium in the midaxillary line (Finometer, Finapres Medical Systems, Amsterdam, the Netherlands). Finometer measurements were checked with absolute BP measurements obtained by an automated sphygmomanometer (SunTech, Medical Instruments, Raleigh, NC, USA). Respiratory movements were monitored using a strain gauge pneumograph placed in a stable position around the abdomen (Pneumotrace, UFI, Morro Bay, CA, USA). The ECG signal, respiratory signal and BP waveform were sampled at 1000 Hz (Powerlab, ADInstruments, Bella Vista, NSW, Australia) and beat-to-beat values of HR, systolic BP, diastolic BP and mean BP were stored for off-line analysis (Chart v5.2, ADInstruments).
Experimental procedures
Subjects arrived at the laboratory a minimum of 2 h following a light meal and had abstained from caffeinated beverages for 12 h and strenuous physical activity and alcohol for at least 24 h. Subjects were seated in a semi-recumbent position on a medical exam table and following instrumentation a 20 min rest period was conducted after which resting trials of NP and NS were performed as described above.
Heart rate variability analyses
Indices of cardiac autonomic control were derived using time and frequency domain analysis of spontaneous oscillations in HR. Time domain analysis of heart rate variability was performed using the square root of the mean of the sum of successive differences in R-R interval (RMSSD), which is recommended for the estimation of short-term high-frequency variability of HR (Task force 1996). Frequency domain analysis of heart rate variability was performed using fast Fourier transformation, and power spectral density calculated at the high frequency range (HF; 0.15–0.4 Hz) (Fisher et al. 2009). It is generally accepted that HF power predominantly represents cardiac parasympathetic tone (Malliani et al. 1991; Parati et al. 1995; Task Force, 1996), but this notion has been challenged (Taylor et al. 2001). Although there is some suggestion that power at the low frequency range (0.04–0.15 Hz) can suffice as an index of cardiac sympathetic activity (1996; Pagani et al. 1997), a significant parasympathetic component has also been demonstrated (Malliani et al. 1991; Task Force, 1996). The ratio between LF and HF has been proposed as a measure of the balance between the cardiac and sympathetic control of the heart (Task Force, 1996; Pagani et al. 1997), but this remains questionable (Taylor & Studinger, 2006). Given the lack of a satisfactory index of cardiac SNA, in the present study indices of cardiac parasympathetic tone were principally utilized (i.e. RMSSD and HF power).
Parasympathetic blockade and CBR latency (protocol 2)
To investigate the normal contribution of parasympathetic nerve activity to CBR response latency, NP and NS were applied before and after cardiac muscarinic blockade with glycopyrrolate in young subjects. These studies were added to better understand how known age-related reductions in parasympathetic nerve activity influence CBR response latency (Davies, 1975; Lipsitz et al. 1990; Taylor et al. 1995; Poller et al. 1997; Oberhauser et al. 2001; Stratton et al. 2003).
Instrumentation
Arterial BP was measured by a catheter (1.1 mm i.d., 20 gauge) placed in the brachial artery of the non-dominant arm and connected to a transducer (Baxter, Uden, the Netherlands) positioned at the level of the right atrium in the midaxillary line. A catheter (1.2 mm i.d., 18 gauge) was inserted into the median antecubital vein for the administration of the study drug. HR was monitored using a lead II ECG. Respiratory movements were monitored using a strain gauge pneumograph placed in a stable position around the abdomen. The signals were connected to a Dialogue 2000 monitor (IBC-Danica, Copenhagen, Denmark) interfaced with a personal computer equipped with customized data acquisition software for the beat-to-beat recording of variables. Both the ECG signal and BP waveforms were sampled at 200 Hz, and real-time beat-to-beat values of HR, systolic BP, diastolic BP and mean BP were stored for off-line analysis.
Experimental procedures
Subjects were seated in a semi-recumbent position on a hospital bed, and following instrumentation a 20 min rest period was conducted. Resting trials of NP and NS were performed. In this group, trials of NP and NS were repeated after cardiac muscarinic blockade, which was achieved via stepwise infusions of 0.2 mg of glycopyrrolate. Complete blockade was determined when consecutive doses of 0.2 mg caused no further increases in HR (group average dose of 12.6 ± 1.6 μg kg−1).
Data and statistical analysis
A 5 min data segment was used for the calculation of baseline cardiovascular variables and heart rate variability. Reflex changes in HR and mean BP were calculated from pre-stimulus values and the response plotted on a beat-to-beat scale with peak response latency defined as the elapsed time to the peak response beat, as described previously (Potts & Raven, 1995; Yamazaki & Sone, 2005). The time to peak change in HR and mean BP in response to individual trials of NP and NS were averaged for each subject and then combined to provide a group mean. Data were tested for normal distribution by the Shapiro–Wilk test. Statistical comparisons of physiological variables between young and older groups were made using Student's t test for independent samples. Equality of variances was assessed using Levine's test and adjustments were made when appropriate. Comparisons between control and glycopyrrolate conditions were made using paired t tests. For non-normally distributed data, a non-parametric Mann–Whitney U test (young vs. older) or Wilcoxon's sign-rank test (control vs. glycopyrrolate) was used. Correlation coefficients relating peak cardiac response latency and indices of cardiac autonomic control derived using heart rate variability analysis were obtained using Spearman's correlation analysis. Statistical significance was set at P < 0.05. Results are presented as means ± standard error of the mean (s.e.m.). Analyses were conducted using SPSS v. 13.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Ageing and CBR latency (protocol 1)
Baseline characteristics
The mean age difference between young and older subjects was 39 years (Table 1). There were no significant age-group differences in body mass index, systolic BP, pulse pressure or HR (P > 0.05), while mean BP and diastolic BP were lower in the young group (P < 0.05).
CBR unloading and age
Figure 1A shows original records from young and older individuals illustrating the temporal pattern of the beat-to-beat mean BP and HR responses to CBR unloading with NP. Group summary data are provided in Fig. 2. The time to peak change in mean BP (P= 0.35) and HR (P= 0.35) in response to NP was similar in the young and older subjects. Likewise, no age group differences were found in the number of beats taken to reach the peak mean BP (P= 0.40) and HR (P= 0.16) response to NP. However, the magnitude of the HR response to NP was attenuated in the older group (Δ+8 ± 1 vs.Δ+3 ± 0.4 beats min−1, young vs. older; P < 0.05), whereas the magnitude of the mean BP response to NP was not significantly different between the groups (Δ+7 ± 1 vs.Δ+6 ± 1 mmHg, young vs. older; P= 0.48).
Figure 1. Temporal pattern of beat-to-beat mean blood pressure and heart rate responses to acute carotid baroreceptor unloading and loading.
Original records from young (filled circles) and older subjects (open circles) illustrating the temporal pattern of beat-to-beat mean blood pressure (mean BP) and heart rate responses to acute carotid baroreceptor unloading (neck pressure, NP; A) and loading (neck suction, NS; B). Peak responses are highlighted with an arrow.
Figure 2. Peak heart rate and mean blood pressure responses elicited by acute carotid baroreceptor unloading.
Summary data showing the characteristics of the peak heart rate (A) and mean blood pressure (mean BP; B) responses elicited by acute carotid baroreceptor unloading (neck pressure) in young and older subjects. Values are means ±s.e.m. *Significantly different from young (P < 0.05).
CBR loading and age
Original records illustrating the temporal pattern of the beat-to-beat mean BP and HR responses to CBR loading with NS in young and older individuals, are shown in Fig. 1B. Group summary data are provided in Fig. 3. The time to peak change in mean BP and HR in response to NS was significantly prolonged in the older subjects. Similarly, the number of beats taken to reach the peak response was greater in the older group, compared with the young group (35% and 104% slower mean BP and HR response in older subjects, P < 0.05 vs. young). The magnitude of the HR response to NS was significantly attenuated in the older group (Δ−11 ± 2 vs.Δ−6 ± 1 beats min−1, young vs. older; P < 0.05), whereas the magnitude of the mean BP response (Δ−11 ± 1 vs.Δ−15 ± 2 mmHg, young vs. older; P < 0.05) to NS was significantly greater in the older group.
Figure 3. Peak heart rate and mean blood pressure responses elicited by acute carotid baroreceptor loading.
Summary data showing the characteristics of the peak heart rate (A) and mean blood pressure (mean BP; B) responses elicited by acute carotid baroreceptor loading (neck suction) in young and older subjects. Values are means ±s.e.m. *Significantly different from young (P < 0.05).
Heart rate variability and CBR cardiac latency
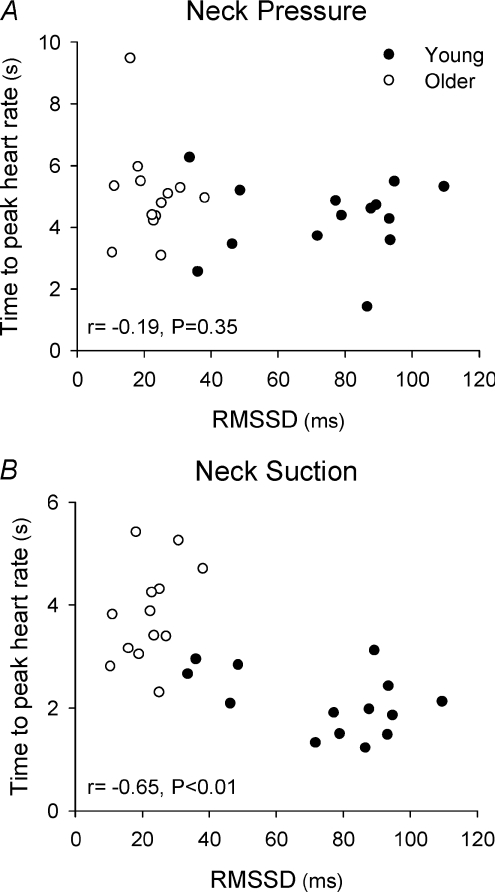
Time and frequency domain indices of heart rate variability for young and older groups are shown in Table 2. RMSSD, LF power, HF power and total power were significantly lower in the older group (P < 0.05 vs. young). The latency of the peak HR response to NP was not observed to be related to the calculated indices of cardiac parasympathetic tone, RMSSD (r=−0.19, P= 0.35; Fig. 4) or HF power (r=−0.18, P= 0.35). In contrast, a significant relationship was found between the peak latency of the HR response to NS and both RMSSD (r=−0.65, P < 0.01; Fig. 4) and HF power (r=−0.65, P < 0.01).
Table 2.
Time and frequency domain indices of heart rate variability in young and older subjects (protocol 1), and young subjects under control conditions and following glycopyrrolate (protocol 2)
| Protocol 1 |
Protocol 2 |
|||
|---|---|---|---|---|
| Young | Older | Control | Glycopyrrolate | |
| RR interval (ms) | 997 ± 25 | 998 ± 31 | 995 ± 70 | 590 ± l0† |
| RMSSD (ms) | 75 ± 6 | 24 ± 3* | 51 ± 9 | 3 ± 1† |
| LF power (ms2) | 1406 ± 390 | 375 ± 93* | 767 ± 189 | 4 ± 1† |
| HF power (ms2) | 1506 ± 291 | 180 ± 31* | 877 ± 289 | 3 ± 1† |
| Total power (ms2) | 2911 ± 636 | 554 ± 110* | 1644 ± 445 | 7 ± 2† |
Values are means ±s.e.m. RMSSD, square root of the mean of the sum of successive differences; LF power, low frequency power spectral density (0.04–0.15 Hz); HF power, high frequency power spectral density (0.15–0.4 Hz).
Significantly different from young (P < 0.05).
Significantly different from control (P < 0.05).
Figure 4.
Relationship between RMSSD, an index of cardiac parasympathetic tone, and the latency of the peak heart response to neck pressure (A) and neck suction (B)
Parasympathetic blockade and CBR latency (protocol 2)
Baseline characteristics
As expected, HR was significantly increased by cardiac parasympathetic blockade with glycopyrrolate (63 ± 5 vs. 102 ± 2 beats min−1, control vs. glycopyrrolate, P < 0.05). In addition, mean BP was slightly increased with glycopyrrolate (84 ± 3 vs. 89 ± 2 mmHg, control vs. glycopyrrolate, P < 0.05). Glycopyrrolate significantly reduced RR interval, RMSSD, LF power, HF power, and total power (P < 0.05 vs. control; Table 2).
CBR unloading with parasympathetic blockade
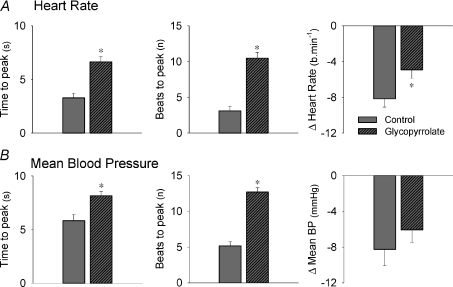
The time to the peak change in mean BP and HR in response to NP was significantly delayed with glycopyrrolate (P < 0.05; Fig. 5). Furthermore, the magnitude of the HR response to NP was significantly attenuated with glycopyrrolate (P < 0.05 vs. control), whereas the mean BP response to NP was not different from control conditions (P > 0.05).
Figure 5. Peak heart rate and mean blood pressure responses elicited by acute carotid baroreceptor unloading under control conditions and following glycopyrrolate.
Summary data showing the characteristics of the peak heart rate (A) and mean blood pressure (mean BP; B) responses elicited by acute carotid baroreceptor unloading (neck pressure) in young subjects under control conditions (grey bar) and following glycopyrrolate (grey and striped bar). Values are means ±s.e.m. *Significantly different from control (P < 0.05).
CBR loading with parasympathetic blockade
The time to the peak change in mean BP and HR in response to NS was significantly prolonged with glycopyrrolate (P < 0.05; Fig. 6). Following glycopyrrolate administration the magnitude of the HR response to NS was significantly attenuated (P < 0.05 vs. control), while the magnitude of the mean BP response was unchanged.
Figure 6. Peak heart rate and mean blood pressure responses elicited by acute carotid baroreceptor loading in young subjects under control conditions and following glycopyrrolate.
Summary data showing the characteristics of the peak heart rate (A) and mean blood pressure (mean BP; B) responses elicited by acute carotid baroreceptor loading (neck suction) in young subjects under control conditions (grey bar) and following glycopyrrolate (grey and striped bar). Values are means ±s.e.m. *Significantly different from control (P < 0.05).
Discussion
The major finding of the present study is the age-related delay in the time to the peak cardiovascular response to carotid baroreceptor loading (i.e. simulated carotid hypertension with NS), but similar temporal response to carotid baroreceptor unloading (i.e. simulated carotid hypotension with NP) in young and older subjects. Notably, the slower time to peak HR and BP responses to carotid baroreceptor loading observed in older individuals was related to reductions in indices of resting cardiac parasympathetic tone. Furthermore, cardiac parasympathetic blockade in young subjects significantly prolonged the timing of peak cardiovascular responses to carotid baroreceptor loading. Overall, our findings suggest that older individuals exhibit sluggish cardiovascular responses to simulated carotid hypertension, which may be due, in part, to age-related reductions in resting cardiac parasympathetic tone.
Previous work in young subjects has observed delayed carotid–cardiac responses during heat stress (Yamazaki & Sone, 2005) and dynamic exercise (Strange et al. 1990; Linnarsson et al. 1996; Sundblad & Linnarsson, 1996). These studies suggested that such delays were attributable to the reductions in cardiac parasympathetic nerve activity (Keyl et al. 2001) and/or the increases in SNA (Yang et al. 1994; Sundblad & Linnarsson, 1996) that are typically elicited by these stimuli (Robinson et al. 1966; Mitchell, 1990; Kenney et al. 1995; Niimi et al. 1997). Given that ageing is characterised by both reductions in parasympathetic nerve activity (Davies, 1975; Lipsitz et al. 1990; Taylor et al. 1995; Poller et al. 1997; Oberhauser et al. 2001; Stratton et al. 2003) and increases in SNA (Esler et al. 1995; Seals & Esler, 2000), such changes in cardiac autonomic control may provide a potential mechanism for the age-related prolongation of the peak HR response to carotid baroreceptor loading that we observed. Indeed, age-related increases in carotid–cardiac baroreflex latency were found to be correlated with reductions in indices of resting parasympathetic tone (i.e. RMSSD and HF power). In addition, in young subjects cardiac parasympathetic blockade significantly prolonged the carotid–cardiac latency. Taken together these findings suggest that age-related reductions in parasympathetic tone (Davies, 1975; Lipsitz et al. 1990; Taylor et al. 1995; Poller et al. 1997; Oberhauser et al. 2001; Stratton et al. 2003) may contribute to the delayed peak responses to carotid baroreceptor loading observed in older individuals (Head & McCarty, 1987; Keyl et al. 2001).
Although the importance of parasympathetic nerve activity for cardiac baroreflex control has been well established, an important contribution from the sympathetic nervous system to the age-related slowing of HR responsiveness cannot be completely discounted (Yang et al. 1994; Sundblad & Linnarsson, 1996; Herring & Paterson, 2009). Indeed, previous work in rats has demonstrated that antecedent stimulation of sympathetic nerves to the sinus node markedly attenuated the speed of the bradycardic response to vagal stimuli (Yang et al. 1994). In addition, reflex activation of SNA has been purported to slow the carotid–cardiac baroreflex latency in young subjects (Sundblad & Linnarsson, 1996). Thus, the elevations in resting cardiac SNA reported in older subjects (Esler et al. 1995) may potentially contribute to prolonged latency of the peak reflex bradycardia to NS. Importantly, increases in SNA with age might be expected to influence latency even more given the age-related reductions in parasympathetic nerve activity. In this regard, if cardiac parasympathetic tone is compromised the baroreflex would be limited to modulation of cardiac sympathetic control. Given that changes in cardiac SNA take 2–3 s to have an effect, as compared to <1 s for the cardiac parasympathetic response time (Head & McCarty, 1987), a delay in baroreceptor chronotropic responses might be a sensitive indicator of cardiac parasympathetic abnormalities or changes in the balance between cardiac parasympathetic and sympathetic control (Sundblad & Linnarsson, 1996).
Along with an age-related delay in the peak HR response to simulated carotid hypertension with NS, the time to peak BP was also significantly delayed in older individuals. Importantly, our studies with pharmacological blockade of cardiac parasympathetic activity demonstrated a concomitant prolongation of the time to the peak CBR-mediated HR response as well as the BP response with NS. These findings suggest that the timing of the HR response to NS has an important influence on the timing of the peak BP response. This observation is in agreement with previous work by Ogoh et al. (2002, 2003) who assessed the relative contributions of cardiac output and peripheral vascular conductance to CBR-mediated changes in blood pressure. Of note, these authors observed that HR-induced changes in cardiac output made a significant contribution to the initial BP responses to NP and NS. Thus, removal of the contribution of cardiac output may lead to a delay in baroreflex latency for the control of BP. However, an important caveat to pharmacological blockade of resting parasympathetic nerve activity is the enhancement in resting cardiac output owing to the >40 beats min−1 increase in HR (Stratton et al. 2003). This alteration makes comparisons of the relative contributions of cardiac output and vascular conductance to normal conditions difficult, but clearly demonstrates that reductions in resting parasympathetic nerve activity delay CBR-cardiac latency in young subjects, which may in turn delay the occurrence of the peak BP response to CBR perturbation.
Although cardiac output can contribute importantly to the CBR control of BP, it is well established that sympathetically mediated changes in peripheral vascular conductance are of critical importance (Ogoh et al. 2002, 2003; Fadel, 2008). Age-related increases in resting sympathetic outflow to the periphery have been well documented (Seals & Esler, 2000); however, the mechanisms and functional role of this increase in SNA remain unclear. In the present study we observed a slower time to the peak BP response to NS in older subjects suggesting that the time required to remove the enhanced resting SNA may contribute to the prolonged latency to a hypertensive challenge with age. However, given the numerous steps involved in the arterial baroreflex regulation of BP (e.g. baroreceptor afferent output and conduction, central integration, efferent autonomic activity or end-organ responsiveness to autonomic changes) as well as the multiple alterations that occur in the cardiovascular system with age (e.g. arterial stiffening, reduced M2 muscarinic receptor density and function) (Lakatta, 1993; Poller et al. 1997; Seals & Esler, 2000; Oberhauser et al. 2001; Monahan, 2007), additional studies are needed to better understand the mechanisms by which CBR BP latency is prolonged in older individuals.
In contrast to the age-related prolongation of the cardiovascular responses to NS, we observed a similar time to peak HR and BP in the young and older groups in response to simulated carotid hypotension with NP. This finding is in contrast to previous work in animals which has reported an age-related prolongation in the time to peak BP response to 12 s of bilateral carotid artery occlusion in rats (Ferrari et al. 1991). However, species differences and disparities in the duration and modality of CBR perturbation employed make direct comparisons with the results of the current study difficult. Furthermore, the HR responses to carotid occlusion were notably variable in the older rats and unexpectedly tended to decrease (by Δ−19 ± 13 beats min−1), in contrast to the small but consistent increase in HR with NP that we observed in the older individuals (Δ+2.1 ± 0.2 beats min−1). In addition, Mancia et al. (1984) reported that in older individuals (>54 years) the initial BP responses to 2 min of NP were slower. However, resting BP was notably elevated in the older group examined (mean BP 137 ± 5 mmHg) and as such it is unclear whether the initial sluggish BP responses reported were the result of ageing per se, or were attributable to the hypertensive state (Eckberg, 1979). Thus, the present study is the first to determine the changes that occur in the temporal pattern of HR and BP responses elicited by acute CBR unloading and loading in normotensive older individuals.
We observed an age-related delay in the cardiovascular response to carotid hypertension simulated with NS, but similar temporal response to carotid hypotension simulated with NP in young and older individuals. The reasons for this altered response pattern are not clear, although several points require consideration. First, HR responses to NS are quite rapid in young subjects, occurring in 1–2 s, whereas responses to NP occur with a naturally slower latency on the order of 3–4 s (Fadel et al. 2003). In this regard, it has been suggested that changes in cardiac SNA may play a greater role in evoking increases in HR to carotid baroreceptor unloading contributing importantly to the inherent slower response time (Eckberg, 1980). However, a contribution of parasympathetic nerve activity is clearly present as the HR response latency to NP was longer after glycopyrrolate in young subjects (Fig. 5). A plausible reason why a prolongation in the older subjects was not observed is that there was still adequate parasympathetic tone to evoke a HR response in the normal time window, as heart rate variability indices are higher in older subjects than those following glycopyrrolate in young subjects (see Table 2). Lastly, interactions between mechanical (transduction of pressure into carotid stretch) and neural (transduction of carotid stretch into vagal outflow) aspects of the baroreflex may be different during falls and rises in BP leading to divergent response times (Studinger et al. 2007, 2009). Overall, the complex interplay between these various aspects of integrated baroreflex control may contribute to differential age-related cardiac latency alterations between carotid hypertension and hypotension.
The magnitude of HR responses to NP and NS was significantly attenuated in older individuals, and is likely to be indicative of an age-related reduction in overall carotid–cardiac baroreflex sensitivity which has been previously demonstrated by our group (Fisher et al. 2007) and others (Gribbin et al. 1971; Ebert et al. 1992; Matsukawa et al. 1996; Monahan, 2007). As expected, parasympathetic blockade with glycopyrrolate significantly attenuated the magnitude of the cardiac responses to NP and NS in young subjects, suggesting that age-related reductions in cardiac parasympathetic tone may underlie the attenuated responses observed in older individuals. Interestingly, previous work has suggested that both age-related reductions in cardiac baroreflex sensitivity (Shi et al. 2000) and cholinergic blockade in young adults (Wray et al. 2001) lead to impairments in the ability to compensate for abrupt changes in BP (e.g. standing). However, despite the significant reduction in cardiac responses to NP in the older group in the present study, the magnitude of the BP response was similar between groups, whereas the magnitude of the peak reduction in BP with NS was more marked. This finding was somewhat unexpected given previous contradictory work reporting either impaired (Jones et al. 2001) or preserved (Shi et al. 1996; Brown et al. 2003) arterial baroreflex regulation of BP with increased age. However, in line with our findings, recent work by Studinger et al. (2009) reported that in response to a rise in arterial pressure (i.e. arterial baroreflex loading) the inhibition of sympathetic outflow is enhanced in older adults, compared with younger adults. Thus, in the current study, the more pronounced reduction in BP induced by NS in the older group may be due to a greater ability to inhibit sympathetic outflow to the peripheral vasculature.
We acknowledge that caution must be exercised when using heart rate variability as an index of cardiac autonomic tone, due to the ongoing scientific debate regarding the validity of these measures (Parati et al. 2006; Taylor & Studinger, 2006). However, our observation that widely used indices of cardiac parasympathetic tone (i.e. RMSSD and HF power) (Malliani et al. 1991; Parati et al. 1995; Task Force, 1996) are reduced in older individuals, is well supported by previous studies (Davies, 1975; Lipsitz et al. 1990; Taylor et al. 1995; Poller et al. 1997; Oberhauser et al. 2001; Stratton et al. 2003). Furthermore, the administration of glycopyrrolate to pharmacologically block cardiac parasympathetic tone in the present study mimicked the age-related reductions in heart rate variability indices, and prolonged cardiovascular responses to carotid baroreceptor loading with NS. Collectively, these data suggest an important cardiac parasympathetic nerve activity component to peak baroreflex latency.
An important point to consider is the relationship between measures of baroreflex latency and traditional measures of baroreflex sensitivity. While it is quite possible that there is a relationship between these measures, it is unlikely that they are simply synonymous. Indeed, Gulli et al. (2005) demonstrated that subjects with postural related syncope had delayed baroreflex responses, while baroreflex sensitivity was not different from control subjects. Of note, our group and others have reported reductions in cardiovagal baroreflex sensitivity in older individuals compared with younger subjects (Gribbin et al. 1971; Ebert et al. 1992; Matsukawa et al. 1996; Fisher et al. 2007; Monahan, 2007). However, in the present study baroreflex latency was only delayed in the older subjects with NS, and not with NP. Collectively, these data highlight the importance of studying multiple facets of arterial baroreflex function.
The assessment of the arterial baroreflex in terms of baroreflex sensitivity (i.e. gain) has received considerable clinical attention, mainly due to its prognostic utility (Parati et al. 2006). However, along with the magnitude of the effector response (i.e. sensitivity or gain), the time delay of this response (i.e. latency) is of critical importance for the stability of the arterial baroreflex, or in fact any physiological control system (Mackey & Glass, 1977; Cavalcanti & Belardinelli, 1996). Indeed, computer simulations of baroreflex regulation have demonstrated that an increase in the time delay of the effector response may generate an unstable state of regulation, where cardiovascular oscillations become more complex and chaotic (Madwed et al. 1989; Keyl et al. 2001). Thus, it is possible that an increased time delay of the baroreflex response, as we observed in older individuals, associated with an impaired parasympathetic function, may contribute to an unstable regulation of heart rate and thus precipitate the occurrence of cardiac rhythm and/or blood pressure regulation abnormalities (Shi et al. 2000; Wray et al. 2001; Gulli et al. 2005). Furthermore, ageing-induced delays in baroreflex latency may compromise rapid reflex adjustments to cardiovascular stressors. However, further work is required to establish the utility and prognostic significance of baroreflex latency measurements.
In summary, our data demonstrate age-related alterations in baroreflex latency such that peak cardiovascular responses to carotid baroreceptor loading (i.e. simulated carotid hypertension with NS) are delayed. Furthermore, our findings suggest that such sluggish cardiovascular responses in older subjects may be, in part, due to age-related reductions in resting cardiac parasympathetic tone.
Acknowledgments
The authors appreciate the time and effort expended by all the volunteer subjects. This research is the result of work supported by NIH Grant no. HL-093167 (PJF), and by an American Heart Association Postdoctoral Fellowship Award (JPF).
Glossary
Abbreviations
- BP
blood pressure
- CBR
carotid baroreceptor
- HR
heart rate
- NP
neck pressure
- NS
neck suction
- RMSSD
square root of the mean of the sum of successive differences
- SNA
sympathetic nerve activity
Author contributions
J.P.F. contributed to study design, data acquisition, data analysis, data interpretation and wrote the first draft of the manuscript. A.K. contributed to data acquisition and data analysis. C.N.Y. contributed to data acquisition, data interpretation and critical review of the manuscript. S.O. contributed to data acquisition and data interpretation. P.B.R. contributed to data interpretation and critical review of the manuscript. N.H.S. provided clinical support and contributed to data acquisition and critical review of the manuscript. P.J.F. contributed to study design, data acquisition, data analysis, data interpretation and critical review of the manuscript. All authors approved the final version of the manuscript.
References
- Brown CM, Hecht MJ, Weih A, Neundorfer B, Hilz MJ. Effects of age on the cardiac and vascular limbs of the arterial baroreflex. Eur J Clin Invest. 2003;33:10–16. doi: 10.1046/j.1365-2362.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- Cavalcanti S, Belardinelli E. Modelling of cardiovascular variability using a differential delay equation. IEEE Trans Biomed Eng. 1996;43:982–989. doi: 10.1109/10.536899. [DOI] [PubMed] [Google Scholar]
- Chen J, Wasmund SL, Hamdan MH. Back to the future: the role of the autonomic nervous system in atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:413–421. doi: 10.1111/j.1540-8159.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- Davies HE. Respiratory change in heart rate, sinus arrhythmia in the elderly. Gerontol Clin (Basel) 1975;17:96–100. doi: 10.1159/000245563. [DOI] [PubMed] [Google Scholar]
- Davy KP, Tanaka H, Andros EA, Gerber JG, Seals DR. Influence of age on arterial baroreflex inhibition of sympathetic nerve activity in healthy adult humans. Am J Physiol Heart Circ Physiol. 1998;275:H1768–1772. doi: 10.1152/ajpheart.1998.275.5.H1768. [DOI] [PubMed] [Google Scholar]
- Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ. Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Physiol Heart Circ Physiol. 1992;263:H798–803. doi: 10.1152/ajpheart.1992.263.3.H798. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Carotid baroreflex function in young men with borderline blood pressure elevation. Circulation. 1979;59:632–636. doi: 10.1161/01.cir.59.4.632. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Nonlinearities of the human carotid baroreceptor-cardiac reflex. Circ Res. 1980;47:208–216. doi: 10.1161/01.res.47.2.208. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Kifle YT, Roberts VL. Phase relationship between normal human respiration and baroreflex responsiveness. J Physiol. 1980;304:489–502. doi: 10.1113/jphysiol.1980.sp013338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler MD, Thompson JM, Kaye DM, Turner AG, Jennings GL, Cox HS, Lambert GW, Seals DR. Effects of aging on the responsiveness of the human cardiac sympathetic nerves to stressors. Circulation. 1995;91:351–358. doi: 10.1161/01.cir.91.2.351. [DOI] [PubMed] [Google Scholar]
- Fadel PJ. Arterial baroreflex control of the peripheral vasculature in humans: rest and exercise. Med Sci Sports Exerc. 2008;40:2055–2062. doi: 10.1249/MSS.0b013e318180bc80. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Keller DM, Raven PB. Recent insights into carotid baroreflex function in humans using the variable pressure neck chamber. Exp Physiol. 2003;88:671–680. doi: 10.1113/eph8802650. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Watenpaugh DE, Wasmund W, Olivencia-Yurvati A, Smith ML, Raven PB. Carotid baroreflex regulation of sympathetic nerve activity during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2001;280:H1383–1390. doi: 10.1152/ajpheart.2001.280.3.H1383. [DOI] [PubMed] [Google Scholar]
- Ferrari AU, Daffonchio A, Albergati F, Mancia G. Differential effects of aging on the heart rate and blood pressure influences of arterial baroreceptors in awake rats. J Hypertens. 1991;9:615–621. doi: 10.1097/00004872-199107000-00006. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Ogoh S, Ahmed A, Aro MR, Gute D, Fadel PJ. Influence of age on cardiac baroreflex function during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2007;293:H777–783. doi: 10.1152/ajpheart.00199.2007. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Ogoh S, Junor C, Khaja A, Northrup M, Fadel PJ. Spontaneous baroreflex measures are unable to detect age-related impairments in cardiac baroreflex function during dynamic exercise in humans. Exp Physiol. 2009;94:447–458. doi: 10.1113/expphysiol.2008.044867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribbin B, Pickering TG, Sleight P, Peto R. Effect of age and high blood pressure on baroreflex sensitivity in man. Circ Res. 1971;29:424–431. doi: 10.1161/01.res.29.4.424. [DOI] [PubMed] [Google Scholar]
- Grundy SM. Primary prevention of coronary heart disease: integrating risk assessment with intervention. Circulation. 1999;100:988–998. doi: 10.1161/01.cir.100.9.988. [DOI] [PubMed] [Google Scholar]
- Gulli G, Cooper VL, Claydon VE, Hainsworth R. Prolonged latency in the baroreflex mediated vascular resistance response in subjects with postural related syncope. Clin Auton Res. 2005;15:207–212. doi: 10.1007/s10286-005-0273-8. [DOI] [PubMed] [Google Scholar]
- Head GA, McCarty R. Vagal and sympathetic components of the heart rate range and gain of the baroreceptor-heart rate reflex in conscious rats. J Auton Nerv Syst. 1987;21:203–213. doi: 10.1016/0165-1838(87)90023-3. [DOI] [PubMed] [Google Scholar]
- Herring N, Paterson DJ. Neuromodulators of peripheral cardiac sympatho-vagal balance. Exp Physiol. 2009;94:46–53. doi: 10.1113/expphysiol.2008.044776. [DOI] [PubMed] [Google Scholar]
- Jones PP, Shapiro LF, Keisling GA, Jordan J, Shannon JR, Quaife RA, Seals DR. Altered autonomic support of arterial blood pressure with age in healthy men. Circulation. 2001;104:2424–2429. doi: 10.1161/hc4501.099308. [DOI] [PubMed] [Google Scholar]
- Kenney MJ, Barney CC, Hirai T, Gisolfi CV. Sympathetic nerve responses to hyperthermia in the anaesthetized rat. J Appl Physiol. 1995;78:881–889. doi: 10.1152/jappl.1995.78.3.881. [DOI] [PubMed] [Google Scholar]
- Keyl C, Schneider A, Dambacher M, Bernardi L. Time delay of vagally mediated cardiac baroreflex response varies with autonomic cardiovascular control. J Appl Physiol. 2001;91:283–289. doi: 10.1152/jappl.2001.91.1.283. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT, Jr, Camm AJ, Schwartz PJ. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation. 2001;103:2072–2077. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. 1993;73:413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- Linnarsson D, Sundberg CJ, Tedner B, Haruna Y, Karemaker JM, Antonutto G, Di Prampero PE. Blood pressure and heart rate responses to sudden changes of gravity during exercise. Am J Physiol Heart Circ Physiol. 1996;270:H2132–2142. doi: 10.1152/ajpheart.1996.270.6.H2132. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Mietus J, Moody GB, Goldberger AL. Spectral characteristics of heart rate variability before and during postural tilt. Relations to aging and risk of syncope. Circulation. 1990;81:1803–1810. doi: 10.1161/01.cir.81.6.1803. [DOI] [PubMed] [Google Scholar]
- Mackey MC, Glass L. Oscillation and chaos in physiological control systems. Science. 1977;197:287–289. doi: 10.1126/science.267326. [DOI] [PubMed] [Google Scholar]
- Madwed JB, Albrecht P, Mark RG, Cohen RJ. Low-frequency oscillations in arterial pressure and heart rate: a simple computer model. Am J Physiol Heart Circ Physiol. 1989;256:H1573–1579. doi: 10.1152/ajpheart.1989.256.6.H1573. [DOI] [PubMed] [Google Scholar]
- Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- Mancia G, Grassi G, Bertinieri G, Ferrari A, Zanchetti A. Arterial baroreceptor control of blood pressure in man. J Auton Nerv Syst. 1984;11:115–124. doi: 10.1016/0165-1838(84)90070-5. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Sugiyama Y, Mano T. Age-related changes in baroreflex control of heart rate and sympathetic nerve activity in healthy humans. J Auton Nerv Syst. 1996;60:209–212. doi: 10.1016/0165-1838(96)00057-4. [DOI] [PubMed] [Google Scholar]
- Mitchell JH. J. B. Wolffe memorial lecture. Neural control of the circulation during exercise. Med Sci Sports Exerc. 1990;22:141–154. [PubMed] [Google Scholar]
- Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293:R3–R12. doi: 10.1152/ajpregu.00031.2007. [DOI] [PubMed] [Google Scholar]
- Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Ito H, Sobue G, Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst. 1997;63:61–67. doi: 10.1016/s0165-1838(96)00134-8. [DOI] [PubMed] [Google Scholar]
- Oberhauser V, Schwertfeger E, Rutz T, Beyersdorf F, Rump LC. Acetylcholine release in human heart atrium: influence of muscarinic autoreceptors, diabetes, and age. Circulation. 2001;103:1638–1643. doi: 10.1161/01.cir.103.12.1638. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fadel PJ, Monteiro F, Wasmund WL, Raven PB. Haemodynamic changes during neck pressure and suction in seated and supine positions. J Physiol. 2002;540:707–716. doi: 10.1113/jphysiol.2001.013259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol. 2003;550:317–324. doi: 10.1113/jphysiol.2003.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M, Montano N, Porta A, Malliani A, Abboud FM, Birkett C, Somers VK. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95:1441–1448. doi: 10.1161/01.cir.95.6.1441. [DOI] [PubMed] [Google Scholar]
- Parati G, Mancia G, Di Rienzo M, Castiglioni P. Point: cardiovascular variability is/is not an index of autonomic control of circulation. J Appl Physiol. 2006;101:676–678. doi: 10.1152/japplphysiol.00446.2006. discussion 681–672. [DOI] [PubMed] [Google Scholar]
- Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension. 1995;25:1276–1286. doi: 10.1161/01.hyp.25.6.1276. [DOI] [PubMed] [Google Scholar]
- Poller U, Nedelka G, Radke J, Ponicke K, Brodde OE. Age-dependent changes in cardiac muscarinic receptor function in healthy volunteers. J Am Coll Cardiol. 1997;29:187–193. doi: 10.1016/s0735-1097(96)00437-8. [DOI] [PubMed] [Google Scholar]
- Potts JT, Raven PB. Effect of dynamic exercise on human carotid-cardiac baroreflex latency. Am J Physiol Heart Circ Physiol. 1995;268:H1208–1214. doi: 10.1152/ajpheart.1995.268.3.H1208. [DOI] [PubMed] [Google Scholar]
- Robertson RM, Medina E, Shah N, Furlan R, Mosqueda-Garcia R. Neurally mediated syncope: pathophysiology and implications for treatment. Am J Med Sci. 1999;317:102–109. doi: 10.1097/00000441-199902000-00004. [DOI] [PubMed] [Google Scholar]
- Robinson BF, Epstein SE, Beiser GD, Braunwald E. Control of heart rate by the autonomic nervous systemStudies in man on the interrelation between baroreceptor mechanisms and exercise. Circ Res. 1966;19:400–411. doi: 10.1161/01.res.19.2.400. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Stone HL. The role of the autonomic nervous system in sudden coronary death. Ann N Y Acad Sci. 1982;382:162–180. doi: 10.1111/j.1749-6632.1982.tb55214.x. [DOI] [PubMed] [Google Scholar]
- Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol. 2000;528:407–417. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Gallagher KM, Welch-O’Connor RM, Foresman BH. Arterial and cardiopulmonary baroreflexes in 60- to 69- vs. 18- to 36-yr-old humans. J Appl Physiol. 1996;80:1903–1910. doi: 10.1152/jappl.1996.80.6.1903. [DOI] [PubMed] [Google Scholar]
- Shi X, Wray DW, Formes KJ, Wang HW, Hayes PM, O-Yurvati AH, Weiss MS, Reese IP. Orthostatic hypotension in aging humans. Am J Physiol Heart Circ Physiol. 2000;279:H1548–1554. doi: 10.1152/ajpheart.2000.279.4.H1548. [DOI] [PubMed] [Google Scholar]
- Strange S, Rowell LB, Christensen NJ, Saltin B. Cardiovascular responses to carotid sinus baroreceptor stimulation during moderate to severe exercise in man. Acta Physiol Scand. 1990;138:145–153. doi: 10.1111/j.1748-1716.1990.tb08826.x. [DOI] [PubMed] [Google Scholar]
- Stratton JR, Levy WC, Caldwell JH, Jacobson A, May J, Matsuoka D, Madden K. Effects of aging on cardiovascular responses to parasympathetic withdrawal. J Am Coll Cardiol. 2003;41:2077–2083. doi: 10.1016/s0735-1097(03)00418-2. [DOI] [PubMed] [Google Scholar]
- Studinger P, Goldstein R, Taylor JA. Mechanical and neural contributions to hysteresis in the cardiac vagal limb of the arterial baroreflex. J Physiol. 2007;583:1041–1048. doi: 10.1113/jphysiol.2007.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studinger P, Goldstein R, Taylor JA. Age- and fitness-related alterations in vascular sympathetic control. J Physiol. 2009;587:2049–2057. doi: 10.1113/jphysiol.2009.170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundblad P, Linnarsson D. Slowing of carotid-cardiac baroreflex with standing and with isometric and dynamic muscle activity. Am J Physiol Heart Circ Physiol. 1996;271:H1363–1369. doi: 10.1152/ajpheart.1996.271.4.H1363. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Taylor JA, Hayano J, Seals DR. Lesser vagal withdrawal during isometric exercise with age. J Appl Physiol. 1995;79:805–811. doi: 10.1152/jappl.1995.79.3.805. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Myers CW, Halliwill JR, Seidel H, Eckberg DL. Sympathetic restraint of respiratory sinus arrhythmia: implications for vagal-cardiac tone assessment in humans. Am J Physiol Heart Circ Physiol. 2001;280:H2804–2814. doi: 10.1152/ajpheart.2001.280.6.H2804. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Studinger P. Counterpoint: cardiovascular variability is not an index of autonomic control of the circulation. J Appl Physiol. 2006;101:678–681. doi: 10.1152/japplphysiol.00446.2006. discussion 681. [DOI] [PubMed] [Google Scholar]
- Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–1481. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- Wray DW, Formes KJ, Weiss MS, O-Yurvati AH, Raven PB, Zhang R, Shi X. Vagal cardiac function and arterial blood pressure stability. Am J Physiol Heart Circ Physiol. 2001;281:H1870–1880. doi: 10.1152/ajpheart.2001.281.5.H1870. [DOI] [PubMed] [Google Scholar]
- Yamazaki F, Sone R. Whole-body heating slows carotid baroreflex response in human subjects. Eur J Appl Physiol. 2005;94:690–696. doi: 10.1007/s00421-005-1349-9. [DOI] [PubMed] [Google Scholar]
- Yang T, Senturia JB, Levy MN. Antecedent sympathetic stimulation alters time course of chronotropic response to vagal stimulation in dogs. Am J Physiol Heart Circ Physiol. 1994;266:H1339–1347. doi: 10.1152/ajpheart.1994.266.4.H1339. [DOI] [PubMed] [Google Scholar]