Abstract
Arterial blood pressure can often fall too low during dehydration, leading to an increased incidence of orthostatic hypotension and syncope. Systemic sympathoexcitation and increases in volume regulatory hormones such as angiotensin II (AngII) may help to maintain arterial pressure in the face of decreased plasma volume. Our goals in the present study were to quantify muscle sympathetic nerve activity (MSNA) during dehydration (DEH), and to test the hypothesis that endogenous increases in AngII in DEH have a mechanistic role in DEH-associated sympathoexcitation. We studied 17 subjects on two separate study days: DEH induced by 24 h fluid restriction and a euhydrated (EUH) control day. MSNA was measured by microneurography at the peroneal nerve, and arterial blood pressure, electrocardiogram, and central venous pressure were also recorded continuously. Sequential nitroprusside and phenylephrine (modified Oxford test) were used to evaluate baroreflex control of MSNA. Losartan (angiotensin type 1 receptor (AT1) antagonist) was then administered and measurements were repeated. MSNA was elevated during DEH (42 ± 5 vs. EUH: 32 ± 4 bursts per 100 heartbeats, P= 0.02). Blockade of AT1 receptors partially reversed this change in MSNA during DEH while having no effect in the control EUH condition. The sensitivity of baroreflex control of MSNA was unchanged during DEH compared to EUH. We conclude that endogenous increases in AngII during DEH contribute to DEH-associated sympathoexcitation.
Introduction
Dehydration (DEH) causes arterial blood pressure dysregulation, resulting in an increased incidence of orthostatic hypotension and syncope. Neurohumoral responses to dehydration, which may help to maintain arterial pressure in the dehydrated state, include increased sympathetically mediated peripheral vasoconstriction, as well as increases in circulating levels of hormones such as angiotensin II (AngII). In addition to its volume-regulating influences, AngII has been shown to alter control of the sympathetic nervous system (Guo & Abboud, 1984; Cox & Bishop, 1991; Matsukawa et al. 1991; Fink, 1997; Hasser et al. 2000; Sanderford & Bishop, 2000; Bealer, 2003; McMullan et al. 2007) and, as such, may have a mechanistic role in the effects of DEH on the sympathetic nervous system. Since sympathetic neural mechanisms are key to the regulation of arterial pressure in humans, mechanisms underlying these changes are key to understanding the alterations in blood pressure regulation that occur in dehydrated humans.
In humans, exercise-induced DEH and hyperosmolality induced by saline infusion have been shown to cause alterations in sympathetic and cardiac baroreflex function (Charkoudian et al. 2003, 2005), which may be a protective mechanism to restore and protect arterial pressure. For example, augmented baroreflex sensitivity may allow the system to be more responsive to changes in blood volume and pressure seen during dehydration, allowing the system to respond to such changes before they become excessively detrimental (i.e. before syncope occurs).
Furthermore, there is evidence that AngII influences sympathetic activity (Cox & Bishop, 1991; Fink, 1997; Hasser et al. 2000). In animals, AngII has been shown to alter baroreflex function (Guo & Abboud, 1984; Sanderford & Bishop, 2000) and there is some evidence that it may have a mechanistic role in the changes in baroreflex sensitivity during hyperosmolarity (Bealer, 2003; McMullan et al. 2007). In humans, exogenously administered AngII was shown to augment sympathetic neural responses when the pressor effects of the AngII itself were reversed by simultaneous administration of nitroprusside (Matsukawa et al. 1991). Whether an influence of increased endogenous AngII is causal in dehydration-mediated sympathoexcitation in humans is unknown. Our purpose, therefore, was to quantify the effects of DEH on peripheral sympathetic vasoconstrictor neural activity in humans and to evaluate whether endogenous AngII has a mechanistic role in these changes.
We hypothesized that (1) muscle sympathetic nerve activity (MSNA) and sensitivity of baroreflex control of MSNA are increased during DEH in humans, and that (2) AngII contributes mechanistically to the increase in MSNA and in sympathetic baroreflex sensitivity during DEH. To test our hypotheses, we measured MSNA and calculated sympathetic baroreflex sensitivity before and after blocking angiotensin type 1 receptors (AT1) with losartan in dehydrated humans. We also performed the same investigations in the same individuals on a separate study day in which subjects maintained euhydration (EUH), to test whether losartan has effects on sympathetic activity and baroreflex function independent of dehydration, when angiotensin levels are relatively low.
Methods
Ethical approval
The Mayo Clinic Institutional Review Board approved this project and all procedures. Informed consent was obtained in writing from all subjects for this study which was performed in accordance with the standards of the Declaration of Helsinki.
All experiments were conducted in the Mayo CTSA Clinical Research Unit (CRU). Potential young healthy male and female subjects from Rochester Minnesota and surrounding areas were identified and underwent screening by a nurse study coordinator. Subjects were between the ages of 18 and 35 and were excluded if they were smokers, had a history of hypertension, cardiovascular disease or diabetes mellitus or were on antihypertensive, cardiac, vasoactive or non-steroidal anti-inflammatory medications. Female subjects had a confirmed negative pregnancy test within 48 h of any experiment. Females were studied during the early follicular phase of the menstrual cycle or placebo of oral contraceptive therapy (Minson et al. 2000a,b;). Seventeen eligible subjects (11 male and 6 female) were enrolled in the study and underwent informed consent by a study coordinator and were then each scheduled for two study days approximately one month apart, in random order. On one study day subjects served as their own control under conditions of euhydration, and on a second study day subjects were studied under conditions of dehydration.
Prior to any experiments, each subject had an individual consultation with a CRU dietician to evaluate his or her eating and drinking patterns, and the dietician provided specific rules for the euhydration and dehydration study days. For euhydration, subjects were instructed to maintain normal water intake during the 24 h prior to the study day. Individuals were given specific instruction from CRU dietitians regarding maintenance of euhydration, and were instructed to drink more fluids if they were not drinking enough. Dehydration was achieved by admitting subjects to the CRU 24 h prior to study day 2 for fluid restriction to 10 ml kg−1 as well as limited carbohydrates (< 25% of calories) and salt (2500 mg) under direction of a dietician with the goal of mild to moderate dehydration. The status of hydration was substantiated by laboratory tests including haemoglobin, haematocrit and plasma renin activity, as well as direct measurement of central venous pressure, on the respective study days. In this study we chose fluid restriction as the method of inducing dehydration to avoid the confounding effects of the metabolic and mechanical reflexes, the exercise pressor reflex and increased body temperature when exercise is used to induce dehydration, and the confounding effects of volume infusion when hypertonic infusion is used to mimic osmolar changes of dehydration.
All studies were performed according to an identical study protocol on the EUH day and the DEH day, starting at 07.00 h with the patient in supine position. Subjects were instrumented with ECG electrodes, Finometer continuous non-invasive blood pressure monitor (finger photoplethysmography), a peripherally inserted central catheter in an antecubital vein for measurement of central venous pressure, blood sampling and administration of drugs, and a peroneal nerve microelectrode for microneurography of multiunit muscle sympathetic nerve activity (MSNA) (Delius et al. 1972). Blood was drawn for complete blood count, serum osmolality, plasma renin activity, plasma arginine vasopressin levels and AngII levels. Following instrumentation, baseline MSNA and haemodynamics were recorded for ∼5 min. Pre-intervention baroreflex function was tested by the modified Oxford technique (Ebert & Cowley, 1992; Rudas et al. 1999), to test cardiac and sympathetic baroreflex responses to transient changes in arterial pressure, as follows. Heart rate (HR), arterial blood pressure and MSNA were recorded for 5 min, after which nitroprusside (100 μg) was injected. This was followed 60 s later by a bolus of 150 μg of phenylephrine, after which a further 2 min of HR, arterial blood pressure and MSNA were recorded. Phenylephrine was injected earlier than 60 s if the systolic blood pressure dropped by more than ∼20%. Twenty-five minutes of rest was allowed following the first set of measurements to enable the washout of drugs and a return to baseline state after the modified Oxford test, after which a second pre-intervention modified Oxford baroreflex test was performed.
After two sets of pre-intervention data were obtained, 50 mg of losartan, a selective angiotensin II AT1 receptor antagonist, was administered by mouth with a small amount of water and one hour was given for it to reach peak plasma concentrations (Johnston, 1995). Subsequently, a baroreflex test was performed by the same modified Oxford technique as described above. Twenty-five minutes of rest was once again allowed for drug washout after the baroreflex testing followed by a duplicate post-intervention modified Oxford baroreflex test.
Data analysis and statistics
MSNA, ECG, arterial blood pressure and central venous pressure were recorded at 250 Hz on a personal computer using the WinDaq data acquisition system (Dataq Instruments, Akron, OH, USA), and stored for off-line analysis. Our primary endpoints were MSNA and sensitivity of baroreflex control of MSNA (sympathetic baroreflex) in DEH before and after losartan administration as compared to in the control EUH condition. The MSNA signal in WinDaq was calibrated by allocating an amplitude of 1000 to the largest burst of activity and zero to no activity. Subsequently, MSNA was quantified as bursts per minute (burst frequency (BF)), bursts per 100 heartbeats (burst incidence (BI)) and as total activity, calculated as total integrated area of bursts (Halliwill, 2000). Because heart rate tended to drift up over time (HR was slightly higher at the end of the protocols, which averaged ∼3 h in length), we focused on BI in our MSNA measurements, which controls for changes in heart rate by expressing MSNA as bursts per 100 heartbeats.
Baroreflex control of MSNA was assessed as the relationship between the diastolic blood pressure and the corresponding MSNA burst area during drug boluses (during modified Oxford test), using a custom-designed baroreflex analysis program designed by Halliwill (Halliwill, 2000) which groups diastolic blood pressure values into 3 mmHg bins and evaluates total activity of MSNA as a function of diastolic blood pressure in each bin. This can be graphically displayed with diastolic blood pressure on the x-axis and MSNA on the y-axis. The sensitivity of baroreflex control of MSNA was then determined by the slope of the linear portion of this line and was calculated by logistic regression. The weighted slope of the regression line was used as an index of baroreflex sensitivity, in which bins of diastolic blood pressure with more cardiac cycles have greater weight in the regression calculation than do bins which include fewer cardiac cycles (Halliwill, 2000). We averaged the values for baroreflex sensitivity for the two trials in a given condition (pre- and post-losartan in each study).
We also investigated baroreflex control of HR (cardiac baroreflex). This is best examined by the relationship between systolic blood pressure and RR interval. The sensitivity of baroreflex control of HR is determined by weighted logistic regression of this relationship (Charkoudian et al. 2003, 2005).
All data are presented as mean ± standard error of the mean (s.e.m.). Our two main questions were: (1) whether DEH itself alters the control of sympathetic nerve activity, haemodynamics and blood variables, and (2) whether losartan altered sympathetic nerve activity and haemodynamics. To address each question, Student's paired t test was used, with each subject serving as his or her own control. For all comparisons, P < 0.05 was accepted as statistically significant. We also noted comparisons in which P < 0.10 showed statistical trends.
For each specific comparison, subjects were included if they had both data points being compared and the means and standard errors of the mean (s.e.m.) were calculated for the included subjects. The number (n) of subjects used in the calculation of the mean, s.e.m. and P value is given for each comparison.
Results
Demographics
Subjects had an average age of 24 ± 1 years, an average weight of 73 ± 2 kg, and an average BMI of 24.5 ± 0.7 kg m−2. Five subjects only had partial data because they did not complete two study days. In another five cases the peroneal nerve electrode moved during a study day, leading to some incomplete data (for example, only pre-losartan values on a given study day).
Haemodynamics and blood data in DEH vs. EUH
As shown in Table 1, central venous pressure was decreased and haemoglobin, haematocrit and renin activity were significantly increased in DEH as compared to EUH. Osmolality was unchanged in DEH compared to EUH. AngII showed a trend to be increased in DEH compared to EUH (10.5 vs. 6.4 pg ml−1, P= 0.099 vs. EUH) even though only five subjects had values for AngII from DEH and EUH because of a technical error in the biochemical laboratory which resulted in the loss of several samples.
Table 1.
Haemodynamics and blood data in dehydration (DEH) compared to euhydration (EUH)
| EUH | DEH | P | |
|---|---|---|---|
| CVP (mmHg) | 5.1 ± 0.8 | 2.3 ± 0.5 | 0.002* |
| HR (beats min−1) | 61 ± 2 | 62 ± 2 | 0.29 |
| SBP (mmHg) | 127 ± 5 | 128 ± 3 | 0.39 |
| DBP (mmHg) | 70 ± 3 | 70 ± 2 | 0.40 |
| Pulse pressure (mmHg) | 59 ± 3 | 61 ± 3 | 0.8 |
| Haemoglobin (g dl−1) | 14.2 ± 0.5 | 15.3 ± 0.5 | 0.001* |
| Haematocrit (%) | 40.8 ± 1.4 | 44.3 ± 1.3 | <0.001* |
| Osmolality (mosmol kg−1) | 286 ± 1 | 286 ± 1 | 0.41 |
| Renin activity (ng ml−1 h−1) | 0.8 ± 0.1 | 1.6 ± 0.3 | 0.007* |
| Arginine vasopressin (pg ml−1) | 3.0 ± 1.0 | 4.7 ± 1.3 | 0.17 |
| Angiotensin II (pg ml−1) | 6.4 ± 3.0 | 10.5 ± 2.8 | 0.10 |
P < 0.05. CVP, central venous pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure.
HR and arterial pressure were unchanged in DEH compared to EUH. Average HR was 62 ± 2 beats min−1 in DEH and 61 ± 2 beats min−1 in EUH (P= 0.29 vs. EUH, n= 12), and mean arterial pressure was 88 ± 2 mmHg in DEH and 88 ± 3 mmHg in EUH (P= 0.40 vs. EUH, n= 12). Systolic, diastolic and pulse pressures were not different between conditions (see Table 1). During modified Oxford baroreflex trials, systolic blood pressure decreased ∼15 mmHg below baseline and increased ∼22 mmHg above baseline; diastolic pressure decreased ∼15 mmHg below baseline and increased ∼10 mmHg above baseline. These changes in pressure were not different across trials (P > 0.2 for all; data not shown).
Resting MSNA and baroreflex sensitivities in DEH vs. EUH
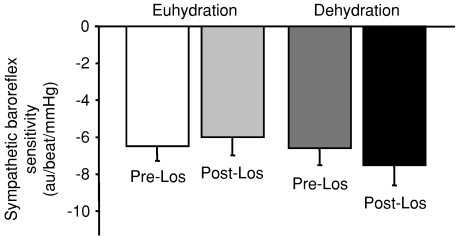
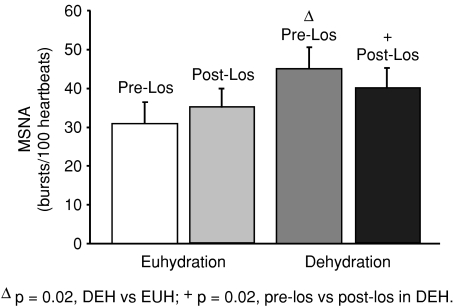
MSNA was significantly increased in DEH compared to EUH (BI 42 ± 5 vs. 32 ± 4 bursts (100 heartbeats)−1 and BF 26 ± 3 vs. 19 ± 2 bursts min−1, P= 0.02 vs. EUH for BI and BF, n= 12). Sensitivity of sympathetic baroreflex control of MSNA was unchanged in DEH compared to EUH (weighted slope −8.1 ± 1.2 vs.−7.0 ± 1.1 au beat−1 mmHg−1, P= 0.24 vs. EUH, n= 12) (Fig. 2). Sensitivity of cardiac baroreflex control of HR was also unchanged in DEH vs. EUH (slope in terms of RR interval 15.0 ± 2.1 vs. 18.1 ± 2.4 ms mmHg−1, P= 0.14 vs. EUH, n= 12).
Figure 2.
Sensitivity of baroreflex control of muscle sympathetic nerve activity (MSNA) during euhydrated (EUH) and dehydrated (DEH) study days, expressed as au beat−1 mmHg−1 (see text for explanation). Sympathetic baroreflex sensitivity was not significantly different between the two study days.
Effects of AngII receptor blockade with losartan in DEH and EUH
Haemodynamics
HR and arterial pressure both increased after losartan in both DEH and EUH. HR increased from 63 ± 3 to 68 ± 3 beats min−1 (P= 0.002 vs. pre-losartan, n= 10) during DEH and from 62 ± 3 to 67 ± 3 beats min−1 (P= 0.002 vs. pre-losartan, n= 10) during EUH. Mean arterial pressure increased from 89 ± 2 to 94 ± 3 mmHg (P= 0.03 vs. pre-losartan, n= 10) during DEH and from 86 ± 4 to 91 ± 4 mmHg (P= 0.05 vs. pre-losartan, n= 10) during EUH. Since these changes were not large, and occurred on both study days, we think they may have been due to a general cardiovascular drift related to lying supine for several hours.
MSNA and baroreflex sensitivities
On the DEH study day, MSNA decreased from 45 ± 6 to 40 ± 5 bursts (100 heartbeats)−1 (P= 0.02 vs. pre-losartan, n= 10) after AngII AT1 receptor blockade with losartan (using burst incidence which corrects for change in heart rate) (Fig. 1). In terms of burst frequency, MSNA during DEH was 28 ± 3 bursts min−1 pre-losartan and 27 ± 4 bursts min−1 post-losartan (P= 0.27, n= 10). In the control EUH condition, there was no significant change when corrected for change in heart rate using burst incidence (31 ± 5 pre-losartan and 35 ± 5 bursts (100 heartbeats)−1 post-losartan, P= 0.13 vs. pre-losartan, n= 10) (Fig. 1). In terms of burst frequency, MSNA during EUH was 19 ± 3 bursts min−1 pre-losartan and 24 ± 3 bursts min−1 post-losartan (P= 0.02, n= 10).
Figure 1.
Resting muscle sympathetic nerve activity (MSNA, bursts (100 heartbeats)−1) on euhydrated (EUH) and dehydrated (DEH) study days, before and after administration of losartan. MSNA was significantly increased in DEH compared to EUH. Furthermore, losartan caused a significant decrease in MSNA only during DEH.
Losartan did not significantly affect sympathetic or cardiac baroreflex sensitivity in either the DEH or EUH condition. As shown in Fig. 2, sympathetic baroreflex sensitivities (weighted slopes) pre- and post-losartan were −6.6 ± 0.9 and −7.5 ± 1.1 au beat−1 mmHg−1, respectively, in DEH (P= 0.09 vs. pre-losartan, n= 10) and −6.5 ± 0.8 and −6.0 ± 1.0 au beat−1 mmHg−1 in EUH (P= 0.19 vs. pre-losartan, n= 9). Cardiac baroreflex sensitivities (in terms of RR interval) pre- and post-losartan were 11.6 ± 1.9 and 12.9 ± 1.8 ms mmHg−1, respectively, in DEH (P= 0.22 vs. pre-losartan, n= 10) and 15.9 ± 2.3 and 15.4 ± 1.5 ms mmHg−1 in EUH (P= 0.81 vs. pre-losartan, n= 10).
Discussion
The major new findings of the present study were twofold: first, we directly quantified the increase in MSNA during dehydration compared to a control, euhydrated study day in the same individuals. Second, we found that losartan, a specific AngII AT1 receptor blocker, partially reversed this dehydration-mediated sympathoexcitation. In previous work, exogenous AngII was shown to augment sympathetic nerve activity in humans (Matsukawa et al. 1991). Importantly, we demonstrate here for the first time that endogenous AngII has a sympathoexcitatory influence in healthy individuals in a dehydrated state.
Confirmation of dehydrated state
Changes in central venous pressure, haemoglobin, haematocrit and renin activity were confirmatory of dehydration by our fluid restriction protocol. Osmolality is a tightly regulated variable and homeostasis of this variable was maintained during this degree of acute dehydration. More severe or prolonged dehydration would probably have been required for this measurement to change with dehydration. This allowed us to study the effect of volume reduction by dehydration on MSNA and baroreflexes independent of changes induced by hyperosmolality, unlike previous studies where dehydration was induced by exercise (Charkoudian et al. 2003). AngII was elevated in the setting of decreased plasma volume with normal arterial pressure and osmolality, but unfortunately we did not have the power to confirm this because of a laboratory error which resulted in the loss of most of the samples. However, plasma renin activity was elevated, which is also consistent with elevated AngII. Arginine vasopressin levels were also unchanged in dehydration. This is in keeping with the unaltered osmolality, as vasopressin levels in humans are tightly linked to plasma osmolality (Stachenfeld et al. 1997).
Elevated MSNA during dehydration: baroreflex mechanisms
Increased MSNA during DEH was an expected finding; however, although it is often assumed that DEH is a ‘hyperadrenergic’ state, there are few examples in the literature in humans of direct comparisons of MSNA between euhydrated and dehydrated states, particularly independent of hyperosmolality. Kimmerly and Shoemaker reported an increase in MSNA during dehydration induced by administration of spironolactone, a diuretic which acts via blockade of aldosterone receptors (Kimmerly & Shoemaker, 2002). In a previous study from our laboratory using exercise-induced dehydration, volume infusion led to a decrease in MSNA (Charkoudian et al. 2003); however, due to the study design, MSNA was not directly compared between euhydrated and dehydrated conditions.
In the present study, the elevation of MSNA in DEH with a normal heart rate and arterial pressure suggests that changes in MSNA, more than changes in HR, are ‘protective’ of arterial pressure during DEH. This is consistent with previous data on the importance of vascular resistance rather than HR to the maintenance of arterial pressure during tilt, although tilting is a more acute arterial pressure challenge (Cooper & Hainsworth, 2002).
The fact that changes in MSNA occurred without changes in arterial pressure during DEH does not rule out potential involvement of the arterial baroreceptors in this sympathoexcitation via an effect of changes in central blood volume (Lacolley et al. 1992; Taylor et al. 1995). Alternatively, our data could suggest baroreflex-independent sympathoexcitation mediated by angiotensin. In this context, directly measured central venous pressure was significantly decreased in DEH compared to EUH in the present study, consistent with a role for cardiopulmonary baroreceptors in influencing resting MSNA in DEH (Johnson et al. 1974). However, our data do not address which specific population of cardiopulmonary mechanoreceptors (e.g. atrial, ventricular, pulmonary vein, etc.) may have been involved in this effect (Hainsworth, 1991).
With regard to the baroreflex, however, there were no statistically significant effects of either DEH or losartan on baroreflex sensitivity. Therefore, although the baroreflex may have contributed to the resting sympathoexcitation, the actual responsiveness of that reflex does not appear to have been altered in our subjects during dehydration. This was surprising as previous human studies have found an inverse relationship between central venous pressure and sympathetic baroreflex sensitivity. Decreases in central venous pressure have been shown to increase the responsiveness of baroreflex control of MSNA (Kimmerly & Shoemaker, 2002) and blood pressure (Shi et al. 1993) and conversely, increases in central venous pressure have been shown to decrease responsiveness of baroreflex control of MSNA (Charkoudian et al. 2003) and arterial pressure (Pawelczyk & Raven, 1989). Our previous study in exercise-induced dehydration, which found a decrease in sympathetic baroreflex sensitivity with post-exercise rehydration, was different from the present study in that osmolality was altered by that protocol (Charkoudian et al. 2003). This may be a possible mechanism by which baroreflex sensitivity was altered in the previous study when it was not in the present study. In keeping with this, increases in plasma osmolality have been shown to enhance baroreflex control of sympathetic activity in humans (Charkoudian et al. 2005; Wenner et al. 2007).
Potential mechanisms for elevated MSNA during dehydration: role of AngII
Consistent with our hypothesis, losartan decreased MSNA in DEH, representing a partial reversal of the increase in MSNA seen in the DEH condition. In contrast, losartan did not decrease MSNA in the EUH condition. Taken together, these data suggest an important role of endogenous AngII via AT1 receptors in increasing MSNA thus defending arterial pressure during DEH. In this context, it is interesting to note that a potential influence of losartan may be to cause a passive vasodilatation (because of its properties to block vascular effects of angiotensin, which is a vasoconstrictor). This effect, which could result in a reflex sympathoexcitation, would be augmented in the DEH condition, when endogenous angiotensin was elevated. With the present study design it would not have been possible to evaluate whether losartan caused a reflex sympathoexcitation (via vasodilatation) in addition to any direct sympathoinhibition. However, this latter possibility would mean that the sympathoinhibitory effect of losartan, which we observed in the dehydrated condition, was actually greater in magnitude than that which we were able to measure in the present study.
In previous work in hypertensive patients, two groups have investigated the effect of AngII AT1 receptor blockade on MSNA. In one study, subjects with essential hypertension receiving eprosartan or losartan for 4 weeks showed no change in MSNA, which is consistent with our findings in EUH (Krum et al. 2006). In a different study, subjects receiving losartan-hydrochlorothiazide demonstrated an increase in MSNA after both 1–2 weeks and 3 months of treatment (Fu et al. 2005a). However, hydrochlorothiazide is a diuretic and volume changes may add a confounding influence to the interpretation of those results with regard to the role of AngII. Furthermore, chronic AT1 receptor blockade in a hypertensive population involves fundamentally different study design and hypothesis testing compared to that of the present study.
Interestingly, in our study there was a trend for sympathetic baroreflex sensitivity to increase after blockade of AngII receptors in DEH when angiotensin was elevated. This suggests that AngII may actually reduce sympathetic baroreflex sensitivity during DEH, which is contrary to our hypothesis. With the current data it is difficult to interpret this trend regarding endogenous AngII and further study is clearly needed.
Effect of angiotensin receptor blockade in euhydrated condition
In contrast to DEH, there was no effect of losartan on MSNA burst incidence in the ‘control’ EUH study day which is in keeping with angiotensin levels being low in healthy individuals. There appeared to be a drift of HR and arterial pressure over time such that HR and arterial pressure were greater after losartan. Since this occurred on both study days, this could be an acute drug effect of losartan or an effect of time. This is in contrast to the effect of losartan on MSNA, which was only seen during the DEH condition when AngII itself was elevated, in that instance suggesting a mechanism via the AT1 receptor.
In this context, one of the challenges related to expression of MSNA data is that bursts of MSNA are linked to the cardiac cycle. As such, an increase in heart rate could cause an increase in MSNA burst frequency (bursts min−1) due to an increase in the number of opportunities (heartbeats) for a burst to occur. In contrast to this idea, longer RR intervals (which would be associated with slower heart rates) have been shown to be associated with an increase in the occurrence of bursts (Sundlof & Wallin, 1978), possibly because a longer cardiac cycle allows for a longer period of disinhibition of sympathetic neural outflow. A third alternative is that any simultaneous changes in heart rate and MSNA burst occurrence could be caused by a separate, unrecognized factor that influences both of these variables independently of each other. In the present study, we wished to control for any potential influence of heart rate (and RR interval) on MSNA burst occurrence by focusing our analyses on data expressed as burst incidence (bursts (100 heartbeats)−1).
As noted above, both heart rate and arterial pressure increased slightly, but significantly, over time on both EUH and DEH study days. We specifically included the EUH study day as a control condition so that we could observe any haemodynamic and neural changes in the absence of dehydration. In the present work, we interpreted the increase in burst frequency after losartan in the EUH condition to be related to this drift in HR (there was no change in burst incidence (bursts (100 heartbeats)−1). In the DEH condition, there was a decrease in burst incidence after losartan, while similarly controlling for changes in heart rate.
An alternative view is that burst frequency represents the total amount of activity (and noradrenaline release) in a given minute, and is therefore more important in terms of control of vascular resistance. We recognize this, but focused on our main question regarding neurophysiological mechanisms controlling MSNA and therefore used burst incidence which controls for changes in heart rate.
Clinical implications
The effects of DEH on sympathetic neural control of the circulation and the mechanisms thereof are important in various clinical contexts, including during dehydration, exercise or fasting, as well as in chronic health conditions associated with intravascular volume depletion, such as hypertension and congestive heart failure. Furthermore, the effects of AngII on the sympathetic nervous system have important implications in patients with chronic illnesses associated with elevated levels of AngII, including congestive heart failure and renovascular hypertension (McMurray & Pfeffer, 2005; Schmieder et al. 2007). The sympathetic neural effects of blockade of AngII AT1 receptors are also important for patients who are treated with these angiotensin receptor-blocking medications.
Perspectives and limitations
Our present study was designed to evaluate the acute effects of AngII receptor blockade during dehydration in otherwise healthy humans. Therefore, it is difficult to compare our results with other studies in which longer-term administration of losartan was used, or involved a different patient group (e.g. hypertensives) (Fu et al. 2005b; Krum et al. 2006). Our results are therefore important in understanding dehydration-associated sympathoexcitation, but may or may not be relevant to other AngII-sympathetic neural interactions. In terms of data interpretation, it is also important to note that we investigated the effects of AngII by acutely blocking AT1 angiotensin receptors during dehydration. The effects of blocking AngII receptors throughout the insult of dehydration may differ from acutely blocking AngII after the subject has already been dehydrated and exposed to increased levels of AngII.
Our EUH study day was included as a control to evaluate haemodynamic and neural variables in the absence of dehydration; however, we did not include time control experiments without losartan. Thus, although we believe the small cardiovascular drift we observed was due to the length of time lying supine (∼3–4 h), we are not able to make this statement based on specific time control data. Additionally, because of technical challenges associated with finding and maintaining a good MSNA signal, we did not have sufficient statistical power to evaluate a potential interaction between hydration status and losartan in our subjects. Ideally, if all subjects had good nerve data for all four trials, we would have been able to evaluate this interaction, but as it was we were only able to evaluate the individual specific paired comparisons (DEH vs. EUH and pre- vs. post-losartan).
Conclusion
We have shown that MSNA increases during water restriction-induced DEH in humans and our data suggest that this sympathoexcitation was more protective of arterial pressure than was HR during DEH. AngII appears to mediate this increase in MSNA during DEH via the AT1 receptors, since blockade of these AngII receptors decreased MSNA during DEH. Sensitivities of the cardiac and sympathetic baroreflexes were unchanged by DEH and losartan. The potential roles of other humoral regulators of volume, such as aldosterone and vasopressin on sympathetic neural mechanisms still need to be investigated. Nonetheless, our present results indicate that in addition to its known volume regulatory effects, AngII appears to provide an additional protective influence on maintenance of blood pressure during dehydration by contributing to some or all of the sympathoexcitation seen in the dehydrated state.
Acknowledgments
These studies were supported by American Heart Association grant 0750036Z and by CTSA UL1 RR024150 from the NIH National Center for Research Resources (NCRR). The contents of the project are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Glossary
Abbreviations
- AngII
angiotensin II
- AT1
angiotensin type 1 receptor
- CRU
clinical research unit
- DEH
dehydration
- EUH
euhydration
- HR
heart rate
- MSNA
muscle sympathetic nerve activity
Author contributions
Each author contributed to the conception and design of the project, analysis and interpretation of data, drafting of the article and revising it critically for important intellectual content and gave final approval of the version to be published.
References
- Bealer SL. Peripheral hyperosmolality reduces cardiac baroreflex sensitivity. Auton Neurosci. 2003;104:25–31. doi: 10.1016/s1566-0702(02)00265-5. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Eisenach JH, Joyner MJ, Roberts SK, Wick DE. Interactions of plasma osmolality with arterial and central venous pressures in control of sympathetic activity and heart rate in humans. Am J Physiol Heart Circ Physiol. 2005;289:H2456–H2460. doi: 10.1152/ajpheart.00601.2005. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Halliwill JR, Morgan BJ, Eisenach JH, Joyner MJ. Influences of hydration on post-exercise cardiovascular control in humans. J Physiol. 2003;552:635–644. doi: 10.1113/jphysiol.2003.048629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper VL, Hainsworth R. Effects of head-up tilting on baroreceptor control in subjects with different tolerances to orthostatic stress. Clin Sci (Lond) 2002;103:221–226. doi: 10.1042/cs1030221. [DOI] [PubMed] [Google Scholar]
- Cox BF, Bishop VS. Neural and humoral mechanisms of angiotensin-dependent hypertension. Am J Physiol Heart Circ Physiol. 1991;261:H1284–H1291. doi: 10.1152/ajpheart.1991.261.4.H1284. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand. 1972;84:65–81. doi: 10.1111/j.1748-1716.1972.tb05158.x. [DOI] [PubMed] [Google Scholar]
- Ebert TJ, Cowley AW., Jr Baroreflex modulation of sympathetic outflow during physiological increases of vasopressin in humans. Am J Physiol Heart Circ Physiol. 1992;262:H1372–H1378. doi: 10.1152/ajpheart.1992.262.5.H1372. [DOI] [PubMed] [Google Scholar]
- Fink GD. Long-term sympatho-excitatory effect of angiotensin II: a mechanism of spontaneous and renovascular hypertension. Clin Exp Pharmacol Physiol. 1997;24:91–95. doi: 10.1111/j.1440-1681.1997.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol. 2005a;289:R109–R116. doi: 10.1152/ajpregu.00013.2005. [DOI] [PubMed] [Google Scholar]
- Fu Q, Zhang R, Witkowski S, Arbab-Zadeh A, Prasad A, Okazaki K, Levine BD. Persistent sympathetic activation during chronic antihypertensive therapy: a potential mechanism for long term morbidity? Hypertension. 2005b;45:513–521. doi: 10.1161/01.HYP.0000158312.63381.c1. [DOI] [PubMed] [Google Scholar]
- Guo GB, Abboud FM. Angiotensin II attenuates baroreflex control of heart rate and sympathetic activity. Am J Physiol Heart Circ Physiol. 1984;246:H80–H89. doi: 10.1152/ajpheart.1984.246.1.H80. [DOI] [PubMed] [Google Scholar]
- Hainsworth R. Reflexes from the heart. Physiol Rev. 1991;71:617–658. doi: 10.1152/physrev.1991.71.3.617. [DOI] [PubMed] [Google Scholar]
- Halliwill JR. Segregated signal averaging of sympathetic baroreflex responses in humans. J Appl Physiol. 2000;88:767–773. doi: 10.1152/jappl.2000.88.2.767. [DOI] [PubMed] [Google Scholar]
- Hasser EM, Cunningham JT, Sullivan MJ, Curtis KS, Blaine EH, Hay M. Area postrema and sympathetic nervous system effects of vasopressin and angiotensin II. Clin Exp Pharmacol Physiol. 2000;27:432–436. doi: 10.1046/j.1440-1681.2000.03261.x. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Rowell LB, Niederberger M, Eisman MM. Human splanchnic and forearm vasoconstrictor responses to reductions of right atrial and aortic pressures. Circ Res. 1974;34:515–524. doi: 10.1161/01.res.34.4.515. [DOI] [PubMed] [Google Scholar]
- Johnston CI. Angiotensin receptor antagonists: focus on losartan. Lancet. 1995;346:1403–1407. doi: 10.1016/s0140-6736(95)92411-6. [DOI] [PubMed] [Google Scholar]
- Kimmerly DS, Shoemaker JK. Hypovolemia and neurovascular control during orthostatic stress. Am J Physiol Heart Circ Physiol. 2002;282:H645–H655. doi: 10.1152/ajpheart.00535.2001. [DOI] [PubMed] [Google Scholar]
- Krum H, Lambert E, Windebank E, Campbell DJ, Esler M. Effect of angiotensin II receptor blockade on autonomic nervous system function in patients with essential hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H1706–H1712. doi: 10.1152/ajpheart.00885.2005. [DOI] [PubMed] [Google Scholar]
- Lacolley PJ, Pannier BM, Slama MA, Cuche JL, Hoeks AP, Laurent S, London GM, Safar ME. Carotid arterial haemodynamics after mild degrees of lower-body negative pressure in man. Clin Sci (Lond) 1992;83:535–540. doi: 10.1042/cs0830535. [DOI] [PubMed] [Google Scholar]
- McMullan S, Goodchild AK, Pilowsky PM. Circulating angiotensin II attenuates the sympathetic baroreflex by reducing the barosensitivity of medullary cardiovascular neurones in the rat. J Physiol. 2007;582:711–722. doi: 10.1113/jphysiol.2007.128983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray JJ, Pfeffer MA. Heart failure. Lancet. 2005;365:1877–1889. doi: 10.1016/S0140-6736(05)66621-4. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Gotoh E, Minamisawa K, Kihara M, Ueda S, Shionoiri H, Ishii M. Effects of intravenous infusions of angiotensin II on muscle sympathetic nerve activity in humans. Am J Physiol Regul Integr Comp Physiol. 1991;261:R690–R696. doi: 10.1152/ajpregu.1991.261.3.R690. [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000a;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation. 2000b;102:1473–1476. doi: 10.1161/01.cir.102.13.1473. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Raven PB. Reductions in central venous pressure improve carotid baroreflex responses in conscious men. Am J Physiol Heart Circ Physiol. 1989;257:H1389–H1395. doi: 10.1152/ajpheart.1989.257.5.H1389. [DOI] [PubMed] [Google Scholar]
- Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol Heart Circ Physiol. 1999;276:H1691–H1698. doi: 10.1152/ajpheart.1999.276.5.h1691. [DOI] [PubMed] [Google Scholar]
- Sanderford MG, Bishop VS. Angiotensin II acutely attenuates range of arterial baroreflex control of renal sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2000;279:H1804–H1812. doi: 10.1152/ajpheart.2000.279.4.H1804. [DOI] [PubMed] [Google Scholar]
- Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM. Renin-angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–1219. doi: 10.1016/S0140-6736(07)60242-6. [DOI] [PubMed] [Google Scholar]
- Shi X, Potts JT, Foresman BH, Raven PB. Carotid baroreflex responsiveness to lower body positive pressure-induced increases in central venous pressure. Am J Physiol Heart Circ Physiol. 1993;265:H918–H922. doi: 10.1152/ajpheart.1993.265.3.H918. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, DiPietro L, Nadel ER, Mack GW. Mechanism of attenuated thirst in aging: role of central volume receptors. Am J Physiol Regul Integr Comp Physiol. 1997;272:R148–R157. doi: 10.1152/ajpregu.1997.272.1.R148. [DOI] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Halliwill JR, Brown TE, Hayano J, Eckberg DL. ‘Non-hypotensive’ hypovolaemia reduces ascending aortic dimensions in humans. J Physiol. 1995;483:289–298. doi: 10.1113/jphysiol.1995.sp020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner MM, Rose WC, Delaney EP, Stillabower ME, Farquhar WB. Influence of plasma osmolality on baroreflex control of sympathetic activity. Am J Physiol Heart Circ Physiol. 2007;293:H2313–H2319. doi: 10.1152/ajpheart.01383.2006. [DOI] [PubMed] [Google Scholar]