Abstract
Phrenic long-term facilitation (pLTF) is a serotonin (5-HT)-dependent augmentation of phrenic motor output induced by acute intermittent hypoxia (AIH). AIH-induced pLTF requires spinal NADPH oxidase activity and reactive oxygen species (ROS) formation. Since 5-HT receptor activation stimulates NADPH oxidase activity in some cell types, we tested the hypothesis that episodic spinal 5-HT receptor activation (without AIH) is sufficient to elicit an NADPH oxidase-dependent facilitation of phrenic motor output (pMF). In anaesthetised, artificially ventilated adult male rats, episodic intrathecal 5-HT injections (3 × 6 μl injections at 5 min intervals) into the cerebrospinal fluid (CSF) near cervical spinal segments containing the phrenic motor nucleus elicited a progressive increase in integrated phrenic nerve burst amplitude (i.e. pMF) lasting at least 60 min post-5-HT administration. Hypoglossal (XII) nerve activity was unaffected, suggesting that effective doses of 5-HT did not reach the brainstem. A single 5-HT injection was without effect. 5-HT-induced pMF was dose dependent, but exhibited a bell-shaped dose–response curve. Activation of different 5-HT receptor subtypes, specifically 5-HT2versus 5-HT7 receptors, may underlie the bell-shaped dose–response curve via a mechanism of ‘cross-talk’ inhibition. Pre-treatment with NADPH oxidase inhibitors, apocynin or diphenylenodium (DPI), blocked 5-HT induced pMF. Thus, episodic spinal 5-HT receptor activation is sufficient to elicit pMF by an NADPH oxidase-dependent mechanism, suggesting common mechanisms of ROS formation with AIH-induced pLTF. An understanding of the mechanisms giving rise to AIH-induced pLTF and 5-HT induced pMF may inspire novel therapeutic strategies for respiratory insufficiency in diverse conditions, such as sleep apnoea, cervical spinal injury or amyotrophic lateral sclerosis.
Introduction
Acute intermittent hypoxia (AIH) elicits a form of serotonin-dependent respiratory plasticity known as long-term facilitation (LTF; Bach & Mitchell, 1996; Feldman et al. 2003; MacFarlane et al. 2008). Phrenic (pLTF) and hypoglossal (XII) LTF are expressed as a progressive and sustained increase in respiratory burst amplitude that persists for hours following AIH. Serotonin (5-HT) is released within the phrenic motor nucleus during each hypoxic episode (Kinkead et al. 2001), activating 5-HT2 receptors on or near phrenic motor neurons and initiating intracellular signalling cascades that give rise to sustained increases in motor neuron activity (Kinkead et al. 1998; Fuller et al. 2001b; Baker-Herman & Mitchell, 2002). Episodic bath application of 5-HT (or 5-HT2 receptor agonist) to neonatal phrenic (Lovett-Barr et al. 2006) or XII (Bocchiaro & Feldman, 2004) motor neurons is sufficient to elicit LTF in vitro, demonstrating that 5-HT receptor activation is both necessary and sufficient for LTF in at least some conditions. Although considerable progress has been made towards an understanding of cellular mechanisms giving rise to pLTF (Feldman et al. 2003; Baker-Herman et al. 2004; Mahamed & Mitchell, 2007; MacFarlane et al. 2008), we still lack a detailed understanding of the role played by 5-HT, particularly in adults.
Spinal reactive oxygen species (ROS) formation is necessary for AIH-induced pLTF (MacFarlane & Mitchell, 2008a), and the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex is a critical source of relevant ROS (MacFarlane et al. 2009). Both hypoxia (Dinger et al. 2007; Rathore et al. 2008) and re-oxygenation (Abramov et al. 2007) are capable of increasing NADPH oxidase activity and ROS formation. Thus, AIH has the requisite characteristics to induce NADPH oxidase activity. On the other hand, 5-HT2 receptor activation stimulates NADPH oxidase activity and ROS formation without changes in oxygenation in renal mesangial cells, suggesting that serotonergic signalling also activates NADPH oxidase in some cell types (Grewal et al. 1999). Episodic (but not continuous) bath application of 5-HT induces long-lasting facilitation of chemo-sensory discharge in ex vivo carotid bodies by a mechanism that requires 5-HT2 receptor activation and NADPH oxidase activity (Peng et al. 2006, 2009). Since hypoxia stimulates raphe serotonergic neurons (Erickson & Millhorn, 1991, 1994; Teppema et al. 1997) and releases 5-HT in the phrenic motor nucleus (Kinkead et al. 2001), 5-HT receptor activation (vs. hypoxia/re-oxygenation) may induce the NADPH oxidase activity necessary for AIH-induced pLTF.
Here we tested the hypotheses that episodic spinal 5-HT receptor activation (without hypoxia/re-oxygenation) induces a long lasting increase in the amplitude of integrated phrenic nerve discharge (phrenic motor facilitation, pMF) in adult rats in vivo, and that it requires spinal NADPH oxidase activity. We demonstrate that episodic spinal 5-HT (without AIH) is sufficient to elicit pMF, although these effects exhibit a bell-shaped dose–response curve. Maximal pMF was observed at 5-HT concentrations below the threshold for the induction of tonic motor neuron activity, suggesting that pMF results from mechanisms distinct from ‘classical’ serotonergic modulation of motor neurons (e.g. Lindsay & Feldman, 1993; Bayliss et al. 1995; McCrimmon et al. 1995; Jacobs & Fornal, 1997; Rekling et al. 2000; Schwarzacher et al. 2002; Hodges & Richerson, 2008). Finally, 5-HT-induced pMF requires NADPH oxidase activity, suggesting that 5-HT receptor activation per se may be the relevant stimulus to NADPH oxidase activity necessary for AIH-induced pLTF in adult rats.
Methods
Ethical approval
Experiments were performed on 3- to 4-month-old male Sprague–Dawley rats (Colony 236, Harlan, Indianapolis, IN, USA). All experiments were approved by the Animal Care and Use Committee at the School of Veterinary Medicine, University of Wisconsin–Madison and are in compliance with the policies and regulations outlined by The Journal of Physiology (Drummond, 2009).
Experimental preparation
All surgical procedures were performed under isoflurane anaesthesia (∼3.5% in 50% O2, balance N2). Rectal temperature was continuously monitored (Fisher Scientific, USA) and regulated with a temperature controlled surgical table. An O2 sensor (TED 60T, Teledyne Analytical Instruments, City of Industry, CA, USA) was used to monitor inspired O2 concentration throughout the experiment; inspired gas levels for O2 and CO2 were varied using flow meters. Following induction of anaesthesia with isoflurane, the tail vein was catheterised (24 gauge, Surflo, Elkton, MD, USA) and the rat was given an injection of 3 ml kg−1 of lactated Ringer solution (Baxter, Deerfield, IL, USA) to maintain fluid balance and minimise deviations in the base excess. To maintain base-excess levels throughout surgery and experimental protocols, an infusion pump (Cole-Palmer, Vernon Hills, IL, USA) provided a slow continuous intravenous infusion (∼4 ml kg−1 h−1) of a solution containing lactated Ringer, Hetastarch (6%; Hospira Inc., Lake Forest, IL, USA) and sodium bicarbonate (8.4%; Hospira), mixed in a 10: 10: 1 ratio (respectively).
Following tests to ensure adequate anaesthesia (toe-pinch, corneal reflexes), rats were tracheotomised for artificial ventilation (tidal volume, 2.5 ml; Rodent Ventilator, model 683; Harvard Apparatus, South Natick, MA, USA) and bilaterally vagotomised through a midline ventral neck incision. A catheter was inserted into the right femoral artery and connected to a pressure transducer (Gould Pressure Transducer, P23, USA) to monitor blood pressure and sample blood for gas analyses. Blood samples taken at various time points were analysed for arterial partial pressures of O2 (PaO2) and CO2 (PaCO2), as well as pH and base-excess using a blood gas analyser (ABL 500, Radiometer, Copenhagen, Denmark). The left phrenic and XII nerves were dissected using a dorsal approach, cut distally and de-sheathed. Once the nerves were isolated, they were covered with saline-soaked cotton wool for the duration of the remainder of surgical preparation (i.e. during placement of intrathecal catheters) to prevent desiccation. To enable placement of the intrathecal catheters, a 1-segment laminectomy was performed at C2 and a silicone catheter (outer diameter, 0.6 mm; Access Technologies, Skokie, IL, USA) was inserted through a limited durotomy. The catheter was advanced ∼2 mm caudally so that its tip was positioned at the anterior edge of C4 as described previously (Baker-Herman & Mitchell, 2002; MacFarlane et al. 2009). The catheter was primed with serotonin (or vehicle) solutions prior to placement. During dissection of the nerves, the rat was slowly converted to urethane anaesthesia (∼1.8 g kg−1) while at the same time, the isoflurane was slowly withdrawn.
When the surgical preparation was complete, the nerves were submerged in mineral oil and placed on bipolar silver recording electrodes. Once nerve signals were observed, the rats were subject to neuromuscular blockade with an intravenous injection of pancuronium bromide (1 mg kg−1; Sicor Pharmaceuticals, Irvine, CA, USA) and allowed ∼1 h for electroneurograms and blood pressure to stabilise. Nerve activity was amplified (gain, 10,000; A-M systems, Everett, WA, USA), bandpass-filtered (100 Hz to 10 kHz), and integrated (CWE 821 filter; Paynter, Ardmore, PA, USA; time constant, 50 ms). The integrated signal was digitised and recorded using the WINDAQ data acquisition system (DATAQ Instruments, Akron, OH, USA) and subsequently analysed using custom-designed software on a LabView platform (National Instruments, Austin, TX, USA). At the end of each experiment, the rat was humanely killed by urethane overdose in accordance with our animal protocol approval.
Drugs and vehicles
Serotonin hydrochloride (Sigma-Aldrich, St Louis, MO, USA) was dissolved in artificial cerebral spinal fluid (aCSF), which had been bubbled with a gas mixture consisting of 95% O2 and 5% CO2. A small amount of the NADPH oxidase inhibitor, apocynin (Sigma-Aldrich), was weighed, dissolved in dimethylsulphoxide (DMSO) and diluted in bubbled aCSF (1: 1000, DMSO: aCSF) for a final concentration of 600 μm. Diphenylenodium (DPI; Sigma-Aldrich, MO, USA) was prepared in a similar manner, but with a 1: 100 mixing ratio of DMSO: aCSF to achieve a final concentration of 1 mm. Methysergide maleate (Sigma-Aldrich), a broad spectrum 5-HT receptor antagonist, was dissolved in saline and administered intravenously (4 mg kg−1); 10 min after injection, the apnoeic and recruitment thresholds were determined, followed by a further 30 min of baseline nerve recording prior to AIH. The 5-HT7 antagonist, SB269970 (Sigma-Aldrich) was dissolved in DMSO and frozen in 100 μl aliquots; on the day of the experiment, the aliquot was thawed and mixed with 400 μl aCSF at a final concentration of 5 mm. aCSF consisted of NaCl (120 mm), KCl (2.9 mm), CaCl2.2H2O (2 mm), MgCl2.6H2O hexahydrate (2 mm), NaHCO3 (23 mm) and glucose (10 mm) dissolved in double-distilled water.
Experimental protocols
To establish baseline nerve activity, rats were ventilated with 50% O2 to establish 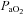 of ∼250 mmHg; CO2 was added to the inspired gas as necessary to regulate arterial
of ∼250 mmHg; CO2 was added to the inspired gas as necessary to regulate arterial 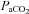 . After a stabilisation period of 1 h, the apnoeic CO2 threshold was determined by progressively lowering inspired CO2 until rhythmic phrenic activity ceased; the CO2 recruitment threshold was then determined by progressively raising the inspired CO2 until rhythmic activity resumed. The end- tidal
. After a stabilisation period of 1 h, the apnoeic CO2 threshold was determined by progressively lowering inspired CO2 until rhythmic phrenic activity ceased; the CO2 recruitment threshold was then determined by progressively raising the inspired CO2 until rhythmic activity resumed. The end- tidal  was then set 2–3 mmHg above the CO2 recruitment threshold (see Bach & Mitchell, 1996). End-tidal
was then set 2–3 mmHg above the CO2 recruitment threshold (see Bach & Mitchell, 1996). End-tidal 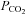 was monitored throughout the experiment using a flow-through capnograph (Novametrix, Wallingford, CT, USA) placed in the ex-current section of the endotracheal Y-tube.
was monitored throughout the experiment using a flow-through capnograph (Novametrix, Wallingford, CT, USA) placed in the ex-current section of the endotracheal Y-tube. 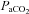 was maintained within 1.5 mmHg of baseline throughout the experiment by manipulating inspired CO2. Blood gases and arterial blood pressure were monitored and corrected as necessary. Negative base excess values less than −3 mEq l−1 were corrected with intravenous injections of sodium bicarbonate, and progressive reductions in blood pressure were offset by constant intravenous infusion, or less frequently supplemented with intravenous bolus injections of lactated Ringer solution. Phrenic and XII nerve activity were recorded continuously. Blood samples (0.3 ml in a heparinised glass syringe) were collected once baseline nerve recordings had stabilised, and then again at 30 and 60 min post-episodic 5-HT injections. Measurements of phrenic and XII burst amplitude and frequency were evaluated in 1 min periods immediately prior to each blood sample.
was maintained within 1.5 mmHg of baseline throughout the experiment by manipulating inspired CO2. Blood gases and arterial blood pressure were monitored and corrected as necessary. Negative base excess values less than −3 mEq l−1 were corrected with intravenous injections of sodium bicarbonate, and progressive reductions in blood pressure were offset by constant intravenous infusion, or less frequently supplemented with intravenous bolus injections of lactated Ringer solution. Phrenic and XII nerve activity were recorded continuously. Blood samples (0.3 ml in a heparinised glass syringe) were collected once baseline nerve recordings had stabilised, and then again at 30 and 60 min post-episodic 5-HT injections. Measurements of phrenic and XII burst amplitude and frequency were evaluated in 1 min periods immediately prior to each blood sample.
Initial experiments were designed to determine dose-dependent effects of episodic intrathecal injections of 5-HT on phrenic nerve activity (i.e. pMF). Three consecutive intrathecal injections of 5-HT (6 μl per injection at 5 min intervals) were administered immediately after the baseline blood sample and nerve signals had stabilised. The following concentrations were used: 50–100 μm (n= 6), 500 μm (n= 6), 1 mm (n= 9), 10 mm (n= 5) and 100 mm (n= 7). Since 1 mm 5-HT was the most effective concentration for episodic injections, additional rats were used with this same concentration to test if 5-HT-induced pMF (1) required patterned application of 5-HT, (2) was the result of 5-HT receptor activation, (3) required NADPH oxidase activity and (4) required sustained NADPH oxidase activity for maintenance of pMF. To confirm that 5-HT-induced pMF required patterned application, rats received a single intrathecal injection (1 mm, 6 μl; n= 5) of 5-HT (Fig. 1B). To confirm that 5-HT-induced pMF resulted from spinal 5-HT receptor activation, rats (n= 5) were pre-treated with an intravenous injection of methysergide maleate (4 mg kg−1) ∼40 min prior to episodic 5-HT. The methysergide dose was chosen because it was previously shown to inhibit AIH-induced pLTF (Bach & Mitchell, 1996). To test if 5-HT-induced pMF was NADPH oxidase dependent, two rat groups received either a single bolus injection of apocynin (600 μm, 15 μl; n= 6) or DPI (1 mm, 15 μl, n= 5) via a second intrathecal catheter ∼20 min prior to episodic 1 mm 5-HT. DPI was chosen because its mechanism of action on NADPH oxidase activity is distinct from apocynin (Hancock & Jones, 1987; O’Donnell et al. 1993; Heumüller et al. 2008); the same drug doses used in the current study were previously shown to inhibit AIH-induced pLTF (MacFarlane et al. 2009). To test if NADPH oxidase activity is necessary to maintain (versus initiate) 5-HT-induced pMF, one group of rats (n= 5) was injected with apocynin (600 μm, 15 μl) 5 min after episodic 5-HT injections.
Figure 1. Representative neurograms from rats that received intrathecal injections of 1 mm 5-HT.
A, episodic 5-HT (3 × 6 μl, 5 min intervals) elicited a significant increase in integrated phrenic (i.e. phrenic motor facilitation, pMF), but not hypoglossal (XII) nerve burst amplitude for at least 60 min post-injections. Inset: expanded 60 s traces of neural activity during and following each injection. Note that there was no effect of 5-HT injections on rhythmic or tonic phrenic motor activity. B, single intrathecal 5-HT injections (6 μl; 1 mm) were not sufficient to elicit pMF. An open arrow indicates 5-HT injections. C, for comparison, a neurogram illustrating phrenic long-term facilitation (pLTF) following acute intermittent hypoxia (AIH); AIH consisted of 3 × 5 min hypoxic episodes with 5 min intervals (10% O2; black bars). Note pLTF following AIH is comparable in magnitude and time-course to pMF following episodic 1 mm 5-HT-injections. Arterial blood (see also Table 1) was sampled and assessed for 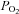 ,
, 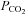 and pH at baseline and 30 and 60 min after episodic 5-HT or AIH.
and pH at baseline and 30 and 60 min after episodic 5-HT or AIH.
The magnitude of 5-HT-induced pMF was concentration dependent, but exhibited a bell-shaped concentration–response curve with a constant injection volume (see Fig. 2A); thus, we tested the hypothesis that the inability of high concentrations (i.e. 100 mm) of 5-HT to elicit pMF resulted from the activation of different 5-HT receptors. We reasoned that antagonism of such receptors, particularly of the 5-HT7 subtype, should restore 5-HT-induced pMF. To test this hypothesis we injected the highest concentration of 5-HT (100 mm; which consistently failed to elicit pMF; Fig. 2A and also 4A) into rats (n= 5) pre-treated with an intrathecal injection of the 5-HT7 antagonist, SB269970 (5 mm; 12 μl injection volume; 10 min prior to episodic 5-HT). This SB269970 concentration was chosen because it enhances AIH-induced pLTF, suggesting that 5-HT7 receptor activation during AIH constrains mechanisms of pLTF (Hoffman MS and Mitchell GS, unpublished observations).
Figure 2. Concentration-dependent effects of episodic intrathecal 5-HT injections (3 × 6 μl) on integrated phrenic (A) and XII (B) nerve amplitude at 60 min post-injection.
At progressively higher 5-HT concentrations, pMF increased, reaching a peak at 1 mm. At concentrations higher than 1 mm, pMF decreased and was no longer significant at 100 mm, thus revealing a bell-shaped dose–response curve. Open bar in A indicates the average effect of a single 1 mm injection of 5-HT. There was no effect of intrathecal 5-HT on XII motor activity at any concentration (see also Fig. 1A). Values are mean ± 1 s.e.m.; n values for each treatment group are indicated in parentheses. *Significant difference from baseline; #significant difference from time control (TC) group (P < 0.05).
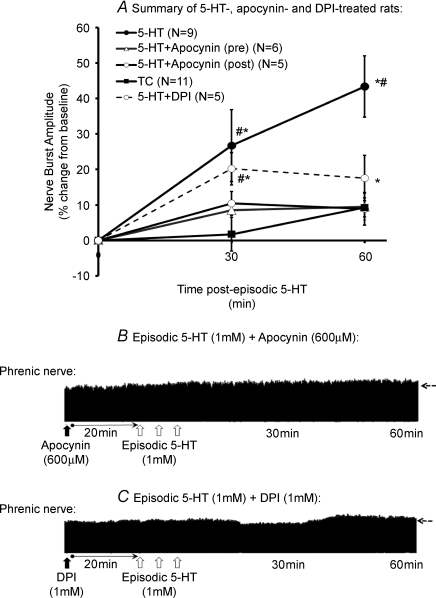
Figure 4. Effects of NADPH oxidase inhibitors, apocynin and DPI, on 5-HT-induced pMF.
A, episodic 5-HT-induced pMF (3 × 6 μl; 1 mm; filled symbols) in rats that were pre-treated with an intrathecal injection of the NADPH oxidase inhibitors, apocynin (12 μl; 600 μm) or DPI (12 μl; 1 mm). Note, apocynin and DPI blocked episodic 5-HT-induced pMF. In a separate group, rats received intrathecal apocynin 5 min post-episodic 5-HT, which significantly attenuated pMF at 60 min. Values are means, ± 1 s.e.m. Note, the time control group (TC) in A consists of pooled data for all control groups (see methods) *Significant difference from baseline values; #significant difference from apocynin and DPI-treated groups (P < 0.05). B and C, also shown are two representative phrenic neurograms in rats that were pre-treated with either apocynin (B) or DPI (C) prior to episodic 1 mm 5-HT. In B and C, open arrows indicate an injection of 1 mm 5-HT (6 μl); filled arrow indicates injection of apocynin (B) or DPI (C). Blood samples (and nerve activity) were assessed at 30 and 60 min post-episodic 5-HT, as indicated. Dashed horizontal arrows at 60 min extrapolate the baseline nerve burst amplitude.
Time-matched control experiments were performed in rats that received episodic aCSF (3 × 6 μl, n= 2), apocynin (600 μm, 15 μl) or DPI (1 mm, 15 μl) + episodic aCSF (n= 3), SB269970 (5 mm, 15 μl) + aCSF (n= 4) or methysergide (4 mg kg−1) + episodic aCSF (n= 2), i.e. without episodic 5-HT. In these experiments, nerve activity was monitored for the duration of a normal protocol. None of these ‘sham treatments’ elicited detectable pMF, although methysergide caused a significant and sustained increase in phrenic nerve burst amplitude as reported previously (Bach & Mitchell, 1996; Baker-Herman & Mitchell, 2002). Because there were no differences among time-control groups, these rats were pooled into a single time control (TC) group for further analyses.
Statistical analysis
Peak integrated nerve burst amplitude and frequency (bursts per minute) of phrenic and XII nerve activity were averaged in 1 min bins at each recorded data point (baseline, 30 and 60 min post-episodic serotonin). Values were expressed as a percentage change from baseline. For changes in phrenic and XII nerve activity, within-rat comparisons were made with baseline; comparisons were also made with TC rats at all time points. Statistical comparisons were made for time and drug treatment using two-way, repeated measures ANOVA with Bonferroni's post hoc test to make individual comparisons. Differences were considered significant at P < 0.05. All values are expressed as means ± 1 s.e.m.
Results
Values for body temperature (Tb), arterial blood gases (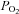 and
and 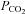 ) and pH are provided in Table 1 for various treatment groups. Body temperature,
) and pH are provided in Table 1 for various treatment groups. Body temperature, 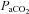 and blood pH were kept constant throughout the experiment for each group. However, there was a small, but significant, decrease in
and blood pH were kept constant throughout the experiment for each group. However, there was a small, but significant, decrease in 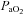 and mean arterial pressure (MAP) for each treatment group, including time control rats. We don't anticipate that these changes in
and mean arterial pressure (MAP) for each treatment group, including time control rats. We don't anticipate that these changes in 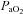 and MAP contribute to the magnitude of pMF because they were small, they occurred in time-control animals and in rats that did not express pMF. We also observed a small tendency for an upward drift in respiratory frequency in virtually all treatment groups, although it was more pronounced in animals that received the highest concentration (100 mm) of 5-HT. Nevertheless, it is unlikely that spinally applied 5-HT reached brainstem regions where it could affect respiratory frequency because: (1) there was no effect on XII nerve activity (Figs 1A and 2B), and (2) time control rats (which did not receive 5-HT) also tended to increase frequency.
and MAP contribute to the magnitude of pMF because they were small, they occurred in time-control animals and in rats that did not express pMF. We also observed a small tendency for an upward drift in respiratory frequency in virtually all treatment groups, although it was more pronounced in animals that received the highest concentration (100 mm) of 5-HT. Nevertheless, it is unlikely that spinally applied 5-HT reached brainstem regions where it could affect respiratory frequency because: (1) there was no effect on XII nerve activity (Figs 1A and 2B), and (2) time control rats (which did not receive 5-HT) also tended to increase frequency.
Table 1.
Body temperature (Tb), blood gas values and respiratory frequency (fR) for various experimental treatment groups before (i.e. baseline) and 60 min after episodic 5-HT
| Group | BW (g) | Tb (°C) | pH |
 (mmHg) (mmHg) |
 (mmHg) (mmHg) |
MAP (mmHg) | fR (breaths min−1) | |
|---|---|---|---|---|---|---|---|---|
| Baseline | TC | 359 ± 9 | 37.7 ± 0.1 | 7.350 ± 0.005 | 44.4 ± 0.5 | 288.7 ± 2.4 | 107.8 ± 5.1 | 46.1 ± 1.3 |
| 5-HT (1 mm) | 373 ± 4 | 38.1 ± 0.2 | 7.374 ± 0.011 | 42.9 ± 0.7 | 273.2 ± 6.0 | 107.8 ± 4.7 | 44.3 ± 2.5 | |
| + apocynin (pre) | 373 ± 8 | 38.1 ± 0.2 | 7.337 ± 0.005 | 43.3 ± 0.9 | 280.6 ± 4.0 | 113.2 ± 4.2 | 46.3 ± 1.0 | |
| + DPI | 368 ± 8 | 37.4 ± 0.1 | 7.345 ± 0.013 | 46.4 ± 2.2 | 285.0 ± 8.9 | 114.5 ± 3.9 | 42.5 ± 1.7 | |
| + methysergide | 393 ± 7 | 38.1 ± 0.2 | 7.337 ± 0.013 | 45.9 ± 1.5 | 285.7 ± 9.0 | 120.8 ± 8.7 | 47.1 ± 2.8 | |
| + apocynin (post) | 377 ± 13 | 38.0 ± 0.1 | 7.321 ± 0.018 | 48.9 ± 0.8 | 288.7 ± 6.0 | 102.6 ± 11.3 | 45.4 ± 2.2 | |
| 5-HT (100 mm) | 364 ± 10 | 38.2 ± 0.3 | 7.326 ± 0.012 | 43.3 ± 0.7 | 272.9 ± 5.3 | 120.8 ± 5.2 | 40.4 ± 1.4 | |
| + SB269970 | 368 ± 14 | 37.6 ± 0.2 | 7.358 ± 0.008 | 45.5 ± 1.3 | 291.4 ± 6.9 | 98.8 ± 4.0 | 43.7 ± 1.1 | |
| 60 min | TC | — | 37.9 ± 0.1 | 7.350 ± 0.006 | 44.4 ± 0.4 | 281.0 ± 2.1 | 99.6 ± 5.4 | 48.8 ± 0.6 |
| 5-HT (1 mm) | — | 38.0 ± 0.2 | 7.368 ± 0.009 | 43.3 ± 0.9 | 263.1 ± 4.8 | 96.8 ± 4.1 | 50.9 ± 2.0 | |
| + apocynin (pre) | — | 38.0 ± 0.2 | 7.341 ± 0.006 | 43.3 ± 1.0 | 271.6 ± 2.4 | 100.6 ± 6.6 | 50.1 ± 0.9 | |
| + DPI | — | 37.3 ± 0.2 | 7.356 ± 0.012 | 46.4 ± 2.4 | 276.6 ± 7.0 | 101.1 ± 3.5 | 45.0 ± 1.6 | |
| + methysergide | — | 38.3 ± 0.2 | 7.338 ± 0.013 | 46.0 ± 2.0 | 273.0 ± 6.7 | 108.8 ± 6.4 | 50.5 ± 1.5 | |
| + apocynin (post) | — | 37.9 ± 0.1 | 7.324 ± 0.023 | 48.7 ± 0.8 | 289.2 ± 4.8 | 93.1 ± 6.8 | 47.9 ± 3.1 | |
| 5-HT (100 mm) | — | 38.0 ± 0.3 | 7.313 ± 0.016 | 43.6 ± 0.9 | 272.4 ± 5.4 | 104.6 ± 5.1 | 47.1 ± 1.1 | |
| + SB269970 | — | 37.8 ± 0.1 | 7.358 ± 0.010 | 45.5 ± 1.3 | 280.5 ± 6.4 | 86.7 ± 5.9 | 51.7 ± 1.3 | |
| Δ(60 min − baseline) | TC | — | 0.1 | 0.001 | 0.0 | −7.7* | −8.2* | 2.4* |
| 5-HT (1 mm) | — | −0.1 | −0.006 | 0.4 | −10.2* | −11.0* | 6.2* | |
| + apocynin (pre) | — | −0.1 | 0.004 | −0.1 | −9.0* | −12.6* | 3.4* | |
| + DPI | — | −0.1 | 0.011 | 0.0 | −8.3 | −13.4* | 2.4 | |
| + methysergide | — | 0.2 | 0.001 | 0.2 | −12.6* | −12.0* | 3.4* | |
| + apocynin (post) | — | −0.1 | 0.004 | −0.3 | 0.5 | −9.6 | 2.5 | |
| 5-HT (100 mm) | — | −0.2 | −0.013 | 0.3 | −0.5 | −16.1* | 6.7* | |
| + SB269970 | — | 0.1 | 0.001 | 0.0 | −11* | −12.0* | 8.0* |
Values are means ± 1 s.e.m. Note, included in the table are the groups that were pre- and also post-treated with apocynin. Drugs: apocynin, 600 μm; DPI, 1 mm; methysergide, 4 mg kg−1 (intravenously); SB269970, 5 mm. Also provided is the calculated mean difference between baseline and the 60 min time point for each treatment group.
Significant difference between 60 min and baseline.
Episodic 5-HT is sufficient to induce pMF
Serotonin (5-HT) applied episodically (3 injections, 6 μl each, 5 min intervals) into the cerebrospinal fluid (CSF) of the cervical spinal cord caused a concentration-dependent, progressive and sustained increase in the amplitude of integrated phrenic nerve burst activity (i.e. pMF). When observed, this effect lasted at least 60 min following the final 5-HT injection (Figs 1A and 2A). The magnitude of pMF (quantified at 60 min post-injection) was larger at progressively higher 5-HT concentrations up to 1 mm (43 ± 9%, n= 9, Figs 1A and 2A). However, at higher concentrations, pMF magnitude declined, demonstrating a bell-shaped dose–response curve. In general, concentrations effective at inducing pMF did not cause acute changes in phrenic nerve activity immediately following injection; higher concentrations that failed to elicit pMF frequently coincided with increased tonic phrenic activity which is typically regarded as a hallmark of serotonergic modulation of respiratory motor neurons (McCrimmon et al. 1995; Rekling et al. 2000).
A representative experimental trace illustrating the effects of episodic 1 mm 5-HT on phrenic nerve activity is shown in Fig. 1A. The magnitude and time course of 5-HT-induced pMF (∼45%) is similar to AIH-induced long-term facilitation (Fig. 1C); indeed, although there are strain and sub-strain differences in the magnitude of AIH-induced LTF (Fuller et al. 2001a), the most recent report by us using this same strain (Sprague–Dawley) indicated 46% pLTF following AIH (MacFarlane et al. 2009). At concentrations higher than 1 mm 5-HT, pMF magnitude progressively decreased, completely disappearing at 100 mm (0.7 ± 10.3%, n= 7, Fig. 2A). There was no significant effect of episodic spinal 5-HT on XII nerve activity at any concentration used (Figs 1A and 2B), suggesting that 5-HT had not spread to the brainstem in sufficient quantity to elicit XII motor facilitation. Thus, we believe that intrathecal 5-HT injections reached effective concentrations only in spinal regions associated with the phrenic motor nucleus.
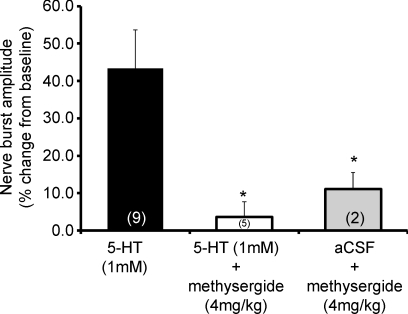
A single intrathecal injection of 5-HT (1 mm, 6 μl) also failed to elicit pMF (Fig. 1B). Episodic 5-HT-induced pMF was blocked by pre-treatment with an intravenous injection of methysergide (Fig. 3).
Figure 3. Magnitude of pMF following episodic 5-HT (3 × 6 μl; 1 mm) or vehicle (aCSF) and the effects of pre-treatment with an intravenous injection of the broad spectrum 5-HT receptor antagonist methysergide maleate (4 mg kg−1).
Values are means ± 1 s.e.m. for 60 min post-episodic 5-HT; n values for each treatment group are indicated in parentheses. *Significant difference from 1 mm 5-HT group (P < 0.05).
Serotonin-induced pMF requires NADPH oxidase activity for initiation and maintenance
Pre-treatment with intrathecal apocynin (600 μm, 12 μl) or DPI (1 mm, 12 μl) blocked episodic 5-HT (1 mm) induced pMF (9.5 ± 3.7%, n= 6; and 11.0 ± 8.2%, n= 5, respectively; Fig. 4A–C). Neither apocynin nor DPI had any effect on baseline phrenic nerve activity, nor did they elicit an apparent facilitation in time-control experiments (Fig. 4A). When apocynin was administered 5 min after the third 5-HT injection, pMF was observed 30 min post-injection (20.2 ± 6.4%, n= 5), with a magnitude similar to 5-HT-treated rats at this same time point (26.7 ± 4.5%, n= 9, Fig. 4A). However, 60 min following episodic 5-HT, pMF was significantly blocked (17.6 ± 6.4%; P < 0.05), suggesting that continued NADPH oxidase activity is necessary to maintain pMF.
Bell-shaped dose–response results from co-activation of multiple receptor subtypes
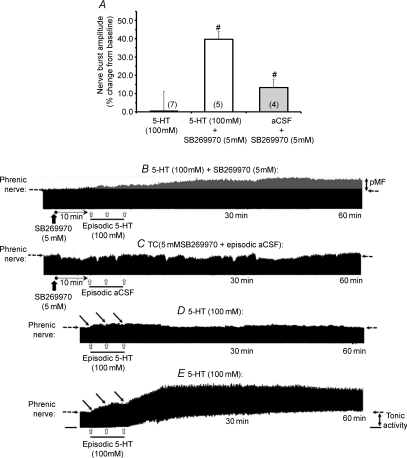
Since 5-HT concentrations above 1 mm (6 μl) decreased pMF magnitude, we investigated whether this effect may relate to co-activation of opposing 5-HT receptor subtypes. While there are multiple candidate receptor subtypes, we hypothesised that the 5-HT7 receptor mediates this inhibitory effect because these receptors constrain the magnitude of AIH-induced pLTF (Hoffman MS and Mitchell GS, unpublished observations). Thus, we pre-treated rats with a 5-HT7 receptor antagonist and injected episodic 5-HT at a concentration of 100 mm (6 μl). Pre-treatment with the 5-HT7 antagonist SB269970 (5 mm) restored pMF following episodic 100 mm 5-HT injections (39.7 ± 3.4%, n= 5, Fig. 5A and B) to levels comparable with the peak concentration of 1 mm (43.3 ± 8.7%, Fig. 2A). SB269970 by itself had no effect on phrenic nerve activity in time-control (n= 4) experiments (Fig. 5A and C).
Figure 5. Effects of episodic intrathecal 100 mm 5-HT injections (3 × 6 μl, 5 min intervals) on pMF with or without pre-treatment with the 5-HT7 antagonist, SB269970 (5 mm; 12 μl).
A, although 100 mm 5-HT did not elicit pMF, SB269970 pre-treatment fully restored 5-HT induced pMF. Values are means ± 1 s.e.m. at 60 min following episodic 5-HT; #significant difference from 100 mm 5-HT treated animals (P < 0.05); n values for each treatment group are given in parentheses. Representative phrenic neurograms are also provided from a rat in which pMF was restored by pre-treatment with SB269970 prior to episodic 100 mm 5-HT (B) and a time control (TC) rat treated with SB269970 prior to aCSF (C). Additional neurograms illustrate variable neuromodulatory effects immediately following 100 mm 5-HT injections on phrenic nerve activity (D and E). Note, in some (3 of 7) cases (D), each 5-HT injection rapidly increased integrated phrenic nerve activity (open arrows), followed by a return to baseline levels within minutes; on the other hand, increased phrenic nerve activity was sometimes (4 of 7) associated with a profound increase in tonic expiratory activity that lasted >60 min post-injections. Dashed horizontal arrows at the 60 min time point extrapolates baseline nerve burst amplitude. pMF was never observed in rats where 100 mm 5-HT injections caused short-term neuromodulatory effects on phrenic nerve activity.
Neuromodulatory effects of episodic 5-HT on phrenic nerve activity
5-HT concentrations that elicited pMF (up to 1 mm) had no immediate effects on spontaneous phrenic nerve activity (i.e. ‘classical’ neuromodulation; McCrimmon et al. 1995). However, at higher concentrations, 5-HT had variable effects on tonic phrenic activity. On many occasions (3 of 7), 100 mm 5-HT caused a rapid (seconds) and robust increase in phrenic nerve activity that lasted many minutes (Fig. 5D and E); the response frequently (4 of 7), though not always (e.g. Fig. 5D), exhibited increased tonic phrenic activity during the expiratory phase of the respiratory cycle (Fig. 5E). When observed, tonic phrenic activity was accompanied by a small, but sustained, decrease in arterial blood pressure (data not shown). In rats pre-treated with the 5-HT7 antagonist (SB269970), the effects of 100 mm 5-HT on tonic phrenic activity were attenuated (Fig. 5B). Thus, we observed a negative correlation between the extent of neuromodulatory activity on phrenic nerve activity and the magnitude of pMF; specifically, pMF was not associated with the short-term, neuromodulatory effects of 5-HT.
Discussion
Here, we demonstrate that spinal 5-HT receptor activation (without hypoxia/re-oxygenation) is sufficient to elicit long-lasting phrenic motor facilitation (pMF) in adult rats. This form of phrenic motor facilitation requires episodic 5-HT receptor activation and exhibits a bell-shaped dose–response curve. The down-slope of the dose–response curve most likely results from co-activation of multiple serotonin receptor subtypes that interact via ‘cross-talk inhibition;’ we suggest that the relevant serotonin receptor subtypes are 5-HT2 and 5-HT7 receptors. Serotonin-induced pMF does not require ‘classical’ neuromodulatory actions of 5-HT on spontaneous phrenic nerve activity, but requires spinal NADPH oxidase activity for its induction and maintenance. These findings advance our understanding of phrenic long-term facilitation, a frequently studied model of spinal respiratory plasticity induced by acute intermittent hypoxia (Mitchell et al. 2001; Feldman et al. 2003; Mahamed & Mitchell, 2007). Such an understanding may aid in the development of novel therapies to treat diverse ventilatory control disorders, including obstructive sleep apnoea, spinal injury and motor neuron disease.
Effective 5-HT concentration following intrathecal injections
Although 5-HT concentrations used to elicit pMF are reported as the concentration of injected solutions (with a constant 6 μl volume), the actual concentration in the ventral spinal cord at the level of phrenic motor neurons is expected to be considerably lower. Brumley and colleagues (2007) utilised 5-HT-sensitive micro-electrodes placed in lamina VIII of the lumbar spinal cord in anaesthetised rats to determine changes in 5-HT concentration in response to dorsal spinal 5-HT administration. Although they detected a sharp rise in intra-spinal 5-HT concentration within 30 s of serotonin administration, the peak intra-spinal concentration reached values of only 0.001% (i.e. ∼10 nm) of the exogenously applied level (1 mm). At concentrations lower than 1 mm, a rapid return of 5-HT to baseline levels was observed, even during sustained applications, presumably due to the efficiency of intrinsic 5-HT re-uptake mechanisms. Thus, considering the small intrathecal injection volumes (6 μl) and the limited range of 5-HT concentrations used in our study, we anticipate that the actual concentration within the phrenic motor nucleus is orders of magnitude lower than the concentrations delivered, both due to dilution in the spinal extracellular fluid and the avid re-uptake of 5-HT by serotonergic terminals and astro-glia (Brumley et al. 2007). Since available evidence suggests that extracellular 5-HT concentrations in the phrenic motor nucleus in the present study were low, and that intrathecal injections caused a pulse of 5-HT deep within the spinal cord, we believe that episodic 5-HT injections caused episodic (versus continuous or incrementing) 5-HT receptor activation.
Episodic spinal 5-HT receptor activation is sufficient to induce pMF
Episodic spinal 5-HT injections at concentrations less than 100 mm elicited pMF lasting at least 60 min post-injection, an effect similar in magnitude and time course to AIH-induced phrenic long-term facilitation (pLTF; Fig. 1). On the other hand, XII nerve activity was unaffected by spinal injections, suggesting that persistent changes in phrenic nerve activity resulted from spinal 5-HT receptor activation, and that effective serotonin concentrations did not reach the brainstem. XII activity has been used previously as an ‘internal control’ to test for unintended drug distribution (Baker-Herman & Mitchell, 2002; MacFarlane et al. 2009).
Single 5-HT injections did not elicit pMF, indicating that repetitive 5-HT receptor activation is a requirement, consistent with the pattern sensitivity of AIH-induced pLTF (Baker & Mitchell, 2000; Baker et al. 2001). These data are also consistent with reported effects of episodic 5-HT on facilitation of integrated phrenic (Lovett-Barr et al. 2006) and XII (Bocchiaro & Feldman, 2004) nerve activity using in vitro experimental preparations from neonatal rats, and 5-HT induced sensory-motor long-term facilitation in Aplysia (Mauelshagen et al. 1998).
5-HT-induced pMF requires spinal NADPH oxidase activity for induction and maintenance
Pre-treatment with DPI or apocynin attenuated 5-HT induced pMF, demonstrating that spinal NADPH oxidase activity is necessary in its underlying mechanism. These findings are similar to AIH-induced pLTF, which also requires spinal 5-HT receptor activation (Fuller et al. 2001b; Baker-Herman & Mitchell, 2002) and ROS formation (MacFarlane et al. 2008) via NADPH oxidase activity (MacFarlane et al. 2009). Indeed, key subunits of the NADPH oxidase complex are localised in putative phrenic motor neurons (MacFarlane et al. 2009), and the NADPH oxidase complex has been implicated in multiple forms of neuroplasticity, including hippocampal LTP (Kishida et al. 2006; see Kishida & Klann, 2007) and sensory facilitation of the carotid body (Peng et al. 2006, 2009). Since pMF elicited by episodic spinal 5-HT receptor activation (without hypoxia/re-oxygenation) shares common features with AIH-induced pLTF, 5-HT receptor activation during AIH is most likely the relevant stimulus to pLTF versus repetitive hypoxia/re-oxygenation per se, at least in the experimental conditions most frequently studied (Mitchell et al. 2001; Baker-Herman & Mitchell, 2008; MacFarlane et al. 2008).
NADPH oxidase activity is necessary for pMF maintenance as demonstrated by the reversal of pMF when apocynin is delivered to the intrathecal space after episodic 5-HT administration. When apocynin was administered 5 min post-episodic 5-HT, significant pMF was observed at 30 but not 60 min post-injection. Reversal of pMF by apocynin post-treatment likely results from a combination of factors, including the diffusion dynamics of apocynin to the target region, or a ‘slow’ action of apocynin on NADPH oxidase. Thus, signalling cascades mediating 5-HT induced pMF appear to require persistent NADPH oxidase activity to maintain the plasticity. This conclusion contrasts with the role of 5-HT receptor activation per se, which is necessary to initiate, but not maintain, AIH-induced pLTF (Fuller et al. 2001b). The requirement for persistent NADPH oxidase activity to maintain AIH-induced pLTF has not been investigated. Nevertheless, because of similarities between 5-HT induced pMF and AIH-induced pLTF, we hypothesise that 5-HT receptor activation stimulates the NADPH oxidase activity necessary for AIH-induced pLTF, and that persistent NADPH oxidase activity is necessary to maintain AIH-induced pLTF.
The processes leading to persistent NADPH oxidase activity are not known, but could involve serotonin-dependent phosphorylation of the cytosolic NADPH oxidase subunits (such as p47phox and p67phox); phosphorylation of these subunits is necessary for activation of the NADPH oxidase complex (Valko et al. 2007). We propose that persistent ROS formation is necessary to inhibit the activity of serine/threonine protein phosphatases that constrain, and possibly reverse, AIH-induced pLTF (Wilkerson et al. 2007; MacFarlane et al. 2008). Apocynin is thought to interfere with translocation of phosphorylated p47phox subunits to the membrane, where it normally binds to and activates membrane-bound subunits, subsequently increasing ROS formation (Valko et al. 2007). By inhibiting NADPH oxidase activity with apocynin following episodic 5-HT receptor activation, impaired ROS formation presumably restores the phosphatase constraint, thereby preventing further development of, or even reversing, 5-HT induced pMF or AIH-induced pLTF (MacFarlane et al. 2008).
Dose-response of 5-HT induced pMF
We hypothesise that the bell shaped dose–response curve of 5-HT induced pMF results from co-activation of different receptor subtypes, leading to ‘cross-talk inhibition’ between their respective cellular cascades. The 5-HT2 receptor family is relevant to pLTF/pMF initiation since episodic intrathecal injections of 5-HT2 receptor agonists elicit pMF (MacFarlane & Mitchell, 2008b) and 5-HT2 receptor activation is necessary for AIH-induced pLTF (Kinkead et al. 1998; Fuller et al. 2001b; Baker-Herman & Mitchell, 2002). Furthermore, 5-HT2 receptors are abundant in respiratory motor nuclei, including the XII (Kubin & Volgin, 2008) and phrenic motor pools (Basura et al. 2001). At higher concentrations of 5-HT (>1 mm), however, other serotonergic receptors may play progressively greater roles in determining the expression of pMF, including Gi (5-HT1/5) and/or Gs-coupled (5-HT4/6/7) metabotropic receptors (Hannon & Hoyer, 2008). These receptors may initiate processes that converge on and inhibit 5-HT2-dependent pMF. This ‘cross-talk inhibition’ between G-protein coupled receptors and their downstream signalling processes has been described in the literature (Robinson-White & Stratakis, 2002), and has been proposed to be an important mechanism regulating AIH-induced pLTF (Hoffman & Mitchell, 2008). Since pre-treatment with a 5-HT7 receptor antagonist enhances AIH-induced pLTF, these receptors constrain pLTF, most likely by a PKA-dependent mechanism (Hoffman & Mitchell, 2008). Thus, the declining phase of the bell-shaped concentration–response curve may involve progressively greater 5-HT7 receptor activation. Similar concentration-dependent effects of 5-HT on synaptic plasticity occur in sensory inputs to the spinal dorsal horn. At low concentrations of 5-HT, ineffective (i.e. ‘silent’) glutamatergic synapses in the spinal dorsal horn became functional, whereas higher concentrations were inhibitory due to co-activation of 5-HT1A/7 receptors (Li & Zhuo, 1998).
Neuromodulatory effects of 5-HT are not necessary for pMF
The observation that 5-HT induced pMF was greatest at concentrations too low to elicit the ‘classical’ neuromodulatory actions of 5-HT on phrenic motor neurons (McCrimmon et al. 1995; Rekling et al. 2000) is of interest in several important respects. Although this dissociation is difficult to explain, it does suggest that ‘classical neuromodulation’ and pMF arise from distinct cellular mechanisms. The observation may indicate that different 5-HT receptor subtypes underlie pMF and classical neuromodulation, respectively. On the other hand, it may be that low levels of 5-HT2 receptor activation elicit pMF, whereas greater activation elicits neuromodulation and, in turn, inhibits subsequent development of pMF. These competing hypotheses remain to be explored. Regardless, the dissociation between pMF and the tonic, neuromodulatory effects of 5-HT suggest that future studies of 5-HT induced facilitation must carefully consider the concentration of 5-HT (or agonists) used, and the potential impact of the tonic, neuromodulatory actions of 5-HT receptor activation (e.g. Monteau et al. 1990; Berger et al. 1992; Bocchiaro & Feldman 2004; Peng et al. 2006; Brandes et al. 2006).
Although we did not investigate which 5-HT receptor subtype caused tonic phrenic activity, others have implicated 5-HT1A/2C (Kubin et al. 1992) or 5-HT2A receptors (Lindsay & Feldman, 1993; DiPasqual et al. 1997). Here, we observed that the 5-HT7 receptor antagonist SB269970 attenuated the tonic activity, suggesting a prominent role for this receptor type in mediating phrenic neuromodulation. Overall, the variability in the responses to 5-HT (or agonists) could depend on several factors including the concentrations used, the selectivity of agonists for the target receptors, species differences, the neural populations under investigation, and the experimental preparation (in vitro versus in vivo, developmental age, etc.).
Conclusions
We provide the first in vivo evidence that spinal serotonin receptor activation is sufficient to elicit long-lasting phrenic motor facilitation in adult rats via an NADPH oxidase (presumably ROS)-dependent mechanism. These data are consistent with previous reports concerning the role of spinal serotonin receptors (Fuller et al. 2001b; Baker-Herman & Mitchell, 2002) and NADPH oxidase (MacFarlane et al. 2009) in AIH-induced pLTF, suggesting that both forms of plasticity share common mechanisms. Thus, since hypoxia stimulates raphe serotonergic neurons (Erickson & Millhorn, 1994) and 5-HT release in respiratory motor nuclei (Kinkead et al. 2001), we suggest that the relevant stimulus to AIH-induced pLTF is repetitive serotonin receptor activation versus hypoxia/re-oxygenation per se.
The bell-shaped dose–response curve indicates that 5-HT-induced pMF depends on the relative activation of different 5-HT receptor subtypes, possibly invoking ‘cross-talk inhibition’. Furthermore, since pMF is not observed if the 5-HT concentration used induced tonic, neuromodulatory effects on phrenic nerve activity, we suggest that mechanisms of pMF and classical neuromodulation in respiratory motor neurons are distinct. The diversity of serotonin receptor subtypes and the ways in which they determine different motor neuron behaviours (e.g. neuromodulation versus plasticity; Mitchell & Johnson, 2003) could be important, enabling the respiratory neural networks to mount appropriate responses when confronted with varied challenges.
Acknowledgments
This work was funded by the National Institutes of Health (RO1 HL-080209) and a Parker B. Francis Fellowship awarded to P.M.M. by the Francis Family Foundation. We thank Brad Hodgeman for technical assistance. The computer software used for data analysis was custom-designed and kindly provided by Safraaz Mahamed.
Glossary
Abbreviations
- AIH
acute intermittent hypoxia
- CSF
cerebrospinal fluid
- DPI
diphenylenodium
- MAP
mean arterial pressure
- ROS
reactive oxygen species
- XII
hypoglossal
- pLTF
phrenic long-term facilitation
- pMF
phrenic motor facilitation
Author contributions
Both authors contributed to the conception, design, and analysis of experiments including interpretation of data and drafting/revising the final version of the article approved for publication. All experiments were carried out at University of Wisconsin, Madison, Wisconsin, USA.
References
- Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27:1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol Neurobiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol. 2000;15:215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TL, Fuller DD, Zabka AG, Mitchell GS. Respiratory plasticity: differential actions of continuous and episodic hypoxia and hypercapnia. Respir Physiol Neurobiol. 2001;129:25–35. doi: 10.1016/s0034-5687(01)00280-8. [DOI] [PubMed] [Google Scholar]
- Baker-Herman T, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol. 2008;162:8–17. doi: 10.1016/j.resp.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Basura GJ, Zhou SY, Walker PD, Goshgarian HG. Distribution of serotonin 2A and 2C receptor mRNA expression in the cervical ventral horn and phrenic motoneurons following spinal cord hemisection. Exp Neurol. 2001;169:255–263. doi: 10.1006/exnr.2001.7682. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Umemiya M, Berger A. Inhibition of N- and P-type calcium currents and the after-hyperpolarization in rat motoneurons by serotonin. J Physiol. 1995;485:635–647. doi: 10.1113/jphysiol.1995.sp020758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneurons excitability by serotonin. Neurosci Lett. 1992;143:164–168. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci U S A. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes IF, Zuperku EJ, Stucke AG, Jakovcevic D, Hopp FA, Stuth EAE. Serotonergic modulation of inspiratory hypoglossal motoneurons in decerebrate dogs. J Neurophysiol. 2006;95:3449–3459. doi: 10.1152/jn.00823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumley MR, Hentall ID, Pinzon A, Kadam BH, Blythe A, Sanchez FJ, Taberner AM, Noga BR. Serotonin concentrations in the lumbosacral spinal cord of the adult rat following microinjection or dorsal surface application. J Neurophysiol. 2007;98:1440–1450. doi: 10.1152/jn.00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinger B, Hea L, Chen J, Liua X, Gonzalez C, Obeso A, Sanders K, Hoidal J, Stensaas L, Fidone S. The role of NADPH oxidase in carotid body arterial chemoreceptors. Respir Physiol Neurobiol. 2007;157:45–54. doi: 10.1016/j.resp.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPasquale D, Lindsay A, Feldman JL, Monteau R, Hilaire G. Serotonergic inhibition of phrenic motoneurons activity: an in vitro study in neonatal rat. Neurosci Lett. 1997;230:29–32. doi: 10.1016/s0304-3940(97)00469-2. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Fos-like protein is induced in neurons of the medulla oblongata after stimulation of the carotid sinus nerve in awake and anaesthetized rats. Brain Res. 1991;567:11–24. doi: 10.1016/0006-8993(91)91430-9. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotonergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Baker TL, Behan M, Mitchell GS. Expression of hypoglossal long-term facilitation differs between substrains of Sprague–Dawley rat. Physiol Genomics. 2001a;4:175–181. doi: 10.1152/physiolgenomics.2001.4.3.175. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Selected contribution: phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001b;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Grewal JS, Mukhin YV, Garnovskaya MN, Raymond JR, Greene EL. Serotonin 5-HT2A receptor induces TGF-β1 expression in mesangial cells via ERK: proliferative and fibrotic signals. Am J Physiol Renal Physiol. 1999;276:F922–930. doi: 10.1152/ajprenal.1999.276.6.F922. [DOI] [PubMed] [Google Scholar]
- Hancock JT, Jones OT. The inhibition by diphenyleneiodonium and its analogues of superoxide generation by macrophages. Biochem J. 1987;242:103–107. doi: 10.1042/bj2420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008;164:222–232. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Mitchell GS. 2008 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2008. Spinal PKA inhibits phrenic long-term facilitation following acute intermittent hypoxia. Program No. 571.8. [Google Scholar]
- Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol. 1997;7:820–825. doi: 10.1016/s0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci. 1998;18:8436–8443. doi: 10.1523/JNEUROSCI.18-20-08436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Bach KB, Johnson SM, Hodgeman BA, Mitchell GS. Plasticity in respiratory motor control: intermittent hypoxia and hypercapnia activate opposing serotonergic and noradrenergic modulatory systems. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:207–218. doi: 10.1016/s1095-6433(01)00393-2. [DOI] [PubMed] [Google Scholar]
- Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antiox Redox Signal. 2007;9:233–244. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida KT, Hoeffer CA, Hu D, Pao M, Holland SM, Klann E. Synaptic plasticity deficits and mild memory impairments in mouse models of chronic granulomatous disease. Mol Cell Biol. 2006;26:5908–5920. doi: 10.1128/MCB.00269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- Kubin L, Volgin DV. Developmental profiles of neurotransmitter receptors in respiratory motor nuclei. Respir Physiol Neurobiol. 2008;164:64–71. doi: 10.1016/j.resp.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Zhuo M. Silent synapses and nociception in mammalian spinal cord. Nature. 1998;393:695–698. doi: 10.1038/31496. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurons by serotonin. J Physiol. 1993;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Barr MR, Mitchell GS, Satriotomo I, Johnson SM. Serotonin-induced in vitro long-term facilitation exhibits differential pattern sensitivity in cervical and thoracic inspiratory motor output. Neuroscience. 2006;142:885–892. doi: 10.1016/j.neuroscience.2006.06.036. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. Respiratory long-term facilitation following intermittent hypoxia requires reactive oxygen species formation. Neuroscience. 2008a;152:189–97. doi: 10.1016/j.neuroscience.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. NADPH oxidase activity is necessary for phrenic motor facilitation induced by 5-HT2B receptor activation. FASEB J. 2008b;21:918.10. [Google Scholar]
- MacFarlane PM, Satriotomo I, Windelborn JA, Mitchell GS. NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. J Physiol. 2009;587:1931–1942. doi: 10.1113/jphysiol.2008.165597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Wilkerson JE, Lovett-Barr MR, Mitchell GS. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol. 2008;164:263–271. doi: 10.1016/j.resp.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol. 2007;92:27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- Mauelshagen J, Sherff CM, Carew TJ. Differential induction of long-term synaptic facilitation by spaced and massed applications of serotonin at sensory neuron synapses of Aplysia californica. Learn Mem. 1998;5:246–256. [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Dekin MS, Mitchell GS. Glutamate, GABA and serotonin in ventilatory control. In: Dempsey JA, Pack AI, editors. Lung Biology in Health and Disease. Vol. 79. New York: Marcel-Dekker; 1995. pp. 151–218. Regulation of Breathing: Central Nervous Control. [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited Review: intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Monteau R, Morin D, Hennequin S, Hilaire G. Differential effects of serotonin on respiratory activity of hypoglossal and cervical notoneruons: an in vitro study on the newborn rat. Neurosci Lett. 1990;111:127–132. doi: 10.1016/0304-3940(90)90356-e. [DOI] [PubMed] [Google Scholar]
- O’Donnell BV, Tew DG, Jones OT, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J. 1993;290:41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol. 2006;576:289–295. doi: 10.1113/jphysiol.2006.116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK, Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci. 2009;29:4903–4910. doi: 10.1523/JNEUROSCI.4768-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCɛ signalling axis in pulmonary artery smooth muscle cells. Free Rad Biol Med. 2008;45:1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong X-W, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-White A, Stratakis CA. Protein kinase A signalling: ‘cross-talk’ with other pathways in endocrine cells. Ann NY Acad Sci. 2002;968:256–270. doi: 10.1111/j.1749-6632.2002.tb04340.x. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Pestean A, Günther S, Ballanyi K. Serotonergic modulation of respiratory motoneurons and interneurons in brainstem slices of perinatal rats. Neurosci. 2002;115:1247–1259. doi: 10.1016/s0306-4522(02)00540-7. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–89. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wilkerson JER, MacFarlane PM, Hoffman MS, Mitchell GS. Respiratory plasticity following intermittent hypoxia: roles of protein phosphatases and reactive oxygen species. Biochem Soc Trans. 2007;35:1269–1272. doi: 10.1042/BST0351269. [DOI] [PubMed] [Google Scholar]