Abstract
Ageing is characterized by a decline in muscle mass that could be explained by a defect in the regulation of postprandial muscle protein metabolism. Indeed, the stimulatory effect of food intake on protein synthesis and its inhibitory effect on proteolysis is blunted in old muscles from both animals and humans. Recently, low grade inflammation has been suspected to be one of the factors responsible for the decreased sensitivity of muscle protein metabolism to food intake. This study was undertaken to examine the effect of long-term prevention of low grade inflammation on muscle protein metabolism during ageing. Old rats (20 months of age) were separated into two groups: a control group and a group (IBU) in which low grade inflammation had been reduced with a non-steroidal anti inflammatory drug (ibuprofen). After 5 months of treatment, inflammatory markers and cytokine levels were significantly improved in treated old rats when compared with the controls: −22.3% fibrinogen, −54.2%α2-macroglobulin, +12.6% albumin, −59.6% IL6 and −45.9% IL1β levels. As expected, food intake had no effect on muscle protein synthesis or muscle proteolysis in controls whereas it significantly increased muscle protein synthesis by 24.8% and significantly decreased proteolysis in IBU rats. The restoration of muscle protein anabolism at the postprandial state by controlling the development of low grade inflammation in old rats significantly decreased muscle mass loss between 20 and 25 months of age. In conclusion, the observations made in this study have identified low grade inflammation as an important target for pharmacological, nutritional and lifestyle interventions that aim to limit sarcopenia and muscle weakness in the rapidly growing elderly population in Europe and North America.
Introduction
Ageing is characterized by a gradual loss of muscle proteins (sarcopenia), which is ultimately responsible for decreased mobility and autonomy. Sarcopenia also reduces the ability of the elderly to cope with nutritional, infectious or traumatic stresses, whose incidence increases in ageing. Proteins in skeletal muscle undergo a continuous process of synthesis and degradation. Thus, sarcopenia should be due to an imbalance between rates of protein turnover. Skeletal muscle protein synthesis decreases in the post-absorptive (PA) state and increases in the postprandial (PP) state, while protein breakdown follows the inverse pattern. In adults, net positive protein balance in the PP state and net negative protein balance in the PA state cancel each other and allow maintaining muscle mass. There is strong evidence that the stimulatory effect of food intake on protein synthesis and its inhibitory effect on proteolysis is blunted in older muscles from both animals (Mosoni et al. 1995; Dardevet et al. 2002; Combaret et al. 2005) and humans (Guillet et al. 2004; Cuthbertson et al. 2005). It has been hypothesized that this impairment may be responsible for muscle wasting during ageing. However, the cause of this impairment is still unknown and under question.
Levels of inflammatory markers, such as interleukin-6 (IL6) and C reactive protein (CRP), increase slightly with ageing, and these higher levels are correlated with disability and mortality in humans (Harris et al. 1999; Bautmans et al. 2005). Even if the increase is moderate, higher levels of cytokines and CRP increase the risk of muscle strength loss (Schaap et al. 2006) and are correlated with lower muscle mass in healthy older persons (Visser et al. 2002). We have recently shown that old ‘healthy’ rats also exhibit a moderate increase of plasma inflammatory markers such as α2-macroglobulin, fibrinogen or IL6 concentrations (Balage et al. 2009). Furthermore, we and others have shown that the presence of this low grade inflammation, characterized by an increase of pro-inflammatory markers and cytokines (i.e. α2-macroglobulin and IL6), was correlated with a decrease of muscle protein synthesis in humans (Toth et al. 2005) and an impaired postprandial stimulation of muscle protein synthesis in old rats (Balage et al. 2009). Pro-inflammatory cytokines increase the synthesis of prostaglandin-E2 (PGE2) in many cells types (Perkins & Kniss, 1997) by inducing the activation of cyclooxygenase 2 (COX2), a rate-limiting enzyme in the synthesis of PGE2 from arachidonic acid. A decrease of PGE2 production has been shown to preserve protein synthesis and to decrease protein degradation in skeletal muscle in highly inflamed rat models (Mendez et al. 1992; Tisdale, 1996; Whitehouse et al. 2001). Therefore, a modulation of COX2 activity during ageing may be beneficial to counteract the deleterious effects of low grade inflammation on the modulation of muscle protein metabolism by food intake. To test this hypothesis, a non-steroidal anti-inflammatory drug (NSAID) which inhibits cyclooxygenase activities, including COX2, was used in this study i.e. ibuprofen. Furthermore, recent evidence also suggests that ibuprofen has additional anti-inflammatory properties due to modulation of leucocyte activity by reducing cytokine production (see Rainsford, 2003) for a review) or inhibition of NF-κB activation. These additional properties of ibuprofen are particularly of interest as pro-inflammatory cytokines impair skeletal muscle protein synthesis stimulation by decreasing the mammalian target of rapamycin (mTOR) signalling pathways (Lang et al. 2002) and as NF-κB activation has been linked to muscle proteolysis stimulation (see Tisdale, 2005 for a review). In this study, we evaluated the long-term effect of ibuprofen in old rats: (1) on the development of low grade inflammation (cytokines, acute phase proteins), (2) on the regulation of muscle protein synthesis and proteolysis by food intake and (3) on muscle mass itself.
Methods
Animals and experimental design
The experiments were conducted in accordance with the National Research Council's Guidelines for the Care and Use of Laboratory Animals (DSV-63-08). Old Wistar rats (n= 70 final, 20 months old) were housed under controlled environmental conditions (temperature, 22°C; 12 h dark period starting at 0800 h). The rats were given free access to water and commercial laboratory chow (UAR A04, Usine d’Alimentation Rationnelle, Villemoisson, France) containing 19% protein, 3% fat, 59% carbohydrates, water, fibres, vitamins and minerals. The 20-month-old rats were separated into two groups with similar body weight, food intake, and also plasma α2-macroglobulin, fibrinogen and albumin levels (Table 1). Indeed, we have recently shown that α2-macroglobulin levels, like C reactive protein in humans, are strong predictors of persistent low grade inflammatory status in old rats (Mayot et al. 2007). One group received the regular commercial laboratory chow (control group n= 47 (55 at beginning, 8 deaths after 5 months)) and the other group received the commercial laboratory chow supplemented with ibuprofen (700 mg kg−1 diet, IBU; n= 23 (25 at beginning, 2 deaths after 5 months)). This supplementation allowed a daily NSAID intake of 30 mg (kg body weight)−1. Ageing rat population is very heterogeneous and only 50% of the rats developed a low grade inflammation between 20 and 25 months (Balage et al. 2009). In order to be representative of this population, the control group was comprised of 2 times more animals than the treated group in which the inflammation was controlled by the treatment. Animals were studied for 5 months; body weight and food intake were recorded every week. Once a month, a blood sample was withdrawn to assess plasma fibrinogen, α2-macroglobulin and albumin levels.
Table 1.
Food intake, fibrinogen, α2 macroglobulin and albumin levels
| Age | 20 months | 21 months | 22 months | 23 months | 24 months | 25 months |
|---|---|---|---|---|---|---|
| Food intake | ||||||
| C | 27.0 ± 0.7 | 27.7 ± 0.7 | 29.8 ± 0.6 | 28.5 ± 0.6 | 29.0 ± 0.5 | 29.3 ± 0.8 |
| IBU | 27.1 ± 0.7 | 28.3 ± 0.6 | 27.4 ± 1.2 | 29.5 ± 0.7 | 28.1 ± 1.4 | 28.9 ± 1.0 |
| Fibrinogen (g l−1) | ||||||
| C | 4.19 ± 0.15a | 4.52 ± 0.17ab | 4.43 ± 0.15ab | 4.44 ± 0.13ab | 4.74 ± 0.19b | 4.47 ± 0.19ab |
| IBU | 4.00 ± 0.16a | 3.93 ± 0.21*ab | 3.72 ± 0.17*ab | 3.97 ± 0.19*ab | 4.17 ± 0.20*a | 3.47 ± 0.19*b |
| α2 macroglobulin (mg l−1) | ||||||
| C | 68.5 ± 8.5a | 131.6 ± 23.8ab | 135.3 ± 18.1ab | 198.1 ± 23.2bc | 253.3 ± 35.2cd | 294.0 ± 60.8d |
| IBU | 78.0 ± 15.3ab | 53.7 ± 7.2*a | 75.1 ± 12.6*ab | 97.1 ± 14.1*b | 98.1 ± 13.0*b | 134.6 ± 58.9*b |
| Albumin (g l−1) | ||||||
| C | 17.71 ± 0.58a | 16.10 ± 0.40bc | 16.48 ± 0.35ab | 16.49 ± 0.37ab | 14.63 ± 0.53d | 15.00 ± 0.57cd |
| IBU | 17.83 ± 0.39a | 17.38 ± 0.51ab | 17.11 ± 0.37ab | 16.80 ± 0.53ab | 16.49 ± 0.65*b | 16.89 ± 0.70*ab |
C, control group (n= 47); IBU, old rats treated with ibuprofen (n= 23). *Significantly different from the control value at the same time. Different letters indicate values significantly different over time in the same group.
Plasma acute-phase protein measurement and cytokines assay
Plasma fibrinogen was measured by turbidimetry on a Cobas Mira analyser (ABX Diagnostics, Montpellier, France). Concentration is expressed as g human equivalent l−1 since human fibrinogen was used as reference (Ingen, Rungis, France). Plasma albumin and α2-macroglobulin were measured by single radial immunodiffusion. Plasma TNFα, IL1β, IL6, MCP1 (monocyte chemo-attractant protein 1) and PAI-1 (plasminogen activator inhibitor-1) were assayed by ELISA (Linco, LincoPlex, MI, USA).
Protein synthesis measurement
At the end of the experimental study, the rats were fasted overnight. The next morning, they were separated into two groups with similar inflammation status and studied either in the post-absorptive (PA) or in the postprandial state (PP) (150–180 min after a 1 h feeding period of the same diet they received for the last 5 months). Protein synthesis was assessed with the flooding dose method. As described and validated by Dardevet et al. (2002), each rat was injected intravenously with l-[1-13C] phenylalanine (99%) (50 μmol (100 g body weight)−1), 45 min before the time of killing. Animals were then anaesthetised with pentobarbital sodium (6 mg (100 g body weight)−1); blood was rapidly collected and centrifuged at 3000 g for 10 min. Hind limb muscles and liver were excised, weighed, frozen in liquid nitrogen, and stored at −80°C until analysis.
Free and bound-phenylalanine enrichments were determined and measured as previously described (Dardevet et al. 2002). Measurement of free phenylalanine enrichment was done as its t-butyldimethylsilyl (TBDMS) derivative by gas chromatography electron impact mass spectrometry (GC-MS). This was done using a HP-5890 gas chromatograph coupled to a HP-5972 organic mass spectrometer quadrupole (Hewlett-Packard, Paris, France). The ions m/z 336 and 337 were monitored. Enrichment of [l-13C] phenylalanine into proteins (muscle proteins and albumin) was measured as its N-acetyl-propyl derivatives by gas chromatography–combustion–isotope ratio mass spectrometry (GC-C-IRMS, Micromass Isochrom II, Fisons Instruments, Middlewitch, UK).
Calculations of protein synthesis rates
Protein fractional synthesis rate (FSR, in % per day) was calculated from the formula:
where Sb is the enrichment at time t (minus natural basal enrichment of protein) of the protein-bound phenylalanine, t is the incorporation time in days, and Sa is the mean enrichment of free tissue phenylalanine between time 0 and time t. The mean Sa enrichment was the Sa (t1/2) value calculated from the linear regression obtained in tissue between time 0 and time t. For albumin synthesis, the mean Sa enrichment was the Sa (t1/2) minus the excretion time of albumin from liver estimated at 20 min.
Amino acid measurements
An aliquot of plasma (700 μl) was homogenized in 7 volumes of trichloroacetic acid (0.6 mol l−1) containing 2.5% of thiodiglycol. Norleucine (2.9 mmol l−1) was added as internal standard. Samples were incubated on ice for 20 min and centrifuged at 8000 g for 15 min at 4°C. This procedure was repeated once and pooled supernatants were passed through columns of cation exchange resin (AG 50W-X8, 100–200 mesh, Bio-Rad, Richmond, CA, USA). Purified amino acids eluted from the column by 4 mol l−1 NH4OH were dried and reconstituted in 1 ml of 0.1 mol l−1 lithium acetate buffer, pH 2.2. Amino acid concentrations were then determined using an automated amino acid analyser (HPLC System, BioTEK instruments).
In vitro rates of muscle proteolysis
Epitrochlearis muscles from rats were quickly excised and rinsed in Krebs–Henselheit bicarbonate buffer ((mm): NaCl 120, KCl 4.8, NaHCO3 25, KH2PO4 1.2 and MgSO4 1.2, pH 7.4), supplemented with 5 mm Hepes, 5 mm glucose and 0.1% BSA (Dardevet et al. 2000). Muscles were then transferred to plastic tubes containing 1.5 ml of fresh buffer saturated with a 95% O2–5% CO2 gas mixture. After 30 min of pre-incubation, muscles were transferred to a fresh medium of identical composition and further incubated for 1 h. Rates of protein breakdown were measured by following the rates of tyrosine release into the medium in the presence of 0.5 mm cycloheximide, which blocks protein synthesis (Combaret et al. 2005). Protein degradation was expressed in nanomoles of tyrosine released in the medium per milligram muscle per hour. Muscle protein content was measured according to the bicinchoninic acid procedure.
Measurement of muscle signalling pathways linked to protein synthesis and proteolysis
The musculature is comprised of mainly fast-twitch muscles. Measurement of protein synthesis or proteolysis (e.g. in vitro rates of proteolysis and in vivo protein levels, signalling etc.) often needs to be performed in several muscles due to the limited amounts of material in rodents. Regarding muscle proteolysis, we have previously shown that these parameters were co-ordinately regulated in different muscles that mainly are composed of type IIa,b fibres (e.g. gastrocnemius, epitrochlearis and tibialis anterior) (Combaret et al. 2004). Similarly, regarding protein synthesis, we have shown that lack of response to food intake was present in all the muscles we have already studied (i.e. gastrocnemius, epitrochlearis, soleus and tibialis anterior) (Dardevet et al. 2000, 2002; Rieu et al. 2003; Balage et al. 2009). Then because gastrocnemius muscle is representative of the fast-twitch muscles fibre type, signalling pathways assessment has been performed on this muscle. Muscle was homogenized at 4°C in 7 volumes of lysis buffer: 20 mm Hepes (pH 7.4), 100 mm potassium chloride, 0.2 mm EDTA, 2 mm EGTA, 1 mm DTT, 50 mm NaF, 50 mm glycerophosphate, 0.1 mm PMSF, 1 mm benzamidine and 0.5 mm sodium vanadate, using a Polytron homogenizer. The homogenate was centrifuged at 10 000 g at 4°C for 10 min. Total protein concentration was determined using a commercial protein assay reagent (Bio-Rad).
For determination of S6K1, S6rp, 4EBP1, Foxo3a and their phosphorylation state, samples containing equal amounts of proteins were separated by SDS-PAGE and transferred to PVDF membrane (Millipore). Membranes were incubated overnight with specific antibodies against: S6K1, p-S6K1 (Thr389), p-S6K1 (Thr421/S424), S6rp, p-S6 (Ser240/244), p-S6 (Ser235/236), Foxo3a, p-Foxo3a (Ser253) (Cell Signaling, Beverly, MA, USA) and 4EBP1 (Bethyl Laboratories). The signal was detected by enhanced chemiluminescence (ECL; GE Healthcare) after incubation with a secondary antibody conjugated with horseradish peroxidase (Cell Signaling). Blots were exposed to high performance chemiluminescence films (GE Healthcare), the films were scanned and identified bands were quantified by densitometry using Image J 1.41o (NIH, USA).
Statistical analysis
Data are expressed as means ±s.e.m. and analysed by XLStat (Addinsoft NY, USA, version 7.5.2). Differences in food intake, fibrinogen, albumin and α2 macroglobulin levels during the 5 months of the experimental period were assessed by a one-way repeated-measure analysis of variance to test the time effect on these parameters. For protein synthesis and inflammation markers at 25 month old, statistical evaluation of the data was performed by one-way ANOVA to analyse the treatment effect or a two-way ANOVA to analyse the effect of treatment and nutritional status. When a significant overall effect was detected, differences among individual means were assessed with the Fisher's test to determine significant differences. A Student's t test was performed when appropriate. The level of significance was set at P < 0.05 for all statistical tests.
Results
Animal characteristics
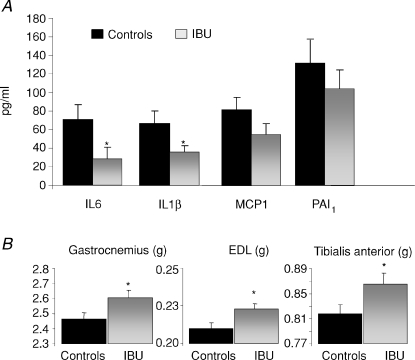
During ageing (20 to 25 months of age), control rats exhibited a slight increase of fibrinogen levels associated with a significant increase of α2-macroglobulin (4.3-fold) and a significant decrease of plasma albumin levels (−15%) (Table 1). This demonstrated the development of a spontaneous low grade inflammatory state in old rats. When treated with ibuprofen (IBU), fibrinogen levels did not increase over the 5 month experimental period, albumin levels remained constant, and α2-macroglobulin levels only increased by 1.7 times (Table 1). Food intake was similar in both groups during the 5 months of the treatment period. At 25 months old, treated rats showed a lower concentration in fibrinogen (−22.3%, P < 0.05) and α2-macroglobulin (−54.2%, P < 0.05) and higher albumin concentration (+12.6%, P < 0.05) when compared to the controls. In order to further characterize the inflammation state of each group, other inflammatory markers were measured at 25 months of age. The IBU rats showed a decrease of plasma IL6 and IL1β levels (−59.6% and −45.9%, respectively; P < 0.05) compared to the control rats (Fig. 1) with no modification in MCP1 and PAI1 levels. The TNFα levels remained undetectable in both groups (i.e. levels less than 4.88 pg ml−1; not shown).
Figure 1.
Plasma cytokines, inflammatory markers (A) and hind limb muscle mass (B) in 25-month-old rats treated or not with the NSAID ibuprofen for 5 months. Values are means ±s.e.m., n= 47 (controls) and n= 23 in ibuprofen-treated rats. *Significantly different from controls values (P < 0.05).
Liver and spleen weights were not significantly different between the two groups (15.13 ± 0.43 and 15.33 ± 0.32 g, 1.41 ± 0.06 and 1.47 ± 0.05 g, for liver and spleen in IBU and controls, respectively). After 5 months of treatment, IBU rats exhibited a significant increase in muscle mass when compared to the controls (Fig. 1). This increase was recorded for gastrocnemius, extensor digitorum longus (EDL) and tibialis anterior muscles. Despite this increase in muscle mass, the decrease of total body weight over the 5 months of the study was similar in both groups (−105 ± 9 and –98 ± 27 g in controls and IBU, respectively).
Protein synthesis and muscle proteolysis
Protein synthesis and proteolysis were assessed at the post-absorptive and the postprandial states. At 25 months of age, animals of both groups were fasted overnight. The next day, rats either received food or received no food for 1 h. Food consumption during the 1 h feeding period was not different between the control and IBU groups (9.72 ± 0.90 vs. 10.23 ± 0.60 g, respectively). There was no difference in the resulting postprandial plasma amino acid concentrations observed at killing (90–120 min after feeding) between the control and the IBU groups (Table 2). Any difference in protein metabolism recorded could not therefore be attributed to different amino acid availability between the groups.
Table 2.
Postprandial amino acid concentration (μm) following 1 h feeding in controls and old rats treated with ibuprofen (IBU)
| Controls |
IBU |
|||
|---|---|---|---|---|
| Mean | s.e.m. | Mean | s.e.m. | |
| Methionine | 9.5 | 0.7 | 12.2 | 1.5 |
| Isoleucine | 46.3 | 1.9 | 39.3 | 1.3 |
| Leucine | 64.4 | 2.4 | 61.4 | 2.3 |
| Tyrosine | 185.3 | 7.3 | 159.7 | 8.1 |
| Phenylalanine | 236.8 | 7.0 | 221.8 | 9.0 |
| Histidine | 51.4 | 2.7 | 49.6 | 1.3 |
| Aspartic acid | 18.7 | 1.3 | 12.1 | 1.3 |
| Threonine | 187.5 | 7.6 | 190.5 | 5.9 |
| Serine | 187.6 | 6.6 | 189.5 | 9.7 |
| Asparagine | 31.1 | 1.8 | 29.0 | 1.5 |
| Glutamic acid | 288.4 | 17.9 | 247.6 | 16.2 |
| Glutamine | 226.0 | 15.9 | 195.8 | 9.5 |
| Glycine | 224.3 | 12.3 | 189.4 | 12.6 |
| Proline | 131.9 | 8.3 | 153.1 | 9.0 |
| Lysine | 283.0 | 9.3 | 255.7 | 12.7 |
| Valine | 108.9 | 4.3 | 99.1 | 4.5 |
| Alanine | 496.5 | 21.1 | 484.4 | 16.6 |
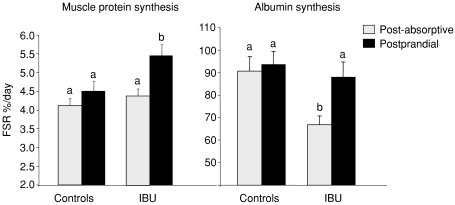
Muscle protein synthesis was unchanged at the post-absorptive state between the two groups (4.11 ± 0.18 vs. 4.38 ± 0.19% day−1 in controls and IBU rats, respectively) (Fig. 2). Food intake had no effect on muscle protein synthesis in control rats whereas it increased muscle protein synthesis significantly by 24.8% in IBU rats (P < 0.05). Post-absorptive albumin synthesis was significantly decreased by 26.2% (P < 0.05) in IBU rats compared to controls. Food intake had only a significant stimulatory effect on albumin synthesis in the IBU rats (+31.5%, P < 0.05) (Fig. 2).
Figure 2. Fractional synthesis rate of muscle and albumin protein synthesis in controls and ibuprofen-treated 25-month-old rats (IBU) that were food deprived (post-absorptive (PA)) or refed (postprandial).
Values are means ±s.e.m. (controls: PA, n= 23; PP, n= 24; and IBU, PA, n= 11; PP, n= 12). Values with different letters are significantly different (P < 0.05).
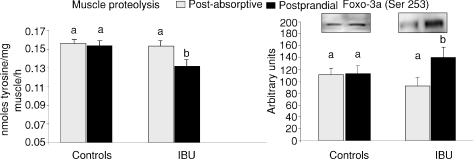
Figure 3 shows also that muscle total proteolysis was similar in controls and IBU rats at the post-absorptive state. Food intake had no effect on total muscle proteolysis in control rats whereas it significantly decreased muscle proteolysis in the rats treated with ibuprofen.
Figure 3. Muscle proteolysis and Foxo3A phosphorylation state in controls and ibuprofen-treated 25-month-old rats (IBU) that were food deprived (post-absorptive) or refed (postprandial).
Values are means ±s.e.m. (controls: PA, n= 23; PP, n= 24; and IBU, PA, n= 11; PP, n= 12) and representative Western blots are shown. Values with different letters are significantly different (P < 0.05).
Muscle signalling pathways activation
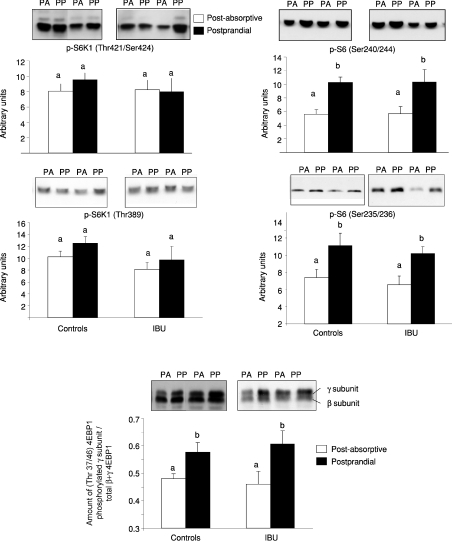
The amount of skeletal muscle S6 kinase 1 (S6K1), S6 ribosomal protein (S6), 4E binding protein 1 (4EBP1) and forkhead box O3a (Foxo3a) was unchanged whether old rats were treated or not with ibuprofen for 5 months (results not shown). Food intake leads to the activation of the mTOR signalling pathways characterized by an increased phosphorylation and activity of S6K1, S6 or 4EBP1, three downstream proteins directly linked to the initiation of muscle protein synthesis. Phosphorylation of skeletal muscle S6K1 on either threonine 421/serine424 or threonine 389 was not different in controls or IBU groups at the post-absorptive state. The S6K1 phosphorylation on both sites did not increase after feeding in either controls or IBU old rats (Fig. 4). By contrast, food intake significantly increased the phosphorylation of S6 on serine 240/244 and serine 235/236 to the same extent in both groups (Fig. 4). Similarly, food intake increased the phosphorylation of 4EBP1 to the same extent in the controls or IBU old rats (Fig. 4). Phosphorylation of Foxo3a on Ser253 was similar in controls and IBU old rats at the post-absorptive state. However, after food intake, a significant increase of phosphorylated Foxo3a was shown in the treated group whereas it remained unchanged in the controls (Fig. 3).
Figure 4. Phosphorylation state of skeletal muscle signalling pathways proteins S6 kinase 1, ribosomal protein S6, 4E binding protein 1 in the post-absorptive (PA) and the postprandial state (PP) from controls and ibuprofen-treated (IBU) 25-month-old rats.
Values are means ±s.e.m. (controls: PA, n= 23; PP, n= 24; and IBU, PA, n= 11; PP, n= 12) and representative Western blots are shown. Values with different letters are significantly different (P < 0.05).
Discussion
Ageing is characterized by a decrease of muscle mass named sarcopenia. We have previously shown a correlation between the development of a low grade inflammation and an impaired postprandial stimulation of muscle protein synthesis (Balage et al. 2009). The present study showed that the prevention of this low grade inflammation by a chronic administration of ibuprofen maintained the anabolic effect of food intake on the regulation of muscle protein metabolism in old rats. Moreover, and very interestingly, the modulation of this low grade inflammation during ageing has been associated with a significant decrease in muscle mass loss in old rats. Taken together, our results demonstrated for the first time a direct link between the well-known micro-inflammation or ‘inflamm-ageing’ and the development of sarcopenia. Ibuprofen did not have side effects since food intake was not affected by the treatment in our study and since no hepatic or gastrointestinal lesions had been observed at the time of the killing of the animal. It is important to note that ibuprofen was incorporated in the pellets and was ingested systematically with food over a 24 h period (ad libitum feeding). Our observations are consistent with those of Antezana et al. (2003) who had previously shown that ibuprofen up to 240 mg kg−1 day−1 is well tolerated in rats and there was no systemic toxicity established.
The mechanisms leading to sarcopenia are still unclear. However, this muscle loss results from an imbalance between rates of protein synthesis and degradation. This imbalance is not obvious when basal rates of protein turnover are measured (Mosoni et al. 1995; Volpi et al. 2001) but is detected in the postprandial state in old rats (Mosoni et al. 1995; Combaret et al. 2005) and elderly humans (Arnal et al. 1999) fed a normal protein meal. This imbalance results in a daily small muscle protein loss, leading in the long term to muscle wasting in elderly. In our study, the restoration of the regulation of protein metabolism in the postprandial state is associated with decreased levels of IL6 and IL1β. This suggests a direct deleterious effect of cytokines on muscle protein synthesis stimulation and muscle proteolysis inhibition by food intake. Muscle protein synthesis is under the control of factors and protein kinases (mTOR, S6K1, S6, 4EBP1) involved in the signalling pathways that control translation initiation of proteins. Interestingly, this signalling pathway has been shown to be correlated with muscle protein synthesis stimulation by food intake and amino acids. Hence, we can hypothesize that low grade inflammation, even with a slight increased cytokine production, renders this signalling pathway within skeletal muscle insensitive to food intake. In the present study, we showed that muscle S6K1 and S6rp phosphorylation levels were not affected despite the prevention of the development of the low grade inflammation. The S6K1 phosphorylation was not increased by food intake in aged muscle but its downstream target (i.e. the ribosomal protein S6) was similarly phosphorylated with food intake regardless of the inflammatory state. Reiter et al. (2005) showed that the phosphorylation of S6K1 was maximally increased 60 min after re-feeding fasted rats, and returned toward control values within 180 min. In contrast, phosphorylation of ribosomal protein S6 was maintained through the 180 min time point. Therefore, because muscle samples in the current study were collected 150–180 min after food intake, ribosomal protein S6 phosphorylation should be a good indicator of mTOR signalling pathway activation. Furthermore, phosphorylation of 4EBP1 (a downstream effector of mTOR but whose phosphorylation is independent of S6K1 activation) was no different between the two groups of rats in the present study. A specific experimental design to assess the activation of this muscle signalling pathway should be performed in order to conclude definitively on the effect of low grade inflammation on the mTOR/S6K1/S6rp activation. However, our results seemed to demonstrate that an alteration of this signalling pathway by low grade inflammation was not involved in the blunted stimulation of muscle protein synthesis by food intake in the elderly. Similarly, McCarthy et al. (2004) have shown that the preservation of muscle mass by ibuprofen in a highly inflamed rat model (i.e. tumour-bearing mice) was also independent of the S6K1 activation. The intracellular mechanisms, by which low grade inflammation and/or ibuprofen regulated muscle protein synthesis, remain to be determined and elucidated.
We showed, as others have, that normal ageing is characterized by a decreased plama albumin level associated with an increase of its hepatic synthesis (Horbach et al. 1989; Papet et al. 2003). These results may be surprising but it has been shown that during acute or chronic inflammation, hypoalbuminaemia can be mainly explained by a higher flux of albumin toward the extravascular pool where the protein is probably catabolized (Ruot et al. 2003). The albumin synthesis would then be increased in order to maintain as much as possible the level of this protein in the vascular compartment (Ruot et al. 2003). We showed that this might be the case during ageing with an increase of the synthesis rate of albumin or fibrinogen that are exclusively produced by the liver (Papet et al. 2003; Balage et al. 2009). In the present study, we indeed demonstrated that albumin synthesis was lowered when low grade inflammation was decreased compared with the control group (−26%). Interestingly, the fractional synthesis rate recorded in the treated rats was similar to the fractional synthesis rate of albumin previously assessed in adult rats (Papet et al. 2003). It may thus be hypothesized that the decreased amino acid utilization for the hepatic protein synthesis in IBU old rats may have improved their utilization to synthesized muscle proteins and participated in maintaining muscle mass during the ibuprofen treatment.
As observed with muscle protein synthesis, muscle proteolysis was insensitive to food intake in old rats whereas the decrease of the low grade inflammation in the treated group allowed restoration of its inhibitory effect. It is not surprising since cytokines (i.e. TNFα and IL6) have been shown to be involved in the increase of muscle proteolysis seen in a different acute inflammation model (Voisin et al. 1998). NF-κB is a well-known intracellular factor involved in the cytokine signalling pathways and its expression has been shown to be significantly increased in elderly compared to young adults (Cuthbertson et al. 2005). In the present study, the decrease of IL6 and IL1β with the ibuprofen treatment may have decreased the activation of muscle NF-κB. This allowed the restoration of the effect of food intake on muscle proteolysis. The link between the activation of NF-κB and the regulation of muscle proteolysis is still under question. However, in the present study, the recovery of muscle proteolysis inhibition after food intake in the treated old rats was correlated to the phosphorylation of Foxo3a on Ser253. Degradation of muscle protein, especially the contractile components, is believed to result from the activation of the ubiquitin–proteasome proteolytic pathway (Attaix et al. 2005). In this proteolytic pathway, the atrogin-1/MAFbx is a muscle-specific E3 ubiquitin ligase known to be specifically involved in muscle atrophy and to be induced by Foxo3a (Sandri et al. 2004; Stitt et al. 2004). In growing muscles, the activated PI3K–Akt signalling pathway (i.e. postprandial state) phosphorylates Foxo3 that becomes sequestered in the cytosol. This prevents it from entering the nucleus and prevents the transcription of the atrogin-1/MAFbx. In the present study, the recovery of muscle proteolysis inhibition after food intake in the IBU rats may originate from the restored ability of Foxo3a to be phosphorylated by the PI3K–Akt signalling pathway. This may result in the postprandial inhibition of the ubiquitin–proteasome proteolytic pathway known to be unresponsive to nutrients in older rat muscle (Combaret et al. 2005). To support this hypothesis, Crossland et al. (2008) showed that LPS infusion induced increases in muscle tumour necrosis factor-α and interleukin-6 parallelled by reduced cytosolic Foxo3 phosphorylation. These changes were accompanied by significant increases in muscle atrophy F-box mRNA.
In conclusion, our results provide convincing evidence that the blunting of increases in muscle protein synthesis and decreases in proteolysis that occur in the postprandial state in ageing rats and human beings can be restored by the use of NSAIDs. The mechanism by which they operated is independent of the mTOR/S6K1 signalling pathway. Low grade inflammation is therefore an important target to prevent sarcopenia and limit muscle strength loss and reduce fall risk in the rapidly growing elderly population in western countries.
Acknowledgments
We thank Arlette Cissoire, Christian Lafarge from the UE231 (INRA Clermont-Ferrand-Theix, France) for their excellent assistance during animal experimentations. We also thank C. Sornet and V. Mahé for technical assistance. This project was supported by a grant from the French Ministry of Research (UNH1019).
Glossary
Abbreviations
- COX2
cyclo-oxygenase 2
- CRP
C reactive protein
- 4EBP1
4E binding protein 1
- Foxo3a
forkhead box O3a
- IL6
interleukin-6
- IL1β
interleukin-1β
- MCP1
monocyte chemo-attractant protein 1
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor-κB
- NSAID
non-steroidal anti-inflammatory drug
- PA
post-absorptive
- PAI-1
plasminogen activator inhibitor-1
- PGE2
prostaglandin-E2
- PP
postprandial
- S6rp
ribosomal protein S6
- S6K1
S6 kinase 1
- TNFα
tumor necrosis factor α
Author contributions
All authors have contributed to all of the following: conception and design or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; final approval of the version to be published.
References
- Antezana DF, Clatterbuck RE, Alkayed NJ, Murphy SJ, Anderson LG, Frazier J, et al. High-dose ibuprofen for reduction of striatal infarcts during middle cerebral artery occlusion in rats. J Neurosurg. 2003;98:860–866. doi: 10.3171/jns.2003.98.4.0860. [DOI] [PubMed] [Google Scholar]
- Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, et al. Protein pulse feeding improves protein retention in elderly women. Am J Clin Nutr. 1999;69:1202–1208. doi: 10.1093/ajcn/69.6.1202. [DOI] [PubMed] [Google Scholar]
- Attaix D, Mosoni L, Dardevet D, Combaret L, Mirand PP, Grizard J. Altered responses in skeletal muscle protein turnover during aging in anabolic and catabolic periods. Int J Biochem Cell Biol. 2005;37:1962–1973. doi: 10.1016/j.biocel.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Balage M, Averous J, Rémond D, Bos C, Pujos-Guillot E, Mosoni L, et al. Presence of low-grade inflammation impaired postprandial stimulation of muscle protein synthesis in old rats. J Nutr Biochem. 2009 doi: 10.1016/j.jnutbio.2009.01.005. 2009 Apr 13. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Bautmans I, Njemini R, Lambert M, Demanet C, Mets T. Circulating acute phase mediators and skeletal muscle performance in hospitalized geriatric patients. J Gerontol A Biol Sci Med Sci. 2005;60:361–367. doi: 10.1093/gerona/60.3.361. [DOI] [PubMed] [Google Scholar]
- Combaret L, Dardevet D, Rieu I, Pouch MN, Bechet D, Taillandier D, et al. A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle. J Physiol. 2005;569:489–499. doi: 10.1113/jphysiol.2005.098004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combaret L, Taillandier D, Dardevet D, Béchet D, Rallière C, Claustre A, et al. Glucocorticoids regulate mRNA levels for subunits of the 19 S regulatory complex of the 26 S proteasome in fast-twitch skeletal muscles. Biochem J. 2004;378:239–246. doi: 10.1042/BJ20031660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossland H, Constantin-Teodosiu D, Gardiner SM, Constantin D, Greenhaff PL. A potential role for Akt/FOXO signalling in both protein loss and the impairment of muscle carbohydrate oxidation during sepsis in rodent skeletal muscle. J Physiol. 2008;586:5589–5600. doi: 10.1113/jphysiol.2008.160150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signalling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Balage M, Grizard J. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr. 2000;130:2630–2635. doi: 10.1093/jn/130.11.2630. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Bayle G, Prugnaud J, Pouyet C, Grizard J. Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-supplemented meal. J Nutr. 2002;132:95–100. doi: 10.1093/jn/132.1.95. [DOI] [PubMed] [Google Scholar]
- Guillet C, Prod’homme M, Balage M, Gachon P, Giraudet C, Morin L, et al. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004;18:1586–1587. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Horbach GJMJ, Van Bezooijen CFA. Hepatic protein metabolism and aging. In: Woodhouse KW, Yelland C, James OFW, editors. Liver: Metabolism and Ageing. Eurage (Rijswijk); 1989. pp. 95–116. [Google Scholar]
- Lang CH, Frost RA, Nairn AC, MacLean DA, Vary TC. TNF-α impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol Endocrinol Metab. 2002;282:E336–E347. doi: 10.1152/ajpendo.00366.2001. [DOI] [PubMed] [Google Scholar]
- McCarthy DO, Whitney P, Hitt A, Al-Majid S. Indomethacin and ibuprofen preserve gastrocnemius muscle mass in mice bearing the colon-26 adenocarcinoma. Res Nurs Health. 2004;27:174–184. doi: 10.1002/nur.20019. [DOI] [PubMed] [Google Scholar]
- Mayot G, Vidal K, Martin JF, Breuille D, Blum S, Obled C, et al. Prognostic values ofa2-macroglobulin, fibrinogen and albumin in regards to mortality and frailty in old rats. Exp Gerontol. 2007;42:498–505. doi: 10.1016/j.exger.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Mendez B, Ling PR, Istfan NW, Babayan VK, Bistrian BR. Effects of different lipid sources in total parenteral nutrition on whole body protein kinetics and tumor growth. JPEN J Parenter Enteral Nutr. 1992;16:545–551. doi: 10.1177/0148607192016006545. [DOI] [PubMed] [Google Scholar]
- Mosoni L, Valluy MC, Serrurier B, Prugnaud J, Obled C, Guezennec CY, Patureau Mirand P. Altered response of protein synthesis to nutritional state and endurance training in old rats. Am J Physiol Endocrinol Metab. 1995;268:E328–E335. doi: 10.1152/ajpendo.1995.268.2.E328. [DOI] [PubMed] [Google Scholar]
- Papet I, Dardevet D, Sornet C, Bechereau F, Prugnaud J, Pouyet C, Obled C. Acute phase protein levels and thymus, spleen and plasma protein synthesis rates differ in adult and old rats. J Nutr. 2003;133:215–219. doi: 10.1093/jn/133.1.215. [DOI] [PubMed] [Google Scholar]
- Perkins DJ, Kniss DA. Tumor necrosis factor-a promotes sustained cyclooxygenase-2 expression: attenuation by dexamethasone and NSAIDs. Prostaglandins. 1997;54:727–743. doi: 10.1016/s0090-6980(97)00144-5. [DOI] [PubMed] [Google Scholar]
- Rainsford KD. Discovery, mechanisms of action and safety of iIbuprofen. Int J Clin Pract. 2003;135:3–8. [PubMed] [Google Scholar]
- Reiter AK, Crozier SJ, Kimball SR, Jefferson LS. Meal feeding alters translational control of gene expression in rat liver. J Nutr. 2005;135:367–375. doi: 10.1093/jn/135.3.367. [DOI] [PubMed] [Google Scholar]
- Rieu I, Sornet C, Bayle G, Prugnaud J, Pouyet C, Balage M, et al. Leucine-supplemented meal feeding for ten days beneficially affects postprandial muscle protein synthesis in old rats. J Nutr. 2003;133:1198–1205. doi: 10.1093/jn/133.4.1198. [DOI] [PubMed] [Google Scholar]
- Ruot B, Papet I, Bechereau F, Denis P, Buffiere C, Gimonet J, et al. Increased albumin plasma efflux contributes to hypoalbuminemia only during early phase of sepsis in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R707–R713. doi: 10.1152/ajpregu.00483.2002. [DOI] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophyrelated ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SMF, Deeg DJH, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:U82–U90. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- Tisdale MJ. Inhibition of lipolysis and muscle protein degradation by EPA in cancer cachexia. Nutrition. 1996;12:S31–S33. doi: 10.1016/0899-9007(96)90015-5. [DOI] [PubMed] [Google Scholar]
- Tisdale MJ. The ubiquitin-proteasome pathway as a therapeutic target for muscle wasting. J Support Oncol. 2005;3:209–217. [PubMed] [Google Scholar]
- Toth MJ, Matthews DE, Tracy RP, Previs MJ. Age-related differences in skeletal muscle protein synthesis: relation to markers of immune activation. Am J Physiol Endocrinol Metab. 2005;288:E883–E891. doi: 10.1152/ajpendo.00353.2004. [DOI] [PubMed] [Google Scholar]
- Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: The health ABC study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- Voisin L, Breuillé D, Ruot B, Rallière C, Rambourdin F, Dalle M, et al. Cytokine modulation by PX differently affects specific acute phase proteins during sepsis in rats. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1412–R1419. doi: 10.1152/ajpregu.1998.275.5.R1412. [DOI] [PubMed] [Google Scholar]
- Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286:1206–1212. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AS, Smith HJ, Drake JL, Tisdale MJ. Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res. 2001;61:3604–3609. [PubMed] [Google Scholar]