Abstract
Caffeine is commonly used clinically to treat apnoeas and unstable breathing associated with premature birth. Caffeine antagonizes adenosine receptors and acts as an efficient respiratory stimulant in neonates. Owing to its persistent effects on adenosine receptor expression in the brain, neonatal caffeine administration also has significant effects on maturation of the respiratory control system. However, since adenosine receptors are critically involved in sleep regulation, and sleep also modulates breathing, we tested the hypothesis that neonatal caffeine treatment disrupts regulation of sleep and breathing in the adult rat. Neonatal caffeine treatment (15 mg kg−1 day−1) was administered from postnatal days 3–12. At adulthood (8–10 weeks old), sleep and breathing were measured with a telemetry system and whole-body plethysmography respectively. In adult rats treated with caffeine during the neonatal period, sleep time was reduced, sleep onset latency was increased, and non-rapid eye movement (non-REM) sleep was fragmented compared to controls. Ventilation at rest was higher in caffeine-treated adult rats compared to controls across sleep/wake states. Hypercapnic ventilatory responses were significantly reduced in caffeine-treated rats compared to control rats across sleep/wake states. Additional experiments in adult anaesthetized rats showed that at similar levels of arterial blood gases, phrenic nerve activity was enhanced in caffeine-treated rats. This study demonstrates that administration of caffeine in the neonatal period alters respiratory control system activity in awake and sleeping rats, as well as in the anaesthetized rats, and also has persistent disrupting effects on sleep that are apparent in adult rats.
Introduction
Apnoeas and unstable breathing are the leading causes of hospitalisation and morbidity in infants born prematurely. Caffeine is commonly administered clinically to treat apnoeas of prematurity (Bhatt-Mehta & Schumacher, 2003). Being an efficient and relatively safe respiratory stimulant, caffeine is often administered chronically (i.e. days to weeks) depending on the gestational age at birth (Hascoet et al. 2000). The long-term impact of this treatment on the newborn is under investigation (Finer et al. 2006) and a recent placebo-controlled study demonstrated that 2-year-old children previously treated with caffeine following premature birth have higher rates of survival and less neurodevelopmental deficits than newborns receiving placebo (Schmidt et al. 2007). These results are reassuring but the potential impact of perinatal caffeine treatment on central nervous system development is not known, especially at ages beyond early developmental stages in either human or animal models.
Sleep is strongly modulated by adenosine and its receptors (Basheer et al. 2004; Radulovacki, 1985) and acute caffeine, a non-specific adenosine receptor antagonist, promotes wakefulness in adults (Landolt et al. 1995; Huang et al. 2005) and in premature newborns (Hayes et al. 2007). In rats, chronic caffeine administration during the first week following birth augments adenosine receptor expression in the brain, including the thalamus, and this effect persists for several weeks and up to months after cessation of treatment (Etzel & Guillet, 1994). Because adenosine neuromodulation contributes to the regulation of sleep/wake behaviour (Basheer et al. 2004; Halassa et al. 2009), it is plausible that adenosine receptor overexpression due to neonatal caffeine treatment affects sleep/wake state regulation in the adult, although this has never been tested. Such a study is important given increasing evidence that abnormalities in sleep and its regulation are associated with significant morbidity and mortality.
We have previously shown in rats that caffeine administration during the immediate postnatal period modifies the performance of the respiratory control system measured later in life (for a review see Montandon et al. 2008b). For example, neonatal caffeine treatment (NCT; 15 mg kg−1 day−1) in rats from postnatal days 3–12 persistently decreases ventilatory sensitivity to adenosine receptor antagonists when measured in the same animals when juveniles (Montandon et al. 2007). NCT also produces a sex-specific increase in the hypoxic respiratory chemoreflex that is related to an enhancement in adenosine A2A receptor expression in the rat carotid body (Montandon et al. 2008a). These findings, obtained in awake and anaesthetized rats, are relevant because male sex and disruption of chemoreflexes are common hallmarks of sleep-disordered breathing in humans (Smith & Pacchia, 2007). However, given that sleep exerts significant effects on breathing and its variability (Phillipson & Bowes, 1986), and most breathing disorders are exacerbated by sleep (White, 2006), it is important to determine if caffeine treatment in the neonatal period has long-term effects on breathing and sleep in adult rats.
The present study tested the hypothesis that administration of caffeine during the neonatal period disrupts the regulation of sleep/wake states and the control of breathing when measured in the same rats as adults. Since the level of maturation of the central nervous system in newborn rats in the first weeks of life is similar to a premature newborn human between 20 and 40 weeks post-conception (Clancy et al. 2007), we administered caffeine everyday from postnatal day 3 to 12 to mimic as closely as possible in an animal model the exposure experienced by premature human infants. We then evaluated the long-term effects of neonatal caffeine treatment in the adult; a study otherwise not feasible in humans. Since hypercapnia strongly influences both sleep and breathing and is importantly involved in the protective arousal response to chemoreceptor stimulation (Phillipson & Bowes, 1986), we also evaluated NCT-induced effects on sleep and breathing during both room air breathing and hypercapnic respiratory challenge in these freely behaving rats. Additional studies were performed in anaesthetized rats to determine if the changes observed in freely behaving rats persisted under anaesthesia. Importantly, this preparation also allows control over arterial blood gases. Moreover, if differences in respiration between NCT and control rats persist in anaesthesia, this would provide further evidence that exposure to caffeine in the neonatal period produces long-term alterations in respiratory network activity that are not due to potential behavioural effects of caffeine treatment (e.g. heightened arousal or alertness) that may influence breathing in the non-anaesthetized state.
Methods
Neonatal caffeine treatment
The experimental protocols were approved by the Animal Care Committee at Laval University in accordance with the Canadian Council on Animal Care guidelines and complied with the policies of The Journal of Physiology (Drummond, 2009). Experiments were performed on 30 male Sprague–Dawley rats (Charles Rivers, St-Constant, Québec, Canada) maintained in standard conditions (21°C, 12: 12 h dark–light cycle; lights on at 07.00 h). Caffeine (15 mg kg−1) was administered orally each day from postnatal day 3 to 12 (caffeine citrate, Sabex, Boucherville, QC, Canada) in a volume of 0.05 ml (10 g body weight)−1 (neonatal caffeine treatment, NCT, as previously described in Montandon et al. 2006). This dose regimen yields plasma caffeine levels of ∼13 mg l−1 (Montandon et al. 2006) which correspond to levels measured in premature newborns subjected to caffeine treatment clinically (Leon et al. 2007). A control group received the same volume of vehicle (water, control group). To avoid litter-specific effects, NCT was administered to half the pups of each litter and vehicle to the remaining pups. Oral administration started at postnatal day 3 (to minimize damage to the oesophagus) and ended at postnatal day 12. This developmental window was selected because, during the first weeks of life, the CNS of rats goes through similar developmental stages as the premature infant between 20 and 40 weeks post-conception (Clancy et al. 2007). It is noteworthy that caffeine treatment in preterm babies rarely begins at birth and usually ends at post-conceptional age of 40 weeks (Hascoet et al 2000).
Study 1: Effects of NCT on sleep and breathing – freely behaving adult rats
Chronic instrumentation for recording sleep/wake states
At adulthood (8–10 weeks old), rats in both control and NCT groups (n= 8 each) were implanted under isoflurane anaesthesia (3–3.5% inspired isoflurane, 50% oxygen, balance N2) with a radio transmitter (model F50-EET, Data Sciences International, St-Paul, MN, USA) for continuous measurements of the electroencephalogram (EEG), neck electromyogram (neck EMG), and body temperature (Tbody) in freely behaving rats as described previously (Stephenson et al. 2001). Mid-line incisions were made in the scalp and the abdomen to expose the skull and peritoneal cavity, respectively. A transmitter was placed inside the peritoneum and sutured behind the internal wall of the cavity with non-absorbable 3-0 silk. The electrode leads were tunnelled subcutaneously from the abdominal cavity to the neck and the abdomen was closed and sutured with absorbable 3-0 vicryl. The rat was placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA, USA), the head was immobilized with blunt ear bars and a mask was placed over the snout for administration of isoflurane. Two telemetry electrodes were sutured to the dorsal neck muscle for neck EMG. Three holes were then drilled into the skull and two EEG wires and a reference electrode were attached with stainless steel screws (size 0–80 × 1/16, Plastic One Inc., Roanoake, VA, USA). The EEG electrodes were placed approximately 2 mm to the right and 2 mm anterior to bregma, and 2 mm to the left and 3 mm posterior to bregma. The reference electrode was placed 3 mm to the left and 3 mm anterior to bregma. Dental acrylic was used to affix electrodes and screws to the skull. The skin incision was then closed with 3-0 vicryl suture. Post-surgical care consisted of three subcutaneous injections of anti-inflammatory drug (ketoprofen, 2 mg kg−1), immediately after surgery and at 24 h and 48 h post-operatively.
Rats recovered from surgery for at least 1 week before measurements of sleep and breathing were performed. On the day of the experiment, the animal was placed in the plethysmographic chamber at 09.00 h for 1 h of acclimation and room air (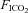 = 0,
= 0, 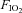 = 0.21, balance N2) was delivered to the chamber at a flow rate of 2 l min−1. At 10.00 h, the EEG, neck EMG, Tbody and breathing signals were recorded simultaneously for 3 h. At 13.00 h, a hypercapnic gas mixture (
= 0.21, balance N2) was delivered to the chamber at a flow rate of 2 l min−1. At 10.00 h, the EEG, neck EMG, Tbody and breathing signals were recorded simultaneously for 3 h. At 13.00 h, a hypercapnic gas mixture (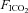 = 0.05,
= 0.05, 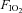 = 0.2, balance N2) was delivered to the chamber for 1 h. Signals were digitized and recorded at 1 kHz with a data acquisition system (WinDAQ, DATAQ Instruments Inc, Akron, OH, USA). Breathing was measured by whole body plethysmography according to standard methods (Montandon et al. 2006).
= 0.2, balance N2) was delivered to the chamber for 1 h. Signals were digitized and recorded at 1 kHz with a data acquisition system (WinDAQ, DATAQ Instruments Inc, Akron, OH, USA). Breathing was measured by whole body plethysmography according to standard methods (Montandon et al. 2006).
Determination of sleep/wake states
EEG and neck EMG recordings (Fig. 1) were analysed visually in 20 s epochs to determine sleep/wake states according to standard criteria for rats (Morrison et al. 2003). Mean integrated EMG amplitude and EEG frequencies in the following frequency bands: δ2 (0.5–2 Hz), δ1 (2–4 Hz), θ (4–7.5 Hz), α (7.5–13.5 Hz), β1 (13.5–20 Hz), β2 (20–30 Hz). They were analyzed using customized scripts under Matlab 6 (The Mathworks Inc., Natick, MA, USA) to determine levels of motor activity and depth of sleep, respectively (Fig. 2). Ratio of high to low frequencies (i.e. the β2/δ1 ratio) was also calculated for each epoch as an index of relative EEG activation (Hamrahi et al. 2001). The percentage of time spent in each sleep/wake state, i.e. wakefulness, non rapid-eye-movement (non-REM) sleep, and rapid-eye-movement (REM) sleep, was also calculated. Sleep onset latencies were defined as the length of time for the rat to fall asleep after at least 1 min of wakefulness. The number of episodes of each sleep/wake state was also calculated for each hour. Mean duration of sleep episodes were also calculated for each sleep/wake state.
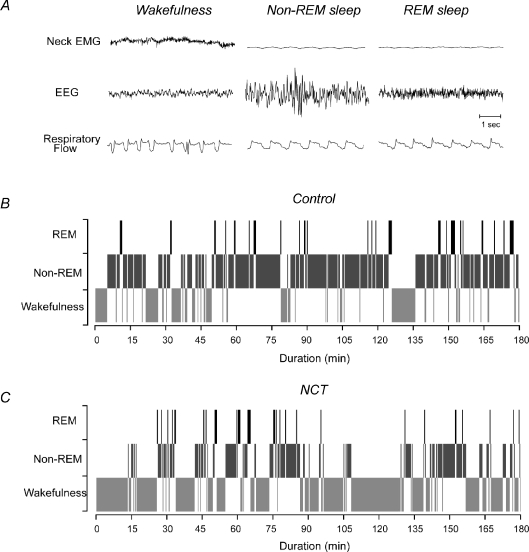
Figure 1. Respiratory activity and sleep/wake pattern in the freely behaving adult rat.
Representative tracings for neck EMG, EEG and respiratory flow in a control rat during room air breathing (A). Distribution of sleep/wake pattern in a control (B) and a NCT rat (C) recorded for 3 h. In B and C, 20 s periods of wakefulness, non-REM sleep and REM sleep are shown by light grey, dark grey and black shading.
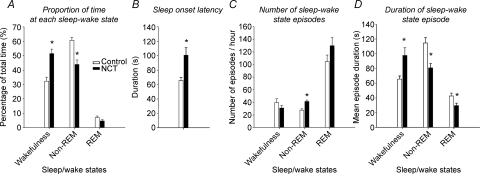
Figure 2. Proportion of time spent in each sleep/wake state (A), sleep onset latency (B), number of sleep episodes (C), and duration of sleep episodes (D) during room air breathing in control (n= 8) and NCT (n= 8) rats.
Note the increase in wakefulness and decrease in non-REM sleep in NCT rats compared to control, mainly due to decrease in duration of non-REM sleep episodes. In addition, NCT increased sleep onset latency and increased the number of non-REM sleep episodes, indicating sleep fragmentation given the reduced non-REM sleep time. Data are shown as means ±s.e.m.*P < 0.05 compared to control.
Ventilatory recordings
Respiratory frequency (fR), tidal volume (VT), and minute ventilation (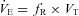 ) were calculated according to standard methods (Montandon et al. 2006; Drorbaugh & Fenn, 1955) using customized scripts under Matlab 6 and were averaged for each 20 s epoch corresponding to the prevailing sleep/wake state periods. Barometric pressure, flow rate, chamber temperature and humidity were also measured to express VT (BTPS) in ml. VT and
) were calculated according to standard methods (Montandon et al. 2006; Drorbaugh & Fenn, 1955) using customized scripts under Matlab 6 and were averaged for each 20 s epoch corresponding to the prevailing sleep/wake state periods. Barometric pressure, flow rate, chamber temperature and humidity were also measured to express VT (BTPS) in ml. VT and 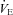 were normalized according to body weight.
were normalized according to body weight.
Study 2: Effects of NCT on respiratory control mechanisms – anaesthetized adult rat
Animal preparation
The effects of NCT on respiratory control were assessed in anaesthetized, vagotomised, neuromuscularly blocked, and artificially ventilated adult rats as previously described (Montandon et al. 2008a). Accordingly, phrenic nerve activity was recorded in adult (8–12 weeks old) rats (control: n= 7; NCT: n= 7). Anaesthesia was first induced with isoflurane (3% of isoflurane, 50% oxygen, balance N2), body temperature was maintained between 37°C and 38°C, and venous and arterial femoral catheters were inserted. The trachea was cannulated and the rat was artificially ventilated with the same gas mixture. The phrenic nerve was isolated unilaterally and the vagus nerves were cut bilaterally. Once surgical interventions were completed, the transition from isoflurane to urethane anaesthesia began by slowly injecting urethane intravenously and decreasing isoflurane progressively. The final urethane dose was 1.6 g kg−1 (i.v. in distilled water). The transition from isoflurane to urethane anaesthesia took approximately 20 min; at the end of the transition, the rat was neuromuscularly blocked with pancuronium bromide (2.5 mg kg−1, i.v.). Appropriate level of analgesia was evaluated throughout the transition by monitoring paw withdrawal (before pancuronium) and every 10 min during the entire experiment by evaluating cardiovascular and respiratory responses to toe pinch. If necessary, supplemental doses of urethane were administered (0.2 g kg−1i.v.).
Recordings
Phrenic nerve activity was recorded with a bipolar silver recording electrode and was amplified (AM-Systems, Everett, WA, USA), band-pass filtered (100 Hz to 10 kHz), and fed to a moving averager (model MA-821, CWE Inc., Ardmore, PA, USA) before being digitized and recorded at 2 kHz with a data acquisition system (IOX, EMKA Technologies, Falls Church, VA, USA). Blood pressure was also continuously recorded at a sample rate of 2 kHz for subsequent analysis and blood samples were analysed for arterial CO2 pressure (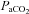 ), arterial O2 pressure (
), arterial O2 pressure (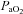 ), and pH (model ABL-5, Radiometer Copenhagen, Brønshøj, Denmark). In these experiments, phrenic activity, blood gases, blood pressure and heart rate were evaluated under baseline conditions (
), and pH (model ABL-5, Radiometer Copenhagen, Brønshøj, Denmark). In these experiments, phrenic activity, blood gases, blood pressure and heart rate were evaluated under baseline conditions (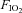 = 0.5) and during hypercapnia. After a stabilization period of 60 min under hyperoxic conditions, the CO2 apnoeic threshold for inspiratory (phrenic) activity was determined by mechanically hyperventilating the rats until phrenic nerve activity ceased. A blood sample was then taken to establish
= 0.5) and during hypercapnia. After a stabilization period of 60 min under hyperoxic conditions, the CO2 apnoeic threshold for inspiratory (phrenic) activity was determined by mechanically hyperventilating the rats until phrenic nerve activity ceased. A blood sample was then taken to establish 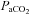 at apnoeic threshold (CO2 apnoeic threshold). The rate of the ventilator was then decreased progressively until phrenic activity returned and
at apnoeic threshold (CO2 apnoeic threshold). The rate of the ventilator was then decreased progressively until phrenic activity returned and 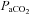 was set 2–3 mmHg above
was set 2–3 mmHg above 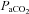 at apnoeic threshold. This defined baseline phrenic activity and was recorded for 4 min. Then,
at apnoeic threshold. This defined baseline phrenic activity and was recorded for 4 min. Then, 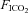 in the gas mixture was slowly increased to reach a
in the gas mixture was slowly increased to reach a 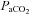 5 mmHg and 10 mmHg above
5 mmHg and 10 mmHg above 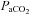 measured at baseline. Increasing arterial CO2 by 10 mmHg above baseline roughly corresponds to the level of stimulation achieved by exposing awake rats to 5% CO2 (Kinkead et al. 2001). At the end of the protocol,
measured at baseline. Increasing arterial CO2 by 10 mmHg above baseline roughly corresponds to the level of stimulation achieved by exposing awake rats to 5% CO2 (Kinkead et al. 2001). At the end of the protocol, 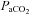 was increased by more than 30 mmHg to produce maximal phrenic nerve activity. Mean ‘steady-state’ values for peak integrated phrenic nerve activity, phrenic burst frequency, mean arterial blood pressure, and heart rate were obtained by averaging data over a 4 min period for each CO2 level. Absolute (non-normalized) values of phrenic nerve activity are presented to show the effects of NCT on baseline respiratory activity. Since the activity at baseline was different between groups, hypercapnic data (responses) are also expressed as a percentage from baseline, as well as a percentage of maximal activity. Killing was performed by overdose with urethane.
was increased by more than 30 mmHg to produce maximal phrenic nerve activity. Mean ‘steady-state’ values for peak integrated phrenic nerve activity, phrenic burst frequency, mean arterial blood pressure, and heart rate were obtained by averaging data over a 4 min period for each CO2 level. Absolute (non-normalized) values of phrenic nerve activity are presented to show the effects of NCT on baseline respiratory activity. Since the activity at baseline was different between groups, hypercapnic data (responses) are also expressed as a percentage from baseline, as well as a percentage of maximal activity. Killing was performed by overdose with urethane.
Statistical analysis
In Study 1, to determine the sleep-dependant effect of NCT on breathing and sleep during room air breathing, statistics were performed using two-way analysis of variance with repeated measures (ANOVA-RM, treatments × states, with states as the repeated factor) and post hoc analyses were realized with Student's t tests when appropriate (JMP 7, SAS Institute Inc., Cary, NC, USA). To test the sleep-dependant effect of NCT on responses to hypercapnia, three-way ANOVA-RMs were also performed (treatments × states × hypercapnia, with states and hypercapnia as repeated factors) and two-way ANOVA-RMs were subsequently performed to determine the effect of NCT on responses to hypercapnia at each sleep/wake state. In Study 2, one-way ANOVA were performed to determine the effect of NCT under baseline conditions, and two-way ANOVA to determine the effect of NCT on hypercapnic responses followed by Student's t tests when appropriate. P values in the text indicate post hoc results, unless otherwise mentioned. Data were considered significantly different when P < 0.05.
Results
Study 1: Effects of NCT on sleep and breathing – freely behaving adult rats
Sleep/wake states
NCT reduces non-REM sleep. To test whether exposure to caffeine during the neonatal period altered sleep patterns in adult rats, we measured EEG and neck EMG activities during room air breathing (Fig. 1A). Sleep recordings during the 3 h sessions showed that freely behaving NCT rats spent more time in wakefulness and less time in non-REM sleep compared to control rats (Fig. 1B–C). Group data in Fig. 2 confirmed that NCT alters sleep time (P < 0.0001, two-way ANOVA-RM) by significantly decreasing time spent in non-REM sleep (from 61 ± 2% to 44 ± 3%, P= 0.001) and increasing time spent in wakefulness (from 32 ± 3% to 51 ± 3%, P= 0.0007), with no effects on REM sleep (from 7 ± 1% to 5 ± 1%, P= 0.091). In addition to the decreased non-REM sleep, NCT significantly increased sleep onset latency (mean = 52%, P= 0.006, Fig. 2B). Despite the overall reduced non-REM sleep, NCT significantly increased the number of episodes of non-REM sleep by 50% (P= 0.007, Fig. 2C), indicating a sleep disrupting effect of NCT producing more non-REM sleep episodes. The number of episodes of REM sleep was not affected by NCT (P= 0.15, Fig. 2C). NCT altered episode duration (P < 0.0001, two-way ANOVA-RM, Fig. 2D) by significantly increasing mean duration of wakefulness episodes (mean = 53%, P= 0.009), decreasing mean duration of non-REM sleep episodes (mean = 29%, P= 0.0036), and reducing duration of REM sleep episodes (mean = 31%, P= 0.028).
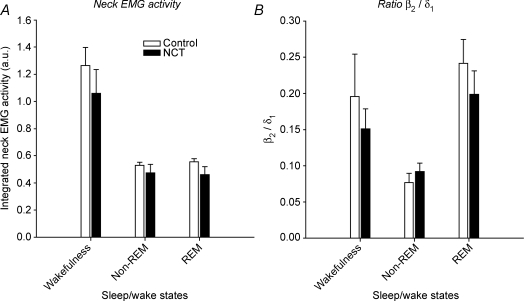
To determine whether NCT altered the depth of sleep in adult rats, further analyses of the neck EMG and EEG frequencies were performed. Although integrated neck EMG activity decreased from wakefulness to non-REM (P= 0.0003, Fig. 3A) and to REM sleep (P= 0.0004), the magnitude of these changes did not differ between control and NCT rats (P= 0.72, two-way ANOVA-RM, Fig. 3A). The relative level of EEG activation assessed as the ratio β2/δ1 decreased in non-REM sleep compared to wakefulness (P= 0.007, Fig. 3B), although this effect was similar in control and NCT rats (P= 0.60, two-way ANOVA-RM, Fig. 3B).
Figure 3. Integrated neck EMG activity (A) and the ratio of β2 (20–30 Hz) to δ1 (2–4 Hz) EEG frequencies (B) in control (open bars) and neonatal caffeine treated (NCT, filled bars) rats for each sleep/wake state.
Note the similar changes in neck muscle activity and β2/δ1 ratio across sleep/wake states in control and NCT rats. Values are shown as means ±s.e.m.
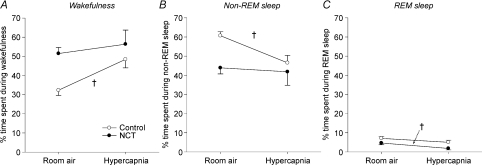
NCT decreases the sleep-disrupting effects of hypercapnia on sleep. We evaluated the effect of hypercapnia, an arousing as well as a ventilatory stimulus, on sleep patterns. In control rats, hypercapnia significantly increased the proportion of time spent in wakefulness (from 32 ± 3% to 48 ± 4%, P= 0.007, Fig. 4A) and significantly decreased the time spent in non-REM sleep (from 61 ± 2% to 47 ± 4%, P= 0.005, Fig. 4B). There was no effect of hypercapnia on the proportion of time spent during REM sleep in control rats (from 7 ± 1% to 5 ± 1%, P= 0.20, Fig. 4C). Importantly, comparison between control and NCT rats showed that NCT blunted the disrupting effect of hypercapnia on sleep/wake activity (P= 0.039, three-way ANOVA-RM, treatments × states × hypercapnia). Indeed, NCT per se was such a powerful disruptor of sleep by itself that application of hypercapnia had no additional effects on wakefulness (P= 0.55, two-way ANOVA-RM) and non-REM sleep (P= 0.80, two-way ANOVA-RM), despite a slight decrease of time spent during REM sleep in NCT rats (from 5 ± 1% to 2 ± 1%, P= 0.001, two-way ANOVA-RM). Moreover, the level of sleep disruption produced by NCT per se during room air breathing was equivalent to the level produced in control rats by hypercapnia (Fig. 4A–C).
Figure 4. Changes in proportion of time spent in each sleep/wake states between room air and hypercapnia in control (n= 8) and NCT rats (n= 8).
Data are shown as means ±s.e.m.†Significant changes due to hypercapnia (P < 0.05) in control or NCT rats.
Breathing across sleep/wake states
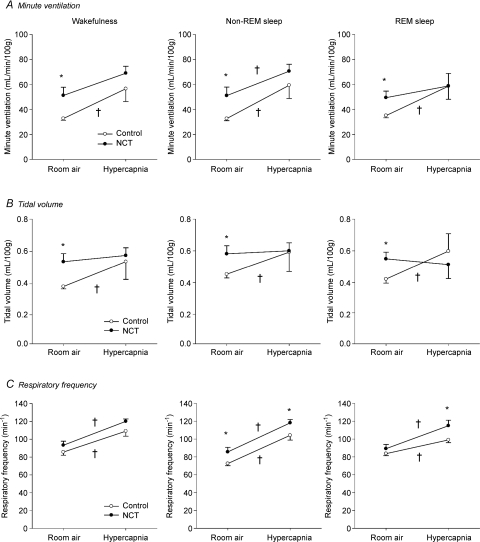
NCT increases minute ventilation during room air breathing. We determined whether NCT alters ‘normal’ breathing across sleep/wake states by measuring ventilation with whole-body plethysmography at each sleep/wake state during room air breathing. NCT rats significantly increased minute ventilation compared to controls (Fig. 5A) during wakefulness (mean increase = 57%, P= 0.015), non-REM sleep (mean increase = 56%, P= 0.021), and REM sleep (mean increase = 41%, P= 0.021), an effect not dependant on the prevailing sleep/wake state (P= 0.874, two-way ANOVA-RM). The higher ventilation of NCT rats was mainly due to larger tidal volumes (mean increases of 40%, 28%, and 30%; P= 0.010, P= 0.037, and P= 0.022; for wakefulness, non-REM, and REM sleep respectively; Fig. 5B), again not dependant on the prevailing state (P= 0.92, two-way ANOVA-RM). Tidal volume measured during transitions between states was not significantly different in NCT rats compared to controls (P= 0.45, Table 1). Tidal volume measurements are corrected with the equation provided by Drorbaugh & Fenn (1955) which uses humidity, barometric pressure, and body and chamber temperatures (Table 1). Note that chamber temperature was higher in NCT rats (mean increase = 1.8°C, P= 0.044) compared to controls, whereas the other parameters were not affect by NCT. When tidal volumes were not normalized according to body weight, volume changes due to NCT were lower (mean increases of 27%, 18% and 20%; P= 0.039, P= 0.083 and P= 0.049; for wakefulness, non-REM and REM sleep, respectively), again not dependant on the prevailing state (P= 0.91, two-way ANOVA-RM). Although NCT did not change significantly body weight (mean body weights in controls 597 ± 29 g and in NCT 556 ± 29 g, P= 0.33), it slightly decreased it in some animals which contributed to further increase tidal volume in NCT rats. Respiratory frequency was not different between control and NCT rats during wakefulness (P= 0.20) and REM sleep (P= 0.31), but it was significantly higher during non-REM sleep (increase of 18%, P= 0.039, Fig. 5C).
Figure 5. Minute ventilation (A), tidal volume (B), and respiratory frequency (C) for each sleep/wake state during room air and hypercapnic breathing in control (open circles) and NCT (filled circles) rats.
Data are shown as means ±s.e.m.†Significant changes due to hypercapnia (P < 0.05) in control or NCT rats. *Significant differences in mean values between control and NCT rats (P < 0.05).
Table 1.
Environmental variables and tidal volumes in control and NCT freely behaving rats at different sleep/wake states: wakefulness, non-REM sleep, REM sleep and transitions between states
| Control rats (n= 8) |
NCT rats (n= 8) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Wake | Non-REM | REM | Transitions | Wake | Non-REM | REM | Transitions | |
| Body Weight (g) | 597 ± 27 | 556 ± 31 | ||||||
| Patm (mmHg) | 759 ± 1 | 759 ± 1 | 759 ± 1 | 759 ± 1 | 755 ± 2 | 755 ± 2 | 755 ± 2 | 755 ± 2 |
| Tbody (°C) | 36.6 ± 0.2 | 36.5 ± 0.2 | 36.5 ± 0.2 | 36.8 ± 0.3 | 36.9 ± 0.2 | 36.8 ± 0.2 | 36.7 ± 0.2 | 36.7 ± 0.2 |
| Tchamber (°C) | 24.0 ± 0.6 | 24.0 ± 0.6 | 24.0 ± 0.6 | 24.0 ± 0.6 | 25.8 ± 0.5* | 25.8 ± 0.5* | 25.8 ± 0.5* | 25.8 ± 0.5* |
| Humidity (%) | 82 ± 9 | 82 ± 9 | 82 ± 9 | 82 ± 9 | 74 ± 11 | 74 ± 11 | 74 ± 11 | 74 ± 11 |
| VT (ml) | 2.3 ± 0.2 | 2.7 ± 0.1 | 2.5 ± 0.1 | 2.6 ± 0.8 | 2.9 ± 0.2* | 3.2 ± 0.2* | 3.0 ± 0.2* | 2.8 ± 0.5 |
| VT/100 g (ml (100 g)−1) | 0.38 ± 0.01 | 0.45 ± 0.02 | 0.42 ± 0.03 | 0.48 ± 0.13 | 0.54 ± 0.05* | 0.58 ± 0.05* | 0.55 ± 0.04* | 0.53 ± 0.06 |
These variables are used to calculate tidal volume according to Drorbaugh & Fenn (1955). Patm, atmospheric pressure; Tbody, body temperature; Tchamber, chamber temperature; Humidity, humidity inside the chamber; VT, tidal volume; VT/100 g, normalized tidal volume per 100 g. Values are expressed as means ±s.e.m.
Significantly different from respective control value with P < 0.05.
NCT decreases the effects of hypercapnia on minute ventilation. In control rats, hypercapnia increased minute ventilation by 98%, 103% and 91% during wakefulness, non-REM sleep and REM sleep, respectively (P= 0.037, P= 0.026 and P= 0.045, respectively, Fig. 5A) with the increases in ventilation being similar across sleep/wake states (P= 0.74, two-way ANOVA-RM). The enhancements of minute ventilation observed in control rats were due to significant increases in tidal volumes at all sleep/wake states (Fig. 5B), with these stimulating effects also not depending upon the prevailing sleep/wake state (P= 0.54), i.e. they occurred across all sleep/wake states. Respiratory frequency also contributed to the significant enhancement of minute ventilation during non-REM sleep, but not during wakefulness or REM sleep (Fig. 5C). In NCT rats, however, the CO2-mediated enhancements of minute ventilation were reduced compared to control rats (Fig. 5A). Indeed, comparison between control and NCT rats showed that NCT blunted the stimulating effect of hypercapnia on minute ventilation at each sleep/wake state (P= 0.037, P= 0.025 and P= 0.024 for wakefulness, non-REM and REM sleep, respectively, two-way ANOVA-RM), with these effects being similar across sleep/wake states (P= 0.96, three-way ANOVA, treatments × states × hypercapnia). In fact, NCT per se was such a powerful activator of ventilation by itself that subsequent addition of hypercapnia produces a lesser increase in ventilation, and little change in tidal volume compared to controls. Indeed the level of tidal volume produced by NCT per se during room air breathing was equivalent to that produced in control rats by hypercapnia (Fig. 5B). The stimulatory effects of hypercapnia on tidal volume were significantly reduced in NCT rats compared to control rats during non-REM and REM sleep (P= 0.030 and P= 0.011, respectively, two-way ANOVA-RM, Fig. 5B) with these effects being similar across sleep/wake states (P= 0.85, three-way ANOVA, treatments × states × hypercapnia). The increases in respiratory frequency in response to hypercapnia were similar between control and NCT rats at each sleep/wake state (P= 0.85, P= 0.77 and P= 0.09, for wakefulness, non-REM and REM sleep, respectively, two-way ANOVA-RM, Fig. 5C).
Study 2: Effects of NCT on respiratory control mechanisms – anaesthetized adult rat
Additional studies were performed in anaesthetized rats to determine if the changes observed in freely behaving rats persisted under anaesthesia, this latter preparation allowing for control over arterial blood gases. Rats were vagotomised and artificially ventilated to control arterial blood gases. They breathed a hyperoxic (50% of oxygen) gas mixture to reduce the contribution of peripheral chemoreceptors since NCT alters the hypoxic respiratory response of anaesthetized and freely behaving adult rats (Montandon et al. 2008a).
Baseline activity
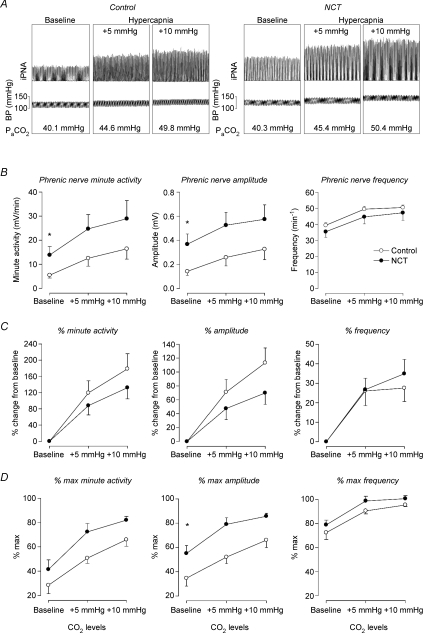
To assess effects of NCT on phrenic nerve activity under baseline conditions, we first compared absolute values in control and NCT rats (Fig. 6A). Minute phrenic nerve activity (i.e. the product of phrenic nerve amplitude and frequency) was significantly higher in anaesthetized NCT rats than in control rats at baseline (mean increase = 153%, P= 0.044, Fig. 6B). This increase in minute activity was mainly due to higher mean phrenic nerve amplitude in the NCT rats compared to the controls (mean increase = 158%, P= 0.030, Fig. 6B). Mean phrenic nerve frequency was not different between control and NCT rats at baseline (P= 0.154, Fig. 6B). During baseline, phrenic nerve amplitude normalized with maximal phrenic nerve amplitude was also higher in NCT compared to control rats (P= 0.014, Fig. 6D). These observations are in agreement with the increased minute ventilation and tidal volume observed in freely behaving conscious NCT rats compared to controls (Fig. 5A–B).
Figure 6. Phrenic nerve activity in the anaesthetized adult rat.
A, representative integrated phrenic nerve activity and arterial blood pressure of control and NCT rats at different CO2 levels (i.e. baseline, +5, and +10 mmHg above baseline). B, mean absolute values of integrated phrenic nerve minute activity, phrenic nerve amplitude, and phrenic nerve frequency at baseline, +5 and +10 mmHg in control (open circles, n= 7) and NCT (neonatal caffeine treatment, filled circles, n= 7) rats. C, percentage changes compared to baseline in response to hypercapnia of phrenic nerve minute activity, amplitude, and frequency. D, percentage of CO2 maximal phrenic activity for minute activity, amplitude and frequency. Values are expressed as means ±s.e.m.*Mean values significantly (P < 0.05) different from controls. iPNA, integrated phrenic nerve activity. BP, blood pressure.
Hypercapnia
For these additional studies in control and NCT rats, we also measured phrenic nerve activity at different CO2 levels. Application of CO2 increased minute phrenic nerve activity (P= 0.007, one-way ANOVA with hypercapnia as repeated factor) by an effect on phrenic amplitude (P= 0.037, one-way ANOVA) and phrenic nerve frequency (P= 0.012, one-way ANOVA) in control rats (Fig. 6B). Comparison between control and NCT rats shows that NCT did not affect the responses to hypercapnia in absolute (Fig. 6B) and percentage baseline (Fig. 6C), or percentage maximal (Fig. 6D) values (P > 0.93, two-way ANOVAs), despite a non-significant tendency to reduce the phrenic nerve amplitude response to hypercapnia (Fig. 6C).
Haemodynamic parameters
Table 2 shows that there was no change in blood gas variables between control and NCT rats at apnoea threshold, baseline, +5 and +10 mmHg of CO2 (P > 0.286, P > 0.093, P > 0.152 and P > 0.262, respectively). Mean arterial blood pressures were similar at baseline, +5 and +10 mmHg of CO2 in control and NCT rats (P= 0.947, P= 0.466 and P= 0.246, respectively). Heart rates were also similar in both groups at baseline (P= 0.96) and +5 mmHg (P= 0.13), whereas at +10 mmHg it was higher in NCT rats than in controls (P= 0.028). Two-way ANOVAs demonstrated that NCT had no effect on the cardiovascular responses to hypercapnia (P > 0.43). Body weight did not differ between control and NCT adult rats.
Table 2.
Arterial blood gases, pH, mean arterial blood pressure, heart rate during apnoea, baseline, +5 mmHg and +10 mmHg of CO2 in control and NCT anaesthetized adult male rats
| Control rats (n= 7) |
NCT rats (n= 7) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Apnoea† | Baseline | +5 mmHg | +10 mmHg | Apnoea† | Baseline | +5 mmHg | +10 mmHg | |
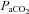 (mmHg) (mmHg) |
36.1 ± 2.2 | 39.6 ± 1.8 | 44.6 ± 1.9 | 49.3 ± 2.0 | 36.8 ± 2.1 | 39.7 ± 2.2 | 45.5 ± 1.8 | 50.3 ± 1.5 |
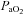 (mmHg) (mmHg) |
181 ± 13 | 173 ± 9 | 165 ± 9 | 170 ± 13 | 203 ± 15 | 204 ± 14 | 193 ± 16 | 193 ± 14 |
| pH | 7.38 ± 0.01 | 7.36 ± 0.01 | 7.32 ± 0.01 | 7.30 ± 0.01 | 7.39 ± 0.02 | 7.37 ± 0.02 | 7.33 ± 0.01 | 7.29 ± 0.01 |
| MAP (mmHg) | — | 106 ± 7 | 112 ± 6 | 112 ± 6 | — | 107 ± 13 | 118 ± 6 | 124 ± 8 |
| Heart rate (min−1) | — | 367 ± 30 | 373 ± 31 | 360 ± 25 | — | 364 ± 39 | 431 ± 10 | 433 ± 11* |
| Body weight (g) | 620 ± 19 | 613 ± 20 | ||||||
Arterial blood gases in this column correspond to values at apnoeic threshold when phrenic nerve activity ceases. 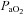 , arterial oxygen pressure;
, arterial oxygen pressure; 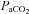 , arterial carbon dioxide pressure; MAP, mean arterial blood pressure. Values are expressed as means ±s.e.m.
, arterial carbon dioxide pressure; MAP, mean arterial blood pressure. Values are expressed as means ±s.e.m.
Significantly different from respective control value with P < 0.05.
Discussion
The present study determined the long-term impact of chronic caffeine administration (10 consecutive days, one per day) during a sensitive period of development in the neonate on sleep and respiratory phenotypes subsequently measured in the same animals as adults. Results obtained using telemetry in freely behaving rats showed that, during room air breathing, NCT rats spent more time in wakefulness, spent less time in non-REM sleep, have longer sleep onset latencies, have shorter sleep episodes, and have a more fragmented sleep compared to controls. To put the magnitude of this effect into perspective, the level of sleep disruption observed in the NCT rats was similar to that observed in control rats undergoing stimulation with 5% of CO2. Assessment of respiratory activity during sleep by whole-body plethysmography also showed that the basal ventilation of NCT rats was significantly higher than controls across all sleep/wake states, owing mainly to an increase in tidal volume. Again, to put the magnitude of the effect into perspective, the level of increased ventilation and tidal volume in the NCT rats was similar to that observed in control rats in the presence of 5% CO2. These effects of NCT on breathing persisted under anaesthesia and were not secondary to blood gas changes since NCT increased ‘neural ventilation’ by a similar effect on phrenic inspiratory motor activity. However, ventilatory measurements performed during hypercapnia showed that NCT reduced the ventilatory response to hypercapnia in all sleep/wake states, an effect not observed under anaesthesia. Overall, these results are consistent with the hypothesis that administration of caffeine during the neonatal period disrupts regulation of sleep and awake states and the control of breathing in the adult. Although thorough understanding of the functional and clinical impact of these results is beyond the scope of the present study, our results nonetheless raise important questions given that a large population of premature newborns receive caffeine for several weeks to months to treat apnoeas of prematurity, and that the long-term outcomes of this treatment are still unknown (Finer et al. 2006).
NCT reduces sleep time and increases wakefulness
Room air
Control rats spent at least 60% of the time in non-REM sleep and 6% in REM sleep, values that correspond to other studies using the same strain of rat (Lu et al. 2000). In NCT rats, however, sleep time was reduced, mainly because of reduced overall amount of non-REM sleep, and increased wakefulness. Non-REM sleep in NCT rats was also more fragmented compared to control rats. Despite these changes in sleep/wake pattern, the depth of non-REM sleep was not overly affected by NCT, at least indicated by the ratio of high to low frequencies in the EEG signal and by neck EMG activity. Interestingly, comparable effects have been observed in premature newborn humans that received theophylline, an adenosine receptor antagonist similar to caffeine, as a treatment for apnoea of prematurity. One month after chronic theophylline treatment, these newborns present less quiet sleep than non-treated controls (Thoman et al. 1985). Although the latter study did not measure sleep in adults but only in infants, the result nonetheless demonstrates that chronic treatment with an adenosine receptor antagonist can induce changes in sleep regulation that persist well beyond cessation of treatment. In mice, however, opposite long-term alterations of sleep have been observed following chronic caffeine administration during gestation (Sinton et al. 1981). The fact that the caffeine treatment was performed during gestation and that the plasma concentration of caffeine might be different between fetus and newborn pup directly exposed to caffeine makes it difficult to compare results between the two studies. The mechanisms responsible for the effects of NCT on long term sleep regulation are unknown. However, since adenosine plays a pivotal role in sleep/wake regulation (Basheer et al. 2004) and chronic caffeine administration during early life enhances adenosine A1 receptor expression in various brain structures, such as the thalamus (Etzel & Guillet, 1994), and modifies the roles of adenosine A1 and A2A receptors in regulating breathing in the juvenile rats (Montandon et al. 2007), it is likely that these results reflect NCT-related disruption of adenosinergic neurotransmission within central nervous system regions that play a key role in sleep regulation.
Hypercapnia
In control rats, hypercapnia decreased time spent in non-REM sleep and fragmented non-REM sleep as evidenced by more non-REM sleep episodes. This effect is expected given that hypercapnia is an arousing stimulus as well as a ventilatory stimulus (Horner et al. 2002). In NCT rats, however, hypercapnia did not affect the proportion of time spent in each sleep/wake state as sleep was already disrupted to a similar degree by NCT alone. Overall, therefore, hypercapnia had no cumulative adverse effects on non-REM sleep, but it almost abolished REM sleep.
Breathing during sleep in NCT rats
Critique of methods
Resting ventilation was larger in NCT than in control animals across sleep/wake states, an effect mainly related to a greater tidal volume, except during non-REM sleep where NCT rats present higher respiratory frequency. This result was unanticipated since such a difference was not observed in our previous studies performed on ‘awake’ adult animals, at least as judged by visual assessment of arousal state (Bairam et al. 2008; Montandon et al. 2006, 2008a). Moreover, tidal volume measurements reported here were generally lower than those reported in our previous studies (Montandon et al. 2006, 2008a). Accuracy of tidal volume measurements is the subject of much debate (Enhorning et al. 1998), yet this method remains the best non-invasive method to measure breathing in unrestrained animals (Mortola & Frappell, 1998). Although experiments were performed by the same investigator, other factors may help explain the differences in tidal volume data between studies. In addition to the fact that the software used for data acquisition and analysis differed between studies, it is conceivable that placing a larger telemetry probe used for monitoring sleep and temperature (5 ml; present study) inside the rat's abdomen affected breathing more than the smaller probe used to monitor temperature alone (1.2 ml; previous studies). While these factors may explain (at least in part) the differences between studies, their contribution to differences between control and NCT rats mentioned previously is likely to be minimal.
Proper assessment of sleep/wakes states by electroencephalographic criteria in the present study made it possible to eliminate ventilatory recordings during sleep/wake state transitions (Table 1), which helped reduce data variability, and ventilation was recorded over a 3 h period, which allowed for more reliable and stable recordings than the short periods in previous studies. However, long periods of ventilatory recordings, combined with the fact that NCT rats spent more time awake when they are highly active (as shown in Fig. 3), is likely to have produced more heat than in control rats, which may explain the increased chamber temperature observed in NCT rats. According to the equation provided by Drorbaugh & Fenn (1955) to correct tidal volume measurements, an increased chamber temperature augments the calculated tidal volume. For instance, an increase of 1.8°C in chamber temperature enhances tidal volume by approximately 10% (Mortola & Frappell, 1998) explaining in part the magnitude of the increase in tidal volume observed in NCT rats. While these differences highlight the importance of assessing sleep/wake states and respiratory behaviour in conscious animals using standardized electrophysiological criteria over long periods, they also reveal the importance of environmental monitoring when rats are kept inside the plethysmographic chamber for a long period of time. But more importantly, they highlight the value of complementary approaches (e.g. electrophysiology) in interpreting tidal volume data. While it is difficult to accurately determine the extent to which the larger tidal volume of NCT rats is due to an overestimation or other potential factors such as changes in airway resistance due to NCT (Bellingham & Berger, 1994), results from phrenic nerve recording tend to support plethysmography data and indicate that the higher resting ventilation in NCT rats is related to a higher diaphragm motor output.
Increased ventilation in NCT rats
The elevated ventilation measured in the NCT rats, compared to the controls, may result in changes in 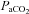 and
and 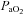 . However, previous measurements of
. However, previous measurements of 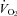 and
and 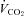 showed no differences between NCT and control rats (Montandon et al. 2006, 2008a), although we accept that the limitations described above for the measurements of ventilation in those previous studies also apply to the measurements of metabolic rate and the reported lack of difference. Although simultaneous recording of sleep and breathing by telemetry and plethysmography provides a non-invasive and reasonably accurate assessment of respiratory control in wakefulness and sleep (i.e. avoiding any physical constraints and restraints of the animal), the lack of arterial blood gas measurements in the conscious freely behaving rats remains an important limitation of this study; without such measurements, it is not possible to know whether differences in arterial blood gases contributed to the increase in baseline breathing. We did not measure arterial blood gases in the conscious rats because we wanted to avoid the possible (and potentially significant) confounding effects of additional surgical intervention (i.e. arterial cannulation) and the restraint required to take blood samples via a tether and cannula system. It is also unknown if the NCT rats would respond differently to these effects and so influence the measurement of arterial blood gases, and for these reasons measurements of blood gases were made in the anaesthetized rats.
showed no differences between NCT and control rats (Montandon et al. 2006, 2008a), although we accept that the limitations described above for the measurements of ventilation in those previous studies also apply to the measurements of metabolic rate and the reported lack of difference. Although simultaneous recording of sleep and breathing by telemetry and plethysmography provides a non-invasive and reasonably accurate assessment of respiratory control in wakefulness and sleep (i.e. avoiding any physical constraints and restraints of the animal), the lack of arterial blood gas measurements in the conscious freely behaving rats remains an important limitation of this study; without such measurements, it is not possible to know whether differences in arterial blood gases contributed to the increase in baseline breathing. We did not measure arterial blood gases in the conscious rats because we wanted to avoid the possible (and potentially significant) confounding effects of additional surgical intervention (i.e. arterial cannulation) and the restraint required to take blood samples via a tether and cannula system. It is also unknown if the NCT rats would respond differently to these effects and so influence the measurement of arterial blood gases, and for these reasons measurements of blood gases were made in the anaesthetized rats.
Inspiratory (phrenic) motor output recording in NCT and control rats
To address the issue of potential changes in blood gases between experimental groups, we used an anaesthetized, neuromuscularly blocked, vagotomised, and artificially ventilated rat preparation, which made it possible to control arterial blood gases while monitoring phrenic activity as a neural correlate of breathing. In anaesthetized adult rats, even though baseline arterial blood gases were similar between groups, phrenic minute activity of NCT rats was higher than controls. Therefore, the NCT-related enhancement of ventilation was, at least in great part, mediated by an increase in the amplitude of inspiratory motor activity which indicates that the higher basal ventilatory activity in the conscious NCT rats may reflect a larger motor output rather than differences only related to the method of measurement (plethysmography). Currently, the precise mechanisms underlying the augmentation of inspiratory motor activity and tidal volume are unknown, but several hypotheses may be suggested. (i) Increased metabolic rate could augment baseline ventilation in the NCT rats. (ii) For the same descending neural drive, changes at the neuromuscular junction in the NCT rats could facilitate respiratory motor activity. (iii) Reduced gas exchange efficiency in the NCT rats would require higher tidal volume to meet similar metabolic demand. (iv) Adenosine A2A receptors are present on phrenic motoneurons of adult rats and their activation elicits persistent enhancement of phrenic nerve activity (Golder et al. 2008). Therefore, augmentation of excitatory adenosine A2A receptor expression in respiratory motor (phrenic) or pre-motor neurons perhaps in consequence of the antagonism in the neonatal period might contribute to the high ventilation observed in NCT rats. With this scenario, the same descending neural drive would yield a greater motor output (and thus tidal volume).
Breathing during hypercapnia in NCT rats
In control rats, hypercapnia increased ventilation owing to higher tidal volume and respiratory frequency. The same response occurred in NCT rats, but because of the higher basal ventilation, the ventilatory response to CO2 was reduced. Indeed the level of ventilation observed in the NCT rats under basal conditions was similar to that observed in controls under CO2 stimulation. Similar to what was obtained in freely behaving rats, results in anaesthetized rats showed that NCT increased minute activity; however in this preparation the hypercapnic respiratory challenge increased similarly phrenic nerve activity in control and NCT rats. This latter discrepancy might be due to several differences between conscious and anaesthetized preparations. Anaesthetized rats were vagotomised and maintained hyperoxic during hypercapnic exposure. The effects of vagotomy may differ between groups; however, since NCT rats have a stronger hypoxic ventilatory response that is likely mediated by NCT-related differences in adenosinergic neurotransmission within the carotid body (Montandon et al. 2008a), NCT-related differences in carotid body function might explain discrepancies between results obtained in freely behaving and anaesthetized hyperoxic preparations. Note that it is also possible that a component of the decreased ventilatory response to hypercapnia in the NCT rats in the freely behaving condition was via a behavioural response, such as an increased alertness or ‘wakefulness stimulus’ (Phillipson & Bowes, 1986).
Clinical perspective
Comparison of central nervous system development between rats and humans indicates that the level of neurological maturity observed in the rat during the immediate postnatal period is comparable to that in humans during the third trimester of pregnancy (Clancy et al. 2007). Despite the limitations inherent to animals models, our results show that a course of caffeine treatment during early postnatal life leads to changes in sleep and respiratory regulation that persist well into adulthood. These results raise important questions concerning the long-term consequences of this pharmaceutical intervention which is common clinical practice to treat apnoea of prematurity (Millar & Schmidt, 2004; Leon et al. 2007). Should the effects of NCT observed in this study elicit comparable effects in humans, it could contribute to the aetiology of insomnia.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (operating grant MOP-81101 to A.B.) and the Foundation for Research into Children's Diseases. R.K. holds a Canada Research Chair in Respiratory Neurobiology. G.M. was supported by awards from the Foundation for Research into Children's Diseases, the J.-and-J.-L.-Levesque Perinatalogy Chair, and the Canadian Lung Association.
Glossary
Abbreviations
- EMG
electromyogram
- EEG
electroencephalogram
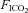
fraction of inspired carbon dioxide
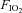
fraction of inspired oxygen
- fR
respiratory frequency
- NCT
neonatal caffeine treatment
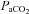
arterial partial pressure of carbon dioxide
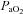
arterial partial pressure of oxygen
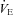
minute ventilation
- VT
tidal volume
Author contributions
G.M. contributed to the conception, design, analysis, interpretation of data, drafting, revision and final approval of the article. R.L.H. contributed to the analysis and interpretation of the data, the revision of the article and final approval of the version to be published. R.K contributed to the conception, design, analysis, and interpretation of the data, as well as the drafting, revision and final approval of the article. A.B. contributed to the conception, design of the study, revision and final approval of the article. The experiments were performed in the St-Francois d’Assise Hospital in Quebec, Canada.
References
- Bairam A, Montandon G, Joseph V, Lajeunesse Y, Kinkead R. Enhancement of the breathing frequency response to hypoxia by neonatal caffeine treatment in adult male rats: The role of testosterone. Respir Physiol Neurobiol. 2008;165:261–265. doi: 10.1016/j.resp.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Berger AJ. Adenosine suppresses excitatory glutamatergic inputs to rat hypoglossal motoneurons in vitro. Neurosci Lett. 1994;177:143–146. doi: 10.1016/0304-3940(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Bhatt-Mehta V, Schumacher RE. Treatment of apnea of prematurity. Paediatr Drugs. 2003;5:195–210. doi: 10.2165/00128072-200305030-00006. [DOI] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enhorning G, van SS, Lundgren C, Vargas I. Whole-body plethysmography, does it measure tidal volume of small animals? Can J Physiol Pharmacol. 1998;76:945–951. doi: 10.1139/cjpp-76-10-11-945. [DOI] [PubMed] [Google Scholar]
- Etzel BA, Guillet R. Effects of neonatal exposure to caffeine on adenosine A1 receptor ontogeny using autoradiography. Brain Res Dev Brain Res. 1994;82:223–230. doi: 10.1016/0165-3806(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Finer NN, Higgins R, Kattwinkel J, Martin RJ. Summary proceedings from the apnea-of-prematurity group. Pediatrics. 2006;117:S47–S51. doi: 10.1542/peds.2005-0620H. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci. 2008;28:2033–2042. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrahi H, Chan B, Horner RL. On-line detection of sleep-wake states and application to produce intermittent hypoxia only in sleep in rats. J Appl Physiol. 2001;90:2130–2140. doi: 10.1152/jappl.2001.90.6.2130. [DOI] [PubMed] [Google Scholar]
- Hascoet JM, Hamon I, Boutroy MJ. Risks and benefits of therapies for apnoea in premature infants. Drug Saf. 2000;23:363–379. doi: 10.2165/00002018-200023050-00002. [DOI] [PubMed] [Google Scholar]
- Hayes MJ, Akilesh MR, Fukumizu M, Gilles AA, Sallinen BA, Troese M, Paul JA. Apneic preterms and methylxanthines: arousal deficits, sleep fragmentation and suppressed spontaneous movements. J Perinatol. 2007;27:782–789. doi: 10.1038/sj.jp.7211820. [DOI] [PubMed] [Google Scholar]
- Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs.diaphragm muscle response to CO2 in rats. J Appl Physiol. 2002;92:878–887. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Dupenloup L, Valois N, Gulemetova R. Stress-induced attenuation of the hypercapnic ventilatory response in awake rats. J Appl Physiol. 2001;90:1729–1735. doi: 10.1152/jappl.2001.90.5.1729. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Dijk DJ, Gaus SE, Borbely AA. Caffeine reduces low-frequency delta activity in the human sleep EEG. Neuropsychopharmacology. 1995;12:229–238. doi: 10.1016/0893-133X(94)00079-F. [DOI] [PubMed] [Google Scholar]
- Leon AE, Michienzi K, Ma CX, Hutchison AA. Serum caffeine concentrations in preterm neonates. Am J Perinatol. 2007;24:39–47. doi: 10.1055/s-2006-958163. [DOI] [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar D, Schmidt B. Controversies surrounding xanthine therapy. Semin Neonatol. 2004;9:239–244. doi: 10.1016/j.siny.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Montandon G, Bairam A, Kinkead R. Long-term consequences of neonatal caffeine on ventilation, occurrence of apneas, and hypercapnic chemoreflex in male and female rats. Pediatr Res. 2006;59:519–524. doi: 10.1203/01.pdr.0000203105.63246.8a. [DOI] [PubMed] [Google Scholar]
- Montandon G, Bairam A, Kinkead R. Neonatal caffeine induces sex-specific developmental plasticity of the hypoxic respiratory chemoreflex in adult rats. Am J Physiol Regul Integr Comp Physiol. 2008a;295:R922–R934. doi: 10.1152/ajpregu.00059.2008. [DOI] [PubMed] [Google Scholar]
- Montandon G, Kinkead R, Bairam A. Disruption of adenosinergic modulation of ventilation at rest and during hypercapnia by neonatal caffeine in young rats: role of adenosine A1 and A2A receptors. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1621–R1631. doi: 10.1152/ajpregu.00514.2006. [DOI] [PubMed] [Google Scholar]
- Montandon G, Kinkead R, Bairam A. Adenosinergic modulation of respiratory activity: developmental plasticity induced by perinatal caffeine administration. Respir Physiol Neurobiol. 2008b;164:87–95. doi: 10.1016/j.resp.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Nolan P, Horner RL. GABAA receptor antagonism at the hypoglossal motor nucleus increases genioglossus muscle activity in NREM but not REM sleep. J Physiol. 2003;548:569–583. doi: 10.1113/jphysiol.2002.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola JP, Frappell PB. On the barometric method for measurements of ventilation, and its use in small animals. Can J Physiol Pharmacol. 1998;76:937–944. doi: 10.1139/cjpp-76-10-11-937. [DOI] [PubMed] [Google Scholar]
- Phillipson EA, Bowes G. Control of breathing during sleep. In: Fishman AP, Cherniack NS, Widdicombe JG, Geiger SR, editors. Handbook of Physiology, section 3, The Respiratory System, vol II, Control of Breathing. Bethesda, MD, USA: American Physiological Society; 1986. pp. 649–989. [Google Scholar]
- Radulovacki M. Role of adenosine in sleep in rats. Rev Clin Basic Pharm. 1985;5:327–339. [PubMed] [Google Scholar]
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- Sinton CM, Valatx JL, Jouvet M. Increased sleep time in the offspring of caffeine-treated dams from two inbred strains of mice. Neurosci Lett. 1981;24:169–174. doi: 10.1016/0304-3940(81)90243-3. [DOI] [PubMed] [Google Scholar]
- Smith ML, Pacchia CF. Sleep apnoea and hypertension: role of chemoreflexes in humans. Exp Physiol. 2007;92:45–50. doi: 10.1113/expphysiol.2006.033753. [DOI] [PubMed] [Google Scholar]
- Stephenson R, Liao KS, Hamrahi H, Horner RL. Circadian rhythms and sleep have additive effects on respiration in the rat. J Physiol. 2001;536:225–235. doi: 10.1111/j.1469-7793.2001.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoman EB, Davis DH, Raye JR, Philipps AF, Rowe JC, Denenberg VH. Theophylline affects sleep-wake state development in premature infants. Neuropediatrics. 1985;16:13–18. doi: 10.1055/s-2008-1052537. [DOI] [PubMed] [Google Scholar]
- White DP. The pathogenesis of obstructive sleep apnea: advances in the past 100 years. Am J Respir Cell Mol Biol. 2006;34:1–6. doi: 10.1165/rcmb.2005-0317OE. [DOI] [PubMed] [Google Scholar]