ABSTRACT
Microvascular compression of the vestibulocochlear nerve is known to cause disabling tinnitus and vertigo. A review of the literature shows that the compression is usually located in the cerebellopontine angle, and that it is usually caused by an artery. The authors add the case of a 46-year-old man with venous compression of the vestibulocochlear nerve inside the internal auditory canal (IAC). The patient presented with a 2-year history of recurrent attacks of disabling vertigo and intermittent high-frequency tinnitus on the right side. Magnetic resonance images showed a small, contrast-enhancing lesion in the fundus of the right IAC, which was suspicious for vestibular schwannoma. During surgical exploration, a large venous loop was found extending into the IAC and compressing the vestibulocochlear nerve. The vessel was mobilized and rerouted out of the IAC. The presumed vestibular schwannoma at the cochlear fossa was left in situ. The patient's symptoms resolved immediately after surgery. Hearing was unchanged postoperatively. On follow-up, there has been no growth of the contrast-enhancing lesion in the IAC for 3 years so far.
Disabling vertigo can also be caused by venous microvascular compression of the vestibulocochlear nerve inside the IAC and may be treated successfully by microvascular decompression. A sensitive, conservative approach to lesions in the fundus may be justified in the presence of an additional, more prominent pathology that causes compression of the vestibulocochlear nerve.
Keywords: Acoustic neuroma, microvascular decompression, tinnitus, vertigo, vestibulocochlear nerve, vestibular schwannoma
The anatomy of the neurovascular complex of the cerebellopontine angle is highly variable. This variability has been attributed to the relatively late development of the anterior inferior cerebellar artery (AICA) and the posterior inferior cerebellar artery (PICA) from the primitive lateral basilovertebral anastomosis. Particularly with respect to the relation of the posterior fossa vessels to the facial-vestibulocochlear nerve complex, a large number of variants exists.1,2,3,4
Clinical observations associated with the close proximity of the vessels to the cranial nerves led to the definition of a variety of different cranial nerve compression syndromes.5 Vascular compression of the vestibulocochlear nerve may lead to different symptoms, including tinnitus, hearing loss, disabling vertigo, and imbalance. There have been attempts to link the character of symptoms to the anatomical location of the vestibulocochlear nerve compression site, but to date conclusive data are not yet available.6,7,8,9,10 Microvascular decompression (MVD) of the vestibulocochlear nerve has been reported to be an efficient treatment option with high success rates of up to 80% for several vestibulocochlear compression syndromes.8,11,12,13 Vestibulocochlear nerve microvascular compression syndromes described in the literature have been attributed to arterial compression of nervous structures. In contrast to trigeminal neuralgia (TN) with symptomatic compression located almost exclusively at the nerve root entry zone, MVD for the vestibulocochlear nerve has successfully been performed for vessels compressing the nerve over its entire segment in the cerebellopontine angle. Intracanalicular neurovascular conflict surgically treated by MVD, however, has only been described in one report, which identified an arterial loop pressing at the nerve as the cause of pulsatile tinnitus.14
The present case adds another facet to the literature on intracranial microvascular compression syndromes. In our patient, the vestibulocochlear nerve was compressed by a venous loop inside the internal auditory canal (IAC). Interestingly, it was this compression, not a coexisting small intracanalicular lesion, that was suspicious for vestibular schwannoma, which caused the patient's symptoms.
CASE REPORT
History and Examination
A 46-year-old man presented at our clinic with a 2-year history of recurrent vertigo. During the first attack, an initial period of acute severe vertigo lasting several minutes was immediately followed by sensorineural hearing loss and imbalance. The patient was treated with intravenous infusions according to the protocol proposed by Stennert,15 and symptoms resolved completely. The second episode of severe vertigo occurred 18 months later, accompanied by right-sided aural fullness and high-frequency nonpulsatile tinnitus, this time without hearing impairment. Six months after the second episode, the patient started to suffer from recurrent episodes of severe vertigo lasting from several minutes to hours, which were incapacitating and significantly impaired the patient's ability to work or carry on with his regular activities. Symptoms were lessened with bed rest. Both frequency and intensity of the vertigo attacks increased over the following months. Two months before surgery, the attacks occurred at least several times a week, sometimes daily. The patient also experienced fluctuating high-frequency tinnitus and increasing aural fullness on the right side, both of which were constantly present before surgery. The patient was not hypertensive. His neurological examination was normal. Preoperative audiometric testing showed no significant hearing impairment (Fig. 1). Preoperative vestibular testing was normal. Further medical treatment of the condition was not successful. Repeated magnetic resonance (MR) images showed a small contrast-enhancing lesion of ~;5 mm in diameter in the right anterior IAC (Fig. 2). Radiographically, the lesion appeared to be consistent with an intracanalicular vestibular schwannoma. The patient's symptoms seemed to fit as well, as these tumors typically present with tinnitus and vertigo.16,17 Because of his increasing disability due to a continuous increase in the frequency and intensity of the vertigo attacks, the patient opted for surgical exploration.
Figure 1.
Pure-tone audiometry of the right ear at initial presentation of the patient.
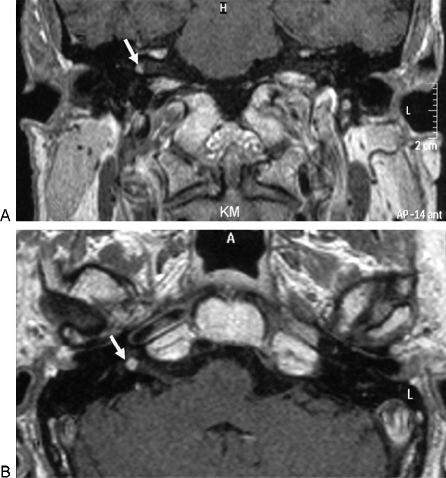
Figure 2.
Preoperative T1-weighted magnetic resonance images showing small contrast-enhancing lesion in the right internal auditory canal (arrows). (A) Coronary section. (B) Axial section.
Operation
The patient underwent a right lateral suboccipital approach. The posterior wall of the IAC was removed to the level of the crus commune of the posterior and lateral semicircular canal using a high-speed drill. After opening the dural sheath of the IAC, the intact arachnoid membrane was exposed. On microscopic inspection, a large vein was found entering the internal auditory meatus and looping into the canal for 7 mm, thereby compressing the vestibulocochlear nerve. The vessel could be followed to the pontomedullary junction. Its contact to the vestibular nerve extended from the porus to the middle of the IAC. The vein displaced the vestibulocochlear nerve cranially (Fig. 3A). After dissection of the arachnoid membrane, the vessel was mobilized and rerouted from the IAC into the cerebellopontine angle (Fig. 3B, C). To avoid recurrent luxation of the venous loop into the IAC, Teflon felt was interposed (Fig. 3D). The presumed vestibular schwannoma was covered by portions of the vestibulocochlear nerve and hidden from direct view. Because of the major vascular compression that had been encountered, it was decided not to explore the fundus any further. The posterior aspect of the IAC was closed with muscle and fibrin glue.
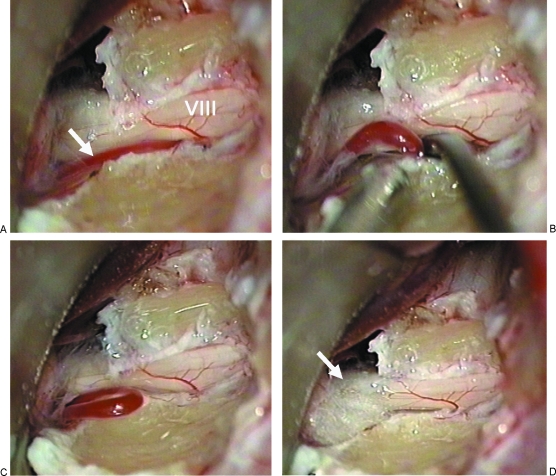
Figure 3.
Intraoperative photographs. (A) Initial exposure of the vestibulocochlear nerve in the internal auditory canal (IAC). A vessel was found, that compressed and displaced nerve VIII from the bottom of the IAC (arrow). (B, C) The vessel is dissected from the nerve and relocated outside the internal auditory meatus. Further dissection reveals a venous loop extending from the cerebellopontine angle to the IAC. (D) Teflon felt (arrow) is placed between the vessel and the nerve at the meatus to prevent recurrence of vascular compression.
Postoperative Course
The patient tolerated the intervention well. No new neurological deficits occurred postoperatively. Audiometric testing was performed 5 days after the surgery and showed unimpaired hearing (Fig. 4). The patient's symptoms resolved completely. On follow-up examinations 3, 6, and up to 39 months postoperatively, the patient had not experienced any further vertigo attacks. He went back to work and resumed his daily activities unimpaired. Right-sided aural fullness and tinnitus were no longer present. Audiometric testing showed unimpaired hearing function at all times during follow-up. Follow-up MR images obtained 3 months postoperatively and in regular 6-to-12 month intervals thereafter showed an unchanged contrast-enhancing lesion in the fundus of the IAC (Fig. 5A, B). Axial 3D-constructive interference in steady state (CISS) images demonstrated tumor invasion of the cochlear fossa (Fig. 5C). Magnetic resonance angiography failed to demonstrate a vascular malformation in the fundus. Because the patient had excellent hearing postoperatively and complete resolution of vertigo and tinnitus, it was agreed that no further intervention should be performed at this time, and he was scheduled for regular follow-up.
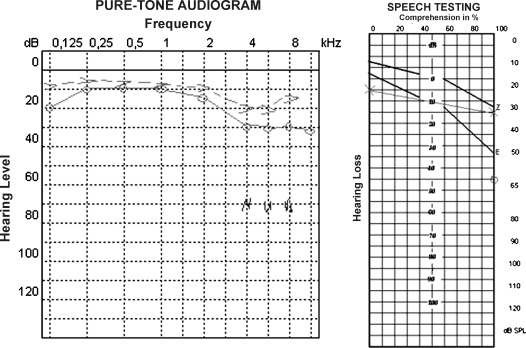
Figure 4.
Results of pure-tone audiometry and speech discrimination threshold of the right ear after the surgical intervention.
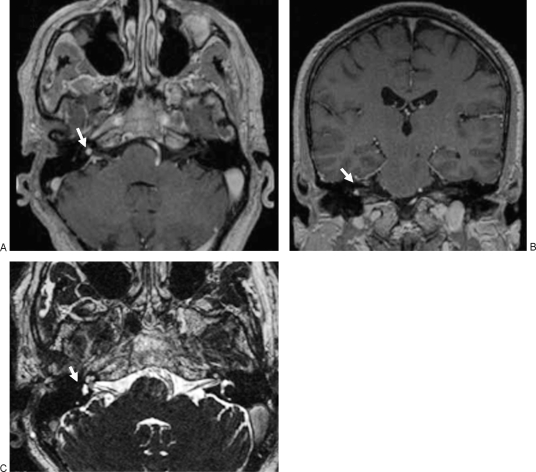
Figure 5.
Follow-up magnetic resonance (MR) images obtained 3 months after surgery. Axial (A) and coronal (B) T1-weighted MR images with contrast shows persistent contrast-enhancing lesion in the fundus of the internal auditory canal (arrows). (C) Axial 3D-constructive interference in steady state (CISS) image demonstrates that the tumor obstructs the cochlear fossa on the right side (arrow).
DISCUSSION
Over the past decades, neurosurgical research and clinical experience have linked tinnitus, vertigo, and hearing loss to microvascular compression of the vestibulocochlear nerve. Microvascular compression of the vestibulocochlear nerve in the cerebellopontine angle has been shown to be caused by the AICA or PICA and/or their branches, which display a highly variable course in the posterior fossa.1,6,18 Several surgeons have attempted MVD in other cranial rhizopathies, such as TN and hemifacial spasm, which have reportedly led to relief of symptoms in a high percentage of patients. Many reports have been published that focus on MVD of the vestibulocochlear nerve in the area of the cerebellopontine angle by isolating the nerve from the compressing vessel with Teflon felt or muscle grafts.9,10,12,13,19,20
Reports of large operative series of MVD for TN have mentioned the presence of venous compression of the trigeminal nerve (alone or in association with arterial compression) in a fraction of the cases. Venous neurovascular compression was found to be involved in ~;20 to 28% of TN cases, whereas pure venous compression of the trigeminal nerve causing TN was present in a very small subpopulation and ranged between ~;3 and 6% of all cases.21,22 To date, there have not been any reports focusing on the role of venous compression in vestibulocochlear nerve neurovascular compression syndromes. In the present case, the site of compression was inside the IAC and caused by a venous loop. The largest vein encountered in this part of the cerebellopontine angle is the vein of the cerebellopontine fissure,23 which usually passes from the petrosal surface of the cerebellum above the facial and vestibulocochlear nerves to join other tributaries of the superior petrosal sinus.
In our case, the venous loop ran caudal to the nerves to displace them cranially. Still, it appears likely that the compressing vein was a variant of the vein of the cerebellopontine fissure.
Elevated IAC pressure caused by intracanalicular vestibular schwannomas has been suspected to cause early vestibular symptoms and hearing loss.24 In this case, elevated IAC pressure was caused by a venous anomaly. The progressive high-frequency tinnitus experienced by the patient may have been indicative of beginning cochlear impairment. Removal of the vessel from the IAC led to complete resolution of symptoms.
Although indications for vestibulocochlear nerve MVD in the cerebellopontine angle have been discussed previously in reports of larger patient series,19,20,22 the present case, along with the observation of arterial compression in the IAC,14 adds another perspective. It can be argued that exploration of the vestibulocochlear nerve should be continued into the IAC if no clear neurovascular conflict in the cerebellopontine angle is found.
Before the intervention, distinction between vestibulocochlear symptoms caused by vascular compression or a fundal tumor was not possible in this case, especially because the intermittent nature of the symptoms on initial presentation of the patient is not typical for vascular compression syndromes. On the other hand, it is usually an arterial vessel that causes the symptoms. Considering the rapidly increasing frequency of symptoms and their disabling character, surgical treatment was definitely indicated. However, although MVD of the vestibulocochlear nerve successfully relieved the symptoms of the patient in this case, pre- and postoperative imaging studies clearly showed a contrast-enhancing lesion in fundus of the IAC. Although the large vessel compressing the vestibulocochlear nerve appeared to provide ample cause for the patient's symptoms during surgery, the outcome with respect to the remaining lesion was not clear. Only the small size of the presumed tumor kept us from risky further surgical exploration. As it has turned out, after 3 years the patient is symptom free; therefore, the decision not to risk further surgery appears to be sustainable.
Magnetic resonance imaging obtained 3 months after surgery included CISS images and demonstrated the tumor in the IAC fundus more clearly. The images obtained from 3D CISS have previously been reported to be superior to T1-weighted images in detecting lesions in the IAC fundus because fundal enhancement may be weak on T1-weighted contrast images.25,26,27 Other authors have even suggested to routinely apply this combination in vestibular schwannoma diagnostic workup.5,8 Although preoperative MR angiography might have identified the venous loop in the IAC, it was not helpful in the differential diagnosis of the fundal lesion. Being asymptomatic after surgery and considering the very small lesion at the fundus of the IAC, the patient has chosen to have the lesion followed by MRI. It has not grown at all thus far, suggesting that another vascular lesion, such as another vessel loop or a small AVM, has to be taken into account.
The preferential treatment for small intracanalicular vestibular schwannomas has long been a matter of debate. Conservative management, microsurgery via middle fossa, or the retrosigmoid approach and radiosurgery provide different treatment options. Which of these options is suggested to the patient is still frequently based on the physician's background and personal experience. Microsurgical treatment to achieve long-term hearing preservation has often been considered, and numerous reports have been published, some of them with excellent hearing preservation with up to 80% serviceable hearing postoperatively,16,28,29,30,31,32 but so have radiosurgical interventions as well as a wait-and-scan attitude.
Recently, efforts have been made to find prognostic factors that might help to compare the different treatment options. One of the most deleterious morphological factors for hearing preservation in vestibular schwannoma surgery appears to be the obliteration of the fundus of the IAC and invasion of the cochlear fossa by the tumor.26,27,33,34
Against this background, the intraoperative decision to treat the neurovascular conflict and manage the patient's presumed tumor conservatively appears to be a valid choice.
CONCLUSION
Disabling vertigo can be caused by venous microvascular compression of the vestibulocochlear nerve in the IAC and may be treated successfully by MVD. Moreover, in the presence of venous compression of the vestibulocochlear nerve and a small tumor in the fundus of the IAC, disabling vertigo and tinnitus may be relieved by MVD alone. Thus, a sensitive, conservative approach to lesions in the fundus may be justified in the presence of an additional, more prominent pathology that causes compression of the vestibulocochlear nerve.
Magnetic resonance imaging should include sequences that are capable of demonstrating vascular anomalies in patients with persistent tinnitus and vertigo, even in the presence of small, enhancing intracanalicular lesions.
REFERENCES
- Kim H N, Kim Y, Park I, Kim G, Chung I. Variability of the surgical anatomy of the neurovascular complex of the cerebellopontine angle. Ann Otol Rhinol Laryngol. 1990;99:288–296. doi: 10.1177/000348949009900408. [DOI] [PubMed] [Google Scholar]
- Martin R G, Grant J L, Peace D, Theiss C, Rhoton A L. Microsurgical relationships of the anterior inferior cerebellar artery and the facial-vestibulocochlear nerve complex. Neurosurgery. 1980;6(5):483–507. doi: 10.1227/00006123-198005000-00001. [DOI] [PubMed] [Google Scholar]
- Padget D. The development of the cranial arteries in the human embryo. Contrib Embryol. 1948;32:207–261. [Google Scholar]
- Rhoton A L., Jr Microsurgical anatomy of the posterior fossa cranial nerves. Clin Neurosurg. 1979;26:398–462. doi: 10.1093/neurosurgery/26.cn_suppl_1.398. [DOI] [PubMed] [Google Scholar]
- Jannetta P J. Observations on the etiology of trigeminal neuralgia, hemifacial spasm, acoustic nerve dysfunction and glossopharyngeal neuralgia. Definitive microsurgical treatment and results in 117 patients. Neurochirurgia (Stuttg) 1977;20:145–154. doi: 10.1055/s-0028-1090369. [DOI] [PubMed] [Google Scholar]
- Bertrand R A, Molina P, Hardy J. Vestibular syndrome and vascular anomaly in the cerebello-pontine angle. Acta Otolaryngol. 1977;83:187–194. doi: 10.3109/00016487709128832. [DOI] [PubMed] [Google Scholar]
- De Ridder D, Møller A, Verlooy J, Cornelissen M, De Ridder L. Is the root entry/exit zone important in microvascular compression syndromes? Neurosurgery. 2002;51(2):427–433. doi: 10.1097/00006123-200208000-00023. [DOI] [PubMed] [Google Scholar]
- De Ridder D, Ryu H, Møller A R, Nowé V, de Heyning P Van, Verlooy J. Functional anatomy of the human cochlear nerve and its role in microvascular decompressions for tinnitus. Neurosurgery. 2004;54:381–388. doi: 10.1227/01.neu.0000103420.53487.79. [DOI] [PubMed] [Google Scholar]
- Ryu H, Yamamoto S, Sugiyama K, Nishizawa S, Nozue M. Neurovascular compression syndrome of the eighth cranial nerve. Can the site of compression explain the symptoms? Acta Neurochir (Wien) 1999;141(5):495–501. doi: 10.1007/s007010050330. [DOI] [PubMed] [Google Scholar]
- Schwaber M K, Hall J W. Cochleovestibular nerve compression syndrome. I. Clinical features and audiovestibular findings. Laryngoscope. 1992;102:1020–1029. doi: 10.1288/00005537-199209000-00012. [DOI] [PubMed] [Google Scholar]
- Møller M B, Møller A R, Jannetta P J, Jho H D, Sekhar L N. Microvascular decompression of the eighth cranial nerve in patients with disabling positional vertigo: selection criteria and operative results in 207 patients. Acta Neurochir (Wien) 1993;125:75–82. doi: 10.1007/BF01401831. [DOI] [PubMed] [Google Scholar]
- Brackmann D E, Kesser B W, Day J D. Microvascular decompression of the vestibulocochlear nerve for disabling positional vertigo: the House Ear Clinic experience. Otol Neurotol. 2001;22:882–887. doi: 10.1097/00129492-200111000-00029. [DOI] [PubMed] [Google Scholar]
- Okamura T, Kurokawa Y, Ikeda N, et al. Microvascular decompression for cochlear symptoms. J Neurosurg. 2000;93:421–426. doi: 10.3171/jns.2000.93.3.0421. [DOI] [PubMed] [Google Scholar]
- De Ridder D, De Ridder L, Nowé V, Thierens H, de Heyning P Van, Møller A. Pulsatile tinnitus and the intrameatal vascular loop: why do we not hear our carotids? Neurosurgery. 2005;57(6):1213–1217. doi: 10.1227/01.neu.0000186035.73828.34. [DOI] [PubMed] [Google Scholar]
- Michel O, Jahns T, Joost-Enneking M, Neugebauer P, Streppel M, Stennert E. The Stennert antiphlogistic-rheologic infusion schema in treatment of cochleovestibular disorders. HNO. 2000;48(3):182–188. doi: 10.1007/s001060050030. [DOI] [PubMed] [Google Scholar]
- Samii M, Matthies C, Tatagiba M. Intracanalicular acoustic neurinomas. Neurosurgery. 1991;29(2):189–198. doi: 10.1097/00006123-199108000-00004. [DOI] [PubMed] [Google Scholar]
- Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery. 1997;40(1):1–9. doi: 10.1097/00006123-199701000-00001. [DOI] [PubMed] [Google Scholar]
- Bachor E, Selig Y, Jahnke K, Rettinger G, Karmody C. Vascular variations of the inner ear. Acta Otolaryngol. 2001;121(1):35–41. doi: 10.1080/000164801300006245. [DOI] [PubMed] [Google Scholar]
- Møller M B. Results of microvascular decompression of the eighth nerve as treatment for disabling positional vertigo. Ann Otol Rhinol Laryngol. 1990;99(9 Pt 1):724–729. doi: 10.1177/000348949009900911. [DOI] [PubMed] [Google Scholar]
- Møller M B, Møller A R, Jannetta P J, Jho H D. Vascular decompression surgery for severe tinnitus: selection criteria and results. Laryngoscope. 1993;103:421–427. doi: 10.1002/lary.5541030410. [DOI] [PubMed] [Google Scholar]
- Sindou M, Howeidy T, Acevedo G. Anatomical observations during microvascular decompression for idiopathic trigeminal neuralgia (with correlations between topography of pain and site of the neurovascular conflict). Prospective study in a series of 579 patients. Acta Neurochir (Wien) 2002;144(1):1–12. discussion 12–13. doi: 10.1007/s701-002-8269-4. [DOI] [PubMed] [Google Scholar]
- Matsushima T, Huynh-Le P, Miyazono M. Trigeminal neuralgia caused by venous compression. Neurosurgery. 2004;55(2):334–337. discussion 338–339. doi: 10.1227/01.neu.0000129552.87291.87. [DOI] [PubMed] [Google Scholar]
- Rhoton A L, Jr, Tedeschi H. Microsurgical anatomy of acoustic neuroma. Neurosurg Clin N Am. 2008;19:145–174. doi: 10.1016/j.nec.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Lapsiwala S B, Pyle G M, Kaemmerle A W, Sasse F J, Badie B. Correlation between auditory function and internal auditory canal pressure in patients with vestibular schwannomas. J Neurosurg. 2002;96(5):872–876. doi: 10.3171/jns.2002.96.5.0872. [DOI] [PubMed] [Google Scholar]
- Casselman J W, Kuhweide R, Deimling M, Ampe W, Dehaene I, Meeus L. Constructive interference in steady state-3DFT MR imaging of the inner ear and cerebellopontine angle. AJNR Am J Neuroradiol. 1993;14:47–57. [PMC free article] [PubMed] [Google Scholar]
- Dubrulle F, Ernst O, Vincent C, Vaneecloo F M, Lejeune J P, Lemaitre L. Cochlear fossa enhancement at MR evaluation of vestibular schwannoma: correlation with success at hearing-preservation surgery. Radiology. 2000;215:458–462. doi: 10.1148/radiology.215.2.r00ma20458. [DOI] [PubMed] [Google Scholar]
- Kocaoglu M, Bulakbasi N, Ucoz T, et al. Comparison of contrast-enhanced T1-weighted and 3D constructive interference in steady state images for predicting outcome after hearing-preservation surgery for vestibular schwannoma. Neuroradiology. 2003;45:476–481. doi: 10.1007/s00234-003-1006-0. [DOI] [PubMed] [Google Scholar]
- Brookes G B, Woo J. Hearing preservation in acoustic neuroma surgery. Clin Otolaryngol Allied Sci. 1994;19(3):204–214. doi: 10.1111/j.1365-2273.1994.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Gharabaghi A, Samii A, Koerbel A, Rosahl S K, Tatagiba M, Samii M. Preservation of function in vestibular schwannoma surgery. Neurosurgery. 2007;60(2, Suppl 1):ONS124–ONS127. doi: 10.1227/01.NEU.0000249245.10182.0D. [DOI] [PubMed] [Google Scholar]
- Haines S J, Levine S C. Intracanalicular acoustic neuroma: early surgery for preservation of hearing. J Neurosurg. 1993;79(4):515–520. doi: 10.3171/jns.1993.79.4.0515. [DOI] [PubMed] [Google Scholar]
- Møller P, Myrseth E, Pedersen P, Larsen J L, Krakenes J, Moen G. Acoustic neuroma—treatment modalities. Surgery, gamma-knife or observation? Acta Otolaryngol Suppl. 2000;543:34–37. [PubMed] [Google Scholar]
- Thomsen J, Charabi S, Tos M, Mantoni M, Charabi B. Intracanalicular vestibular schwannoma - therapeutic options. Acta Otolaryngol Suppl. 2000;543:38–40. doi: 10.1080/000164800453900. [DOI] [PubMed] [Google Scholar]
- Selesnick S H, Rebol J, Heier L A, Wise J B, Gutin P H, Lavyne M H. Internal auditory canal involvement of acoustic neuromas: surgical correlates to magnetic resonance imaging findings. Otol Neurotol. 2001;22:912–916. doi: 10.1097/00129492-200111000-00034. [DOI] [PubMed] [Google Scholar]
- Somers T, Casselman J, de Ceulaer G, Govaerts P, Offeciers E. Prognostic value of magnetic resonance imaging findings in hearing preservation surgery for vestibular schwannoma. Otol Neurotol. 2001;22:87–94. doi: 10.1097/00129492-200101000-00017. [DOI] [PubMed] [Google Scholar]