ABSTRACT
Ewing's sarcoma involving the sinonasal cavity and anterior skull base is very rare. The purpose of this article is to present our experience with two such cases, which were both treated with combined chemotherapy and proton beam radiation therapy. The patients were selected from a retrospective medical record analysis that was conducted of all patients who were diagnosed with Ewing's sarcoma of the sinonasal cavity at the Massachusetts Eye & Ear Infirmary/Massachusetts General Hospital Cranial Base Center from 2004 to 2008. One of the patients underwent pretreatment endoscopic subtotal resection to facilitate proton beam radiation therapy. Response to treatment was assessed, post-treatment endoscopic biopsies were performed to assure eradication of disease, and treatment complications were recorded. Both patients completed chemotherapy and proton beam radiation therapy without complication. One patient completed treatment in December 2006 and remains disease free. The second patient completed treatment in March 2007 and remains disease free. Both patients developed headaches accompanied by frontal sinus opacification after treatment that required endoscopic drainage. After endoscopic drainage, the opacification and symptoms resolved.
Keywords: Ewing's sarcoma, anterior skull base, proton beam, endoscopic surgery
Ewing's sarcoma is the second most common primary malignant tumor of bone found in children between the ages of 10 and 15 years old, second only to osteosarcoma.1 Ewing's sarcoma is typically an aggressive, poorly differentiated tumor. It accounts for 4 to 6% of all primary bone tumors,1,2 and it affects the bones of the skull or face in only 1 to 4% of cases.3 Paranasal sinus involvement is rare, and skull base involvement has been infrequently reported.3 A review of the current literature revealed the cases listed in Table 1.3,4,5,6,7,8,9,10,11,12,13,14,15 During the past 25 years, the prognosis for patients with Ewing's sarcoma without metastasis at presentation has drastically improved. Long-term survival has improved from 15 to 50%. This increase in survival is primarily due to a multimodality approach to therapy with a combination of surgery, radiotherapy, and chemotherapy.16,17,18,19,20,21,22,23,24,25,26
Table 1.
Reported Cases of Sinonasal Ewing's Sarcoma
| # of Patients | Symptoms at Presentation | Location | Therapy | Follow-up | |
|---|---|---|---|---|---|
| Chemo, chemotherapy; RT, radiation therapy; NED, no evidence of disease; CFR, craniofacial resection. | |||||
| Fernandez et al, 19744 | 2 | Not reported | Maxillary sinus | Chemo RT | Not reported |
| Strong et al, 19795 | 3 | Not reported | Maxillary sinus | Chemo RT | 1 Died 1 NED 23 years 1 NED 4 years |
| Hossfeld et al, 19826 | 1 | Not reported | Maxillary sinus | Chemo RT | Not reported |
| Siegal et al, 19873 | 5 | Facial swelling | 4 Maxillary sinus 1 Ethmoid | RT | NED 7 years |
| Pontius and Sebek, 19817 | 1 | Epistaxis Nasal obstruction | Nasal cavity Maxillary Ethmoid | CFR RT | NED 2 years |
| Woodruff et al, 19888 | 1 | Visual loss | Ethmoid | Chemo RT | In treatment at publication |
| Lane and Ironside, 19909 | 1 | Eye swelling and diplopia | Ethmoid with orbit extension | Chemo CFR | Not reported |
| Csokonai et al, 200110 | 1 | Not reported | Nasal cavity Maxillary sinus | Denker operation RT | NED 3 years |
| Harman et al, 200332 | 1 | Epistaxis Nasal obstruction | Nasal cavity, intracranial extension | Chemo RT | In treatment at publication |
| Aferzon et al, 200311 | 1 | Rhinorrhea Nasal obstruction Epistaxis | Ethmoid | Chemo RT | NED 3 years |
| Windfuhr, 200412 | 1 | Visual loss Facial swelling | Maxillary sinus Orbital involvement | Chemo RT | NED 17 months |
| Howarth et al, 200413 | 1 | Facial swelling | Ethmoid Orbital involvement | Chemo | In treatment at publication |
| Coskun et al, 200514 | 1 | Cheek swelling | Maxillary sinus | Chemo RT | NED 12 months |
| Kawabata et al, 200815 | 1 | Cheek swelling | Maxillary sinus | Chemo RT | NED 20 months |
The use of proton beam therapy as a part of the treatment of Ewing's sarcoma of the sinonasal cavity and skull base has not been examined extensively in the literature. Standard photon radiotherapy can result in marked morbidity when treating paranasal sinus malignancies because of radiosensitive adjacent structures, including the globe, optic nerves, optic chiasm, and brainstem. Photons reach maximal dose at the skin followed by a gradually attenuating dose with increasing depth of penetration. This results in a significantly excessive dose to normal structures proximal to and distal to the target and a large dose gradient inhomogeneity over the target. Protons, on the other hand, have a physical advantage over photons because most of the dose is deposited near the end of its maximal range, with a rapid drop-off within millimeters after this narrow localized high-dose region, also known as the Bragg peak.
The Bragg peaks of multiple proton beams of decreasing energy are superimposed to provide uniform coverage of the target volume. The result is less entrance dose as well as lack of exit dose to nearby structures. This superior physical property of protons potentially allows for dose escalation, as well as decreased acute and late radiation sequelae. In sinonasal and skull base malignancies, protons offer the advantage of sparing critical adjacent structures such as the globe, optic nerve, lacrimal gland, pituitary, and intracranial structures.27
We have employed proton beam therapy as part of a multimodality treatment plan at the Massachusetts Eye and Ear Infirmary (MEEI)/Massachusetts General Hospital (MGH) Cranial Base Center for treatment of Ewing's sarcoma of the paranasal sinuses and skull base. Patients are evaluated by a multidisciplinary team consisting of neurosurgery, otolaryngology–head and neck surgery, radiation oncology, and medical oncology. The current protocol at our institution for patients with traditional resectable disease or in cases where surgery would not cause significant morbidity is chemotherapy followed by surgical resection. For disease that has been deemed unresectable or when surgery would result in significant morbidity (i.e., orbital exenteration), chemotherapy followed by proton beam radiotherapy is employed. For some patients, subtotal resection before proton beam radiotherapy has been employed to help reduce the necessary proton treatment volume or alleviate obstructive sinus symptoms.
MATERIALS AND METHODS
Two patients diagnosed with Ewing's sarcoma involving the anterior skull base were identified by a review of records of patients treated at MEEI and MGH Cranial Base Center. Institutional Review Board approval was obtained. Each patient was seen by a multidisciplinary team that included a radiation oncologist, medical oncologist, otolaryngologist, and neurosurgeon. All medical records, including imaging reports, chemotherapy, and radiation therapy records, were reviewed retrospectively. Extracted information included patient demographics, symptoms at diagnosis, radiographic data, pathological data, treatment received, treatment complications, tumor recurrence, and patient survival.
CASE 1
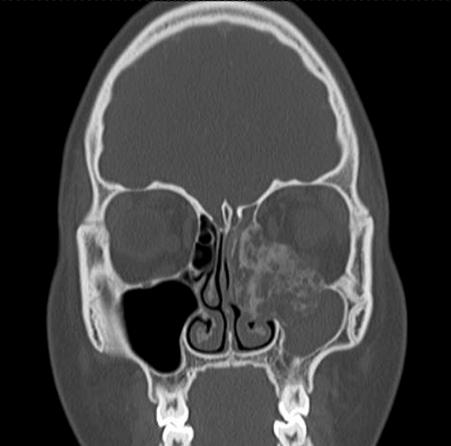
An otherwise healthy 15-year-old female presented to her physician for increasing left nasal congestion and swelling in her left eye. She was experiencing some vague headaches and dizziness for several months before the development of the ocular symptoms, but she did not seek medical attention for these symptoms. She did not have any complaints of diplopia or loss of vision. On initial presentation, her physical examination was significant for proptosis of her left eye. Endoscopic nasal examination revealed a vascular-appearing mass filling the middle meatus. Imaging studies were obtained. The computed tomography (CT) scan showed a large, heterogeneous, enhancing mass with calcifications in the left ethmoid sinus, extending to the skull base and eroding the medial wall and floor of the orbit. The left maxillary sinus was opacified with mucous (Fig. 1). An endoscopic biopsy was performed, which revealed Ewing's sarcoma. Immunohistochemical studies showed that the tumor cells were positive for vimentin and CD99 and focally for epithelial membrane antigen (EMA), and negative for keratin, desmin, muscle-specific actin, myogenin, myo-D1, and FLI-1. Fluorescence in situ hybridization (FISH) analysis revealed a translocation involving EWSR1. Initial metastatic workup, including chest CT scan, did not show evidence of metastatic disease. She was started on a chemotherapy regimen of vincristine, Adriamycin, and Cytoxan. She developed a seizure during her second cycle of chemotherapy and was started on Keppra, after which she did not have recurrence of seizure activity. Her chemotherapy regimen then involved alternating ifosfamide (IF), etoposide (ET) chemotherapy with vincristine, Adriamycin, and Cytoxan every 3 weeks.
Figure 1.
Preoperative coronal computed tomography (CT) scan of patient 1 showing involvement of the left maxillary sinus, ethmoid sinus, skull base, and orbit.
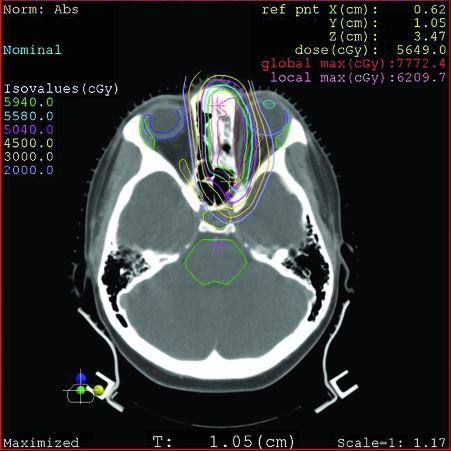
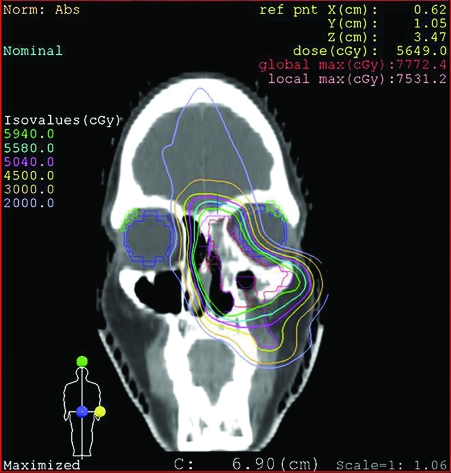
After completing five cycles of chemotherapy, the patient had moderate response. She was referred to our institution for local consolidation with proton beam radiation therapy. Because of severe obstructive sinusitis, she underwent endoscopic subtotal resection before the start of radiation therapy to improve sinus function and to facilitate proton beam radiation therapy. Her postoperative course was unremarkable. She completed 8 weeks of proton beam radiation therapy to a total dose of 45 GyE (Gy equivalent of protons to 1 Gy of photons) at 1.8 GyE per fraction to the prechemotherapy and preresection extent of disease, followed by a cone down to residual disease to a dose 59.4 GyE (Figs. 2 and 3). She completed treatment on December 19, 2006 and is currently disease free. One year after completion of treatment, she developed frontal headaches, and a CT scan showed frontal sinus opacification. Endoscopic sinus surgery was performed for drainage of the frontal sinus, which revealed thick mucus. Biopsies of the frontal recess and the area of the original tumor were performed at the same time, and there was no evidence of residual tumor. The frontal opacification and her headaches resolved after the endoscopic sinus surgery and she is currently doing well.
Figure 2.
Axial computed tomography (CT) proton beam radiation planning for patient 1, showing full treatment dosage to the tumor with decreased dosage to the orbit.
Figure 3.
Coronal computed tomography (CT) proton beam radiation planning for patient 1, showing full treatment dosage to the tumor with decreased dosage to orbit and frontal lobe.
CASE 2
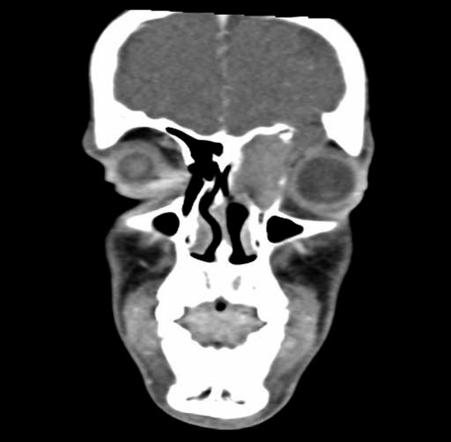
An otherwise healthy 17-year-old male presented to his physician with slowly increasing left-sided proptosis. In the 6 months before diagnosis he complained of increasing sinonasal symptoms of left nasal obstruction and drainage. He was treated with several courses of antibiotics, which did not improve his symptoms. Eventually, he began to develop proptosis. On initial examination, he was noted to have significant orbital proptosis and inferior deviation of the globe. His visual acuity was intact, but he experienced diplopia with upward gaze. Endoscopic examination revealed a vascular mass in the left middle meatus. A CT scan showed a soft tissue mass centered in the left ethmoid cavity, with extension to the skull base and erosion of the superior lamina papyracea and extension into the orbit (Fig. 4). The frontal sinus was also opacified, and there was erosion of the lateral aspect of the superior frontal sinus from mucocele formation. An endoscopic biopsy revealed Ewing's sarcoma. Immunohistochemical studies revealed the tumor cells to be negative for actin, desmin, and S100. Staining for CD99 was strong and diffuse. A FISH analysis was done and confirmed rearrangement of the EWSR1 gene at 22 q12. Metastatic workup including whole body magnetic resonance imaging (MRI), and bone scan did not show any evidence of metastatic disease. He received vincristine, doxorubicin, and cyclophosphamide, alternating with VP-16 and IF. This was followed by a course of proton beam radiation therapy to a dose of 45 GyE at 1.8 GyE per fraction to the initially involved area and a cone down to a total dose of 55.8 GyE to the postchemotherapy extent of disease. His treatment was completed in March of 2007, and he is currently disease free. In October of 2007, he was noted to have a persistent frontal sinus mucocele and ethmoid opacification despite regression of the tumor, and he underwent endoscopic sinus surgery for drainage of the frontal sinus mucocele and biopsy of the residual opacification. Biopsy did not show evidence of persistent disease. The frontal mucocele was adequately drained, and there has been no evidence of residual frontal opacification on repeat imaging.
Figure 4.
Preoperative coronal computed tomography (CT) scan of patient 2 showing involvement of the left maxillary sinus, ethmoid sinus, skull base, and orbit, with a frontal sinus mucocele involving the lateral frontal sinus.
DISCUSSION
Ewing's sarcoma was originally named for James Ewing in 1921, who initially described the tumor as a diffuse endothelioma. It is now considered to be a tumor within the primitive neuroectodermal tumor class.28 In the Intergroup Ewing's Sarcoma Study (IESS), Ewing's sarcoma comprised ~4% of the primary bone tumors of the head and neck. In this review, the most commonly involved site in the head and neck was the skull (11 of 29), followed by the mandible and maxilla. Only one patient had tumor involving the ethmoid sinus. In this group, typical symptoms were related to mass effect of the tumor.3 The most common presenting symptom was a mass or swelling at the site of the tumor. Other symptoms reported were related to ocular or cranial nerve involvement, such as oculomotor dysfunction or proptosis.3 There are very few reported cases of Ewing's sarcoma involving the paranasal sinuses. A review of the literature revealed 14 articles, the majority of which are case reports. Details of these cases are listed in Table 1.3,4,5,6,7,8,9,10,11,12,13,14,15
The diagnosis is made by pathological analysis.28 Ewing's sarcoma is one of the small, blue, round cell tumors of childhood. Histologically, the differential diagnosis includes lymphoma, rhabdomyosarcoma, neuroblastoma, and primitive neuroectodermal tumor (PNET).29,30,31,32 In addition to histochemical analysis, molecular testing is often necessary to identify signature translocations involving the EWS gene (balanced translocation involving chromosomes 11 and 22). These translocations are detectable with both reverse transcriptase polymerase chain reaction (RT-PCR) and fluorescence in situ hybridization (FISH) in formalin-fixed, paraffin-embedded tissue. Bridge, et al reported 100% sensitivity and specificity for a commercial EWS probe, whereas RT-PCR had a sensitivity of 54% and specificity of 85%.33
The prognosis for this tumor has progressively improved over the last decade due to a combination of increased awareness and recognition of the diagnosis as well as the improvement in multimodality therapy. For patients with Ewing's sarcoma of the head and neck, tumors arising in the maxilla or mandible have had the best overall prognosis.3
Treatment usually consists of multimodality therapy incorporating chemotherapy, radiation therapy, and surgery. Overall, the use of chemotherapy and radiation therapy has greatly improved disease-free survival. The Cooperative Ewing's Sarcoma Studies 1981 (CESS-81) compared three treatment regimens: surgical resection, primary radiation therapy, and combination surgery and radiation therapy, with 5-year survival rates being 54, 43, and 68%, respectively. However, a follow-up study looking at 3-year follow-up by the same group (CESS-86) showed no statistical difference within the treatment groups (62 to 67%), thus advocating potential for radiation alone when surgery would lead to significant morbidity. The authors concluded that combined local treatment (surgery and radiation) improved locoregional control and probably improved survival in high-risk patients.34
The use of adjuvant chemotherapy has been shown to have positive effects. The IESS-II reported a disease-free survival rate of 68% with their protocol using adjuvant vincristine, Adriamycin, and cyclophosphamide (VACA).35 The use of neoadjuvant IF and ET has been shown to be effective in patients who have relapsed after treatment with VACA; however, the addition of IF and ET to the VACA regimen has not been shown to have any additional advantage.36
Based on the available literature and our experience with Ewing's sarcoma localized to the sinonasal cavity and skull base, a multimodality treatment regimen is the treatment of choice. Initial chemotherapy is followed by either surgical resection, radiation therapy, or a combination of both, depending on the location of the tumor at initial presentation. If the tumor is thought to be surgically resectable without significant morbidity, surgery is suggested after completion of chemotherapy. If the tumor is thought to be unresectable or if surgery would result in significant morbidity, proton beam radiation therapy is used for local control.
In our two cases of Ewing's sarcoma involving the sinonasal cavity and anterior cranial base, a rarely reported entity, neither patient had evidence of metastatic disease at the time of presentation. Because surgical resection for both patients would have potentially required anterior craniofacial resection with orbital exenteration to achieve en bloc resection with negative margins, the decision by our multimodality group was to proceed with induction chemotherapy followed by proton beam radiation therapy for local control. One patient underwent preoperative endoscopic subtotal removal to improve the radiation field and facilitate adequate sinus drainage during radiation therapy. She had no postsurgical sequelae, radiation therapy was not delayed, and the proton treatment volume in the maxillary sinus was significantly reduced.
In the group of patients, where total surgical resection would result in significant morbidity, subtotal resection via an endoscopic approach (with minimal morbidity) might be useful to reduce the proton treatment volume necessary. In addition, for patients with tumors involving the sinonasal cavity, endoscopic sinus surgery to facilitate sinus drainage and minimize sinus obstruction and infection might also be a helpful part of the treatment regimen. Both patients underwent endoscopic sinus surgery after completion of treatment. This was performed to confirm there was no evidence of persistent disease at the site of the original tumor, but the surgery also addressed blockage of the frontal outflow tract concurrently. Both patients had resolution of frontal headaches after the endoscopic sinus surgery, and there has been no evidence of recurrent frontal sinus opacification.
CONCLUSION
Ewing's sarcoma is a primary bone tumor that occurs rarely in the bones of the face and skull. Involvement of the sinonasal cavity and anterior skull base is even rarer, and there are very few reported cases in the literature. In young patients presenting with a sinonasal mass, it must be considered as part of the differential diagnosis. Definitive diagnosis depends on histopathology, and endoscopic biopsy can be used for this purpose. Treatment of Ewing's sarcoma involves a combination of chemotherapy for systemic treatment followed by local treatment with surgery or radiation therapy. In the case of Ewing's sarcoma involving the skull base, surgery would often result in significant morbidity, requiring craniofacial resection and orbital exenteration. Therefore, radiation therapy for local control should be considered. Proton beam radiotherapy has excellent application for sinonasal malignancies because it limits morbidity to the surrounding structures, including the globe, optic nerves, and intracranial cavity. Proton beam radiotherapy has been successfully used at our institution as part of a multimodality approach for the treatment of Ewing's sarcoma of the sinonasal cavity and anterior skull base, and should be considered for treatment of this rare malignancy.
NOTES
This report was presented at the 7th International Conference on Head and Neck Cancer, San Francisco, California, July 21, 2008.
REFERENCES
- Dahlin D C, Coventry M B, Scanlon P W. Ewing's sarcoma: a critical analysis of 165 cases. J Bone Joint Surg Am. 1961;43-A:185–192. [PubMed] [Google Scholar]
- Spjut H J, Dorfman H D, Fechner R E, Ackerman L V. Tumors of bone and cartilage. In: Atlas of Tumor Pathology. 2nd series, fasc 5. Washington, DC: Armed Forces Institute of Pathology; 1971. p. 317.
- Siegal G P, Olivier W R, Reinus W R. Primary Ewing's sarcoma involving the bones of the head and neck. Cancer. 1987;60:2829–2840. doi: 10.1002/1097-0142(19871201)60:11<2829::aid-cncr2820601139>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Fernandez C H, Lindberg R D, Sutox W W, Samuels M L. Localized Ewing's Sarcoma – treatment and results. Cancer. 1974;34(1):143–148. doi: 10.1002/1097-0142(197407)34:1<143::aid-cncr2820340121>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Strong L C, Herson J, Osborne B M, Sutow W W. Risk of radiation-related subsequent malignant tumors in survivors of Ewing's sarcoma. J Natl Cancer Inst. 1979;62(6):1401–1406. [PubMed] [Google Scholar]
- Hossfeld D K, Seeber S, Siemers E, Schmidt C G, Scherer E. Early results of combined modality therapy of patients with Ewing's sarcoma. Recent Results Cancer Res. 1982;80:124–127. doi: 10.1007/978-3-642-81685-7_21. [DOI] [PubMed] [Google Scholar]
- Pontius K I, Sebek B A. Extraskeletal Ewing's sarcoma arising in the nasal fossa. Light- and electron-microscopic observations. Am J Clin Pathol. 1981;75(3):410–415. doi: 10.1093/ajcp/75.3.410. [DOI] [PubMed] [Google Scholar]
- Woodruff G, Thorner P, Skarf B. Primary Ewing's sarcoma of the orbit presenting with visual loss. Br J Ophthalmol. 1988;72(10):786–792. doi: 10.1136/bjo.72.10.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane S, Ironside J W. Extra-skeletal Ewing's sarcoma of the nasal fossa. J Laryngol Otol. 1990;104(7):570–573. doi: 10.1017/s0022215100113192. [DOI] [PubMed] [Google Scholar]
- Csokonai L V, Liktor B, Arato G, Helffrich F. Ewing's sarcoma in the nasal cavity. Otolaryngol Head Neck Surg. 2001;125(6):665–667. doi: 10.1067/mhn.2001.119486. [DOI] [PubMed] [Google Scholar]
- Aferzon M, Wood W E, Powell J R. Ewing's sarcoma of the ethmoid sinus. Otolaryngol Head Neck Surg. 2003;128(6):897–901. doi: 10.1016/S0194-59980300452-2. [DOI] [PubMed] [Google Scholar]
- Windfuhr J P. Primitive neuroectodermal tumor of the head and neck: incidence, diagnosis, and management. Ann Otol Rhinol Laryngol. 2004;113(7):533–543. doi: 10.1177/000348940411300705. [DOI] [PubMed] [Google Scholar]
- Howarth K L, Khodaei I, Karkanevatos A, Clarke R W. A sinonasal primary Ewing's sarcoma. Int J Pediatr Otorhinolaryngol. 2004;68(2):221–224. doi: 10.1016/j.ijporl.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Coskun B U, Cinar U, Savk H, Basak T, Dadas B. Isolated maxillary sinus Ewing's sarcoma. Rhinology. 2005;43(3):225–228. [PubMed] [Google Scholar]
- Kawabata M, Yoshifuku K, Sagara Y, Kurono Y. Ewing's sarcoma/primitive neuroectodermal tumour occurring in the maxillary sinus. Rhinology. 2008;46(1):75–78. [PubMed] [Google Scholar]
- Jaffe N, Traggis D, Salian S, Cassady J R. Improved outlook for Ewing's sarcoma with combination chemotherapy (vincristine, actinomycin D and cyclophosphamide) and radiation therapy. Cancer. 1976;38:1925–1930. doi: 10.1002/1097-0142(197611)38:5<1925::aid-cncr2820380510>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Rosen G, Caparros B, Nirenberg A, et al. Ewing's sarcoma: ten-year experience with adjuvant chemotherapy. Cancer. 1981;47:2204–2213. doi: 10.1002/1097-0142(19810501)47:9<2204::aid-cncr2820470916>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Jurgens H, Exner U, Gadner H, et al. Multidisciplinary treatment of primary Ewing's sarcoma of bone. A 6-year experience of a European Cooperative Trial. Cancer. 1988;61:23–32. doi: 10.1002/1097-0142(19880101)61:1<23::aid-cncr2820610106>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Bacci G, Toni A, Avella M, et al. Long-term results in 144 localized Ewing's sarcoma patients treated with combined therapy. Cancer. 1989;63:1477–1486. doi: 10.1002/1097-0142(19890415)63:8<1477::aid-cncr2820630805>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Burgert E O, Nesbit M E, Garnsey L A, et al. Multimodal therapy for the management of nonpelvic, localized Ewing's sarcoma of bone: Intergroup Study IESS-II. J Clin Oncol. 1990;8:1514–1524. doi: 10.1200/JCO.1990.8.9.1514. [DOI] [PubMed] [Google Scholar]
- Nesbit M E, Gehan E A, Burgert E O, et al. Multimodal therapy for the management of primary nonmetastatic Ewing's sarcoma of bone: a long-term follow-up of the first Intergroup Study. J Clin Oncol. 1990;8:1664–1674. doi: 10.1200/JCO.1990.8.10.1664. [DOI] [PubMed] [Google Scholar]
- Kinsella T J, Miser J S, Waller B, et al. Long-term follow-up of Ewing's sarcoma of bone treated with combined modality therapy. Int J Radiat Oncol Biol Phys. 1991;20:389–395. doi: 10.1016/0360-3016(91)90047-8. [DOI] [PubMed] [Google Scholar]
- Dunst J, Sauer R, Burgers M V, et al. Radiation therapy as local treatment in Ewing's sarcoma. Results of the cooperative Ewing's sarcoma studies CESS 81 and CESS 86. Cancer. 1991;67:2818–2825. doi: 10.1002/1097-0142(19910601)67:11<2818::aid-cncr2820671118>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Hayes F A, Thompson E J, Meyer W H, et al. Therapy for localized Ewing's sarcoma of bone. J Clin Oncol. 1989;7:208–213. doi: 10.1200/JCO.1989.7.2.208. [DOI] [PubMed] [Google Scholar]
- Bacci G, Campanacci M, Pagani P A. Adjuvant chemotherapy in the treatment of clinically localised Ewing's sarcoma. J Bone Joint Surg Br. 1978;60-B:567–574. doi: 10.1302/0301-620X.60B4.711809. [DOI] [PubMed] [Google Scholar]
- Oberlin O, Habrand J L, Zucker J M, et al. No benefit of Ifosfamide in Ewing's sarcoma: a nonrandomized study of the French Society of Pediatric Oncology. J Clin Oncol. 1992;10:1407–1412. doi: 10.1200/JCO.1992.10.9.1407. [DOI] [PubMed] [Google Scholar]
- Patel S, DeLaney T F. Advanced technology radiation therapy for bone sarcomas. Cancer Control. 2008;15(1):21–37. doi: 10.1177/107327480801500104. [DOI] [PubMed] [Google Scholar]
- Ewing J. Diffuse endothelioma of bone. Proc N Y Pathol Soc. 1921;21:17–24. [Google Scholar]
- Batsakis J G, Mackay B, El-Naggar A K. Ewing's sarcoma and peripheral primitive neuroectodermal tumor: an interim report. Ann Otol Rhinol Laryngol. 1996;105:838–843. doi: 10.1177/000348949610501014. [DOI] [PubMed] [Google Scholar]
- Wood R E, Nortje C J, Hesseling P, et al. Ewing's tumor of the jaw. Oral Surg Oral Med Oral Pathol. 1990;69:120–127. doi: 10.1016/0030-4220(90)90280-6. [DOI] [PubMed] [Google Scholar]
- Yalcin S, Turoglu H T, Ozdamar S, Sadikoglu Y, Gurbuzer B, Yenici O. Ewing's tumor of the mandible. Oral Surg Oral Med Oral Pathol. 1993;76(3):362–367. doi: 10.1016/0030-4220(93)90269-a. [DOI] [PubMed] [Google Scholar]
- Harman M, Kiroglu F, Kosem M, et al. Primary Ewing's sarcoma of the paranasal sinus with intracranial extension: Imaging features. Dentomaxillofacial Radiol. 2003;32(5):343–346. doi: 10.1259/dmfr/17098962. [DOI] [PubMed] [Google Scholar]
- Bridge R S, Rajaram V, Dehner L P, Pfeifer J D, Perry A. Molecular diagnosis of Ewing sarcoma/primitive neuroectodermal tumor in routinely processed tissue: a comparison of two FISH strategies and RT-PCR in malignant round cell tumors. Mod Pathol. 2006;19:1–8. doi: 10.1038/modpathol.3800486. [DOI] [PubMed] [Google Scholar]
- Fiorillo A, Tranfa F, Canale G, et al. Primary Ewing's sarcoma of the maxilla, a rare and curable localization: report of two new cases, successfully treated by radiotherapy and systemic chemotherapy. Cancer Lett. 1996;103(2):177–182. doi: 10.1016/0304-3835(96)04210-3. [DOI] [PubMed] [Google Scholar]
- Burgert E O, Jr, Nesbit M E, Jr, Gehan E A, et al. Multimodal therapy for the management of nonpelvic, localized Ewing's sarcoma of bone: Intergroup Study IESS-II. J Clin Oncol. 1990;8:1514–1524. doi: 10.1200/JCO.1990.8.9.1514. [DOI] [PubMed] [Google Scholar]
- Bacci G, Picci P, Ferrari S, et al. Neoadjuvant chemotherapy for Ewing's sarcoma of bone: No benefit observed after adding ifosfamide and etoposide to vincristine, actinomycin, cyclophosphamide, and doxorubicin in the maintenance phase—results of two sequential studies. Cancer. 1998;82(6):1174–1183. doi: 10.1002/(sici)1097-0142(19980315)82:6<1174::aid-cncr24>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]