Abstract
Purpose:
The optimal regimen for the treatment of metastatic colorectal cancer (CRC) remains uncertain. We sought to document clinicians' treatment recommendations and determine the motivation behind them.
Materials and Methods:
A postal questionnaire was sent to all members of the Medical Oncology Group of Australia concerning chemotherapy treatment options in the setting of metastatic CRC.
Results:
The response rate was 59.7% (n = 188). One hundred sixty-two physicians (86%) treated patients with CRC. Of the 162 physicians, 92.6% (n = 150) recommended oxaliplatin-based regimens as first-line treatment for CRC due to perceived superior efficacy (66.9%; n = 107) or toxicity profile (17%; n = 27). Fluorouracil (FU), leucovorin (LV), and oxaliplatin (FOLFOX6) was the most popular regimen (59.3%; n = 98). Calcium and magnesium to prevent oxaliplatin-related neurotoxicity was routinely used by 34.6% of physicians (n = 56) from cycle 1. Despite the lack of phase III data at the time, 8.6% of physicians (n = 14) selected capecitabine and oxaliplatin (XELOX) a preferred first-line regimen; 61.7% of physicians (n = 100) recommended FU, LV, and irinotecan (FOLFIRI) second-line treatment. Concerning LV dose, one third of physicians (33.3%; n = 54) selected 20 mg/m2 and one third of physicians (32.7%, n = 53) selected 200 mg/m2, with 25.3% of physicians (n = 41) using a fixed 50 mg bolus.
Conclusion:
This survey demonstrated considerable variation regarding recommended chemotherapy for patients with metastatic CRC. Of considerable concern is the use of calcium and magnesium based on retrospective data alone. Given that this variation in practice may significantly impact patient outcomes, additional studies are required to improve understanding of physician attitudes and the motivations behind treatment decision making.
Short abstract
Treatment options for colorectal cancer have expanded to include multiple oxaliplatin- and irinotecan-based regimens and more biological/targeted therapies.
Introduction
In developed countries, colorectal cancer (CRC) is one of the most common malignancies and is a leading cause of cancer-related death.1 The last two decades have seen substantial improvements in survival for patients with metastatic CRC treated with chemotherapy and biologic therapies. Treatment options have expanded from fluorouracil (FU) alone to include multiple oxaliplatin- and irinotecan-based regimens (Appendix Table A1, online only) as well as a growing number of biologic/targeted therapies such as bevacizumab, cetuximab, and panitumumab.
When considering treatment for metastatic CRC, chemotherapy options generally fall into three broad groups: FU/leucovorin (LV) or capecitabine alone, oxaliplatin-based regimens, and irinotecan-based regimens. The National Comprehensive Cancer Network practice guidelines describe a wide range of appropriate first-line and second-line options for metastatic CRC and stratify choices based on ability to tolerate intensive therapy.2 Numerous trials in the last decade have studied a large number of regimens, potentially leading to variation in practice and uncertainty as to what constitutes the optimal dose, schedule, and combination of treatment. Studies that have directly compared oxaliplatin- and irinotecan-based regimens3–5 have suggested overall efficacy and rate of severe toxicity to be similar, although toxicity profiles differ. In many instances, therefore, the choice of agents is based on performance status, comorbidities, and patient or physician preferences.
In addition to issues regarding drug sequence, many unresolved questions about dose, schedule, and potential adjuvants remain. One key question concerns the optimal FU regimen to use with oxaliplatin, given that regimens of FU, LV, and oxaliplatin, (FOLFOX4, FOLFOX6, and FOLFOX7) use different doses and schedules of the same chemotherapy drugs, and have all been selected as standard regimens in recent trials.3,5,6 Calcium and magnesium, until recently, seemed a reasonable addition to oxaliplatin-based regimens as a strategy to reduce the incidence of acute and delayed neurotoxicity. Irinotecan may be used as a single agent, at varying doses and in different schedules; alternatively, irinotecan may be used in combination with bolus or infusional FU. For all regimens that incorporate FU, the optimal dose of LV remains uncertain.
Thus, there are numerous options available to oncologists when considering chemotherapy choices for their patients with metastatic CRC. This situation presents an opportunity to explore decision making, motivations, and knowledge base of medical oncologists.
Materials and Methods
Study Population
All medical oncologists and advanced trainee registrars who were members of the Medical Oncology Group of Australia were mailed a survey in mid-2006 (n = 350). The vast majority of practicing Australian medical oncologists are members of this body. Trainees are senior physicians with at least 4 years of postgraduate medical experience (equivalent to fellows before board certification in the United States). The survey was anonymous; identification numbers were used to track responses and allow follow-up of nonresponders. A reminder e-mail was sent close to the cutoff date to all Medical Oncology Group of Australia members. Nonresponders were mailed the survey again.
Physician demographic information included practice setting (public or private); trainee versus consultant oncologist status; years of experience; and proportion of patients observed with CRC. Additional demographic details were not sought to protect anonymity of the clinicians.
Design
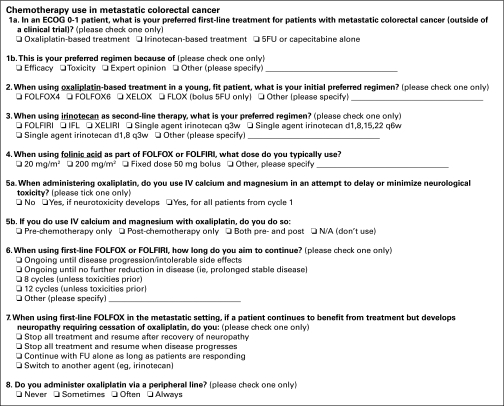
The survey was a clinician-driven project, designed and developed by a panel of oncologists. This was part of a larger survey comprised of three parts. Results from the other parts of the survey have been presented elsewhere.7,8 The survey was pretested with 10 oncologists, and based on feedback, the questionnaire was modified to improve clarity. Clinicians were asked their preferred first-line chemotherapy treatment for fit (Eastern Cooperative Oncology Group [ECOG] performance status 0 to 1) patients with metastatic CRC, the main reason for this choice, their preferred oxaliplatin- and irinotecan-based regimens, and their preferred dose of LV. In addition, questions concerning oxaliplatin-based treatment included the use of intravenous calcium and magnesium and whether oxaliplatin was administered via a peripheral or central venous access device (Appendix Fig A1, online only). Biologic therapies such as cetuximab and bevacizumab are available in Australia but are not subsidized by the Pharmaceutical Benefits Scheme and are not widely available—neither at the time of the survey in 2006 nor at present. Their use was not investigated in this survey.
Figure A1.
Survey questions. ECOG, Eastern Cooperative Oncology Group; 5FU, fluorouracil; LV, leucovorin; FOLFOX, oxaliplatin, LV, and 5FU; XELOX, XELOX, capecitabine, and 5FU; FOLFIRI, irinotecan, LV, and 5FU; IFL, irinotecan, LV, and 5FU; XELIRI, irinotecan and capecitabine; N/A, not applicable.
Analysis
Data analysis was conducted using Stata SE 9.0 (College Station, TX). All P values given are two-sided, and were calculated using the Mantel-Haenszel test, or with Fisher's exact test on occasions that the sample size was small. Responses were compared looking for any significant differences between consultants and trainees, public versus private practices, proportion of patients treated with CRC, and years of practice experience.
Results
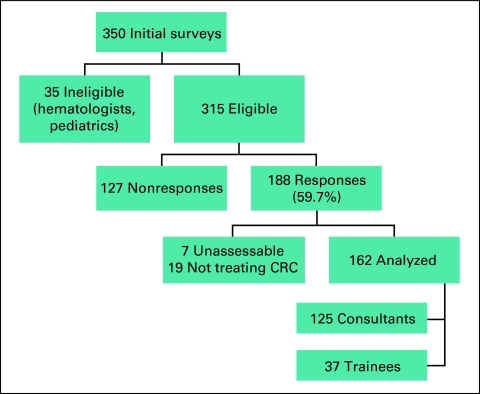
From an initial 350 surveys mailed, 35 respondents were considered ineligible (pediatric oncologists, hematologists, or not in clinical practice). From the remaining 315, 188 clinicians returned the survey (response rate, 59.7%). Of the 188 responses, seven were incomplete, and 19 clinicians stated that they did not treat patients with CRC. Thus, 162 responses remained: 125 consultants and 37 trainees (Fig 1). Of the consultants, 59.2% (n = 74) estimated that patients with CRC represented less than 20% of their practice. Patients with CRC comprised more than 20% of the practices of the remaining 40.8% of consultants (n = 51). This variety in experience with CRC was considered broadly representative of Australian oncologists.
Figure 1.
Survey Responses. CRC, colorectal cancer.
Preferred Chemotherapy Drug Regimen
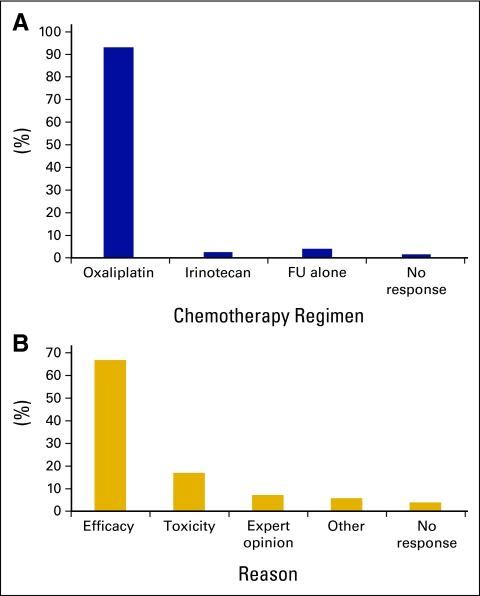
Clinicians were asked about their preferred chemotherapy regimen for a fit (ECOG performance status 0 to 1) patient with metastatic CRC in the first line-setting (Fig 2A). One hundred fifty (92.6%) respondents preferred an oxaliplatin-based regimen, with only 2.5% (n = 4) choosing an irinotecan-based regimen. The most common reason given for choosing this regimen was “efficacy” (66.9%; n = 108), whereas 16.9% (n = 27) selected “toxicity,” and 6.9% (n = 11) chose “expert opinion” (Fig 2). Responses did not differ significantly with level of experience with CRC or according to years of experience, although trainees were more likely to select “expert opinion” (16.2%, n = 6) compared with consultants (4.1%, n = 5; P = .01).
Figure 2.
(A) Preferred first-line treatment for patients with metastatic colorectal cancer; (B) reason for preferred first-line regimen. FU, fluorouracil.
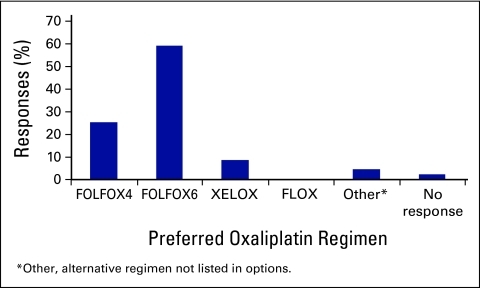
Respondents were given the choice of a number of common oxaliplatin- and irinotecan-based regimens and asked which they generally recommended in the metastatic disease setting for a fit patient. Table A1 outlines the common chemotherapy regimens in use for metastatic CRC. For oxaliplatin-based regimens, the majority (59.3%, n = 98) selected FOLFOX6, which avoids the day 2 chemotherapy bolus (Fig 3). Of the remainder, 25.3% of respondents (n = 41) selected FOLFOX4, and 8.6% of respondents (n = 14) chose capecitabine and oxaliplatin (XELOX). Trainees were equally as likely to choose FOLFOX4 or FOLFOX6, whereas more consultants than trainees selected FOLFOX6 (56% v 32.4%; P = .02). No respondents selected the bolus FLOX regimen.
Figure 3.
Preferred oxaliplatin-based (first-line) regimens. FOLFOX, oxaliplatin, leucovorin (LV), and fluorouracil (FU); XELOX, capecitabine and oxaliplatin; FLOX, FULV and oxaliplatin.
The majority of clinicians (61.7%, n = 100) selected FU, LV, and irinotecan (FOLFIRI) as the preferred irinotecan-based regimens in the second-line setting; the next most common choice was single-agent irinotecan every 3 weeks (14.8%, n = 24). Only eight physicians (4.9%) selected FU, LV, and irinotecan (IFL).
Addressing Oxaliplatin Neuropathy and Administration
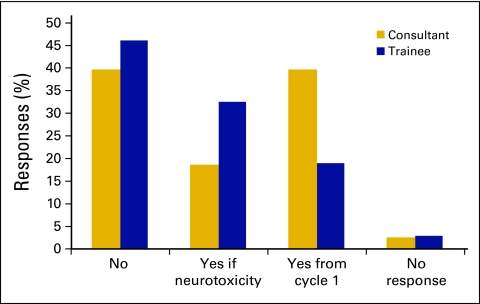
The survey asked respondents to document their use of calcium and magnesium at the time of oxaliplatin administration (Fig 4). Overall, 40.7% of respondents (n = 66) never used calcium and magnesium; 21.6% of respondents (n = 35) used these agents if neurotoxicity developed, and 34.6% of respondents (n = 56) used them in every patient from cycle 1. Consultants overall were more likely than trainees to use calcium and magnesium routinely from cycle 1 (39.5% v 18.9%; P = .02). There were no significant differences depending on level of experience with CRC.
Figure 4.
Use of calcium and magnesium with oxaliplatin: consultant versus trainee status.
Once oxaliplatin-related neuropathy develops, the most common response was to continue with FU alone if there was ongoing disease response (42%, n = 69). Only 5% of respondents (n = 8) said they would restart oxaliplatin once the neuropathy had resolved. Responses did not differ significantly according to physician experience.
The survey also asked about administration of oxaliplatin through a peripheral intravenous cannula versus central access. Whereas 39.5% (n = 64) said they would never use a peripheral cannula, 4.3% of consultants (n = 7) answered that they always followed this practice.
LV Dose
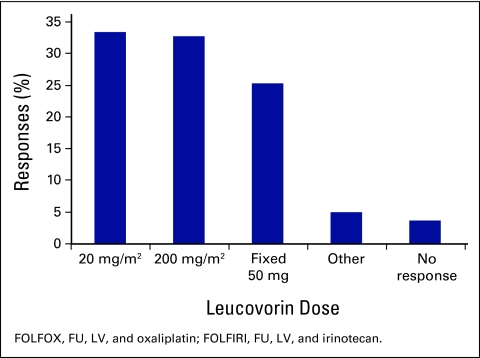
Responses to the question of what dose of LV clinicians used when giving FOLFOX or FOLFIRI (Fig 5) varied widely, with 33.3% of respondents (n = 54) using 20 mg/m2, 32.7% of respondents (n = 53) using 200 mg/m2, and 25.3% of respondents (n = 41) using a fixed 50 mg bolus. There were no significant differences depending on years of experience or proportion of patients seen with CRC.
Figure 5.
Choice of leucovorin dose used in FOLFOX/FOLFIRI. FU, fluorouracil; LV, leucovorin.
Duration of Chemotherapy Treatment
When asked how long they would aim to continue FOLFOX or FOLFIRI, 32.1% of respondents (n = 52) stated they would aim for 12 cycles, 27.8% of respondents (n = 45) would continue until prolonged stable disease, and 21% of respondents (n = 34) selected ongoing chemotherapy until toxicities or disease progression. There were no significant differences based on years of experience or level of experience in treating CRC.
Discussion
This survey highlights the wide variety of choices clinicians face when treating patients with metastatic CRC, not only in chemotherapy regimens, but in adjuncts such as LV and calcium and magnesium infusions. To some extent this variation is expected in a rapidly evolving and complex area; however, it is interesting to explore why physicians are motivated to select one particular regimen instead of another. Potential factors include varying knowledge of the clinical trial literature, the influence of expert opinion, familiarity with particular regimens, or perhaps the influence of pharmaceutical marketing. However, this survey also demonstrates a willingness to embrace strategies that are not well established.
Perhaps the most striking finding is the use of calcium and magnesium prophylaxis for oxaliplatin neuropathy in 2006, based on retrospective data alone, which has since become a controversial issue. Limited evidence at the time suggested that calcium and magnesium might protect against acute and possibly chronic cumulative neurotoxicity.9,10 A 2005 literature review concluded that calcium and magnesium seemed effective in treating and reducing the severity of neuropathic symptoms.11 This survey shows that the use of calcium and magnesium had been widely adopted in Australia despite an absence of phase III data. More recently, the CONCEPT trial, a phase III study, designed to explore the effect of calcium and magnesium prophyplaxis on oxaliplatin neuropathy, was halted due to an interim analysis revealing significantly reduced response rates in the investigational arm.12 A subsequent independent review of scans found no significant difference in responses, however.12a The results of our survey, particularly in light of the CONCEPT data, highlight the importance of evidence-based medicine, in particular using data from randomized phase III trials, to guide decision making.
The results of our survey demonstrated a dominant use of oxaliplatin in first-line therapy, with irinotecan-based regimens recommended for only 2.5% of patients. More than two thirds of respondents indicated that they chose an oxaliplatin-based chemotherapy as first-line treatment for reasons of superior efficacy. This is not inconsistent with currently available data. Some studies have suggested efficacy advantages of oxaliplatin (the oxaliplatin regimen achieved superior outcomes in the N9741 study13); however, many experts attribute this outcome to differences in the FU regimens, and differential crossover to the alternate treatment arm. In the Tournigand study,3 in which a similar FU regimen was employed in both arms and crossover was not compromised, response rates and survival were equivalent. A recent publication describing Australian practice showed a marked increase in the use of first-line oxaliplatin at the time the N9741 study was first reported,14 suggesting that Australian oncologists were significantly influenced by this study. Though it is certainly appropriate clinically to use oxaliplatin as a first-line agent in metastatic CRC, the dominant selection of oxaliplatin for efficacy reasons (rather than toxicity) nevertheless remains largely unsupported by clinical studies. It is possible that the increased use of oxaliplatin might be due to influence from the pharmaceutical industry.
The various oxaliplatin-containing regimens have not been compared directly. Each has been used as a standard in recent randomized studies.3,5,6 Of the infusional regimens, FOLFOX6, which eliminates the day 2 bolus, was the regimen of choice for 60% of oncologists. This may be driven by convenience (for both the patient and the medical system); however, this was not explored in the survey.
The substitution of capecitabine for infusional FU seems a logical choice in the metastatic disease setting where convenience and ease of administration are particularly important. At the time of the survey, phase III studies had shown capecitabine alone to be at least equivalent to infusional FU/LV alone in the adjuvant colon and metastatic colorectal disease settings; however, published results for XELOX in metastatic CRC were from phase II studies only. In this context it is of interest that 14 clinicians (8.6%) selected XELOX as their standard first-line oxaliplatin-based regimen. Subsequent publications after this survey was completed, including three phase III trials, suggest that XELOX is an acceptable alternative to FOLFOX in first-line treatment.15–17
The array of irinotecan-based treatment regimens in the second-line setting is again broad, with the choice of irinotecan alone (multiple doses and schedules) or an FU-containing combination, and the additional options of bolus or infusional FU. Though single-agent irinotecan was used in the pivotal studies demonstrating the impact of this agent in the second-line setting,18–20 the infusional regimen FOLFIRI was the dominant second-line choice of the clinicians surveyed. A recent randomized phase II study involving 55 patients with metastatic CRC treated in the second-line setting compared single-agent irinotecan with or without infusional FU, and showed similar outcomes.21
The optimal dose of LV when combined with FU has long been debated. The available evidence suggests that there are no clear benefits of high-dose compared with low-dose LV.22,23 Outcomes seem equivalent, but toxicity, in particular diarrhea, may be greater with higher doses of leucovorin.23 As a result, FU-alone, FOLFOX4, and FOLFOX6 regimens have been modified to include a lower dose of LV.24 The choices for LV dosing with FOLFOX or FOLFIRI in this study were clearly varied with close to one third of clinicians each choosing 20 mg/m2, 200 mg/m2, or a fixed 50 mg dose (the size of one standard ampoule).
The optimum duration of chemotherapy treatment for any individual patient is uncertain. Though clinical trials may stipulate a given number of chemotherapy cycles, in practice it is more common and may be clinically more appropriate to treat until either disease progression or toxicities intervene. Our results demonstrate a variety of responses regarding the optimal duration of FOLFOX or FOLFIRI treatment. The OPTIMOX1 study demonstrated that a planned oxaliplatin holiday (continuing with FU/LV alone) produced equivalent survival outcomes and reduced toxicity compared with giving continuous FOLFOX.6 The most frequently endorsed approach from our survey was to continue FU/LV alone if neuropathy developed, which does not seem to be harmful to patients, given the results from both the OPTIMOX1 and OPTIMOX2 studies.25
Oxaliplatin is most often administered via a central venous access device, at least partly because most treatment regimens employ infusional FU. Although previously classified as an irritant, oxaliplatin is now considered a vesicant.26 There is a greater risk of extravasation using a peripheral access device. Case reports of vesicant reactions have led authors to suggest that central access is advisable for oxaliplatin administration.26–28 Interestingly, only 2% of clinicians who frequently saw patients with CRC always gave oxaliplatin via peripheral access.
There have been numerous publications describing variations in oncologists' practice, care, and outcomes. An Australian questionnaire of medical professionals' knowledge of pancreatic cancer management revealed a number of differences in perceptions regarding diagnosis and treatment, supporting the development of evidence-based guidelines to allow more uniform management.29 Another Australian survey asked physicians about the role of chemotherapy in metastatic non–small-cell lung cancer and demonstrated significant differences in the perceived role of chemotherapy within the medical oncology community.30 Though the disparities have been well documented, the reasons behind the differences remain more obscure. It remains important to also identify areas where medical decision making itself differs significantly within patient populations. Identification of any disparities is the first step toward addressing this issue and making changes in education or guidelines for clinicians, such that treatment within a population is as uniform and optimal as possible.
Though interesting results have been obtained from this survey, there are a number of limitations. The first of these is the modest response rate despite multiple mail-outs and reminders. As such, it is difficult to know whether the sample is representative of the entire target population. Nevertheless, the survey showed considerable variation, even in the subset of responding medical oncologists. Clearly, this variation would persist even with 100% response rate. We did not include consideration of biologic agents in the survey due to limited use in Australia; though acknowledge that the use of these agents may influence recommendations regarding chemotherapy. Only ECOG performance status 0 to 1 patients were considered: this excludes patients presenting more unwell with metastatic disease, but we particularly targeted the well population to replicate many clinical trial scenarios and to ensure that all choices or regimens represented reasonable possibilities. Patient preference is an important component of chemotherapy decision making: although not specifically listing this as a reason for choice, there was a box identified as “other” available for respondents to list an alternative reason for their first-line chemotherapy choice. Our survey focused on physicians' recommendations.
Additional questions arising from this survey include whether clinicians' use of calcium and magnesium have now dwindled in light of emerging new data; how use of oxaliplatin in the adjuvant disease setting (which did not occur at the time of the survey) affects decisions surrounding chemotherapy choices in the metastatic disease setting; how chemotherapy choices would differ between a well (ECOG 0 to 1) and unwell (ECOG 2 to 3) patient; and neoadjuvant chemotherapy choices in patients being considered for resection of liver metastases. This future research, together with currently available data describing current management practice, will continue to create an improved knowledge base and further guidelines for oncologists with respect to the optimal management of colorectal cancer.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgment
We thank Julie Johns and Ngio Murigu, BioGrid Australia, for assistance with data collection and analysis.
Appendix
Table A1.
Common Chemotherapy Regimens for Metastatic Colorectal Cancer
| Regimen | Reference No. | Description | Cycle Length (weeks) |
|---|---|---|---|
| FOLFOX4 | 31 | Oxaliplatin 85 mg/m2 day 1 | 2 |
| LV 200 mg/m2 days 1-2 | |||
| FU 400 mg/m2 days 1-2 | |||
| FU 600 mg/m2 CIVI 22 hours days 1-2 | |||
| FOLFOX6 | 32 | Oxaliplatin 100 mg/m2 day 1 | 2 |
| LV 400 mg/m2 day 1 | |||
| FU 400 mg/m2 day 1 | |||
| FU 2,400-3,000 mg/m2 CIVI 46 hours days 1-2 | |||
| FLOX | 33 | Oxaliplatin 85 mg/m2 day 1 on weeks 1, 3, 5 | 8 |
| FU 500 mg/m2 bolus weekly, weeks 1-6 | |||
| LV 500 mg/m2 bolus weekly, weeks 1-6 | |||
| XELOX | 34 | Oxaliplatin 130 mg/m2 day 1 | 3 |
| Capecitabine 1 g/m2 twice a day, days 1-14 | |||
| FOLFIRI | 35 | Irinotecan 180 mg/m2 day 1 | 2 |
| LV 200 mg/m2 days 1-2 | |||
| FU 400 mg/m2 bolus days 1-2 | |||
| FU 600 mg/m2 CIVI 22 hours days 1-2 | |||
| IFL | 36 | Irinotecan 100-125 mg/m2 weekly for 4 weeks | 6 |
| LV 20 mg/m2 weekly for 4 weeks | |||
| FU 400-500 mg/m2 bolus weekly for 4 weeks | |||
| XELIRI | 37 | Irinotecan 200-250 mg/m2 day 1 | 3 |
| Capecitabine 1 g/m2 twice a day, days 1-14 |
Abbreviations: LV, leucovorin; FU, fluorouracil; FOLFOX, oxaliplatin, LV, and FU; CIVI, continuous intravenous infusion; FLOX, oxaliplatin, LV, and FU; XELOX, capecitabine and oxaliplatin; FOLFIRI, irinotecan, LV, and FU; IFL, irinotecan, LV, and FU; XELIRI, irinotecan and capecitabine.
References
- 1.Jemal A, Murray T, Ward E, et al: Cancer statistics, 2005. CA Cancer J Clin 55:10-30, 2005 [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Colon Cancer. V. 1.2008. http://www.nccn.org/professionals/physician_gls/PDF/colon.pdf
- 3.Tournigand C, Andre T, Achille E, et al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol 22:229-237, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Goldberg RM, Sargent DJ, Morton RF, et al: A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23-30, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Colucci G, Gebbia V, Paoletti G, et al: Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: A multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol 23:4866-4875, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Tournigand C, Cervantes A, Figer A, et al: OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer—A GERCOR study. J Clin Oncol 24:394-400, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Field K, Kosmider, Jefford M, et al: Chemotherapy (CT) dose adjustment for obese or elderly patients: How often does it occur, and should it? Results of an Australian survey. J Oncol Pract 4:108-113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosmider S, Field K, Jefford M, et al: Surveillance following treatment for colorectal cancer in Australia: Has best practice been adopted by medical oncologists? Intern Med J 38:415-421, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Gamelin L, Boisdron-Celle M, Delva R, et al: Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: A retrospective study of 161 patients receiving oxaliplatin combined with 5-fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res 10:4055-4061, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Muto O, Ando H, Ono T, et al: [Reduction of oxaliplatin-related neurotoxicity by calcium and magnesium infusions]. Gan To Kagaku Ryoho 34:579-581, 2007 [PubMed] [Google Scholar]
- 11.Cersosimo RJ: Oxaliplatin-associated neuropathy: A review. Ann Pharmacother 39:128-135, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Hochster HS, Grothey A, Childs BH: Use of calcium and magnesium salts to reduce oxaliplatin-related neurotoxicity. J Clin Oncol 25:4028-4029, 2007 [DOI] [PubMed] [Google Scholar]
- 12a.Hochster HS, Grothey A: Magnetic resonance imaging versus bone scan in high-risk prostatic carcinoma: Some methodological considerations. J Clin Oncol 26:1189-1190, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Goldberg RM, Sargent DJ, Morton RF, et al: Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: A North American Intergroup Trial. J Clin Oncol 24:3347-3353, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Damianovich D, Adena M, Tebbutt NC: Treatment of 5-fluorouracil refractory metastatic colorectal cancer: An Australian population-based analysis. Br J Cancer 96:546-50, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassidy J: Which first-line treatment is superior in advanced colorectal cancer: XELOX or pviFOX? Nat Clin Pract Oncol 4:280-281, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Rubio E, Tabernero J, Gomez-Espana A, et al: Phase III study of capecitabine plus oxaliplatin versus continuous-infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: Final report of the Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. J Clin Oncol 25:4224-4230, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Martoni AA, Pinto C, Di Fabio F, et al: Capecitabine plus oxaliplatin (xelox) versus protracted 5-fluorouracil venous infusion plus oxaliplatin (pvifox) as first-line treatment in advanced colorectal cancer: A GOAM phase II randomised study (FOCA trial). Eur J Cancer 42:3161-3168, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Cunningham D, Pyrhonen S, James RD, et al: Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 352:1413-1418, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Rougier P, Van Cutsem E, Bajetta E, et al: Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 352:1407-1412, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Rougier P, Bugat R, Douillard JY, et al: Phase II study of irinotecan in the treatment of advanced colorectal cancer in chemotherapy-naive patients and patients pretreated with fluorouracil-based chemotherapy. J Clin Oncol 15:251-260, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Graeven U, Arnold D, Reinacher-Schick A, et al: A randomised phase II study of irinotecan in combination with 5FU/FA compared with irinotecan alone as second-line treatment of patients with metastatic colorectal carcinoma. Onkologie 30:169-174, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Ychou M, Fabbro-Peray P, Perney P, et al: A prospective randomized study comparing high- and low-dose leucovorin combined with same-dose 5-fluorouracil in advanced colorectal cancer. Am J Clin Oncol 21:233-236, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Jäger E, Heike M, Bernhard Het al: Weekly high-dose leucovorin versus low-dose leucovorin combined with fluorouracil in advanced colorectal cancer: Results of a randomized multicenter trial—Study Group for Palliative Treatment of Metastatic Colorectal Cancer Study Protocol 1. J Clin Oncol 14:2274-2279, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Cancer Institute New South Wales: New South Wales Cancer Institute cancer treatment guidelines, https://www.treatment.cancerinstitute.org.au
- 25.Maindrault-Goebel F, Lledo G, Chibaudel B, et al: Final results of OPTIMOX2, a large randomized phase II study of maintenance therapy or chemotherapy-free intervals (CFI) after FOLFOX in patients with metastatic colorectal cancer (MRC): A GERCOR study. J Clin Oncol 25:166s, 2007. (suppl; abstr 4013) [Google Scholar]
- 26.Kennedy JG, Donahue JP, Hoang B, et al: Vesicant characteristics of oxaliplatin following antecubital extravasation. Clin Oncol (R Coll Radiol) 15:237-239, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Foo KF, Michael M, Toner G, et al: A case report of oxaliplatin extravasation. Ann Oncol 14:961-962, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Baur M, Kienzer HR, Rath T, et al: Extravasation of Oxaliplatin (Eloxatin): Clinical Course. Onkologie 23:468-471, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Jefford M, Jennens R, Speer T, et al: Different professionals' knowledge and perceptions of the management of people with pancreatic cancer. Asia-Pacific J Clin Oncol 25:44-51, 2007 [Google Scholar]
- 30.Jennens RR, de Boer R, Irving L, et al: Differences of opinion: A survey of knowledge and bias among clinicians regarding the role of chemotherapy in metastatic non-small cell lung cancer. Chest 126:1985-1993, 2004 [DOI] [PubMed] [Google Scholar]
- 31.de Gramont A, Figer A, Seymour M, et al: Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938-2947, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Maindrault-Goebel F, Louvet C, Andre T, et al: Oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX6). GERCOR. Eur J Cancer 35:1338-1342, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Sørbye H, Dahl O: Nordic 5-fluorouracil/leucovorin bolus schedule combined with oxaliplatin (Nordic FLOX) as first-line treatment of metastatic colorectal cancer. Acta Oncol 42:827-831, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Cassidy J, Tabernero J, Twelves C, et al: XELOX (capecitabine plus oxaliplatin): Active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol 22:2084-2091, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Douillard JY, Cunningham D, Roth AD, et al: Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet 355:1041-1047, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Saltz LB, Cox JV, Blanke C, et al: Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343:905-914, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Delord JP, Pierga JY, Dieras V, et al: A phase I clinical and pharmacokinetic study of capecitabine (Xeloda) and irinotecan combination therapy (XELIRI) in patients with metastatic gastrointestinal tumours. Br J Cancer 92:820-826, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]