Abstract
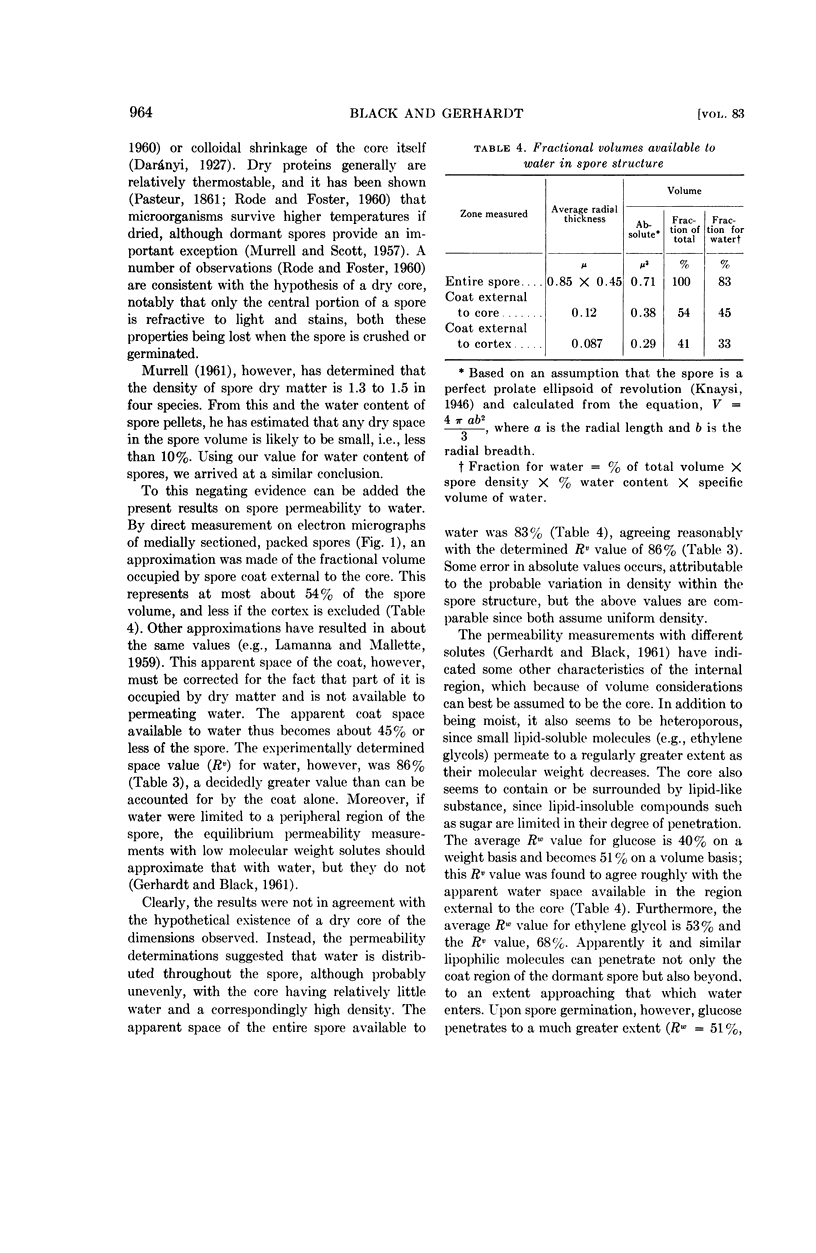
Black, S. H. (The University of Michigan, Ann Arbor) and Philipp Gerhardt. Permeability of bacterial spores. IV. Water content, uptake, and distribution. J. Bacteriol. 83:960–967. 1962.—Dormant and germinated spores of Bacillus cereus strain terminalis were examined for water properties. Respectively, they exhibited a mean density of 1.28 and 1.11 g/ml, a water content of 64.8 and 73.0%, and a total water uptake of 66.6 and 75.6%, based on spore weight, or 86.0 and 83.9%, based on spore volume. The results confirmed a previous report that internal and external water are in virtually complete equilibrium, but refuted a prevailing hypothesis that heat resistance is attributable to a dry core. A model of spore ultrastructure that evolved from the cumulative results pictures a moist, dense, heteroporous core. A new hypothesis is formulated as an explanation for thermostability in spores and possibly in other instances; it postulates the occurrence of an insolubly gelled core with cross-linking between macromolecules through stable but reversible bonds so as to form a high-polymer matrix with entrapped free water.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. I. Characterization of glucose uptake. J Bacteriol. 1961 Nov;82:743–749. doi: 10.1128/jb.82.5.743-749.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. III. Permeation relative to germination. J Bacteriol. 1962 Feb;83:301–308. doi: 10.1128/jb.83.2.301-308.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK S. H., HASHIMOTO T., GERHARDT P. Calcium reversal of the heat susceptibility and dipicolinate deficiency of spores formed "endotrophically" in water. Can J Microbiol. 1960 Apr;6:213–224. doi: 10.1139/m60-023. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. THIOLATION OF PROTEINS. Proc Natl Acad Sci U S A. 1958 Sep 15;44(9):848–853. doi: 10.1073/pnas.44.9.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., BLACK S. H. Permeability of bacterial spores. II. Molecular variables affecting solute permeation. J Bacteriol. 1961 Nov;82:750–760. doi: 10.1128/jb.82.5.750-760.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO T., BLACK S. H., GERHARDT P. Development of fine structure, thermostability, and dipicolinate during sporogenesis in a bacillus. Can J Microbiol. 1960 Apr;6:203–212. doi: 10.1139/m60-022. [DOI] [PubMed] [Google Scholar]
- Henry B. S., Friedman C. A. The Water Content of Bacterial Spores. J Bacteriol. 1937 Mar;33(3):323–329. doi: 10.1128/jb.33.3.323-329.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOFFLER H. Protoplasmic differences between mesophiles and thermophiles. Bacteriol Rev. 1957 Dec;21(4):227–240. doi: 10.1128/br.21.4.227-240.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaysi G. Process of Sporulation in Strain of Bacillus cereus. J Bacteriol. 1946 Feb;51(2):187–197. [PMC free article] [PubMed] [Google Scholar]
- Lewis J. C., Snell N. S., Burr H. K. Water Permeability of Bacterial Spores and the Concept of a Contractile Cortex. Science. 1960 Aug 26;132(3426):544–545. doi: 10.1126/science.132.3426.544. [DOI] [PubMed] [Google Scholar]
- MURRELL W. G., SCOTT W. J. Heat resistance of bacterial spores at various water activities. Nature. 1957 Mar 2;179(4557):481–482. doi: 10.1038/179481a0. [DOI] [PubMed] [Google Scholar]
- RODE L. J., FOSTER J. W. Germination of bacterial spores with alkyl primary amines. J Bacteriol. 1961 May;81:768–779. doi: 10.1128/jb.81.5.768-779.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS K. F., BILLING E. The water and solid content of living bacterial spores and vegetative cells as indicated by refractive index measurements. J Gen Microbiol. 1957 Apr;16(2):418–425. doi: 10.1099/00221287-16-2-418. [DOI] [PubMed] [Google Scholar]
- Rode L. J., Foster J. W. MECHANICAL GERMINATION OF BACTERIAL SPORES. Proc Natl Acad Sci U S A. 1960 Jan;46(1):118–128. doi: 10.1073/pnas.46.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWARTZ M. N., KAPLAN N. O., FRECH M. E. Significance of heat-activated enzymes. Science. 1956 Jan 13;123(3185):50–53. doi: 10.1126/science.123.3185.50. [DOI] [PubMed] [Google Scholar]
- VINTER V. Changes in radioresistance of sporulating cells of Bacillus cereus. Nature. 1961 Feb 18;189:589–590. doi: 10.1038/189589a0. [DOI] [PubMed] [Google Scholar]




