Abstract
Purpose:
To describe patient/family and logistical barriers to participation in university-based, early-phase cancer clinical trials for adults age ≥ 65 years, and to identify influences on their decisions to participate.
Participants and Methods:
In-person surveys were administered to subjects age ≥ 65 years with advanced tumors who had received prior chemotherapy. Subjects were recruited from private medical oncology practices collaborating with the University of Colorado and Moffitt Cancer Center research networks.
Results:
Three hundred individuals (51% age 65 to 74 and 49% age 75 or older) responded. Overall, 60% reported one or more barriers to participation in an early-phase trial; logistical barriers such as driving or time demands (34%) or reluctance to be treated at a university center (21%) were most common. Seniors age 75 or older were more reluctant to be treated at a university center (27% v 14%; P = .005), or concerned about loss of continuity with their primary oncologist (24% v 15%, P = .05). Older seniors were also significantly more reluctant than younger seniors to consider treatments with substantial nausea, vomiting, or fatigue. Older and younger seniors differed little in their preferred sources of information; both age groups emphasized the importance of the primary oncologist (100%), a nurse who provides experimental treatment (93%), other patients (83%) or acquaintances who had received experimental treatment (83%).
Conclusion:
Potential strategies to overcome barriers to enrollment of seniors into early-phase trials include providing more information about trials to community oncologists and prospective enrollees and assisting these individuals in navigating logistical barriers to enrollment.
Short abstract
Potential strategies to overcome barriers to enrollment of seniors into early-phase trials.
Introduction
Cancer is the second leading cause of death among persons 65 years of age and older.1 More than 60% of individuals diagnosed with invasive neoplasms in the United States are 65 years of age or older.2 Because older persons are the fastest growing segment of the population, the elderly will account for an even larger proportion of new cancer diagnoses and cancer deaths in coming decades.3 Despite the substantial burden of cancer in the older population, little is known about issues specific to cancer treatment in this population, such as drug pharmacology, treatment effectiveness, and toxicity, or interactions with comorbid diseases and medications.
This deficit in knowledge arises in large part from the under-representation of older persons in both early-phase (phase I and phase II) and late-phase clinical trials of cancer treatment.4–7 Older patients were under-represented in clinical trials for cancer drug registration for all drugs approved by the US Food and Drug Administration except breast cancer hormone therapy between 1995 and 2002. While adults age ≥ 65, ≥ 70, and ≥ 75 years constituted 60%, 46%, and 31% of the U.S. population with cancer, they represented only 36%, 20%, and 9% of the subjects enrolled in registration trials, respectively.6
Increasing the accrual of older adults into early-phase clinical trials requires knowledge of barriers to enrollment and evaluation of strategies to overcome these barriers. To date, studies assessing age-related barriers have primarily been retrospective reviews of enrollment data from clinical trials and surveys of physicians' perceptions of barriers, rather than assessment of the types, prevalence, and severity of barriers perceived by prospective enrollees themselves.8,9 Further, no studies have characterized barriers to participation in early-phase clinical trials. Knowledge of such barriers is an important first step toward designing and evaluating patient-targeted interventions to improve accrual.
To better characterize remediable patient-level and logistical barriers to participating in early-phase clinical trials, we administered an in-person survey to patients age 65 years and older with advanced cancer. We also compared the prevalence of these barriers among younger seniors (age 65 to 74 years) and older seniors (age 75+ years).
Participants and Methods
Conceptual Framework and Survey Development
We defined a barrier to participation in an early-phase (phase I or phase II) trial as any condition that makes it difficult or impossible for a subject to participate in a clinical trial. Barriers to enrollment in cancer treatment trials in general have been conceptualized in five main categories: physician, patient/family, protocol (eligibility), funding (system), and logistics.10–14 We adopted this conceptual scheme and identified specific age-related concerns in two of these categories—patient/family barriers and logistical barriers—based on clinical experience and informal discussion with national researchers from six sites awarded grants from the National Cancer Institute to assess barriers to clinical trial participation in various vulnerable populations. Patient/family barriers included: concerns about treatment toxicity, caregiver limitations, and lack of knowledge about clinical trials. Other potential patient-level barriers, such as income, actual insurance coverage, comorbid health conditions, or limitations in physical or social functioning, were not assessed, either because they would increase respondent burden or because they would require medical record review that was beyond the scope of the study. Logistical barriers included difficulties with transportation (such as age-related driving concerns), and lack of caregivers to help with transportation. We also assessed patient/family characteristics and attitudes that might enhance interest in enrollment, such as potential benefits to health. In the survey, the concept of early phase clinical trials was explained to respondents as treatment with a new experimental drug in a research study.
Survey questions for the patient/family and logistical domains were written by a phase I/II clinical trialist and geriatric oncologist (M. Basche), a clinical research nurse (M. Persky) and a geriatric clinical research assistant (N. Jackson). The survey was pilot tested with five to 10 oncology clinic nurses and patients and minor revisions in wording were made. The final version of the survey was translated into Spanish. Interviewers at each clinical practice were experienced research assistants who were trained in standard techniques for survey administration on-site by a member of the central research team. A single interviewer in each participating program (Colorado and Moffitt) administered all surveys for the practices in that program.
Identification of Subjects and Eligibility Criteria
Subjects were recruited from community-based medical oncology practices that collaborated with the University of Colorado Cancer Center clinical research network or the H. Lee Moffitt Cancer Center Affiliates Network and had access to a research assistant. Subjects age ≥ 65 years seen in these clinics were identified by office staff and asked to provide Health Insurance Portability and Accountability Act (HIPAA) authorization to be contacted by research staff. It was not possible to determine how many elderly individuals were seen in these busy practices over the study period, because of both logistical and confidentiality concerns. If they agreed to consider participation, the interviewer for that site contacted them by phone, and arranged a personal meeting, typically in the practice at the time of a subsequent clinic visit. Eligibility criteria included: ability to speak English or Spanish, age at least 65 years, and presence of advanced solid tumors (metastatic disease or unresectable solid tumors), lymphoma, or multiple myeloma that had previously been treated with chemotherapy for advanced disease. Eligible patients provided written informed consent and HIPAA authorization. This project was approved by the Colorado Multiple institutional review board, and the University of South Florida institutional review board.
Survey Administration
Face-to-face interviews were conducted in community-based oncology clinics with patients who provided informed consent. Subjects were interviewed alone unless they preferred to be interviewed in the company of a friend or family member. Accrual for the study took place over a 9-month period.
Sample Size Calculation and Statistical Analysis
We were interested in identifying common barriers, which we defined as those reported by more than 20% of survey respondents. Sample size calculations determined that 300 respondents would achieve 80% power to detect a difference of 0.07 between the null hypothesis proportion of .20 and the alternative hypothesis proportion of .27, using a one-sided, binomial hypothesis test with a two-tailed α of .05. We were also interested in identifying differences of 15% or more in the prevalence of barriers between younger seniors and older seniors. A sample size of 300 (with 150 younger seniors and 150 older seniors) also achieved 80% power to detect a difference of .15 when the prevalence of a barrier in younger seniors was .20, using a 2-tailed χ2 test without continuity correction and with an α level of .05.
The prevalence of barriers and other decision-making considerations were described as a proportion with the associated 95% CI. The χ2 test was used to examine the association between patient characteristics and age group and to compare the proportion of perceived barriers, logistic barriers, unacceptable toxicity, and importance of certain decision maker factors between younger seniors and older seniors. A multivariate logistic regression analysis was used to identify independent predictors for the presence of at least one barrier. All analyses were carried in SAS version 9.1 (SAS Institute, Cary, NC).
Results
Patients were recruited until the projected sample of 300 respondents was attained; the number of individuals who declined to enroll was not assessed. Patients were recruited from 10 community oncology practices that collaborated with the University of Colorado Cancer Center, and six practices that collaborated with the H. Lee Moffitt Cancer Center. All interviews were conducted in English. Among the 300 patients interviewed, 154 (51%) were age 65 to 74 years and 146 (49%) were age 75 years or older. Baseline characteristics of the sample are described in Table 1. Most patients were married, white, and had received some college education. The demographic characteristics of the older seniors were similar to that of the younger seniors with the exception of marital status (P = .03).
Table 1.
Characteristics of Patients Participating in the Survey
| Characteristic | Age | P | |||||
|---|---|---|---|---|---|---|---|
| All Respondents | 65-74 | ≥ 75 | |||||
| No. | % | No. | % | No. | % | ||
| No. of patients | 300 | 154 | 146 | ||||
| Sex | |||||||
| Female | 154 | 51.5 | 80 | 52.0 | 74 | 51.0 | .87 |
| Male | 145 | 48.5 | 74 | 48.1 | 71 | 49.0 | |
| Ethnicity | |||||||
| White | 281 | 93.7 | 142 | 92.2 | 139 | 95.2 | .06 |
| African American | 10 | 3.3 | 8 | 5.2 | 2 | 1.4 | |
| Hispanic or Latino | 6 | 2.0 | 4 | 2.6 | 2 | 1.4 | |
| Other* | 3 | 1.0 | 0 | 0.0 | 3 | 2.1 | |
| Marital status | |||||||
| Married | 208 | 69.3 | 116 | 75.3 | 92 | 63.0 | .03 |
| Widowed | 66 | 22.0 | 25 | 16.2 | 41 | 28.1 | |
| Divorced or single | 24 | 8.0 | 13 | 8.4 | 11 | 7.5 | |
| Living with partner | 2 | 0.7 | 0 | 2 | 1.4 | ||
| Highest level of Education | |||||||
| Grade | |||||||
| 1-8 | 18 | 6.0 | 8 | 5.2 | 10 | 6.9 | .88 |
| 9-11 | 17 | 5.7 | 8 | 5.2 | 9 | 6.2 | |
| 12 or GED | 97 | 32.3 | 49 | 31.8 | 48 | 32.9 | |
| College, No. of years | |||||||
| 1-3 | 80 | 26.7 | 40 | 26.0 | 40 | 27.4 | |
| ≥ 4 | 88 | 29.3 | 49 | 31.8 | 39 | 26.7 | |
| Caregiver available when ill | 283 | 94.3 | 146 | 94.8 | 137 | 93.8 | .72 |
| Frequency caregiver is available | |||||||
| Most of the time | 243 | 81.3 | 128 | 83.1 | 115 | 79.3 | .76 |
| Sometimes | 34 | 11.4 | 15 | 9.7 | 19 | 13.1 | |
| Rarely | 5 | 1.7 | 3 | 2.0 | 2 | 1.4 | |
Abbreviation: GED, general equivalency degree.
* Two subjects identified themselves as being a member of two different ethnic groups. They were considered as white in this Table.
One hundred seventy nine patients (60%; 95% CI, 55% to 65%) reported facing at least one barrier to participation in an early phase clinical trial. The only individual barriers noted by more than 20% of respondents were logistical barriers to traveling to the university cancer center (34%; 95% CI, 29% to 38%) and unwillingness to be treated at a university cancer center (21%; 95% CI, 16% to 25%). Specific patient/family concerns and logistical barriers endorsed by subjects are described in Table 2.
Table 2.
Logistical Barriers to Participation in Early-Phase Cancer Treatment Trials
| Logistical Barrier* | Age | Difference | 95% CI | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | 65-74 | ≥ 75 | ||||||||
| No. | % | 95% CI | No. | % | No. | % | ||||
| No. of patients | 102 | 49 | 53 | |||||||
| Driving in bad weather | 72 | 70.6 | 61.8 to 79.4 | 30 | 61.2 | 42 | 79.3 | −18.1 | −35.5 to −0.6 | .05 |
| It takes too long to get there | 67 | 65.7 | 56.5 to 74.9 | 29 | 59.2 | 38 | 71.7 | −12.5 | −30.9 to 5.8 | .18 |
| Driving in the dark | 53 | 52.5 | 42.7 to 62.2 | 20 | 41.7 | 33 | 62.3 | −20.6 | −39.7 to −1.5 | .04 |
| Worried about finding cancer center | 48 | 47.1 | 37.4 to 56.8 | 18 | 36.7 | 30 | 56.6 | −19.9 | −38.9 to −0.9 | .05 |
| Driving in the city | 42 | 41.6 | 32.0 to 51.2 | 19 | 39.6 | 23 | 43.4 | −3.8 | −23.0 to 15.4 | .70 |
| Worried about parking | 37 | 36.3 | 26.9 to 45.6 | 15 | 30.6 | 22 | 41.5 | −10.9 | −29.4 to 7.6 | .25 |
| Driving on the highway | 36 | 35.6 | 26.3 to 45.0 | 18 | 37.5 | 18 | 34.0 | 3.5 | −15.2 to 22.3 | .71 |
| Poor vision | 21 | 20.8 | 12.9 to 28.7 | 9 | 18.8 | 12 | 22.6 | −3.9 | −19.7 to 11.9 | .63 |
| Cannot afford to travel to clinic | 18 | 17.7 | 10.3 to 25.1 | 10 | 20.4 | 8 | 15.1 | 5.3 | −9.5 to 20.2 | .48 |
| Unable to travel because needs to care for family member | 7 | 6.9 | 2.0 to 11.8 | 1 | 2.0 | 6 | 11.3 | −9.3 | −18.7 to 0.1 | .11 |
* Denominator for this Table is the first number of patients who reported logistic barriers (N = 102).
Older seniors more often reported at least one barrier to participation than younger seniors (66% v 54%; P = .04). In addition, older seniors consistently endorsed individual barriers more frequently than younger seniors, although these differences were not always statistically significant (Table 3). Unwillingness to be treated on an early phase trial at a university cancer center, concern about the loss of continuity with their primary oncologist, and concern that they would be treated “like a guinea pig” if they participated in a clinical trial were significantly more common among older seniors. In a multiple logistical regression model that included age category, sex, race/ethnicity, marital status, education, and availability of a caregiver, younger seniors were less likely to identify one or more barriers than older seniors (odd ratio [OR], 0.60; 95% CI, 0.37 to 0.97), while women were more likely to identify one or more barriers than men (OR, 1.87; 95% CI, 1.14 to 3.09).
Table 3.
Prevalence of Perceived Barrier by Age
| Barrier Type/Domain | Age | Difference | 95% CI | P | |||||
|---|---|---|---|---|---|---|---|---|---|
| All Respondents | 65-74 | ≥ 75 | |||||||
| No. | % | No. | % | No. | % | ||||
| No. of patients | 300 | 154 | 146 | ||||||
| At least one barrier | 179 | 59.7 | 83 | 53.9 | 96 | 65.8 | −11.9 | −22.9 to −0.9 | .04 |
| Logistical* | 102 | 34.0 | 49 | 31.8 | 53 | 36.3 | −4.5 | −15.2 to 6.6 | .41 |
| Unwilling to be treated at University cancer center | 62 | 20.7 | 22 | 14.3 | 40 | 27.4 | −13.1 | −22.2 to −4.0 | .005 |
| Loss of continuity with oncologist if treated at university | 58 | 19.3 | 23 | 14.9 | 35 | 24.0 | −9.1 | −18.0 to −0.1 | .05 |
| Concerned that s/he will be treated like a ″guinea pig″ | 56 | 18.7 | 22 | 14.3 | 34 | 23.3 | −9.0 | −17.8 to −0.2 | .05 |
| No insurance coverage for trial | 49 | 16.3 | 21 | 13.6 | 28 | 19.2 | −5.6 | −13.9 to 2.8 | .19 |
* A logistical barrier was defined as a reason for having difficulty getting to the university cancer center to participate in an early-phase trial.
The value that these respondents placed on different sources of information about experimental treatment trials is provided in Table 4. The endorsement of a clinical trial by the patient's oncologist was important to virtually 100% of seniors, regardless of age. The majority of patients also indicated that endorsements by a nurse who treats patients with experimental therapy (93%), family members (69%), and someone they know personally who had received experimental treatment (83%) on a clinical trial would be important to them. Information acquired from the Internet was important to only 32% of respondents. The only significant difference across age groups for patient's ratings of endorsement sources or treatment benefits was that older individuals were less likely to find information from the Internet important (P = .008).
Table 4.
Patient's Ratings of the Importance of Endorsements, Therapeutic Efficacy, and the Impact of Treatment on Functioning When Assessing the Acceptability of an Experimental Therapy
| Decision-Making Factor | Importance Rating* (by age group) | Difference | 95% CI | P | |||||
|---|---|---|---|---|---|---|---|---|---|
| Entire Sample | 65-74 | ≥ 75 | |||||||
| No. | % | No. | % | No. | % | ||||
| No. of patients | 300 | 154 | 146 | ||||||
| Endorsement source | |||||||||
| Primary oncologist | 299 | 99.7 | 153 | 99.4 | 146 | 100 | −0.6 | −1.9 to 0.6 | .99 |
| Nurse who treats patients with the experimental therapy | 278 | 92.7 | 146 | 94.8 | 132 | 90.4 | 4.4 | −1.5 to 10.3 | .14 |
| Spouse/partner | 201 | 67.0 | 110 | 71.4 | 91 | 62.3 | 9.1 | −1.5 to 19.7 | .09 |
| Family | 208 | 69.3 | 112 | 72.7 | 96 | 65.8 | 7.0 | −3.5 to 17.4 | .19 |
| Another patient who has received the experimental therapy | 248 | 82.7 | 130 | 84.4 | 118 | 80.8 | 3.6 | −5.0 to 12.2 | .41 |
| Someone you know personally who has received the experimental therapy | 248 | 82.7 | 132 | 85.7 | 116 | 79.5 | 6.3 | −2.3 to 14.8 | .15 |
| Information from the Internet | 96 | 32.0 | 60 | 39.0 | 36 | 24.7 | 14.3 | 3.9 to 24.7 | .008 |
| Possible benefit from treatment | |||||||||
| Shrinks tumor | 298 | 99.3 | 153 | 99.4 | 145 | 99.3 | 0.0 | −1.8 to 1.9 | .99 |
| Better symptoms | 296 | 98.7 | 151 | 98.1 | 145 | 99.3 | −1.3 | −3.8 to 1.3 | .62 |
| Live longer | 296 | 98.7 | 153 | 99.4 | 143 | 98.0 | 1.4 | −1.2 to 4.0 | .36 |
| Stabilizes tumor | 296 | 98.7 | 152 | 98.7 | 144 | 98.6 | 0.1 | −2.5 to 2.7 | .99 |
| Impact of treatment on social and role functioning | |||||||||
| Needs more care from friends or family | 248 | 82.7 | 130 | 84.4 | 118 | 80.8 | 3.6 | −5.0 to 12.2 | .41 |
| Unable to do the things one enjoys | 232 | 77.3 | 122 | 79.2 | 110 | 75.3 | 3.9 | −5.6 to 13.4 | .42 |
| Unable to take care of spouse or family members | 193 | 64.3 | 104 | 67.5 | 89 | 61.0 | 6.6 | −4.3 to 17.4 | .23 |
| Unable to work | 21 | 7.0 | 14 | 9.1 | 7 | 4.8 | 4.3 | −1.4 to 10.0 | .14 |
* Very important + somewhat important versus not important + other.
These respondents also identified specific areas where knowledge about experimental treatments would be valuable, as summarized in Table 4. The possibility of benefit from treatment was important to almost all respondents, while social factors such as requiring more care from others were also important considerations.
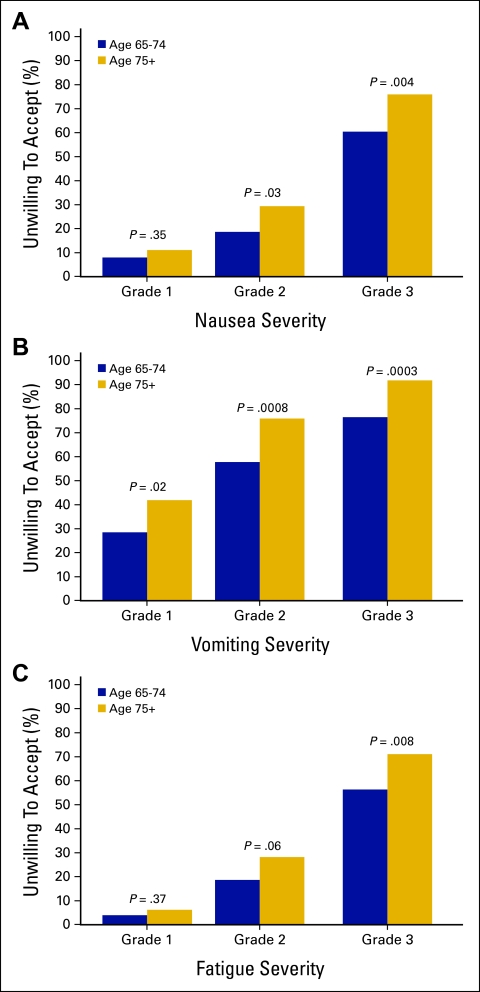
In order to assess the level of treatment toxicity acceptable to patients considering early-phase trials, we asked patients whether they would accept an experimental treatment that was associated with National Cancer Institute Common Toxicity Criteria grade 1, 2, or 3 for nausea, vomiting, or fatigue. As expected, more severe toxicity was associated with greater lack of patient acceptance regardless of age (Figure 1). In addition, older seniors were significantly more likely than younger seniors to report that grade 2 or 3 toxicity would be unacceptable.
Figure 1.
Treatment adverse effects were assessed using National Cancer Institute Common Toxicity grades 1, 2, or 3.
Discussion
Knowledge of patient-perceived barriers to enrollment in early-phase clinical trials, particularly barriers that might be susceptible to interventions, is important for guiding efforts to improve representation of the older population in treatment studies. We found that the majority of seniors reported at least one barrier to participating in a clinical trial. Barriers related to logistics and knowledge were reported most frequently. While older seniors were statistically more likely to report certain barriers, the absolute differences in the proportion of younger and older seniors reporting such barriers were relatively small. In addition, knowledge of key considerations that might influence patient's decisions whether to participate in an early-phase clinical trial is important if we are to offer older patients clinical trials acceptable to them. We found that older and younger seniors had similar views regarding the importance of potential benefits of experimental treatments. Older seniors were less willing to accept moderate and severe toxicity associated with treatment than younger seniors, however.
A recent systematic review of barriers to the recruitment of seniors into cancer clinical trials found that the literature on this topic consisted only of retrospective analyses of enrollment data from specific trials and opinion surveys of oncologists.8 Since that time, a mixed-method survey of 94 elderly patients with cancer, from a single cancer treatment center in Canada, found that 77% of these individuals would consider participation in a trial; older seniors did not differ from younger seniors in their willingness to consider participation.9 Our study, in a larger community sample, extends these observations, and provides substantially more detail about the patient-level and logistical barriers to participation and influences on decision making in this age group. Our findings—that logistical barriers were important (such as the difficulty and time necessary to get to a university cancer center)—suggest that making early-phase clinical trials available in community practices would improve accessibility to these trials. This conclusion is supported by the findings that one-fifth of patients would be unwilling to receive treatment at a university cancer center and one-fifth were concerned about loss of continuity with their primary oncologist if they were treated on a clinical trial at a university.
The results of our survey also suggest that research “navigators” might be useful to patients considering early-phase clinical trials. These navigators could provide the information desired by potential participants, could help coordinate transportation for patients who are willing to be treated at a university cancer center but have logistical barriers, and could improve communication between the comprehensive cancer center and the patient's community oncologist to maintain continuity and provide information about the availability and outcomes of early-phase clinical trials. Research navigators could also assess participant concerns about possible exploitation, and facilitate education for older patients and their families regarding trials and contacts with patients or nurses involved in early phase trials.
We found, as did Townsley,9 that the possibility of therapeutic benefit is important both to older and younger seniors when considering early-phase trials. However, we found that older seniors are less likely to accept risks of moderate or severe toxicity when considering clinical trial participation. These results suggest that trials focused on the development of well-tolerated therapy may be important for increasing representation of the older population in early phase clinical trials.
More than 40% of older respondents reported no barriers to participating in early-phase clinical trials. Considering that older adults are substantially under-represented in such trials, this finding suggests that barriers which we did not assess, such as the failure of community oncologists to offer such trials to older patients, overly restrictive eligibility criteria for protocols, or problems with insurance coverage of experimental treatments, may also play a significant role in the under-representation of older adults in clinical trials. In a case control study conducted at 10 Cancer and Leukemia Group B institutions, Kemeny and colleagues found that older patients were half as likely to be offered a clinical trial as younger patients, a finding that remained significant even after adjustment for disease stage and physical functioning.15
Our findings must be interpreted in the light of several limitations. First, we did not conduct a similar survey in adults younger than age 65, which would have allowed us to compare their barriers to those of seniors. Second, the items in our survey were developed through expert consensus rather than through patient focus groups or individual interviews. As a result, we may have omitted some potential barriers important to these patients. The concept of phase I and phase II treatment trials is difficult to convey succinctly and in easily understandable terms, and thus it is possible that our description of these studies as “treatment with a new experimental drug” may not have sufficiently distinguished these treatment studies from phase III trials. We assessed only the patient-level and logistical barriers to participation, and could not assess the relative importance of these barriers in comparison to barriers at the level of the protocol, the physician, or the health care delivery system. Because of potential respondent burden we could not assess other patient attributes, such as comorbid health conditions or general health status, that might affect willingness to participate in early-phase treatment trials. We did not record the proportion of patients approached for the study who completed the survey. As a result, we cannot assess the representativeness of respondents. This is of potential concern because (as is evident in Table 1) most respondents were white, married, and relatively well educated. It should also be noted, however, that inclusion of patients from community oncology practices probably enhanced the generalizability of our findings beyond prior studies conducted in university-based cancer centers. Finally, it is unclear whether the barriers cited or the factors reported to influence decisions about participation in response to a hypothetical trial would arise in the consideration of enrollment in an actual trial.
Some of these limitations identify important areas for future research, such as qualitative studies to identify other potential patient-level barriers, simultaneous assessment of potential barriers to participation from all five conceptual domains, comparison of barriers between seniors and younger adults, and comparison of survey responses to actual decisions about enrollment in early-phase studies. Overall, our findings suggest that interventions designed to help older patients overcome knowledge and logistic barriers to participation in early-phase clinical trials might enhance accrual of this under-represented population. Randomized trials should be conducted of interventions such as the use of clinical research navigators to assist older patients overcome barriers to early-phase clinical trial participation.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgment
We thank the clinicians and staff from the participating oncology practices in Colorado and Florida for their contributions. Supported by Grant No. 1R21 CA101716 from the National Cancer Institute.
References
- 1.Cancer facts and figures 2007, Atlanta, GA, American Cancer Society, National Media Office, 2007
- 2.Jemal A, Tiwari R, Murray T, et al: Cancer Statistics, 2004. Cancer 54:8-29, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Yancik R, Ries LAG: Aging and cancer in America. Hematology/Oncology Clinics of North America 14:17-23, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Muss HB, Cohen HJ, Lichtman SM: Clinical research in the older cancer patient. Hematol Oncol Clin North Am 14:283-291, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Hutchins LF, Unger JM, Crowley JJ, et al: Underrepresentation of patients 65 years of age and older in cancer treatment trials. N Engl J Med 341:2061-2067, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Talarico L, Chen G, Pazdur R: Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J Clin Oncol 22:4626-4631, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Lewis JH, Kilgore ML, Goldman DP, et al: Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 21:1383-1389, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Townsley CA, Selby R, Siu LL: Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol 23:3112-3124, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Townsley CA, Chan KK, Pond GR, et al: Understanding the attitudes of the elderly toward enrolment into cancer clinical trials. BMC Cancer 6:34, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trimble EL, Carter CL, Cain D, et al: Representation of older patients in cancer treatment trials. Cancer 74:2208-2214, 1994. (suppl 7) [DOI] [PubMed] [Google Scholar]
- 11.Winn RJ: Obstacles to the accrual of patients to clinical trials in the community setting. Semin Oncol 21:112-117, 1994 [PubMed] [Google Scholar]
- 12.Benson AB, Pregler JP, Bean JA, et al: Oncologists' reluctance to accrue patients onto clinical trials: An Illinois Cancer Center Study. J Clin Oncol 9:2067-2075, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Lara PN, Higdon R, Lim N, et al: Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol 9:1728-1733, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Yee KWL, Pater JL, Pho L, et al: Enrollment of older patients in cancer treatment trials in Canada: Why is age a barrier? J Clin Oncol 21:1618-1623, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Kemeny MM, Peterson BL, Kornblith AB, et al: Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol 21:2268-2275, 2003 [DOI] [PubMed] [Google Scholar]