Abstract
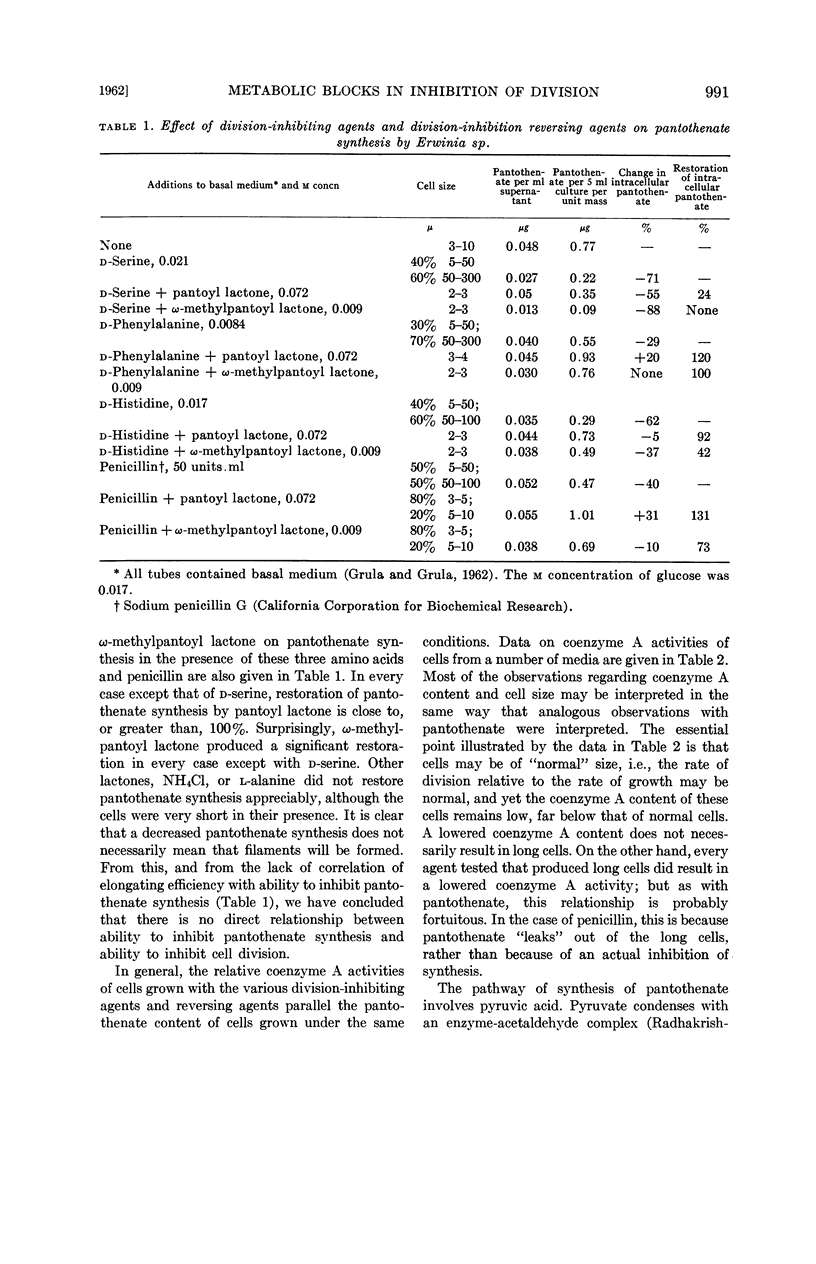
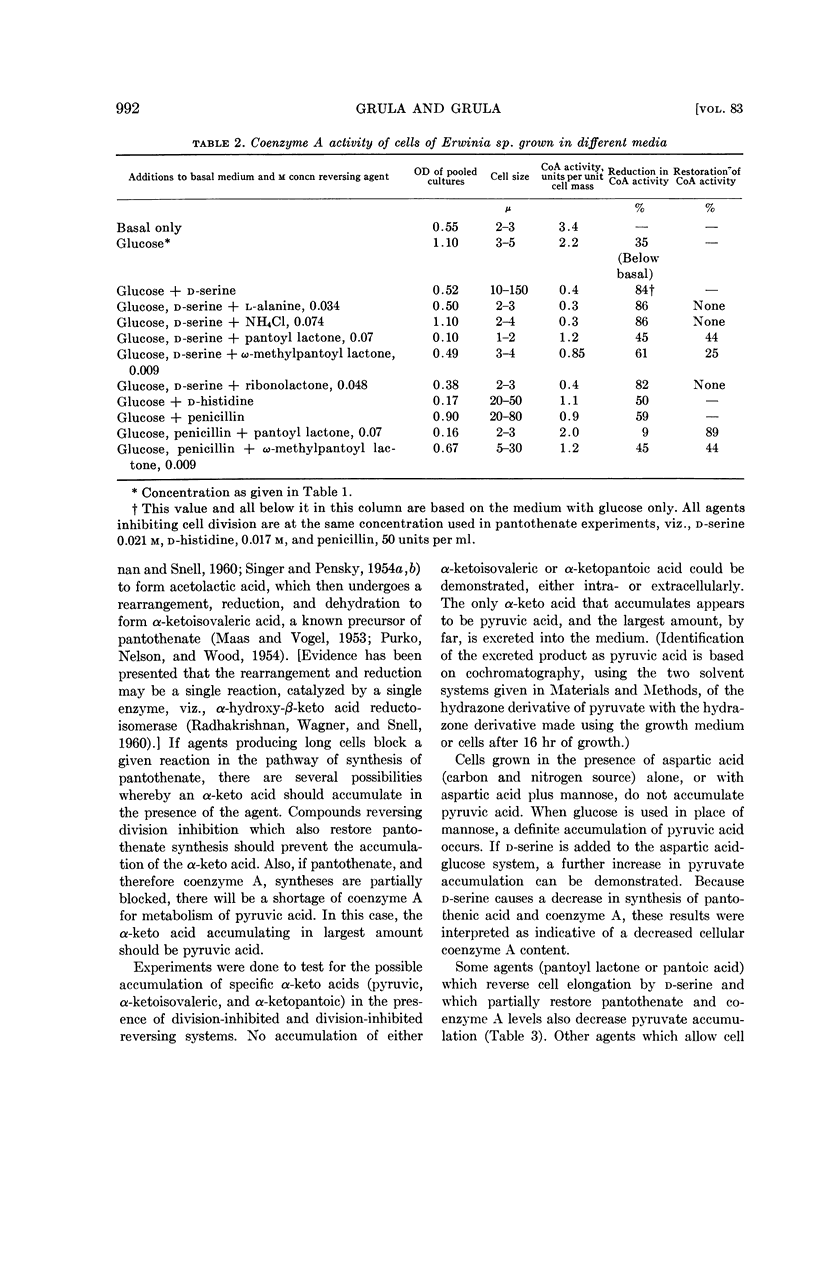
Grula, Mary M. (Oklahoma State University, Stillwater) and E. A. Grula. Cell division in a species of Erwinia. IV. Metabolic blocks in pantothenate biosynthesis and their relationship to inhibition of cell division. J. Bacteriol. 83:989–997. 1962.—Four compounds that inhibit cell division in an Erwinia sp., d-serine, d-histidine, d-phenylalanine, and penicillin, decrease the intracellular pantothenate content of Erwinia at culture ages of 10 and 16 hr. In the case of penicillin, it appears to be the result of excessive leakage from long cells; however, with the three d-amino acids, there is a genuine inhibition of synthesis.
Among agents tested that reverse the inhibition of division, only pantoyl lactone, and to a lesser extent, ω-methylpantoyl lactone, restore intracellular content of pantothenate. This restoration is considerably less effective with d-serine as a division-inhibiting agent than with the others. Other lactones, l-α-alanine, and ammonium chloride are ineffective, or only slightly effective, in restoring pantothenate synthesis. Effects of division-inhibiting compounds and reversing agents upon cellular coenzyme A activity in general parallel their effects on pantothenate synthesis. There is no direct correlation between ability of a compound to reverse cell-division inhibition and ability to restore synthesis of either pantothenic acid or coenzyme A.
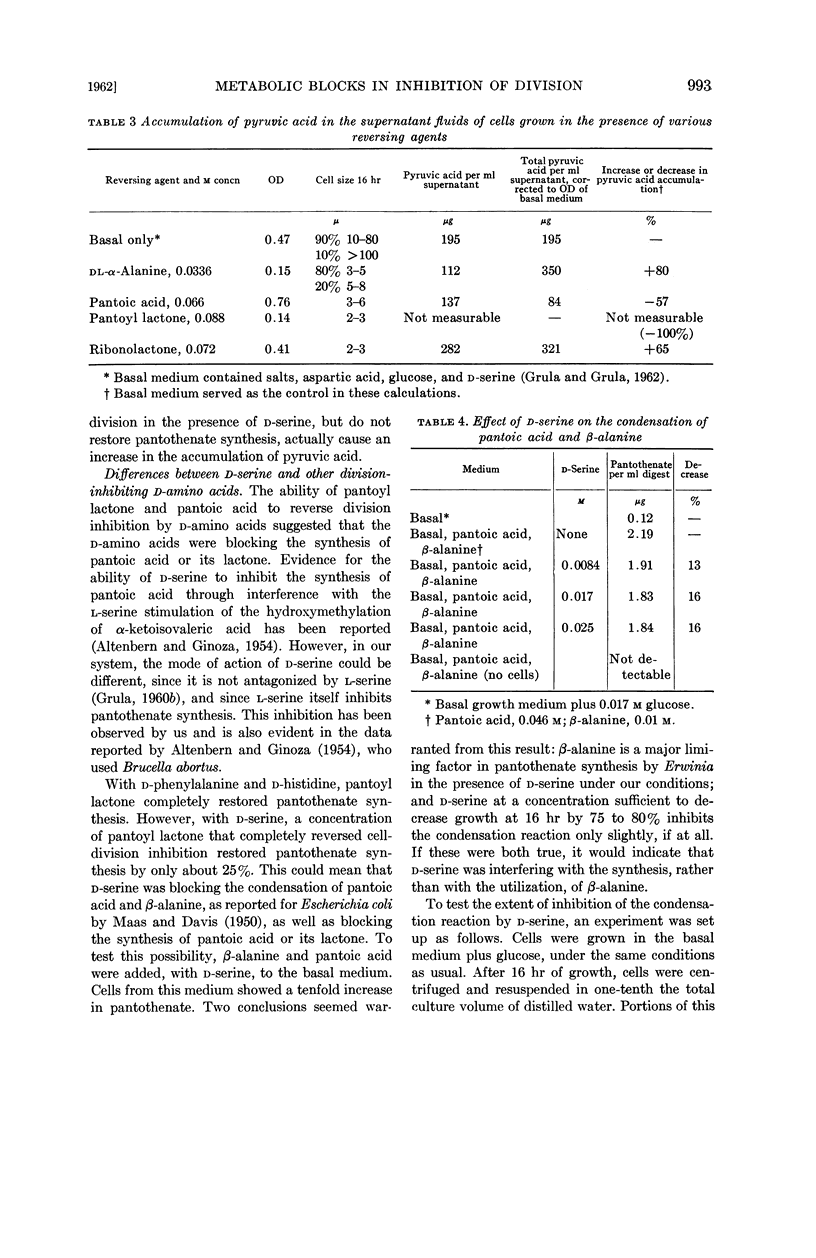
Evidence is presented that d-serine interferes with the utilization of aspartic acid and also blocks synthesis of pantoic acid.
Compounds (including glucose) which tend to produce long cells result in the accumulation of pyruvic acid in the growth medium. Pantoic acid reduces, and pantoyl lactone abolishes completely, this accumulation of pyruvate. Other reversing agents do not abolish the pyruvate accumulation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALTENBERN R. A., GINOZA H. S. Pantothenic acid synthesis by smooth Brucella abortus. J Bacteriol. 1954 Nov;68(5):570–576. doi: 10.1128/jb.68.5.570-576.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen D., Lichstein H. C. STUDIES ON THE ASPARTIC ACID DECARBOXYLASE OF RHIZOBIUM TRIFOLII. J Bacteriol. 1949 Aug;58(2):215–221. doi: 10.1128/jb.58.2.215-221.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINOZA H. S., ALTENBERN R. A. The pantothenate-synthesizing enzyme in cell-free extracts of Brucella abortus, strain 19. Arch Biochem Biophys. 1955 Jun;56(2):537–541. doi: 10.1016/0003-9861(55)90273-3. [DOI] [PubMed] [Google Scholar]
- GRULA E. A. Cell division in a species of Erwinia. I. Inhibition of division by D-amino acids. J Bacteriol. 1960 Sep;80:375–385. doi: 10.1128/jb.80.3.375-385.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRULA E. A. Cell division in a species of Erwinia. I. Initial observations relating to nutritional dependency. J Bacteriol. 1960 Sep;80:369–374. [PMC free article] [PubMed] [Google Scholar]
- GRULA E. A., GRULA M. M. Cell division in a species of Erwinia III. Reversal of inhibition of cell division caused by D-amino acids, penicillin, and ultraviolet light. J Bacteriol. 1962 May;83:981–988. doi: 10.1128/jb.83.5.981-988.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAAS W. K., DAVIS B. D. Pantothenate studies. I. Interference by D-serine and L-aspartic acid with pantothenate synthesis in Escherichia coli. J Bacteriol. 1950 Dec;60(6):733–745. doi: 10.1128/jb.60.6.733-745.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAAS W. K., VOGEL H. J. alpha-Ketoisovaleric acid, a precursor of pantothenic acid in Escherichia coli. J Bacteriol. 1953 Apr;65(4):388–393. doi: 10.1128/jb.65.4.388-393.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PURKO M., NELSON W. O., WOOD W. A. The role of p-aminobenzoate in pantoate synthesis by Bacterium linens. J Biol Chem. 1954 Mar;207(1):51–58. [PubMed] [Google Scholar]
- RADHAKRISHANAN A. N., SNELL E. E. Biosynthesis of valine and isoleucine. 2. Formation of alpha-acetolactate and alpha-aceto-alpha-hydroxybutyrate in Neurospora crassa and Escherichia coli. J Biol Chem. 1960 Aug;235:2316–2321. [PubMed] [Google Scholar]
- RADHAKRISHANAN A. N., WAGNER R. P., SNELL E. E. Biosynthesis of valine and i43soleucine, 3. alpha-Keto-beta-hydroxy acid reductase and alpha-hydroxy-beta-Keto acid reductoisomerase. J Biol Chem. 1960 Aug;235:2322–2331. [PubMed] [Google Scholar]
- SINGER T. P., PENSKY J. Isolation and properties of the carboxylase of wheat germ. J Biol Chem. 1952 May;196(1):375–388. [PubMed] [Google Scholar]
- SINGER T. P., PENSKY J. Mechanism of acetoin synthesis by alpha-carboxylase. Biochim Biophys Acta. 1952 Sep;9(3):316–327. doi: 10.1016/0006-3002(52)90167-4. [DOI] [PubMed] [Google Scholar]
- STRASSMAN M., SHATTON J. B., WEINHOUSE S. Conversion of alpha-acetolactic acid to the valine precursor, alpha,beta-dihydroxyisovaleric acid. J Biol Chem. 1960 Mar;235:700–705. [PubMed] [Google Scholar]


