Abstract
Purpose
Fatigue is one of the most frequently reported and adverse effects of cancer chemotherapy. The present study tested the hypothesis that women's levels of emotional distress at the time of their initial outpatient chemotherapy treatment would predict the severity of their postinfusion fatigue.
Methods
Sixty stage I (32.6%) and II (67.4%) patients with breast cancer (mean age, 44.5 years) who were receiving standard outpatient chemotherapy participated. The independent variable, emotional distress, was assessed for “last night,” “this morning,” and “right now” with a visual analog scale (0 to 100). The dependent variable, post-treatment fatigue (PTF), was assessed (0 to 100) over each of the subsequent 6 days using end-of-day diaries, which also included assessments of distress and nausea (0 to 100). For the statistical analyses, post-treatment fatigue was divided into three phases with means calculated for days 1 through 2 (phase 1), 3 to 4 (phase 2), and 5 to 6 (phase 3).
Results
Consistent with the study hypothesis, patients' pretreatment distress level in the clinic was a significant (P < .001) predictor of PTF. There was also a significant (P < .025) interaction with phase, with distress becoming a predictor of PTF after phase 1. Multivariate analysis indicated that prior levels of distress were not independent predictors of PTF.
Conclusions
This study is the first to demonstrate time-specific effects of pretreatment distress on PTF. Possible mechanisms of these effects now warrant investigation, as do possible benefits of brief interventions to reduce patient distress immediately before treatment.
Introduction
Fatigue is one of the most frequently reported and adverse effects of cancer and its treatment.1,2 Patients' descriptions of cancer-related fatigue (CRF) include a feeling of debilitating tiredness, weakness, lethargy, and malaise3 where the exhaustion felt is disproportionate to the level of physical exertion and not relieved by sleep.4,5 CRF has a substantial negative impact on quality of life.2
CRF is particularly high during and after chemotherapy treatment.6,8 High levels of CRF following chemotherapy infusions have been confirmed with daily assessment methods.9,10 Fatigue typically reaches its maximum over the first few days following treatment infusions and then declines.9,10
One potential contributor to post-treatment fatigue (PTF) that has received little attention is emotional distress, which is particularly high among patients before chemotherapy.11,12 This possibility receives support from the surgery literature, which has documented effects of preoperative distress on postoperative adverse effects including fatigue,11 as well as studies documenting effects of distress before chemotherapy on other adverse effects (eg, nausea) of treatment.13,14
The purpose of the present study was to examine the relationship between emotional distress before chemotherapy and PTF. It was hypothesized that patients with breast cancer with higher levels of distress in the clinic before their first infusion of chemotherapy will have higher levels of fatigue throughout the days following this treatment.
Materials and Methods
Participants
Sixty chemotherapy-naive patients with stage I (32.6%) or II (67.4%) breast cancer who were scheduled to receive standard outpatient chemotherapy (with complete outcome data) were included in this study. As part of a longitudinal study, consecutive patients were referred by their oncologist to a research assistant who confirmed eligibility and obtained informed consent following an institutionally approved protocol. Patients' ages ranged from 29 to 76 years (mean, 44.5 years; standard deviation, 9.0) and they were predominately white (84%) and married or living with their partners (68%).
Patients had either stage I or II breast cancer, were postradical, modified radical, or segmental mastectomy; were at least 18 years of age; and were scheduled to receive their first outpatient adjuvant chemotherapy (IV) treatment. Standard therapy included combinations of cyclophosphamide (C), methotrexate (M), fluorouracil (F), adriamycin (A) and paclitaxel (T), as well as standard antiemetic treatment (eg, 5HT3 agonists). Women who were pregnant or had a serious comorbid condition or tumor were excluded. The preponderance of the sample received a CMF regimen (61.1%); the rest received ACMF (11.1%) or ATC (27.8%) regimens.
Procedure and Measures
In the clinic before their first chemotherapy treatment, patients completed self-report measures of study variables. Medical variables were confirmed by chart review. Emotional distress (“right now”) was assessed with a 10-cm visual analog scale (VAS) on which severity was indicated by placing a mark across a line that was anchored by “not at all emotionally upset” and “as emotionally upset as I could be.” Patients were instructed to mark to the left of the line if they were absolutely not upset at all (coded as zeros). Otherwise, the distance in millimeters from the left end of the line provided the distress score (range, 0 to 100). Pretreatment fatigue was similarly assessed with a VAS for “right now” using the well validated single item measure of overall fatigue from the fatigue severity scale.15,16 Visual analog scales are a validated, efficient and widely used way to assess both distress and self-reported symptoms with minimal burden in clinical settings.17,18 To allow an exploratory evaluation of the temporal specificity of the relationship between pretreatment distress and PTF, patients also completed VAS measures of emotional distress for “last night at home” and “this morning at home.”
Patients were provided with end-of-day “diaries” to assess fatigue severity for 6 days following treatment. Diary instructions were provided and participants were asked to return the diaries at their second infusion. Women were asked to rate their fatigue for the day on a numeric rating scale from 0 (“not at all fatigued”) to 100 (“as fatigued as I could be”) and provide a daily 0 to 100 rating of their distress and nausea to allow analysis of possible relationships to PTF. Such self-reported ratings provide a well validated means of assessment, comparable to VAS approaches, which are both convenient to complete and space saving.19–21
Statistical Analysis
All statistical analyses were conducted with SAS (version 9.1, SAS Institute, Cary, NC) using longitudinal data analysis (generalized linear models). Study variables that could potentially confound the relationship between pretreatment distress and PTF (eg, demographic, medical, and behavioral factors) were evaluated in preliminary analyses. Descriptive statistics are presented in Table 1. Patients' fatigue levels in the clinic before chemotherapy were significantly related to PTF (F1,58 = 17.28, P < .0001) in bivariate analysis and were therefore included as a covariate in subsequent analyses, as appropriate. No significant bivariate relationships (P > .10) were found between PTF and any of the other potential covariates: age, age group (< 49 v ≥ 50), ethnicity, chemotherapy regimen, stage, or sleep duration the night before chemotherapy.
Table 1.
Background Variables
| Patient Characteristics | % of Patients | |
|---|---|---|
| Pretreatment fatigue score | ||
| Mean | 19.2 | |
| SD | 3.3 | |
| Range | 18-64 | |
| Pretreatment distress score | ||
| Mean | 26.9 | |
| SD | 3.7 | |
| Range | 0.100 | |
| Age, years | ||
| Mean | 44.5 | |
| SD | 1.2 | |
| Range | 29-76 | |
| Age group, years | ||
| < 50 | 28.6 | |
| > 50 | 71.4 | |
| Ethnicity | ||
| White | 83.9 | |
| Nonwhite | 16.1 | |
| Marital status, % of patients | ||
| Not married | 32.1 | |
| Married/living with partner | 67.9 | |
| Hours of sleep last night | ||
| Mean | 6.1 | |
| SD | 0.2 | |
| Range | 3-10 | |
| Chemotherapy regimen, % | ||
| CMF | 61.1 | |
| ACMF | 11.1 | |
| ATC | 27.8 | |
| Stage | ||
| I | 32.6 | |
| II | 67.4 |
NOTE. N varies due to missing data. For reference, a recently published study22 found that healthy adults whose levels of current fatigue were assessed using a VAS had a mean fatigue score of 19.7 (of a possible 100). In addition, using an identical VAS measure we have recently found mean distress levels of 38.2 in patients assessed in the clinic prior to breast surgery.23
Abbreviations: CMF, cyclophosphamide-methotrexate-fluorouracil; ACMF, adriamycin-cyclophosphamide-methotrexate-fluorouracil; ATC, adriamycin-paclitaxel-cyclophosphamide; SD, standard deviation.
Results
The women's mean levels of distress “right now” in the clinic before their first treatment infusion was 26.85 (SD = 3.71), which did not significantly differ (P > .10) from their retrospectively reported distress levels (± SD) for “this morning” (26.85 ± 3.71), or “last night” (24.50 ± 3.45).
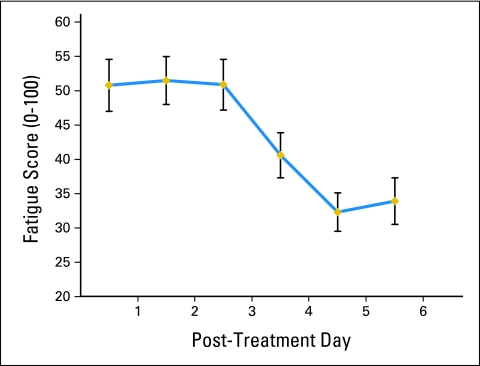
Patients' PTF levels reached a maximum on day 2 and subsequently showed substantial recovery (Fig 1). For statistical purposes (reducing type 1 error) PTF was divided into three phases with means calculated for days 1 to 2 (phase 1), 3 to 4 (phase 2), and 5 to 6 (phase 3) used in all analyses. Repeated measures analyses revealed a significant effect of phase (F2,118 = 22.23, P < .0001) on PTF. Contrasts indicated that phase 2 was significantly lower than phase 1 (F1,59 = 4.13, P < .047), and phase 3 was significantly lower than phase 2 (F1,59 = 33.72, P < .001).
Figure 1.
Descriptive time course of daily post-treatment fatigue.
To test the primary study hypothesis, patients' pretreatment distress in the clinic (predictor variable) was entered into a repeated measures analysis with PTF as the dependent variable, and fatigue in the clinic as a covariate. As presented in Table 2, there was a significant main effect of patients' levels of pretreatment distress on their levels of PTF. There was also a significant main effect of phase and an interaction between pretreatment distress and phase. Post hoc regression analyses indicated that pretreatment distress was not a significant predictor of phase 1 PTF levels (P > .39), but was a significant predictor of phases 2 (P < .002) and 3 (P < .0003) fatigue levels. To explore the temporal specificity of pretreatment distress relative to the infusion, distress levels “last night” and “this morning” were added to the regression model. Distress in the clinic remained a significant predictor, while earlier distress levels did not in the multivariate model (Table 3).
Table 2.
NOTE. Pretreatment Distress Is a Significant Independent Predictor of Post-Treatment Daily Fatigue in a Multivariate Analysis
| F | P | |
|---|---|---|
| Pretreatment fatigue | F1,57 = 28.06 | .001 |
| Pretreatment distress | F1,57 = 11.81 | .001 |
| Phase | F2,114 = 13.43 | .001 |
| Pretreatment distress X phase | F2,114 = 3.29 | .040 |
Dependent variable = post-treatment daily fatigue (0-100).
Table 3.
Selective Relationship Between Distress in the Clinic and Post-Treatment Daily Fatigue
| Bivariate | Multivariate | ||||
|---|---|---|---|---|---|
| F | P | Parameter Estimate | t55 | P | |
| Pretreatment fatigue in the clinic | F1,58 = 37.80 | .001 | 0.39 | 5.00 | .001 |
| Pretreatment distress last night | F1,58 = 7.88 | .006 | −0.02 | −0.24 | .813 |
| Pretreatment distress this morning | F1,58 = 14.30 | .004 | 0.07 | 0.69 | .491 |
| Pretreatment distress in the clinic | F1,58 = 19.50 | .001 | 0.19 | 2.30 | .025 |
NOTE. Dependent variable = post-treatment fatigue.
To explore the possible contribution of patients' levels of post-treatment distress and nausea to the models in which pretreatment distress predicted PTF (ie, phases 2 and 3), separate regression analyses were conducted. For these analyses the daily post-treatment emotional distress and nausea scores on days 3 through 4 and 5 to 6 were averaged to reflect post-treatment phases 2 and 3, as for fatigue. Pretreatment distress remained a significant predictor (P < .005) of fatigue during the third phase of PTF when controlling for pretreatment fatigue, post-treatment phase 3 nausea, and emotional distress. Phase 3 distress was a significant independent predictor of PTF (P < .002), but nausea was not (P > .142).
Discussion
The results of the present study were consistent with the hypothesis. Patients' pretreatment distress levels assessed in the clinic before their first chemotherapy infusion were related to their levels of PTF, assessed daily for 6 days following treatment, controlling for pretreatment fatigue levels. The relationship appeared to be temporally selective, with patients' levels of distress in the clinic environment more predictive than distress that morning or the prior night. The relationship between pretreatment distress in the clinic and PTF also appeared to show temporal selectivity, as a significant relationship was not seen until after the first 2 post-treatment days.
The pattern of daily fatigue levels in the present study was consistent with previous reports indicating that fatigue is highest during the first few days following chemotherapy.9,10 An association between distress and PTF is consistent with previous reports in the literature,22,23 but those studies reported relationships between fatigue and distress measured concurrently following treatment infusions. With such associations, also found in the present study, causality cannot be determined. Distress may cause fatigue, or alternatively, patients' experiences of fatigue may cause distress.2 To our knowledge, the present study is the first to demonstrate that patients' levels of pretreatment distress are predictive of their subsequent levels of PTF, independent of concurrent distress.
To our knowledge, the results of the present study are also the first to suggest that the effects of pretreatment distress on PTF may not be evident over the initial phase of the PTF, but rather come into play over subsequent days. It will be important for future studies to confirm these findings, as such temporally selective relationships would suggest that pretreatment distress may not affect the initial onset and severity of PTF, but rather how well a patient recovers (ie, at what level patients' fatigue will stabilize). It is tempting to speculate that this could reflect a greater variability during the “leveling off” phase of the fatigue response to chemotherapy. Indirect support for such temporally selective effects comes from recent studies documenting effects of patients' levels of distress before chemotherapy infusions on delayed, but not acute, post-treatment nausea.13,14 However, it should be noted that the lack of a relationship between pretreatment distress and the early days of post-treatment fatigue must be interpreted with caution, as the modest sample size limits the power of the study. It is important to note that the relationship between pretreatment distress and patients' PTF across days 5 through 6 remained significant after controlling for concurrent nausea and emotional distress. These findings suggest that pretreatment distress may make a contribution to PTF through pathways beyond effects of pretreatment distress on post-treatment distress or nausea.
Interestingly, it appears that the effect of pretreatment distress on fatigue is time specific, not only with regard to its effect on fatigue, but also with regard to when distress is experienced in relation to treatment. Pretreatment distress immediately before treatment was a better predictor of PTF than was either distress the morning of or the night before treatment. It could be that the measurement of distress immediately before treatment is more reliable, as it is not retrospective. However, confirmation of these findings may also suggest a selective effect of distress at the time of the treatment infusion on PTF. The mechanisms responsible for such effects should be explored in future research. One possibility is that acute distress may affect the blood-brain barrier, allowing cytotoxic agents or active metabolites greater access to the CNS. Consistent with this possibility are studies indicating that acute stress can increase permeability of the blood-brain barrier to a variety of agents.24
Another pathway by which distress could affect fatigue is through effects on cytokines.25 Alterations in cytokine (eg, interleukin-1ra, interleukin-6, neopterin) levels may, in turn, affect the patients' levels of PTF.26,27 Cytokines and related markers have also demonstrated a relationship with the ongoing fatigue reported by breast cancer survivors.28 However, less consistent is the notion that the fatigue witnessed in cancer patients undergoing active chemotherapy treatment is related to an alternation cytokines, and much of it comes from animal models.29 It may be that this association is transient, requiring daily measurement of both cytokines and fatigue to capture it, and future studies should strive to do this. However, in the present study, it remains unclear why this effect is apparent during the recovery period, but not the onset of fatigue.
The present study had several limitations that must be considered when interpreting the results. First, while the diary approach used allows for daily assessments of fatigue, the data are nonetheless retrospective and we have no independent means to confirm the timing of diary entries. Future studies may want to consider ecologic momentary sampling with repeated time-stamped assessments of current fatigue collected electronically across the course of the day.30 Second, though this study was one of the first to assess the effects of pretreatment distress on PTF, while controlling for daily distress, future studies may also want to examine possible effects of pretreatment depression. A third limitation of this study, common to this literature, is the lack of a true baseline fatigue measurement. Although the present study did assess patients' levels of fatigue in the clinic before treatment, assessments during a baseline period before treatment would help to better characterize the effects.
The current results emphasize the importance of additional research to explore the impact of pretreatment distress on PTF. Further studies of the time specificity of distress before patients' initial treatment infusion, for example, could direct attention to novel pathways by which psychological distress could influence PTF. It will also be of interest to examine the impact of distress before subsequent treatments, which is known to be less severe,11,12 on patients' experience of fatigue following those infusions to determine how pervasive these effects may be. Examination of the generalizability of these effects of pretreatment distress across different chemotherapy regimens and types of patients is also important. Finally, a particularly important area for future research to investigate is the possibility that PTF, once considered an outcome that patients must simply endure, may be reduced by interventions to reduce distress before treatment infusions. If supported by the results of randomized clinical trials, prophylactic psychological treatment may one day become an important aspect of standard care for patients with cancer requiring chemotherapy.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgment
Supported by the American Cancer Society Grant No. RSG-010-180-01-PBP and the National Cancer Institute Grant Nos. CA81137 and CA105222.
References
- 1.Boehmke MM: Measurement of symptom distress in women with early-stage breast cancer. Cancer Nurs 27:144-152, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Hofman M, Ryan JL, Figueroa-Moseley CD, et al: Cancer-related fatigue: The scale of the problem. Oncologist 12:4-10, 2007. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 3.Groopman JE: Fatigue in cancer and HIV/AIDS. Oncology (Huntingt) 12:335-351, 1998 [PubMed] [Google Scholar]
- 4.Gutstein HB: The biologic basis of fatigue. Cancer 92:1678-1683, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Jean-Pierre P, Figueroa-Moseley CD, Kohli S, et al: Assessment of cancer-related fatigue: Implications for clinical diagnosis and treatment. Oncologist 12:11-21, 2007. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 6.Hartvig P, Aulin J, Hugerth M, et al: Fatigue in cancer patients treated with cytotoxic drugs. J Oncol Pharm Pract 12:155-164, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Woo B, Dibble SL, Piper BF, et al: Differences in fatigue by treatment methods in women with breast cancer. Oncol Nurs Forum 25:915-920, 1998 [PubMed] [Google Scholar]
- 8.Bower JE, Ganz PA, Desmond KA, et al: Fatigue in long-term breast carcinoma survivors: A longitudinal investigation. Cancer 106:751-758, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Berger AM: Activity/rest patterns and perceived fatigue and sleep quality during the first week of intravenous chemotherapy for early stage breast cancer. Oncol Nurs Forum 22:A54, 1995 [Google Scholar]
- 10.Molassiotis A, Chan CW: Fatigue patterns in Chinese patients receiving chemotherapy. Eur J Oncol Nurs 5:60-67, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Montgomery GH, McClary KA, Bovbjerg DH: Adjuvant therapy for breast cancer and psychological distress. Ann Oncol 7:977-978, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Watson M, Meyer L, Thomson A, et al: Psychological factors predicting nausea and vomiting in breast cancer patients on chemotherapy. Eur J Cancer 34:831-837, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Higgins SC, Montgomery GH, Bovbjerg DH: Distress before chemotherapy predicts delayed but not acute nausea. Support Care Cancer 15:171-177, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Molassiotis A, Yam BM, Yung H, et al: Pretreatment factors predicting the development of postchemotherapy nausea and vomiting in Chinese breast cancer patients. Support Care Cancer 10:139-145, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Kleinman L, Zodet M, Hakim Z, et al: Psychometric evaluation of the fatigue severity scale for use in chronic hepatitis C. Qual Life Res 9:499-508, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Krupp L, LaRocca N, Muir-Nash J, et al: The fatigue severity scale; Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives Neurol 46:1121-1123, 1989 [DOI] [PubMed] [Google Scholar]
- 17.Richardson A: The health diary: An examination of its use as a data collection method. Journal of Advanced Nursing 19:782-791, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Wolfe F: Fatigue assessments in rheumatoid arthritis: Comparative performance of visual analog scales and longer fatigue questionnaires in 7760 patients. J Rheumatol 31:1896-1902, 2004 [PubMed] [Google Scholar]
- 19.Gift AG, Narsavage G: Validity of the numeric rating scale as a measure of dyspnea. Am J Crit Care 7:200-204, 1998 [PubMed] [Google Scholar]
- 20.Breivik EK, Bjornsson GA, Skovlund E: A comparison of pain rating scales by sampling from clinical trial data. Clin J Pain 16:22-28, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Hollen PJ, Gralla RJ, Kris MG, et al: A comparison of visual analogue and numerical rating scale formats for the Lung Cancer Symptom Scale (LCSS): Does format affect patient ratings of symptoms and quality of life? Qual Life Res 14:837-847, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Harboe E, Greve OJ, Beyer M, et al: Fatigue is associated with cerebral white matter hyperintensities in patients with systemic lupus erythematosus. J Neurol Neurosurg Psychiatr: 2007. [Epub ahead of print] [DOI] [PubMed]
- 23.Schnur JB, Bovbjerg DH, David D, et al: Hypnosis decreases presurgical distress in excisional breast biopsy patients. Anesth Analg. (in press) [DOI] [PubMed]
- 24.de Jong JN, Candel MJ, Schouten HC, et al: Course of mental fatigue and motivation in breast cancer patients receiving adjuvant chemotherapy. Ann Oncol 16:372-382, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Irvine D, Vincent L, Graydon JE, et al: The prevalence and correlates of fatigue in patients receiving treatment with cheotherapy and radiotherapy. Cancer Nurs 17:367-378, 1994 [PubMed] [Google Scholar]
- 26.Theoharides TC, Konstantinidou AD: Corticotropin-releasing hormone and the blood-brain-barrier. Front Biosci 12:1615-1628, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Witek-Janusek L, Gabram S, Mathews HL: Psychologic stress, reduced NK cell activity, and cytokine dysregulation in women experiencing diagnostic breast biopsy. Psychoneuroendocrinology 32:22-35, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schubert C, Hong S, Natarajan L, et al: The association between fatigue and inflammatory marker levels in cancer patients: A quantitative review. Brain Behav Immun 21:413-427, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Cleeland CS, Bennett GJ, Dantzer R, et al: Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer 97:2919-2925, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Bower JE, Ganz PA, Aziz N, et al: Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med 64:604-611, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Wood LJ, Nail LM, Perrin NA, et al: The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biol Res Nurs 8:157-169, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Piasecki TM, Hufford MR, Solhan M, et al: Assessing clients in their natural environments with electronic diaries: Rationale, benefits, limitations, and barriers. Psychol Assess 19:25-43, 2007 [DOI] [PubMed] [Google Scholar]