Abstract
Purpose
Determining the optimal starting dose of chemotherapy (CHT) presents a considerable challenge when using body-surface area (BSA)–based dosing, particularly in obese, elderly, or thin patients. We sought to document the range of approaches employed when administering CHT to these patients.
Methods
A questionnaire was developed by a panel of oncologists and mailed to all members of the Medical Oncology Group of Australia.
Results
From 315 oncologists, 188 responded (response rate 59.7%). BSA-based dosing is standard practice for 176 (97.2%) of the responding oncologists. In the adjuvant disease setting, 23 (12.7%) use ideal rather than actual body weight (BW) to calculate BSA, or choose whichever is less. When treating obese patients, only 6.1% of respondents routinely use actual BW. Of the remainder, 69.5% either cap the dose at 2 m2 or use ideal BW. In underweight patients, 95% (n = 171) routinely calculate BSA using actual BW. Forty one respondents (22.7%) routinely reduce dose in the fit elderly.
Conclusion
This analysis of BSA-based CHT dosing methods demonstrates significant variability in practice. Based on evidence from adjuvant studies showing that actual BSA-based dosing is desirable, a substantial number of Australian patients are being underdosed. Further education, together with ongoing research, is required to optimize individualized dosing for efficacy and tolerability.
Introduction
The use of body-surface area (BSA) has been the mainstay of chemotherapy (CHT) dosing in oncology practice for the last half-century. Derived from animal models to estimate appropriate phase I drug doses,1 BSA equates to the two-dimensional surface area of a person's skin.2 However it is apparent that for the most part, the pharmacokinetics and pharmacodynamics of drug administration are related to more than a simple equation derived from height and weight. BSA-based dosing does not account for the marked variations in hepatic or renal function, the proportion of body fat, or multiple other variables involved with drug handling. Indeed, multiple studies using various chemotherapeutic agents have found little correlation between BSA and pharmacokinetic parameters such as clearance or toxicity.2–4 A retrospective review of 33 agents investigated in phase I studies over a 10-year period (1991-2001), found that for only five instances did BSA-based dosing significantly reduce interpatient variability in drug clearance.5 Despite these limitations, BSA-based calculation has become widespread, largely in the absence of a viable alternative.
Three specific patient populations in adult oncology—obese, elderly, and thin—need particular consideration when selecting CHT doses and are an expanding component of oncology practice. It is estimated that 65% of the adult population in the United States is overweight, and 30% are obese.6 Obesity is a global epidemic. In Australia, a 2004-2005 National Health Survey showed that the proportion of adults classified as overweight or obese increased over the previous decade: for men from 52% to 62%, and for women from 37% to 45% (Australian Bureau of Statistics 2005). As advanced cancer can cause anorexia and weight loss, it is also not uncommon to administer CHT to thin or underweight patients. The population in the United States older than 65 years is projected to rise by 100% by 2030 to 68 million—around 20% of the population.7 Australia's population is similarly ageing.8 For the overweight or obese patient, clinicians may compromise effective therapy by dosing according to ideal BW rather than actual BW, potentially reducing the patient's dose by up to 25%, despite little evidence to support this practice.9 Even in the fit older patient, typical age-related changes such as an increase in body fat, decline in total body water, and decreased activity of liver cytochrome P450 enzymes and renal function10 may significantly impact on the pharmacokinetics of CHT administration, and the possibility of increased toxicity again is not accounted for with BSA-based dosing.
The aim of this survey was to quantify and explore some of the methods and issues surrounding BSA-based dosing of CHT and in particular the use and calculation of BSA among Australian medical oncologists.
Methods
Study Population
A nationwide survey was mailed in mid-2006 to all medical oncologists and advanced trainee oncology registrars (fellows) who were members of the Medical Oncology Group of Australia (MOGA), to which the vast majority of Australian medical oncologists and all oncology trainees belong. Three hundred fifty physicians were identified from the membership list of MOGA. The survey was anonymous; identification numbers were used to allow follow-up of nonresponders. To facilitate return of the survey, a reminder email was sent close to the cutoff date.
Design
The survey was an independent, clinician-driven project, designed and developed by a panel of oncologists. An initial evaluation assessing understanding and ease of administration was performed by 10 oncologists. Based on feedback, the questionnaire was modified to improve clarity.
Demographic information was obtained for subsequent analysis, including amount of work in public or private practice, fellow versus consultant oncologist, and years of experience.
Questions included whether actual or ideal BW was used to calculate CHT doses in the adjuvant and metastatic disease settings; what approach was used for the obese or thin patient; and whether routine dose reduction was performed in fit elderly patients. In defining ideal BW, clinicians were given the options of body-mass index (BMI) 20 to 25 kg/m2 (normal as per the World Health Organization11); BMI ≥ 27 kg/m2 (overweight); ‘other’ for an alternative response; and an option of ‘never use’. The option of 27 kg/m2 was included as some charts used to calculate ideal BW are based on this BMI. Respondents were also asked if they would routinely dose reduce for patients who had experienced significant toxicity from prior CHT, and finally whether drug doses were adjusted according to the known size of drug ampoules. Each question had four to five possible responses, and some questions had an ‘other’ option to draft a free text response if none of the answers were applicable for that clinician.
‘Obese’ and ‘thin’ were not specifically defined, but it was assumed that most respondents would be aware of standard classifications (BMI > 30 kg/m2 for obese and < 18.5 kg/m2 for underweight, according to WHO guidelines).11,12 ‘Elderly’ was defined as older than 75 years. ‘Fit’ was not defined but implied a good performance status and adequate major organ function.
Analysis
Data analysis was conducted using Stata SE 9.0 (College Station, TX). All P values are two sided, and were calculated using the Mantel-Haenszel test, or with Fisher's exact test on occasions that the sample size was small.
Results
Demographics
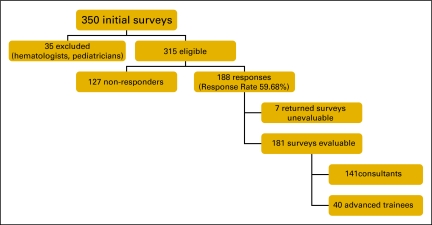
From the full MOGA membership of 350, 35 members were considered ineligible (pediatric trainees, hematologists, or no longer in practice). Of the remaining 315 recipients, 188 responses were received, giving a response rate of 59.7%. Seven questionnaires were unevaluable (not filled out) giving a total of 181 evaluable responses, from 141 consultants and 40 fellows (Figure 1). Of the consultants, 56% worked mostly or always in public practice, and 23.4% were mostly or always in private practice. 48.2% of the consultants had more than 10 years of experience in oncology. The demographic profile of respondents is representative of practicing medical oncologists in Australia.
Figure 1.
Response rate flow chart.
BSA-based dosing.
BSA-based dosing was reported as standard practice by 176 respondents (97.2%), who stated they use this method in all (n = 95; 52.5%) or most (n = 81; 44.7%) cases. An obvious exception was carboplatin dosing, where the Calvert formula is widely used.13
Actual versus ideal body weight.
Clinicians were asked their interpretation of ideal BW. While 32% (n = 58) stated they never use ideal BW, 48 physicians (34% of all physicians) and 23 fellows (57.5% of all fellows) related ideal BW to a BMI of 20 to 25 kg/m2. Of the remainder, 13.8% (n = 25) chose a BMI of 27 as representative of ideal BW.
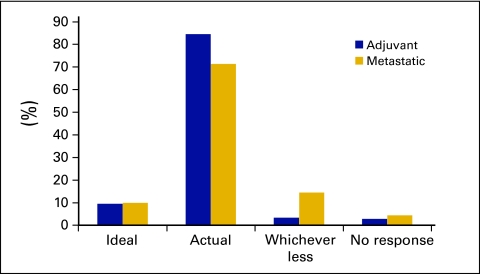
Overall, clinicians were more likely to use actual BW in the adjuvant rather than metastatic disease setting (84.5% in adjuvant v 71% in metastatic disease; P < .01). In the adjuvant disease setting, 84.5% (n = 153) of respondents use actual BW to calculate CHT, 9.4% (n = 17) use ideal BW and 3.3% (n = 6) would choose whichever BW resulted in the lowest dose (Figure 2).
Figure 2.
Body weight used in calculating chemotherapy: adjuvant versus metastatic disease.
In metastatic disease, 71% use actual BW to calculate CHT, with a higher rate among the consultants than the fellows (75.9% v 55.0%, respectively; P = .031). Consistent with this, fellows were more likely than consultants to use whichever gave the lesser dose in the metastatic setting (30% v 9.9%; P = .0018). Differences were not statistically significant when comparing private and public practitioners and level of experience (data not shown).
Obese, thin, and elderly patients (Figures 3 and4).
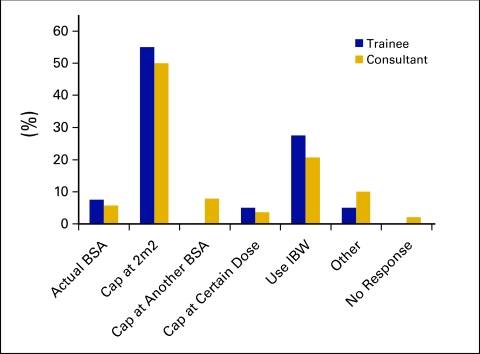
Figure 3.
Approach to chemotherapy dosing in obese patients.
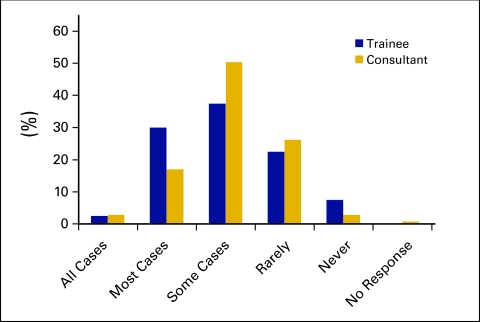
Figure 4.
Dose reduction frequency in fit elderly patients.
Clinicians were asked their typical approach when dosing CHT for the obese patient. The two most common approaches included capping the dose at 2.0 m2 by 92 respondents (50.8%) or using ideal BW by 40 respondents (22.1%). Only 11 respondents (6.1%) routinely used actual BW when calculating CHT doses for obese patients. Responses did not differ significantly with level of experience or public versus private practice. In thin patients, 92.9% (n = 131) of consultants and 100% (n = 40) of fellows routinely use actual BSA, with no significant differences according to level of experience or practice setting (public v private). Respondents were asked if they commonly dose reduce for fit elderly patients (older than 75 years); 32.5% (n = 13) of fellows and 19.8% (n = 28) of consultants would dose reduce in all or most cases (P = .096, not significant).
Prior toxicities.
Clinicians were asked whether CHT dose was routinely reduced in patients who had experienced prior significant toxicity from other agents. Treatment in the adjuvant versus metastatic disease setting was not specified; 50.4% (n = 61) of consultants and 40% (n = 16) of fellows would do this in all or most cases (difference P = .22, not significant).
Ampoule size.
Overall, 18.2% (n = 33) of clinicians adjust doses according to the size of the ampoules or vials of CHT in all or most cases; 49.7% (n = 90) rarely or never adjust doses in this manner. Consultants with more years of experience were more likely to adjust according to ampoule size in all or most cases (31.0% in > 20-years experience, v 7.4% in < 5-years experience; P = .04). There were no significant differences in responses between private and public practitioners.
Discussion
This Australian survey documents clear variation in the use of BSA-based CHT dosing, and explores the individual clinician's approach to two increasingly common scenarios: the overweight patient and the elderly patient. The results need to be considered in the context of current evidence-based guidelines, which, while scant, do suggest that there are areas where best practice is not being employed.
The most striking result is that 12.7% of clinicians, when treating patients in the adjuvant disease setting, used ideal BW, or whatever BW resulted in the lower dose. In obese patients, only 11 clinicians (6.1%) use actual BW; the majority prefer to approximate an ideal BW or to cap at an arbitrary dose or BSA. Given that BSA does not adjust for body fat, clinicians appeared to be concerned about overdosing obese patients and were keen to use some method, regardless of its validity, to compensate. However, even when attempting to do this by using ideal BW, we found wide variation in interpretations of ideal BW, with 13.8% placing ideal BW at a BMI of 27 kg/m2. WHO define this as overweight.11,12 While oncologists may not actually calculate BMI, they may well utilize charts to calculate ideal BW. They should be aware of whether these charts are based on a normal BMI or one reflecting overweight/obesity. This is not the first time that differing preferences surrounding weight selection have been documented. A survey of 52 bone marrow transplant institutions found substantial differences in weight formulas used for dosage calculations, with no single method used in greater than 30% of institutions.14
Where available, evidence strongly supports the use of actual BW in the adjuvant setting for solid organ tumors. For patients with early-stage breast cancer there appears to be a threshold for relative dose intensity below which little or no clinical benefit may be obtained.15,16 One study examining data from a large adjuvant breast cancer trial found that obese women who were routinely dose reduced in cycle 1 experienced inferior failure-free survival, and the use of actual BW did not result in increased toxicities.17 Studies in small-cell lung cancer and bowel cancer, similarly, have found that obesity was not associated with any increase in CHT-related toxicity when using actual BW.18,19
Our survey thus indicates that a substantial proportion of patients may be inappropriately underdosed. This finding has been documented previously—an analysis of International Breast Cancer Study Group trials from 1978 to 1993 showed that in obese patients, 96% of clinicians used ideal BW to calculate CHT, as opposed to 47% in the intermediate BW group and 2% in those with normal BW.20 A retrospective cohort study of nearly 10,000 women undergoing CHT for early-stage breast cancer found that 11% of overweight, 20% of obese, and 37% of severely obese women were routinely dose reduced in the first cycle.21 A prospective registry of 764 patients with breast cancer undergoing adjuvant treatment found that increased BMI was significantly associated with intentionally reduced doses, and multivariate analysis demonstrated that obesity was independently associated with reduced doses.22 In colon cancer, retrospective analysis of a large cohort of patients undergoing adjuvant treatment in National Surgical Adjuvant Breast and Bowel Project trials from 1989 to 1994 found 55% of obese and 73% of morbidly obese had capped doses, compared with 7% of normal weight individuals.23 Obesity in numerous cancer studies has been linked with a poorer prognosis 24–26; if intentionally reduced CHT doses are contributing, this is a concerning finding.
Available data surrounding CHT dosing in underweight individuals is scant. The previously mentioned analysis of National Surgical Adjuvant Breast and Bowel Project trials found that fewer cycles were given in underweight compared with normal weight individuals, and the underweight were more likely to receive less than the planned dose.23 Another analysis from an adjuvant rectal cancer study found that none of the 109 patients considered underweight were dose reduced.19 Grade 3 to 4 diarrhea, leucopenia, and stomatitis were higher in the underweight cohort, but this difference was not significant once adjusted for other predictors of toxicity. Despite scant evidence, it would seem that in the underweight patient using actual BW to calculate CHT dose is appropriate. This is the practice of the majority of our survey respondents.
Considerable heterogeneity was seen in methods of dosing in the elderly. Due to lack of evidence, clinical judgment is paramount in this population, balancing efficacy and toxicity against age-related changes in metabolism. As only around 22% of patients in clinical trials are older than 65 years, and a mere 8% to 13% are older than 70 years, it is difficult to simply apply data concerning regimens and doses from clinical trials to the elderly population.27 Nevertheless, there is increasing awareness that CHT can result in survival and quality-of-life advantages in older patients. In colorectal cancer, a meta-analysis found the benefit of adjuvant treatment was consistent across all age groups including those older than 70 years.28
In metastatic colorectal cancer, another meta-analysis of 22 trials including 629 patients older than 70 years showed equal survival in the older population compared with those younger than 70 years.29
Routine dose reductions in elderly patients are not uncommon. A survey of over 1,200 oncology practices and more than 20,000 patients in the United States, examining women treated with early-stage breast cancer, found that around two thirds of patients older than 65 years received less than 85% of reference dose-intensity.30 The meta-analysis of adjuvant CHT for colon cancer mentioned above found an increase in leucopenia in elderly patients but no increase in febrile neutropenia and overall no justification for less than full doses.28 The obvious issue with routine dose reduction, especially in the adjuvant setting, is that less effective therapy may be given and thus the chance of cure may be reduced. In the fit elderly, over 20% of respondents in our survey would routinely dose-reduce in all or most cases, which appears unjustified and inappropriate.
Nearly 20% of respondents reported that they adjust CHT doses according to size of the ampoule. Presumably this results from concern about the expense of many modern agents, and the reluctance to discard most of an ampoule when a slightly smaller dose may be adequate. Pharmacists, rather than clinicians, may be the predominant health professionals performing this dose adjustment.31,32
While important and interesting results have been obtained, there were a number of limitations of our study. The first of these is the modest response rate of just under 60%. This was despite two mailings and e-mail reminders. An element of bias may thus have affected results as clinicians who chose to respond may have been more interested in CHT dosing strategies. The survey excluded hematologists; as myeloablative CHT is generally not prescribed by medical oncologists in Australia, our discussion focuses on evidence surrounding standard dose CHT for solid organ tumors and should not be extrapolated to the myeloablative setting. Specific questioning asked about the ‘fit’ elderly but did not define ‘fit’. As patients in their seventies and older with cancer have an average of three different comorbidities,33 the interpretation surrounding ‘fitness’ may have differed between clinicians. Limited options were provided to interpret ideal BW which may have influenced the interpretability of these responses, although an ‘other’ section was provided if the options did not fit the clinician's interpretation.
In our survey we asked practice regarding CHT dosing. We did not survey actual practice. It is possible that respondents indicated a safer or more conservative method of dosing. However, the survey was anonymous to encourage reporting of actual practice.
What should change as a result of this survey? Clearly more research, education, and guidelines are warranted, given the discrepancies in dosing choices and the potential impact on outcomes. Given the variation in responses, there appears uncertainty among oncologists regarding what is best practice for these expanding groups of patients. This uncertainty is reflected in the available literature. Alternative CHT dosing methods continue to be explored. These include a modification to BSA-based dosing for obese patients proposed in 2005, using a corrected BSA equation for obese patients with a BMI more than 30 kg/m2, in an attempt to avoid unnecessary drug exposure while maintaining efficacy.34 Other dose adjustment methods for the obese have been described.35 One postulated option for elderly patients is to develop and use elderly-specific regimens rather than routinely dose-reducing standard regimens.36 One study of 92 patients being treated for early-stage breast cancer and aged between 65 and 90 years, adjusted CHT doses according to calculated creatinine clearance and found no age-related differences in toxicities or response; in addition, less dose reductions occurred during treatment.37 Advocates of geriatric oncology recommend the use of the Comprehensive Geriatric Assessment as a tool to guide decisions surrounding CHT dosing and administration in the elderly.38,39 Alternative methods of CHT dosing which have been suggested include fixed dose rates; use of area under the curve; toxicity-adjusted dosing; and incorporating pharmacokinetic and pharmacogenomic profiling.40–43 However, until alternative strategies become widely available and financially feasible, BSA remains the principal, albeit inadequate, method of calculating CHT for cancer patients.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgment
We thank Julie Johns and Ngio Murigu, M Int Hlth, BioGrid Australia, for assistance with data collection and analysis.
References
- 1.Pinkel D: The use of body surface area as a criterion of drug dosage in cancer chemotherapy. Cancer Res 18:853-856, 1958 [PubMed] [Google Scholar]
- 2.Gurney H: Dose calculation of anticancer drugs: A review of the current practice and introduction of an alternative. J Clin Oncol 14:259-611, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Sawyer M, Ratain MJ: Body surface area as a determinant of pharmacokinetics and drug dosing. Invest New Drugs 19:171-177, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Gurney HP, Ackland S, Gebski V, et al: Factors affecting epirubicin pharmacokinetics and toxicity: Evidence against using body surface area for dose calculation. J Clin Oncol 16:2299-2304, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Baker SD, Verweij J, Rowinsky EK, et al: Role of body surface area in dosing of investigational anticancer agents in adults, 1991-2001. J Natl Cancer Inst 94:1883-1888, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Modesitt SC, van Nagell JR Jr: The impact of obesity on the incidence and treatment of gynecologic cancers: A review. Obstet Gynecol Surv 60:683-692, 2005 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization: Overview of ageing, http://www.who.org/ageing/overview.html
- 8.Australian Bureau of Statistics: Australian social trends 2004, http://www.abs.gov.au
- 9.Smith TJ, Desch CE: Neutropeniawise and poundfoolish: Safe and effective chemotherapy in massively obese patients. South Med J 84:883-885, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Balducci L, Extermann M: A practical approach to the older patient with cancer. Curr Probl Cancer 25:6-76, 2001 [PubMed] [Google Scholar]
- 11.World Health Organization: Diet and physical activity, http://www.who.int/dietphysicalactivity/publications/facts/obesity
- 12.World Health Organization: Obesity and overweight, http://www.who.int/mediacentre/factsheets/fs311
- 13.van Warmerdam LJ, Rodenhuis S, ten Bokkel Huinink WW, et al: The use of the Calvert formula to determine the optimal carboplatin dosage. J Cancer Res Clin Oncol 121:478-486, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grigg A, Harun MH, Szer J: Variability in determination of body weight used for dosing busulphan and cyclophosphamide in adult patients: Results of an international survey. Leuk Lymphoma 25:487-491, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Piccart MJ, Biganzoli L, Di Leo A: The impact of chemotherapy dose density and dose intensity on breast cancer outcome: What have we learned? Eur J Cancer 36:S4-10, 2000. (suppl) [DOI] [PubMed] [Google Scholar]
- 16.Budman DR, Berry DA, Cirrincione CT, et al: Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst 90:1205-1211, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Rosner GL, Hargis JB, Hollis DR, et al: Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: Results from cancer and leukemia group B study 8541. J Clin Oncol 14:3000-3008, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Georgiadis MS, Steinberg SM, Hankins LA, et al: Obesity and therapy-related toxicity in patients treated for small-cell lung cancer. J Natl Cancer Inst 87:361-366, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Meyerhardt JA, Tepper JE, Niedzwiecki D, et al: Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: Findings from Intergroup Trial 0114. J Clin Oncol 22:648-657, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Berclaz G, Li S, Price KN, et al: Body mass index as a prognostic feature in operable breast cancer: The International Breast Cancer Study Group experience. Ann Oncol 15:875-884, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Griggs JJ, Sorbero ME, Lyman GH: Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med 165:1267-1273, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Griggs JJ, Culakova E, Sorbero ME, et al: Effect of patient socioeconomic status and body mass index on the quality of breast cancer adjuvant chemotherapy. J Clin Oncol 25:277-284, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Dignam JJ, Polite BN, Yothers G, et al: Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst 98:1647-1654, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Petrelli JM, Calle EE, Rodriguez C, et al: Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control 13:325-332, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Daling JR, Malone KE, Doody DR, et al: Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer 92:720-729, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Senie RT, Rosen PP, Rhodes P, et al: Obesity at diagnosis of breast carcinoma influences duration of disease-free survival. Ann Intern Med 116:26-32, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Hutchins LF, Unger JM, Crowley JJ, et al: Underrepresentation of patients 65 years of age or older in cancer treatment trials. N Engl J Med 341:2061-2067, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Sargent DJ, Goldberg RM, Jacobson SD, et al: A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 345:1091-1097, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Folprecht G, Cunningham D, Ross P, et al: Efficacy of 5-fluorouracil–based chemotherapy in elderly patients with metastatic colorectal cancer: A pooled analysis of clinical trials. Ann Oncol 15:1330-1338, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Lyman GH, Dale DC, Crawford J: Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: A nationwide study of community practices. J Clin Oncol 21:4524-4531, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Dooley MJ, Allen KM, Doecke CJ, et al: A prospective multicentre study of pharmacist initiated changes to drug therapy and patient management in acute care government funded hospitals. Br J Clin Pharmacol 57:513-521, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dooley MJ, Singh S, Michael M: Implications of dose rounding of chemotherapy to the nearest vial size. Support Care Cancer 12:653-656, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Extermann M, Overcash J, Lyman GH, et al: Comorbidity and functional status are independent in older cancer patients. J Clin Oncol 16:1582-1587, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Portugal RD: Obesity and dose individualization in cancer chemotherapy: The role of body surface area and body mass index. Med Hypotheses 65:748-751, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Duffull SB, Dooley MJ, Green B, et al: A standard weight descriptor for dose adjustment in the obese patient. Clin Pharmacokinet 43:1167-1178, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Monfardini S: Prescribing anticancer drugs in elderly cancer patients. Eur J Cancer 38:2341-2346, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Gelman RS, Taylor SGt: Cyclophosphamide, methotrexate, and 5-fluorouracil chemotherapy in women more than 65 years old with advanced breast cancer: The elimination of age trends in toxicity by using doses based on creatinine clearance. J Clin Oncol 2:1404-1413, 1984 [DOI] [PubMed] [Google Scholar]
- 38.Balducci L, Extermann M: Management of cancer in the older person: A practical approach. Oncologist 5:224-237, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Extermann M, Aapro M, Bernabei R, et al: Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 55:241-252, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Chan R, Kerr DJ: Can we individualize chemotherapy for colorectal cancer? Ann Oncol 15:996-999, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Mathijssen RH, Verweij J, de Jonge MJ, et al: Impact of body-size measures on irinotecan clearance: Alternative dosing recommendations. J Clin Oncol 20:81-87, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Gurney H: Developing a new framework for dose calculation. J Clin Oncol 24:1489-1490, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Bergh J: Is pharmacokinetically guided chemotherapy dosage a better way forward? Ann Oncol 13:343-344, 2002 [DOI] [PubMed] [Google Scholar]