Abstract
Root cell division occurs primarily in the apical meristem, from which cells are displaced into the basal meristem, where division decreases and cell length increases before the final differentiation zone. The organization of the root in concentric files implies coordinated division and differentiation of cell types, including the xylem pole pericycle cells, which uniquely can resume division to initiate lateral roots (LR). Here, we show that D-type cyclin CYCD4;1 is expressed in meristematic pericycle protoxylem poles and is required for normal LR density. Cycd4;1 mutants also show a displacement of the apical/basal meristem boundary in the pericycle and longer pericycle basal meristem cells, whereas other cell layers and overall meristem size and root growth are unaffected. Auxin is proposed to separately prepattern and stimulate LR initiation. Stimulation is unimpaired in cycd4;1, suggesting CYCD4;1 requirement for normal spacing but not initiation. Both pericycle cell length and LR density phenotypes of cycd4;1 are rescued by low concentrations of applied auxin, suggesting that the basal meristem has a role in determining LR density. We further show CYCD4;1 is rate-limiting for sucrose-dependent LR formation, since CYCD4;1 expression is sucrose-dependent and wild-type roots fully phenocopy cycd4;1 in sucrose absence. We conclude that CYCD4;1 links meristem pericycle cell behavior to LR density consistent with a basal meristem prepatterning model and that D-type cyclins can confer division potential of defined cell types through cell-specific expression patterns.
Keywords: cell cycle, cell division, plant development
Postembryonic plant development, in contrast to that of animals, is characterized by de novo formation and growth of secondary organs. The activation and maintenance of these developmental programs require integration of cell division and cellular differentiation and is influenced by environmental factors. The root has a broadly cylindrical structure composed of concentric files of cells of distinct identity, whose origin can be traced back to initials adjacent to a small group of rarely dividing cells known as the quiescent center (QC) (1). During root growth, the initials divide, and their progeny are therefore displaced from the QC (2). These cells undergo further rounds of divisions with the result that in the root apical meristem (RAM), cell division is the main contributor to growth, and cell lengths are relatively uniform, since cell division occurs once a critical cell size has been achieved. Subsequently, cells more distal from the QC increase in average length but continue to divide albeit at a lower frequency. This region of combined cell division and elongation has been called the basal meristem (3). Cells exit the basal meristem and enter a zone of rapid elongation where cell division ceases, and cell differentiation is externally observed in the formation of root hairs on specific epidermal cell files.
Lateral roots (LR) result from the reactivation of cell division within the rapid elongation/differentiation zone in specific files of cells within the layer known as the pericycle, which lie adjacent to the xylem poles of the root internal vasculature. Division of these cells then leads to the formation of a primordium, the establishment of a new LR apical meristem, and eventual emergence of the growing LR (4).
Auxin has long been linked to the initiation of LR, since mutants in the auxin response pathway such as iaa14/solitary root lack LR (5), and addition of ectopic auxin at high levels can induce the activation of all xylem pole pericycle cells (6). The specification of xylem pole pericycle cells is dependent on a localized activation of auxin response (7, 8). Two broad models have been proposed to explain the normal patterning and initiation of LR. Recently it has been proposed that a local auxin maximum in pericycle cells of the mature root is sufficient for LR formation (9). A second model suggests that pericycle “founder” cells in the basal meristem are primed by an oscillating auxin response, whereby they are prepatterned to be responsive to a second, independent auxin-requiring (and iaa14/slr-dependent) initiation event in the differentiation zone (8, 10). As mentioned, in addition to this potential priming mechanism involved in normal LR spacing, which appears to be responsible for the responsiveness of a limited number of xylem pole pericycle cells, all such cells have the ability to form LR, since exogenous application of auxin activates pericycle cells to form LR (6, 11–13).
Cell flux through the basal meristem during root growth is determined by the rate of cell division and may have an effect on the number of LR founder cells. As in all eukaryotes, the cell cycle is controlled by cyclin-dependent kinases (CDK) that associate with positive regulators called cyclins (14). The activated cyclin-CDK complexes phosphorylate the retinoblastoma-related protein (RBR), allowing transcription of genes regulated by E2F transcription factors that promote in turn DNA synthesis and commitment to the cell cycle. Cell division in the root meristem has been shown to be dependent on the RBR pathway (15), but as RBR is expressed throughout the root, it is likely that other factors regulate the coordinated division rates of specific cell types or files. Good candidates are the D-type cyclins (CYCDs), which are rate-limiting components for activation of the RBR-E2F pathway, and their expression is under the control of intrinsic and extrinsic signals (16, 17), making them potential developmentally relevant cell cycle activators. Combined loss-of-function mutants in the three CYCD3 genes of Arabidopsis indeed show more rapid exit of cells from the mitotic phase of leaf development, linked to impaired cytokinin responses (16), but the relatively broad expression of these genes in young developing leaves does not indicate whether CYCDs may provide separate control of division of adjacent cell types. Similarly, several CYCDs, including CYCD4;1 are expressed early during germination, and loss of these early expressed CYCD leads to decreased cell division and germination rate (18). Cycd4;1 mutants also have a reduced number of stomata in the hypocotyl epidermis (19), but no other root phenotype has been previously observed. Notably, available microarray data based on cell sorting suggest that the CYCD genes show discrete tissue-specific expression patterns in the root, especially in the root meristem (20, 21), suggesting that individual CYCDs could have specific roles in root growth and development.
Here, we analyze the role of CYCD4;1 in root growth and show that it is expressed in pericycle cells adjacent to the xylem poles. Loss of CYCD4;1 causes an increase in pericycle cell length in the basal meristem zone consistent with reduced cell division rates and a shift toward the root apex of the point at which average pericycle cell lengths start to increase, representing the apical/basal meristem boundary, showing that specific control of the cell cycle in pericycle cells is conferred by CYCD4;1. Cycd4;1 mutants have a reduced LR density, but high levels of ectopic auxin still induce supernumerary LR, and CYCD4;1 expression levels are not affected in slr-1 plants, which together suggest that CYCD4;1 is not involved in the IAA14 pathway of LR initiation but rather in the acquisition of founder cell identity in the basal meristem.
Results
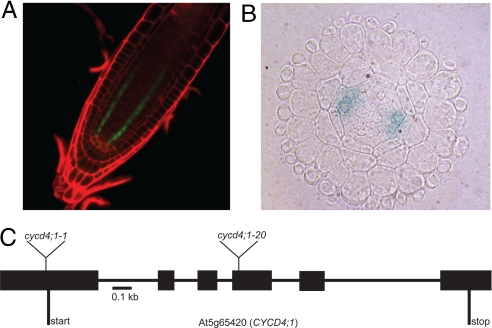
Examining multiple lines carrying CYCD promoters driving a GUS-GFP reporter protein (18), we identified the CYCD4;1 promoter as a pericycle-specific element within the root meristem (Fig. 1A). Cross-sections through the meristem revealed that the CYCD4;1 promoter is not active in all pericycle cell files, but is restricted to the files adjacent to the xylem poles (Fig. 1B). Maximum expression was associated with the cells closest to the QC, and a gradual decline of signal intensity as cells progressed through the meristem was observed. In the maturation zone and older root parts, including the early stages of LR initiation, no CYCD4;1 expression was detected (Fig. 1A).
Fig. 1.
Expression and gene structure of CYCD4;1. (A) The CYCD4;1 promoter is active in the pericycle cells of the root meristem as shown by GFP signal. (B) CYCD4;1 is expressed in the xylem poles of the pericycle in the apical meristem (CYCD4;1 promoter driving GUS reporter). (C) Gene structure of CYCD4;1. Boxes indicate exons, and the position of the T-DNA insertions in the CYCD4;1 mutants are shown. PCR amplicons from homozygote mutant plants were purified and sequenced using insert specific primers, showing that both mutants had tandem head-to-head insertion events within the CYCD4;1 locus. The cycd4;1-1 has a T-DNA insertion at position −25 bp from the initiation codon and cycd4;1-20 in the fourth exon.
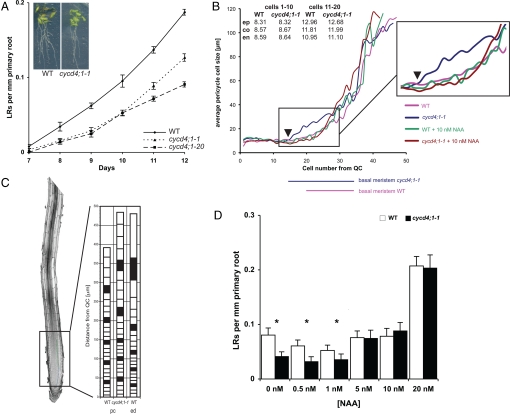
To investigate the role of CYCD4;1, two independent lines containing T-DNA insertions in the CYCD4;1 gene were used. Cycd4;1-1 (18) has an insert just before the start codon, and cycd4;1-20 is an allele with a T-DNA inserted in the fourth exon (Fig. 1C). In neither allele are full-length CYCD4;1 transcripts detected by PCR on cDNA using primers spanning the coding region (data not shown). Using quantitative real-time PCR, cyc4;1-1 and cycd4;1-20 showed 0.8% and 0.4%, respectively, of CYCD4;1 3′ mRNA levels compared to wild-type (WT) levels. Cycd4;1 mutants did not show obvious growth defects, were fully fertile, and in vertical plate assays the primary root growth rate of WT and both cycd4;1 alleles were not significantly different (Fig. S1). However, compared to WT, both cycd4;1 mutants displayed a 30% reduction in LR number (Fig. 2A). Careful examination revealed that this was a consequence of a reduced level of LR primordium formation and not a failure of outgrowth of formed primordia. Complementation tests confirmed that both mutations are allelic and rescued by a genomic fragment encoding the CYCD4;1 gene.
Fig. 2.
The phenotype of the cycd4;1 null-mutants. (A) cycd4;1 show a decreased number of LR. (B) Cell lengths in different zones of the root meristem. Average WT and cycd4;1-1 cell lengths in epidermis (ep), cortex (co), and endodermis (en) for cells 1–10 and 11–20 counted from the QC are shown in upper left table. Main figure: Average pericycle cell lengths are shown for cells 1->40 from QC for WT and cycd4;1 mutants grown on 0 nM and 10 nM exogenous auxin. Note (Inset) that cells of the basal meristem in cycd4;1-1 are enlarged only in the absence of auxin and that the start of net elongation takes place closer to the QC (arrowhead) than in WT. Significant (t test P < 0.05) changes were observed between cycd4;1-1 without NAA and the other condition for cell number 17 to 25. Lines below the x-axis indicate basal meristem regions of WT and cycd4;1-1. The apical meristem and rapid elongation zones lie to the left and right of the basal meristem region, respectively. (C) Cell length and position from the QC of the pericycle cells (pc) in the cycd4;1-1 and WT and epidermis cells in the WT (ed) as comparison. (D) LR density at 10 DAG in WT and cycd4;1-1 upon treatment of increasing levels of auxin. Asterisks indicate differences are significant (t test P < 0.05).
Since CYCDs regulate the G1-S transition and can be rate limiting for cell division in plants (16, 18), we compared cell division in the root meristems of cycd4;1-1 and WT using the mitotic CYCB1;1-GUS marker (22), but neither the overall pattern of cell division nor meristem length (Fig. S2) were affected as determined from the overall distribution of mitotic cells.
We therefore analyzed the sizes and numbers of root cells in different root cell files in more detail, allowing us to determine the lengths of epidermal, cortical, endodermal, and pericycle cells from the initials adjacent to the QC up into the rapid elongation/differentiation zone. We found no significant differences in cell lengths in epidermis, cortex, and endodermis between cycd4;1-1, cycd4;1-20, and WT (Fig. 2B). The pericycle cells in cycd4;1-1 and WT apical meristems were also equal in size, but in cycd4;1-1, we found that elongation in cycd4;1-1 initiates closer to the QC, corresponding to a shift of the boundary of the apical-basal meristem (Fig. 2B). In the mutant, average cell lengths starts to increase from cell number 15 (150 μm from the QC), whereas in WT, the average cell length starts to increase around cell number 20 (200 μm; Fig. 2B). The total number of cells between the QC and the start of the rapid elongation zone in the pericycle layer is unchanged as the inflection point on the cell length curve marking the transition into the rapid elongation zone in both cycd4;1-1 and WT occurs at cell 35 (Fig. 2B). However, this basal meristem-rapid elongation zone transition occurs further in physical distance from the QC (736 μm in cycd4;1-1 and 623 μm in WT), because the average length of pericycle cells across the basal meristem region is larger in cycd4;1-1. This phenotype was also found in cycd4;1-20 and could be rescued in tandem with the LR reduction by complementation of cycd4;1 by the genomic fragment spanning the CYCD4;1 gene (Fig. S3). Taken together, in cycd4;1-1 there are less pericycle cells present in the basal meristem region per unit length relative to the other cell files (Fig. 2C), pericycle cells exit more rapidly from the purely mitotic zone of the RAM, and the length of the pericycle basal meristem is increased because it is composed of larger cells.
Hence, CYCD4;1 appears to control cell length in the pericycle of the basal meristem and affects the formation of LR, and we sought to establish whether these phenotypes are causally linked. It has recently been proposed that particular pericycle cells in the basal meristem become primed as LR founder cells as a consequence of local oscillations in auxin or auxin responses, and that this mechanism controls normal LR specification and spacing (8). Additionally, high levels of applied auxin promote ectopic LR formation (5, 6). Given the effect of loss of CYCD4;1 in the basal meristem, we investigated the relationship between auxin and the cycd4;1 phenotype using a range of concentrations.
1-Napthaleneacetic acid (NAA) was included in the growth medium at concentrations between 0 and 100 nM, and LR density was measured after 10 days of vertical growth (Fig. 2D). At 0–1 nM NAA, the cycd4;1-1 LR density phenotype was manifest. However, at slightly higher concentrations (5–10 nM NAA), LR density of cycd4;1 mutants was restored to WT levels, but no response was observed in WT. Within this range of auxin concentrations, primary root growth was not affected (Fig. S1). As expected, addition of higher levels of auxin increased LR density in WT roots, and a similar total number of LR were also observed in cycd4;1 mutants, demonstrating that auxin responses are not impaired in cycd4;1.
Since LR number was restored in the cycd4;1-1 by the presence of 10 nM NAA, we examined whether this coincided with a restoration of cell length in the mutant's pericycle to WT lengths (Fig. 2B). Cell length analysis of cycd4;1-1 revealed that the distal meristem pericycle cell length was also restored, and this further supports the link between the LR and basal meristem cell length phenotypes of cycd4;1.
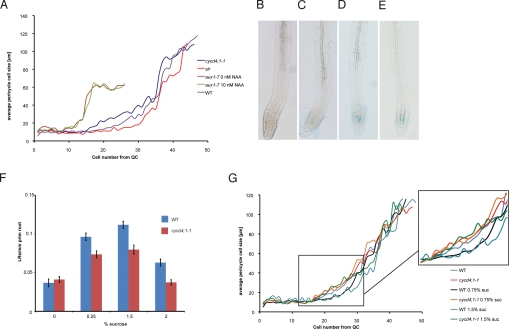
It has been proposed that AUX1 is involved in LR priming through its function as an auxin influx carrier protein (8), and the aux1 mutant has a 30% reduction in LR density (8, 23), similar to that observed in cycd4;1-1. We therefore measured the cell lengths in the meristem of aux1-7 mutant roots and found that pericycle cells also start to elongate prematurely and more rapidly (Fig. 3A). However, unlike cycd4;1 meristems, aux1-7 meristems are greatly reduced in overall length throughout all cell files (data not shown), and pericycle cell expansion appears to be impaired in the rapid elongation zone (Fig. 3A). Although it has been reported that the aux1 LR phenotype can be restored by exogenous application of 10 nM NAA (23), an observation we reconfirmed (data not shown), pericycle cell lengths are not affected in aux1-7 grown on 10 nM NAA, consistent with a lack of responsiveness of the aux1 meristem cells (Fig. 3A). Overall CYCD4;1 transcript levels were also found to be unchanged in aux1 roots (Fig. S3).
Fig. 3.
Effect of auxin and sucrose on the cycd4;1-1 phenotype. (A) Pericycle cell lengths in the aux1 and slr mutants compared to WT and cycd4;1-1. Aux1-7 plants were treated were grown without and with 10 nM NAA. Cell lengths are significantly different (t test P < 0.05) compared to WT for cells 14–26 (both aux1), 17–26 (cycd4;1-1), and 25–30 (slr). (B–E) CYCD4;1 promoter driving GUS-GFP reporter in 10-day-old roots on different sucrose and light conditions. (B) Roots grown for 9 days in the dark (−suc) transferred to the dark (−suc). (C) Roots grown for 9 days in the dark (−suc) transferred to the light (−suc). (D) Roots grown for 9 days in the dark (−suc) transferred to the dark (+suc). (E) Roots grown in the light (+suc) transferred to the light (+suc). (F) LR density at 10 DAG in WT and cycd4;1-1 grown on media containing different sucrose concentrations. (G) Average pericycle cell lengths in WT and cycd4;1-1 grown on media containing different sucrose concentrations. Inset shows enlargement of the basal meristem region of the curves. No significant differences were found between WT on 0.75% and 1.5% sucrose, except for cell numbers 29–31 (t test P < 0.05). No significant changes (t test P < 0.05) were found between WT on 0% sucrose and the cycd4;1-1 conditions. Cell lengths are significantly different (t test P < 0.05) between WT on sucrose and WT without sucrose or the cycd4;1-1 conditions between cells 21–27.
IAA14/SLR is not proposed to be involved in priming mechanisms, but is required in the root differentiation zone for LR initiation in response to auxin, and slr roots are thus devoid of laterals (8). To further investigate the role of basal meristem cell length in LR initiation, we examined pericycle cell length in an slr mutant. Consistent with the proposed action of IAA14/SLR in the differentiation zone and not in the basal meristem, slr-1 roots showed a similar cell length profile to WT in the basal meristem region. We also found that CYCD4;1 transcript levels were unaffected in slr-1 (Fig. S4). Taking together, the lack of CYCD4;1 induction by auxin or alterations in auxin flux and response mutants, and the production of WT levels of LR on addition of higher auxin levels, we conclude that CYCD4;1 is not directly involved in auxin-mediated LR formation, but rather influences LR density through affecting basal meristem pericycle cell length.
LR density also responds to the carbon:nitrate balance (24), and CYCD4;1 mRNA levels in Arabidopsis suspension cultures are dependent on the presence of sucrose (25). To test whether sucrose regulates CYCD4;1 expression in planta, we examined expression in the pCYCD4;1::GUS-GFP reporter line in response to sucrose induction assay. Dark grown plants can be induced to produce sucrose through photosynthesis by shifting plants from dark to light conditions. In dark grown plants without exogenous sucrose, no pCYCD4;1::GUS activity was detected in the root meristem (Fig. 3B). Transfer of the plants to the light, inducing sucrose synthesis, led to detectable CYCD4;1 expression (Fig. 3C). Plants grown continuously in light showed higher levels of CYCD4;1 expression (Fig. 3E). We also tested our reporter line in a comparable assay where sucrose availability was controlled by transferring dark grown plants from media containing no sucrose to media supplemented with sucrose. The transfer onto sucrose containing media induced CYCD4;1 expression in the root meristem (Fig. 3D). Continuous light with exogenous sucrose resulted in strongest expression of CYCD4;1 (Fig. 3E). We conclude that expression from the CYCD4;1 promoter is dependent on sucrose availability in roots.
The dependence of CYCD4;1 expression on sucrose suggested that dark grown plants lacking sucrose may phenocopy cycd4;1. WT and cycd4;1-1 plants were grown on different levels of sucrose and scored for LR emergence after 10 days (Fig. 3F). In WT plants, LR density increases with sucrose levels up to 1.5%, but declines at 2% sucrose. In cycd4;1-1 plants, a similar trend is seen, although overall LR density is reduced compared to WT, except in the absence of sucrose, when LR densities of WT and cycd4;1-1 are equal. Analysis of meristem cell lengths showed that absence of sucrose affects pericycle cell length in the basal meristem, and under these conditions, both WT and cycd4;1 mutants show equivalent cell lengths and meristem boundary positions (Fig. 3G). Whereas WT cell lengths decrease in response to sucrose presence correlating with induction of CYCD4;1 expression and an increased LR density, in the cycd4;1-1 mutant, the distal meristem pericycle cell lengths do not respond to sucrose, and LR density does not respond to the same extent as WT (Fig. 3G). We conclude that sucrose presence is required for both aspects of the cycd4;1 phenotype to be manifest and that the effect of sucrose on pericycle cell length and about half of the sucrose effect on LR density is dependent of CYCD4;1.
Discussion
Root growth depends on the combination of cell production and subsequent cell elongation, processes that are spatially separated in the root meristem. Cell division predominates in the apical meristem, and average cell lengths are constant. Rapid cell expansion occurs with final cell differentiation, but between the apical meristem and rapid elongation/differentiation zones lies an intermediate region known as the basal meristem (8), where average cell lengths increase while divisions still occur. The mechanisms that control and integrate these changes in different cell types of the growing root in response to intrinsic and external signals are mostly unclear, although gravitropism is one well-known example of response to an external signal that causes a temporal and asymmetric change in the cell division-elongation balance, leading to a macroscopic change in root morphology (26). Here, we show that an intrinsic factor, the D-type cyclin, CYCD4;1, affects pericycle cell length independently of other cell layers and influences LR density.
We find that CYCD4;1 is expressed in the RAM within pericycle cells adjacent to the xylem poles. Previous in situ hybridization of roots of Raphanus sativus (radish) showed expression hybridizing to an Arabidopsis CYCD4;1 probe associated with the LR primordium itself (25, 27), but we could not detect any activity of the CYCD4;1 promoter in this region in Arabidopsis, suggesting possible species differences. The expression pattern we describe here and previously (18) is in agreement with the low level of expression and distribution reported for CYCD4;1 from cell sorting and microarray experiments (“digital in situ”), including its expression in aerial tissues (20, 21). Hence, loss of cycd4;1 appears to influence LR density through its expression in the meristem region and not during the subsequent initiation of the LR primordium in the differentiation zone.
Loss of CYCD4;1 function results in an earlier transition of pericycle cells from apical to basal meristem region and a larger average length of pericycle cells in the basal meristem, therefore reducing pericycle cell density in this region. Correlating with these changes in the pericycle basal meristem of cycd4;1 plants, fewer LR are initiated, suggesting that loss of CYCD4;1 expression in the root meristem could interfere with the priming of founder cells in the pericycle (8). Although recent modeling of LR initiation and the role of auxin therein suggests that LR formation can also take place without pericycle cell priming (9) and that the creation of a stable auxin maximum in the mature pericycle is sufficient for LR formation, our observed correlation between pericycle cell length in the basal meristem and normal LR density is consistent with a prepatterning model. We saw a consistent correlation between LR density and basal meristem pericycle cell length in all experiments and treatments, strongly suggesting that these aspects are causally linked. In this case, the prepatterning of LR in the basal meristem would be influenced by the size of the pericycle cells in this region, consistent with a possible cell counting mechanism for LR spacing. It is also possible that loss of CYCD4;1 in the meristem has a permanent effect on pericycle cells that could render them less likely to initiate LR later on.
For example, it has been proposed that xylem pole pericycle cells progress through S phase to a G2 state, whereas other pericycle cells remain in G1 phase (28), and the loss of CYCD4;1 could therefore affect this progression. However, this might be expected to be seen as a subsequent reduced sensitivity to auxin in the stimulation assay, and this was not observed in our analysis of response to exogenous auxin across a range of concentrations. We propose based on our data that the two models of prepatterning versus auxin maxima in mature pericycle are not mutually inconsistent and that both may contribute to the observed pattern of LR in normal plant growth.
The plasticity of plant roots is intimately linked to their functional role as the primary site of nutrient uptake. Thus, changes in nutrient availability have also been noted as key mediators of division and root architecture (11). For instance recent data shows that sucrose availability influences the initiation of LR in response to nitrate levels (29). We show that root expression of CYCD4;1 is dependent on sucrose, as previously reported for the related CYCD2;1 and CYCD3;1 (6, 30) and for CYCD4;1 in suspension cells (25), and that this has important effects within the root meristem in determining the transition boundary between apical and basal meristem with consequential effects on LR density. Moreover we show that the absence of sucrose causes WT roots to phenocopy cycd4;1. Taken together, these data uncover a mechanism of nutrient-regulated LR density whereby sucrose deprivation apparently limits LR initiation through effects on the flux of pericycle cells through the basal meristem.
In addition to the interaction of the cycd4;1 phenotype with sucrose levels, we also observed that the cellular and LR phenotypes of cycd4;1 were completely restored by addition of very low concentrations (5 nM) applied exogenous auxin. This effect may be due to transcriptional responses of other CYCD genes to auxin (31) or to the reported stabilization by auxin of E2Fb protein, the downstream positive effector of CYCD action (32). Taken together, the interplay of the cycd4;1 phenotype with subtle changes in auxin or sucrose levels suggest that it may form part of a network of responses tuning LR density to environmental conditions.
We show here that the analysis of cell lengths through the meristem can reveal information on the position of meristem boundaries in individual cell files. In the cycd4;1 mutant root, only pericycle cells are affected, whereas in the aux1 mutant, which bears several superficial phenotypic similarities, there is a large reduction in overall meristem size and cell expansion is not responsive to 10 nM NAA, suggesting that aux1 effects on LR density are likely to be distinct from those of cycd4;1.
Loss of CYCD4;1 affects cell length in the basal meristem and the boundary between apical and basal meristem of the pericycle, as we detected an earlier transition to the basal meristem in cycd4;1. Despite the significant expression of CYCD4;1 in the apical meristem pericycle cells, we could not detect a change in cell length in this region of cycd4;1, indicating that CYCD4;1 is not rate-limiting for cell division in the apical meristem nor does it directly control cell length. D-type cyclins are described as positive regulators of cell division (33), and we suggest that CYCD4;1 is a rate-limiting cell division regulator specifically in the pericycle of the root basal meristem. Previous analysis of a triple loss-of-function mutant of the CYCD3 family, showed a related phenotype in leaf development with a decrease in cell number and an increase in cell size in lateral organs of the shoot (16). However, in the shoot, the three CYCD3 genes have relatively broad expression in primordia and young developing leaves, whereas the expression and phenotype of CYCD4;1 is highly cell-specific. As the overall rate of root growth in cycd4;1 is unaffected, a lower frequency of cell division in the mutant requires the cells to be larger. Our results show that the boundary between apical and basal meristem can be different and dynamic in different cell types and that division rates and cell sizes of distinct cell files can be autonomously regulated by differential CYCD expression.
Materials and Methods
Plant Material.
WT Arabidopsis thaliana ecotype Columbia (Col-0) were obtained from the Nottingham Arabidopsis Stock Centre. The loss-of-function mutant lines cycd4;1-1 (TAIR accession SALK_015525) and cycd4;1-20 (TAIR accession GK-344D08-016232) were recovered from the SALK (34) and GABI-Kat (35) collections, respectively. Both mutants were backcrossed, and at least 100 F2 progeny were assayed for segregation of the insert using herbicide resistance as a marker (kanamycin resistance was scored for cycd4;1-1 and sulfadiazine resistance for cycd4;1-20). In both F2 populations the marker segregated in a Mendelian manner (three resistant:one sensitive) as expected for a single dominant insertion. Genomic DNA was extracted from resistant plants, and PCRs were performed using insert-specific and flanking gene-specific primers. This genotyping confirmed that the insert was present in all resistant plants analyzed. Identified mutants homozygous for the insertion were then backcrossed at least twice more to create our homozygous lines used in all experiments. Segregating WT lines from the backcross populations were used as WT controls in all growth experiments. Total RNA was extracted to confirm a reduction in the 3′ region of CYCD4;1 transcripts using quantitative real-time PCR. For allelic complementation, homozygous cycd4;1-1 and cycd4;1-20 plants were crossed in both directions. Since each of the mutants contributes a different herbicide resistance, some F1 seed from each cross was scored on media containing both kanamycin and sulfadiazine to test for successful crosses. All F1 progeny tested were resistant to both herbicides, while appropriate controls were all sensitive. Remaining F1 seeds from these crosses were scored for decreases in LR. All F1 plants had a significant decrease in LR number confirming that cycd4;1-1 and cycd4;1-20 describe two independent CYCD4;1 null alleles. Cycd4;1-1 COMP was generated by transforming cycd4;1-1 with a construct carrying the genomic fragment of the CYCD4;1 gene. The start of the CYCD4;1 promoter was chosen from the end of the 3′ UTR from the gene upstream (AT5G65430) using primer ACCAGTTGTTTTCAAGAATTTGCT. The 3′ end of the genomic fragment is until the STOP codon of CYCD4;1 using primer AGAAAGATGTGTATAAGAAGAAGA. aux1-7 (36) and slr (5) were kindly provided by Malcolm Bennett and Ben Scheres, respectively.
Growth Conditions.
Seeds were sterilized in 10% sodium hypochloride and placed on square Petri plates containing Murashige and Skoog (MS) mixture, 1.5% sucrose, 0.5 g/L 2-(N-morpholino) ethanesulfonic acid (Mes), pH 5.8, in 1% agar. Seeds were imbibed in the dark at 4 °C for 3 days, and plates were incubated vertically in Conviron TC30 (Controlled Environments) in a cycle of 8 h dark/16 h white light (170–200 μL m−2s−1) at 22 °C, 70% humidity. For auxin and sucrose treatments, seeds were plated as described above on medium containing NAA or sucrose, respectively.
LR Density Measurements.
Plants were grown as described above and scanned at 1,500 dpi. Emerged LR were counted, and primary root length was measured using the software analysis package, ImageJ.
Expression Analyses.
Plants carrying the pCYCD4;1::GUS-GFP construct were grown as described above. Roots were stained histochemically essentially as described in ref. 37, embedded in Technovit 7100 (Hereaus Kulzer), and 6-μm sections were cut on a Leica RM2145 microtome and examined with a Nikon Optiphot microscope.
Real-Time PCR.
Transcripts were measured using quantitative real-time PCR as described in ref. 38. Total RNA (2 μg) was used to generate cDNA, and relative expression was calculated using the 2-delta-delta CT method (39) using ACTIN2 as reference transcript. CycD4;1 RT primer pair: GCCAGCACAACCAAAGGTAT and CCCATTGGG TGTTTGTGAAC. CDKA;1 RT primer pair: CCGAGCACCAGAGATACTCC and GTTACCCCACGCCATGTATC. ACTIN2 RT primer pair: ACATTGTGCTCAGTGGTGGA and CTGAGGGAAGCAAGAATGGA.
Root Cell Length Measurements.
Root sections were fixed in 50% methanol, 10% acetic acid. After fixation, roots were incubated for 20 min in 1% periodic acid and then for 1 h in 0.18 M sodium bisulfite, 0.15 N hydrochloric acid, and 100 μg/mL propidium iodide. The roots were cleared in chloral hydrate (265 g in 100 mL water). Confocal stacks of roots were obtained with a Zeiss LSM 510 Meta or Zeiss LSM 710 Meta confocal microscope using filter settings for propidium iodide staining, and cell lengths were measured in ImageJ. A minimal of 15 roots per genotype were analyzed.
Supplementary Material
Acknowledgments.
This work was funded by Biotechnology and Biological Sciences Research Council Grant BB/E022383 and the European Research Area in Plant Genomics project “Integrated analysis of plant stem cell function” BB/E024858. J.N., S.M., and L.S. were also partially supported under the FP6 European Union Marie Curie Fellowship Program, contracts MIF1-CT-2004–509962, MEIF-CT-2005–010666, and MEIF-CT-2006–041586.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906354106/DCSupplemental.
References
- 1.Benfey PN, Scheres B. Root development. Curr Biol. 2000;10:R813–R815. doi: 10.1016/s0960-9822(00)00814-9. [DOI] [PubMed] [Google Scholar]
- 2.van den Berg C, Willemsen V, Hage W, Weisbeek P, Scheres B. Cell fate in the Arabidopsis root meristem determined by directional signalling. Nature. 1995;378:62–65. doi: 10.1038/378062a0. [DOI] [PubMed] [Google Scholar]
- 3.Beemster GT, Fiorani F, Inze D. Cell cycle: The key to plant growth control? Trends Plants Sci. 2003;8:154–158. doi: 10.1016/S1360-1385(03)00046-3. [DOI] [PubMed] [Google Scholar]
- 4.Parizot B, et al. Diarch symmetry of the vascular bundle in Arabidopsis root encompasses the pericycle and is reflected in distich lateral root initiation. Plant Physiol. 2008;146:140–148. doi: 10.1104/pp.107.107870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukaki H, Tameda S, Masuda H, Tasaka M. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 2002;29:153–168. doi: 10.1046/j.0960-7412.2001.01201.x. [DOI] [PubMed] [Google Scholar]
- 6.Himanen K, et al. Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell. 2002;14:2339–2351. doi: 10.1105/tpc.004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubrovsky JG, et al. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci USA. 2008;105:8790–8794. doi: 10.1073/pnas.0712307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Smet I, et al. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development. 2007;134:681–690. doi: 10.1242/dev.02753. [DOI] [PubMed] [Google Scholar]
- 9.Laskowski M, et al. Root system architecture from coupling cell shape to auxin transport. PLoS Biol. 2008;6:e307. doi: 10.1371/journal.pbio.0060307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casimiro I, et al. Dissecting Arabidopsis lateral root development. Trends Plants Sci. 2003;8:165–171. doi: 10.1016/S1360-1385(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 11.Nibau C, Gibbs DJ, Coates JC. Branching out in new directions: The control of root architecture by lateral root formation. New Phytol. 2008;179:595–614. doi: 10.1111/j.1469-8137.2008.02472.x. [DOI] [PubMed] [Google Scholar]
- 12.Dubrovsky JG, Gambetta GA, Hernandez-Barrera A, Shishkova S, Gonzalez I. Lateral root initiation in Arabidopsis: Developmental window, spatial patterning, density and predictability. Ann Bot (Lond) 2006;97:903–915. doi: 10.1093/aob/mcj604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boerjan W, et al. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell. 1995;7:1405–1419. doi: 10.1105/tpc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewitte W, Murray JA. The plant cell cycle. Annu Rev Plant Biol. 2003;54:235–264. doi: 10.1146/annurev.arplant.54.031902.134836. [DOI] [PubMed] [Google Scholar]
- 15.Wildwater M, et al. The RETINOBLASTOMA-RELATED gene regulates stem cell maintenance in Arabidopsis roots. Cell. 2005;123:1337–1349. doi: 10.1016/j.cell.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 16.Dewitte W, et al. Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc Natl Acad Sci USA. 2007;104:14537–14542. doi: 10.1073/pnas.0704166104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JA. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science. 1999;283:1541–1544. doi: 10.1126/science.283.5407.1541. [DOI] [PubMed] [Google Scholar]
- 18.Masubelele NH, et al. D-type cyclins activate division in the root apex to promote seed germination in Arabidopsis. Proc Natl Acad Sci USA. 2005;102:15694–15699. doi: 10.1073/pnas.0507581102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kono A, et al. The Arabidopsis D-type cyclin CYCD4 controls cell division in the stomatal lineage of the hypocotyl epidermis. Plant Cell. 2007;19:1265–1277. doi: 10.1105/tpc.106.046763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birnbaum K, et al. Cell type-specific expression profiling in plants via cell sorting of protoplasts from fluorescent reporter lines. Nat Methods. 2005;2:615–619. doi: 10.1038/nmeth0805-615. [DOI] [PubMed] [Google Scholar]
- 21.Nieuwland J, Menges M, Murray JAH. The plant cyclins. In: Inze D, editor. The Cell Cycle Control and Plant Development. Malden, MA: Blackwell; 2007. pp. 31–61. [Google Scholar]
- 22.Colon-Carmona A, You R, Haimovitch-Gal T, Doerner P. Technical advance: Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J. 1999;20:503–508. doi: 10.1046/j.1365-313x.1999.00620.x. [DOI] [PubMed] [Google Scholar]
- 23.Marchant A, et al. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell. 2002;14:589–597. doi: 10.1105/tpc.010354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malamy JE, Ryan KS. Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol. 2001;127:899–909. [PMC free article] [PubMed] [Google Scholar]
- 25.De Veylder L, et al. A new D-type cyclin of Arabidopsis thaliana expressed during lateral root primordia formation. Planta. 1999;208:453–462. doi: 10.1007/s004250050582. [DOI] [PubMed] [Google Scholar]
- 26.Lucas M, Godin C, Jay-Allemand C, Laplaze L. Auxin fluxes in the root apex co-regulate gravitropism and lateral root initiation. J Exp Bot. 2008;59:55–66. doi: 10.1093/jxb/erm171. [DOI] [PubMed] [Google Scholar]
- 27.Engler Jde A, et al. Systematic analysis of cell-cycle gene expression during Arabidopsis development. Plant J. 2009;59:645–660. doi: 10.1111/j.1365-313X.2009.03893.x. [DOI] [PubMed] [Google Scholar]
- 28.Beeckman T, Burssens S, Inze D. The peri-cell-cycle in Arabidopsis. J Exp Bot. 2001;52:403–411. doi: 10.1093/jexbot/52.suppl_1.403. Spec Issue. [DOI] [PubMed] [Google Scholar]
- 29.Little DY, et al. The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues. Proc Natl Acad Sci USA. 2005;102:13693–13698. doi: 10.1073/pnas.0504219102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riou-Khamlichi C, Menges M, Healy JM, Murray JA. Sugar control of the plant cell cycle: Differential regulation of Arabidopsis D-type cyclin gene expression. Mol Cell Biol. 2000;20:4513–4521. doi: 10.1128/mcb.20.13.4513-4521.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oakenfull EA, Riou-Khamlichi C, Murray JA. Plant D-type cyclins and the control of G1 progression. Philos Trans R Soc Lond B Biol Sci. 2002;357:749–760. doi: 10.1098/rstb.2002.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magyar Z, et al. The role of the Arabidopsis E2FB transcription factor in regulating auxin-dependent cell division. Plant Cell. 2005;17:2527–2541. doi: 10.1105/tpc.105.033761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menges M, Murray JA. Plant D-type cyclins: Structure, roles and functions. SEB Exp Biol Ser. 2008;59:1–28. [PubMed] [Google Scholar]
- 34.Alonso JM, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- 35.Rosso MG, et al. An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol Biol. 2003;53:247–259. doi: 10.1023/B:PLAN.0000009297.37235.4a. [DOI] [PubMed] [Google Scholar]
- 36.Pickett FB, Wilson AK, Estelle M. The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol. 1990;94:1462–1466. doi: 10.1104/pp.94.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menges M, Murray JA. Synchronous Arabidopsis suspension cultures for analysis of cell-cycle gene activity. Plant J. 2002;30:203–212. doi: 10.1046/j.1365-313x.2002.01274.x. [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.