Abstract
Objective
Depression and anxiety are associated with autonomic nervous system dysfunction, which may promote the risk of malignant cardiac arrhythmias. This study investigates whether depression and anxiety symptoms are associated with measures of autonomic nervous system dysfunction in patients with implantable cardioverter defibrillators who are at high risk of cardiac rhythm disturbances.
Methods
Patients with an implantable cardioverter defibrillator (ICD) underwent ambulatory electrocardiographic monitoring (n=44, mean age 62.1 ± 9.3 yrs). Depression was assessed using the Beck Depression Inventory and anxiety using the Taylor Manifest Anxiety Scale. Heart rate variability (HRV) was assessed using time (RMSSD, pNN50 and SDNN) and frequency domain measures derived from 24 hour R-R intervals. Multivariate models were adjusted for age, sex, hypertension, diabetes and smoking status.
Results
Defibrillator patients with elevated depression symptoms (n=12) had significantly lower RMSSD (15.25 ± 1.66 ms, vs. 24.97 ± 2.44, p = 0.002) and pNN50 (1.83 ± 0.77 vs. 5.61 ± 1.04, p = 0.006) than defibrillator patients with low depression symptoms (n=32). These associations remained significant after multivariate adjustment for covariates. ICD patients with high anxiety levels (n=10) displayed lower RMSSD (p = 0.013), which became marginally significant when adjusting for covariates (p = 0.069).
Conclusion
Depression and anxiety in defibrillator patients are associated with autonomic nervous system dysfunction indices of reduced parasympathetic control. Autonomic nervous system dysfunction may partially explain the association between depression and anxiety with life-threatening cardiac outcomes in vulnerable patients.
Keywords: depression, anxiety, autonomic nervous system, heart rate variability, implantable cardioverter defibrillator
Epidemiological and clinical research has documented that depression and anxiety are associated with increased risk of coronary artery disease and sudden cardiac death 1–3. Biobehavioral pathways involved in the increased cardiovascular risk associated with depression have been studied extensively 4, and evidence about mechanisms accounting for the elevated cardiovascular risk associated with anxiety is accumulating 5, 6. Depression and anxiety are common in patients with implantable cardioverter defibrillators (ICD) 7–11. Anxiety in ICD patients is associated with impaired quality of life 12, 13 and reduced treatment satisfaction 14, increased pain perception of ICD shocks 15, and possibly with increased risk of ICD discharges 16, 17. Psychological characteristics, such as depression and anger, predict ventricular arrhythmia in ICD patients 17, 18. Autonomic nervous system dysfunction is a plausible common pathway involved in the association between psychological factors and adverse cardiovascular events 19, 20. The present study investigates whether depression and anxiety are associated with altered autonomic nervous system control in coronary artery disease patients with implantable cardioverter defibrillators, who are at high risk of developing malignant cardiac arrhythmias.
Autonomic nervous system dysregulation plays an important role in arrhythmias 21. Heart rate variability (HRV) analysis provides non-invasive markers of autonomic activity 22 and reduced HRV predicts arrhythmia and mortality in post-myocardial infarction patients 23, 24 and malignant arrhythmia in patients with implantable cardioverter defibrillators 25. Depression is associated with reduced HRV in patients with cardiovascular disease 19, 26 and in healthy populations 27–30, although negative findings have also been reported 31, 32. Anxiety disorders such as panic disorder 33 and post-traumatic stress disorder 34 have also been associated with reduced HRV, in addition to trait anxiety 35, phobic anxiety symptoms 36, and worry 37. In contrast to research on depression, very few studies have examined HRV and anxiety in patients with cardiovascular disease 38. Ambulatory ECG monitoring enables investigation of the relationships between psychosocial measures and autonomic nervous system dysfunction in naturalistic conditions. The present study investigates the hypothesis that elevated depression and anxiety levels are related to reduced HRV in defibrillator patients.
METHODS
Participants
Patients with implantable cardioverter defibrillators with documented coronary artery disease (n = 44) were recruited from three clinics in northeastern USA (Arrhythmia Associates, Fairfax, VA; the Veterans Affairs Medical Center, Washington DC, and St. Francis Hospital, Roslyn, NY) between 1998 and 2004. Exclusion criteria for patients were: atrioventricular conduction defects, left bundle-branch block, chronic atrial fibrillation, myocardial infarction < 1 month, unstable angina, class IV heart failure, critical valve pathology, primary cardiomyopathy, use of amiodarone, and age > 80 years. To optimize electrocardiographic assessments beta-adrenergic blocking agents were withheld for ≥ 48 hours when clinically safe. The study was approved by the participating institutional review boards, and all patients gave written informed consent. Power analyses indicate that the present sample of 44 participants is sufficient for the detection of a correlation coefficient of r = 0.40 and a moderate effect size difference (Δ = V2 / σ2) = 0.19) between individuals with high versus low levels of psychological variables.
Demographic and clinical information included age, sex, race, cardiovascular disease risk factors (body mass index (BMI), smoking status, hypertension, diabetes mellitus), cardiac history (myocardial infarction, coronary artery bypass surgery, left ventricular ejection fraction), and ICD characteristics (reason for implantation, duration since device implantation, number of discharges since implantation).
Psychological Measures
Depression
Symptoms of depression were assessed using the original version of the Beck Depression Inventory (BDI) 39, 40. The BDI has been used in a wide range of patients with cardiovascular disorders to assess symptoms of depression 41. Scores range from 0 – 63 with higher scores indicating increased depression severity, with scores ≥10 indicative of the possible presence of depression based on previous validation studies 41, 42.
Anxiety
The Taylor Manifest Anxiety Scale (20 item version) was administered to assess trait anxiety symptoms 43. The Manifest Anxiety Scale is a true-false questionnaire derived from the Minnesota Multiphasic Personality Inventory and includes items such as “I am a high-strung person” and “I work under a great deal of tension”. Scores range from 0–20 with higher scores indicating increased anxiety. For the purposes of this study, the clinical cut-point for elevated anxiety levels was set at ≥ 9, as reported previously 44.
HRV analysis
Patients were monitored for 48 hours using a three-channel electrocardiographic recorder. Analogue signals were digitized at a sampling rate of 1000 Hz (Marquette Medical Systems). Heart rate was continuously assessed over the course of the 48 hour period. HRV was assessed using the General Electric Medical System MARS Personal Computer Workstation with standard electrocardiographic analysis to accurately label ectopic beats and artifact. Tapes were analyzed by trained reviewers who were blinded to clinical and psychological data. If patients had > 20% missing data because of ectopy or artifacts, then they were excluded from analyses. Two patients were excluded from analyses because of excess supraventricular ectopy.
Time domain indices
Time domain indices of HRV included the following measures: the root mean square successive difference of RR intervals (RMSSD), in milliseconds (ms), the percent of successive RR interval differences > 50 ms (pNN50), in percent, and the standard deviation of RR intervals (SDNN) in ms 45. RMSSD and pNN50 primarily reflect parasympathetic influence on the heart and the SDNN reflects all sources of variability in heart rate 45.
Frequency domain indices
Fast Fourier Transformation was used to determine HRV components in the following frequency bands: high frequency (HF; 0.15 – 0.40 Hz, in ln.ms2) and low frequency (LF; 0.04 – 0.15 Hz, in ln.ms2). High frequency HRV primarily reflects parasympathetic modulation of heart rate, whereas LF-HRV reflects both sympathetic and parasympathetic modulation of heart rate 45. The ratio of low frequency to high frequency power was also examined (LF/HF) as an index of sympathovagal balance 45, although this index is associated with some validity concerns 22.
Patients were monitored for 48 hours and HRV was assessed for each of the two consecutive 24-hour observation periods. HRV analyses were based on the minimum value of the two 24-hour periods. Consistent with prior studies 46 correlations between the two days of assessment were high (r-values ranging from 0.59 to 0.92) and no differences in the mean HRV indices over the two days of observation were observed (p-values > 0.10).
Statistical Analysis
Clinical and demographic data are presented as mean ± standard deviation or percentages and HRV data as mean ± standard error of the mean. Comparisons among defibrillator patients were conducted using t-tests or χ2 test as appropriate. Logarithmic transformations were applied to frequency domain HRV data to obtain normal distributions. Associations between depression and anxiety with HRV outcome measures were examined using dichotomized and continuous questionnaire scores. T-tests were used to examine differences between patients with elevated levels of depression symptoms versus patients with low depression levels. Variances were pooled only when equality of variance could be assumed based on Levene’s test of equality. Analyses of covariance were used to adjust for potentially confounding factors (age, sex, hypertension, diabetes mellitus, and smoking status). Analyses for elevated versus low anxiety levels were conducted in the same manner. Associations of continuous depression and anxiety symptoms with HRV indices were examined using Pearson product-moment correlation coefficients and multivariate regression analysis. Two-tailed probabilities were examined and because 5 HRV measures were examined, the Bonferroni-corrected alpha level was set at 0.05 / 5 = 0.01.
RESULTS
Participants
Demographic and clinical characteristics are presented in the left data column of Table 1. Twelve (27.3%) patients had Beck Depression Inventory scores indicative of elevated depression (≥ 10) and 10 patients (22.7%) had Manifest Anxiety Scores suggesting elevated anxiety levels (≥ 9).
Table 1.
Demographic and clinical characteristics of patients with and without symptoms of depression
| Total (N=44) mean±sd; n(%) |
No Depression BDI < 10 (N=32) mean±sd; n (%) |
Depression BDI ≥ 10 (N=12) mean±sd; n (%) |
|
|---|---|---|---|
| Demographics | |||
| Age (years) | 62.1 ± 9.3 | 63.7 ± 9.2 | 58.0 ± 8.6 |
| Sex (Female, n (%) | 3 (6.8%) | 2 (6.2%) | 1 (8.3%) |
| Race African American | 5 (11.4%) | 4 (12.5%) | 1 (8.3%) |
| European American | 39 (88.6) | 28 (87.5%) | 11 (91.7%) |
| Cardiovascular disease risk | |||
| BMI (kg/m2) | 29.5 ± 5.4 | 29.5 ± 5.8 | 29.4 ± 4.7 |
| Current Smoker | 7 (15.9) | 5 (15.6%) | 2 (16.7%) |
| Hypertension | 31 (70.5) | 24 (75.0%) | 7 (58.3%) |
| Diabetes | 12 (27.3) | 8 (25.0%) | 4 (33.3) |
| Cardiac History | |||
| History of myocardial infarction | 37 (82.9%) | 28 (86.2%) | 9 (75.0%) |
| History of bypass surgery | 23 (52.3) | 16 (50.0%) | 7 (58.3%) |
| Ejection fraction (%) | 35.9 ± 12.7 | 34.9 ± (12.8%) | 38.5 ± 12.5 |
| Reason for ICD | |||
| Sudden death | 3 (6.8%) | 1 (3.1%) | 2 (16.7%) |
| Syncope with VT/VF | 15 (34.1%) | 12 (37.5%) | 3 (25.0%) |
| Symptomatic VT | 13 (29.5%) | 10 (31.3%) | 3 (25.0%) |
| VT during Holter | 10 (22.8%) | 8 (25.0%) | 2 (16.7%) |
| Asymptomatic VT | 3 (6.8%) | 1 (3.1%) | 2 (16.7%) |
| Duration since ICD (months) | 13,9 ± 15.4 | 15.4 ± 17.6 | 9.9 ± 6.0 |
| Prior ICD discharges (#) | 1.7 ± 3.3 | 1.6 ± 2.3 | 2.2 ± 5.6 |
| Beta-adrenergic blocking agent | |||
| Not prescribed | 7 (15.9) | 5 (15.6%) | 2 (16.7%) |
| Withheld | 10 (22.7) | 7 (21.9%) | 3 (25.0%) |
| Continued | 27 (61.4) | 20 (62.5%) | 7 (58.3%) |
| Psychological measures | |||
| BDI | 6.5 ± 4.4 | 4.4 ± 2.6 | 12.3 ± 3.0 |
| Taylor | 4,8 ± 3,6 | 4.0 ± 3.2 | 7.0 ± 4.1 * |
VT = ventricular tachycardia; VF = ventricular fibrillation; ICD = implantable cardioverter defibrillator.
Group comparisons wee not significant (p>0.10) for demographic and clinical variables.
p = 0.015 (BDI differences were not tested statistically because this variable was used to create the depression groups).
Patients with low versus elevated depression levels did not significantly differ on demographic or clinical measures, including cardiovascular risk factors, cardiac disease markers, and ICD characteristics (Table 1). Table 2 displays group comparisons related to the presence or absence of anxiety. Anxiety was associated with a history of sudden death reanimation (p = 0.033) and higher left ventricular ejection fraction (p = 0.029).
Table 2.
Characteristics of patients with and without anxiety symptoms
| No Anxiety Taylor < 9 (N=34) mean±sd; n (%) |
Anxiety Taylor ≥ 9 (N=10) mean±sd; n (%) |
|
|---|---|---|
| Demographics | ||
| Age (years) | 62.6 ± 9.3 | 60.6 ± 9.8 |
| Sex (Female, n (%) | 1 (2.9%) | 2 (20.0%) |
| Race African American | 4 (11.8%) | 1 (10.0%) |
| European American | 30 (88.2%) | 9 (90.0%) |
| Cardiovascular disease risk factors | ||
| BMI (kg/m2) | 29.4 ± 5.8 | 29.7 ± 4.2 |
| Current Smoker | 6 (17.6%) | 1 (10.0%) |
| Hypertension | 23 (67.6%) | 8 (80.0%) |
| Diabetes | 10 (29.4%) | 2 (20.0) |
| Cardiac History | ||
| History of myocardial infarction | 30 (87.1%) | 7 (70.0%) |
| History of bypass surgery | 19 (55.9%) | 4 (40.0%) |
| Ejection fraction (%) | 33.5 ± 11.4 | 43.9 ± 14.1 * |
| Reason for ICD | ||
| Sudden death | 0 (0.0%) | 3 (30.0%) * |
| Syncope with VT/VF | 13 (38.2%) | 2 (20.0%) |
| Symptomatic VT | 10 (29.4%) | 3 (30.0%) |
| VT during Holter | 9 (26.4%) | 1 (10.0%) |
| Asymptomatic VT | 2 (5.9%) | 1 (10.0%) |
| Duration since ICD (months) | 13.5 ± 16.3 | 14.9 ± 12.4 |
| Prior ICD discharges (#) | 2.1 ± 3.7 | 0.6 ± 0.9 |
| Beta-adrenergic blocking agent | ||
| Not prescribed | 5 (14.7%) | 2 (20.0%) |
| Withheld | 6 (17.6%) | 4 (40.0%) |
| Continued | 23 (67.6%) | 4 (40.0%) |
| Psychological measures | ||
| Beck Depression Inventory | 5.7 ± 4.0 | 9.7 ± 4.6 * |
| Taylor Manifest Anxiety Scale | 3.2 ± 2.3 | 10.3 ± 1.1 |
VT = ventricular tachycardia; VF = ventricular fibrillation; ICD = implantable cardioverter defibrillator.
Group comparisons wee not significant (p>0.10) for demographic and clinical variables.
= p < 0.05 (Group differences for the Taylor MAS were not tested statistically because this variable was used to create the anxiety groups).
Table 3 displays the interrelationships between the HRV indices, documenting significant interrelationships consistent with the parasympathetic component of these indices. Mean HRV data for this sample were as follows: RMSSD 22.2 ± 12.4 ms, pNN50 = 4.5 ± 5.3 %, SDNN = 102.9 ± 32.7 ms, HF = 4.1 ± 0.9 ln.ms2, LF = 5.2 ± 1.1 ln.ms2, and LF/HF 1.3 ± 0.2.
Table 3.
Correlations between HRV indices in ICD patients.
| RMSSD | PNN50 | SDNN | HF-HRV | LF-HRV | LF/HF | |
|---|---|---|---|---|---|---|
| PNN50 | 0.89 ** | |||||
| SDNN | 0.60 ** | 0.56 ** | ||||
| HF-HRV | 0.64 ** | 0.58 ** | 0.70 ** | |||
| LF-HRV | 0.53 ** | 0.43 * | 0.75 ** | 0.82 ** | ||
| LF/HF | −0.34 * | −0.35 * | −0.10 | −0.43 ** | 0.13 | |
| HR (avg) | −.275 | −.275 | −.502 ** | −.305 * | −.207 | .214 |
RMSSD = root mean square successive difference of RR intervals; PNN50 = percent of successive RR interval differences > 50 ms; SDNN = standard deviation of RR intervals (SDNN); LF = low frequency HRV; HF = high frequency HRV; LF/HF = ratio of LF and HF; HR (avg) = Average heart rate during ambulatory monitoring.
Depression and HRV in defibrillator patients
Associations between depression and HRV indices are shown in Table 4. Patients with elevated depression levels (i.e., BDI score ≥ 10; n= 12) had significantly lower RMSSD (p = 0.002) and pNN50 (0.006) than patients with low depression levels (n = 32). No associations were found between depression status and SDNN. When adjusting for age, sex, hypertension, diabetes and current smoking status, the associations remained significant (RMSSD p = 0.047, PNN50 p = 0.040). Continuous depression scores were also correlated with decreased RMSSD (r = −0.31, p = 0.049, covariate adjusted p = 0.065) and similar associations were found for pNN50 (r = −0.29, p = 0.064, covariate adjusted p = 0.037),
Table 4.
Association between depression and HRV indices of autonomic nervous system activity
| Unadjusted mean | Adjusted mean* | |||||
|---|---|---|---|---|---|---|
| No Depression BDI < 10 mean±s.e.m. |
Depression BDI ≥ 10 mean±s.e,m. |
p a | No Depression BDI < 10 mean±s.e,m. |
Depression BDI ≥ 10 mean±s.e,m. |
p | |
| RMSSD (ms) | 24.97 ± 2.44 | 15.25 ± 1.66 | 0.002 | 24.72 ± 2,19 | 15.85 ± 3.57 | 0.047 |
| pNN50 (%) | 5.61 ± 1.04 | 1.83 ± 0.77 | 0.006 | 5.64 ± 0.93 | 1.75 ± 1.51 | 0.040 |
| SDNN (ms) | 112.0 ± 6.13 | 107.4 ± 10.50 | 0.70 | 110.76 ± 6.27 | 110.86 ± 10.60 | 0.99 |
| HF (ln.ms2) | 4.27 ± 0.18 | 3.71 ± 0.21 | 0.087 | 4.27 ± 0.16 | 3.72 ± 0.27 | 0.092 |
| LF (ln.ms2) | 5.28 ± 0.22 | 5.08 ± 0.31 | 0.62 | 5.30 ± 0.21 | 5.03 ± 0.34 | 0.51 |
| LF/HF | 1.25 ± 0.03 | 1.37 ± 0.05 | 0.061 | 1.26 ± 0.03 | 1.35 ± 0.05 | 0.16 |
adjusted for age, sex, hypertension and diabetes status.
unadjusted p values were calculated using unpooled variances and adjusted degrees of freedom if homogeneity of variance was not present (i.e., Levene’s test for equivalence of variance p < 0.05; RMSSD and PNN50).
HF-HRV tended to be lower in patients with elevated depression symptoms compared to patients with low levels of depressive symptoms (3.7 ± 0.7 ln.ms2 vs. 4.4 ± 1.0 ln.ms2; p = 0.087). LF-HRV did not differ between patients with versus without elevated depression (5.1 ± 1.0 ln.ms2 vs. 5.3 ± 1.2 ln.ms2; p = 0.62), and the LF/HF ratio was marginally higher in ICD patients with elevated depression levels (p = 0.061). Continuous depression levels were not associated with HF-HRV or LF-HRV (r = −0.14 and r = −0.31, respectively, p-values > 0.20).
Anxiety and HRV indices
As shown in Table 5, elevated anxiety was associated with lower RMSSD (p = 0.013). Adjusting for covariates attenuated the significance of this relationship (adjusted p = 0.069). Results were not significant for pNN50 (p = 0.19) and there were no differences in frequency domain indices between the high versus low anxiety groups (p-values > 0.5). The associations of continuous anxiety scores with HRV were in the expected direction but did not reach statistical significance (r-values > − 0.22, p-values > 0.2).
Table 5.
Association between depression and HRV indices of autonomic nervous system activity
| Unadjusted mean | Adjusted mean* | |||||
|---|---|---|---|---|---|---|
| No Anxiety Taylor < 9 mean±s.e,m. |
Anxiety Taylor ≥ 9 mean±s.e,m. |
p a | No Anxiety Taylor < 9 mean±s.e,m. |
Anxiety Taylor ≥ 9 mean±s.e,m. |
p | |
| RMSSD (ms) | 23.97 ± 2.29 | 15.67 ± 2.15 | 0.013 | 24.09 ± 2.09 | 15.24 ± 4.13 | 0.069 |
| pNN50 (%) | 5.08 ± 0.95 | 2.48 ± 1.44 | 0.19 | 5.17 ± 0.91 | 2.16 ± 1.79 | 0.15 |
| SDNN (ms) | 111.67 ± 6.19 | 107.68 ± 9.94 | 0.75 | 112.40 ± 6.00 | 105.29 ± 11.40 | 0.59 |
| HF (ln.ms2) | 4.14 ± 0.16 | 4.03 ± 0.35 | 0.74 | 4.17 ± 0.16 | 3.93 ± 0.31 | 0.50 |
| LF (ln.ms2) | 5.25 ± 0.20 | 5.13 ± 0.40 | 0.78 | 5.27 ± 0.20 | 5.06 ± 0.39 | 0.64 |
| LF/HF | 1.28 ± 0.03 | 1.29 ± 0.07 | 0.85 | 1.28 ± 0.03 | 1.30 ± 0.06 | 0.77 |
adjusted for age, sex, hypertension and diabetes status.
unadjusted p values were calculated using unpooled variances and adjusted degrees of freedom if homogeneity of variance was not present (i.e., Levene’s test for equivalence of variance p < 0.05; RMSSD and PNN50).
Combined Effects of Depression and Anxiety
Depression and anxiety levels were significantly correlated (r = 0.49, p < 0.001). When examining depression and anxiety scores simultaneously as continuous variables using multivariate regression analyses, it was found that depression was more strongly associated with RMSSD (beta = −0.33, p = 0.071) then anxiety (beta = 0.03, p = 0.80). Data for PNN50 revealed a similar pattern (beta depression = −0.32, p = 0.077; beta anxiety = 0.03, p = 0.86).
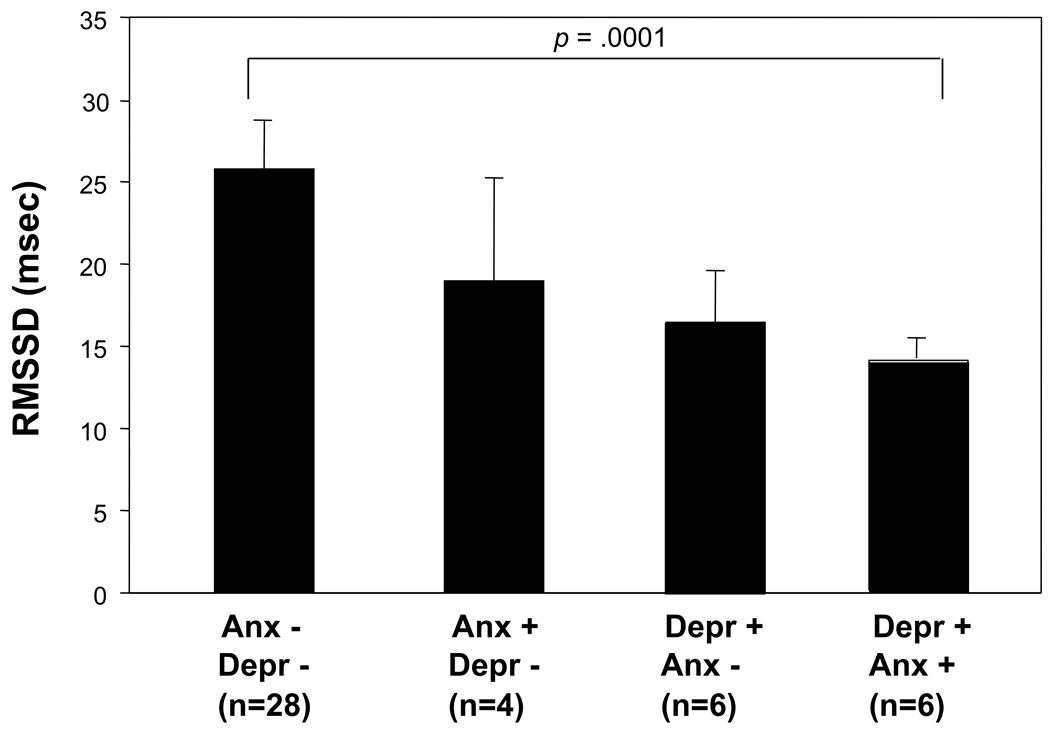
Exploratory analyses were conducted examining the combined effect of depression and anxiety on HRV indices that displayed associations. Six patients displayed elevated depression levels combined with elevated anxiety levels (≥10 on Beck Depression Inventory and ≥ 9 on the Manifest Anxiety Scale), 6 patients had elevated depression and low anxiety, 4 had elevated anxiety and low depression levels, and 28 had low scores on both depression and anxiety scales. Patients with combined elevated depression and anxiety had significantly lower RMSSD (p < 0.001) compared to those with low depression and low anxiety (Figure 1). The ANOVA analyses for linear trend across the 4 groups was also significant (p = 0.047). Results for pNN50 showed the same pattern (group comparison p < 0.001, p trend = 0.042). None of the other subgroup comparisons were significant and differences in SDNN or frequency domain variables were also not significant. Levels of depression in the combined high depression and high anxiety subgroup (12.5 ± 4.6) were not significantly higher than depression levels in the group with elevated depression and low anxiety levels (BDI = 12.2 ± 2.6). The effects of depression and anxiety on RMSSD and PNN50 were additive, and the depression by anxiety interactions on HRV indices were not significant (p-values > 0.20).
Figure 1.
The combined effect of depression and anxiety on HRV indices. Depression and anxiety status was based on previously validated cut-off scores (≥10 for the Beck Depression Inventory and ≥ 9 on the Manifest Anxiety Scale). Patients with combined positive status for both depression and anxiety scores had significantly lower RMSSD (p < 0.001) compared to those with low depression and low anxiety. The ANOVA analyses for trend across the 4 groups was also significant (p = 0.047). Depr = depression, Anx = anxiety; RMSSD = root mean square of successive differences.
DISCUSSION
This study demonstrates that elevated levels of depression and anxiety are related to HRV-based indices of autonomic nervous system dysfunction in patients with implantable cardioverter defibrillators. A shift towards increased sympathetic and reduced parasympathetic nervous system activity may provide a pathophysiological mechanism accounting for the elevated arrhythmic risk in patients with psychological factors associated with elevated cardiovascular risk.
The association between psychological factors and HRV has not previously been examined in patients with high risk of malignant arrhythmias. Associations between depression and HRV tended to be stronger than associations between anxiety and HRV measures. Data analyses based on categorical data identifying relatively high levels of depression and anxiety versus low levels using previously published cut-off values revealed stronger associations with HRV then analyses examining depression and anxiety as continuous variables. These results suggest that psychosocial factors may adversely affect autonomic function only at levels above a certain critical threshold. Further research is needed to examine whether a formal clinical diagnosis of Major Depressive Disorder or specific anxiety disorders better identify cardiac patients at high risk of abnormal autonomic control.
Depression and anxiety often occur in the same patient and exploratory analyses indicate that the combination of high depression with high anxiety was associated with the most pronounced parasympathetic withdrawal as measured by the RMSSD and pNN50 indices. Reduced HRV in the group with elevated levels of both depression and anxiety levels was not merely a function of more severe depression symptoms because depression levels were not higher between the elevated depression subgroups with versus without elevated anxiety levels. These results raise the possibility that anxiety may exacerbate the relationship between depression and reduced HRV. Consistent with these findings are observations in physically healthy depressed individuals, documenting low vagal control during laboratory conditions in the subset of individuals with high state anxiety 35. Furthermore, some evidence suggests that high anxiety and not depression is associated with reduced vagal control among post myocardial infarction patients 47. Symptomatic heterogeneity of HRV within depression 48 and evidence that worry episodes are associated with decreased HRV 49 indicates that attention to individual symptom profiles rather than diagnostic categories may be important. Additional research examining depression and anxiety symptoms is necessary to establish the neurobehavioral mechanisms that integrate central and autonomic nervous system activity.
Clinical variables were not strongly predictive of depression or anxiety. Some support was found for an association between the precipitating circumstances leading to ICD implantation (i.e., sudden death reanimation) with trait anxiety. Other studies have found that increased psychological distress in ICD patients occurs in response to defibrillator shocks 52 53 which was not confirmed in the present study. Somatic symptoms of depression (e.g., fatigue) and anxiety (e.g., palpitations) may occur more frequently in defibrillator patients, and the present data may in part reflect cardiac symptom severity. However, indices of cardiac disease severity such as history of bypass surgery or history of myocardial infarction were not related to psychological measures, and anxiety was unexpectedly associated with better cardiac pump function. Current guidelines for the assessment of depression in patients with cardiac disease indicate that somatic depressive symptoms need to be included in the diagnosis of depression in cardiac patients 54, which is consistent with the present findings suggesting that underlying cardiac disease severity is not likely to be a primary explanatory factor of depression and anxiety in ICD patients.
Time domain HRV measures showed stronger associations with psychological measures than frequency domain measures. One explanation is that time domain assessments collected over several hours are less vulnerable to ECG artifacts and ectopy than frequency domain measures, despite the exclusion of these episodes from the HRV analyses. Breathing frequency may also have affected the results, and some evidence indicates that time domain HRV indices (RMSSD) are less influenced by breathing than frequency domain measures (HF-HRV) 50. It is also possible that frequency domain measures are better tools to document short-term (< 10 min) HRV changes than sustained (8 > hours) markers of autonomic dysfunction. We have previously documented such short-term frequency domain HRV changes precede ambulatory ischemia with mental stress during daily life 51. Future studies may investigate HRV changes to acute psychological challenges during daily life using ambulatory monitoring techniques in ICD patients.
The study has limitations that merit discussion. The present findings are based on cross-sectional analyses in a relatively small sample, thus limiting causal inference. Beta-adrenergic blocking agents were used by 61% of the patients which may have increased the HRV measures 55. This is not a likely explanation because no significant differences in HRV were found between ICD patients taking beta blocking agents versus those who were not on such medications (p-values > 0.2). Prospective examination of psychological factors and HRV in larger samples including a broader range of cardiac patients would be beneficial to precisely determine the magnitude and time-trajectories of depression and anxiety as related to autonomic nervous system indices while adjusting for potentially confounding factors such as cardiac symptom severity and other clinical characteristics.
Psychological characteristics, such as depression and anger, predict ventricular arrhythmia in general coronary artery disease populations and defibrillator patients 17, 18. The present data suggest that assessment of multiple psychological risk factors may improve risk stratification of cardiac patients. This is particularly important in defibrillator patients where high levels of distress are common after implantation and can act as triggers of arrhythmias resulting in device discharges that pose recurrent transient risks 56. The levels of depression and anxiety were relatively low in the present study, and associations with autonomic dysfunction are expected to be even stronger in patients with clinical major depressive disorder and those with anxiety disorders such as panic attacks and post-traumatic stress disorder.
Acknowledgments
This work was supported by grants from the National Heart, Lung and Blood Institute RO1HL047337 and T32HL69751)
Abbreviations
- ICD
implantable cardioverter defibrillators
- HRV
heart rate variability
- BDI
Beck Depression Inventory
- BMI
body mass index
Footnotes
The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the USUHS or the US Department of Defense.
Reference List
- 1.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005 March;131(2):260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 2.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99(16):2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 3.Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med. 2003 March;65(2):201–210. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- 4.Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res. 2002 October;53(4):897–902. doi: 10.1016/s0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 5.Kubzansky LD, Kawachi I, Weiss ST, Sparrow D. Anxiety and coronary heart disease: a synthesis of epidemiological, psychological, and experimental evidence. Ann Behav Med. 1998;20(2):47–58. doi: 10.1007/BF02884448. [DOI] [PubMed] [Google Scholar]
- 6.Kubzansky LD, Davidson KW, Rozanski A. The clinical impact of negative psychological states: expanding the spectrum of risk for coronary artery disease. Psychosom Med. 2005 May;67 Suppl 1:S10–S14. doi: 10.1097/01.psy.0000164012.88829.41. S10–S14. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen SS, van den Broek KC, Sears SF., Jr Psychological intervention following implantation of an implantable defibrillator: a review and future recommendations. Pacing Clin Electrophysiol. 2007 December;30(12):1546–1554. doi: 10.1111/j.1540-8159.2007.00905.x. [DOI] [PubMed] [Google Scholar]
- 8.Maryniak A, Szumowski L, Orczykowski M, Przybylski A, Walczak F. Anxiety and depression among the patients with frequent implantable cardioverter-defibrillator discharges. Int J Cardiol. 2009;132:e80–e81. doi: 10.1016/j.ijcard.2007.08.027. E-pub 2007 November 29. [DOI] [PubMed] [Google Scholar]
- 9.Fritzsche K, Forster F, Schweickhardt A, Kanwischer H, Drinkmann A, Rabung S, Bergmann G, Geibel A, Herrmann-Lingen C. Depressive coping is a predictor for emotional distress and poor quality of life in a German-Austrian sample of cardioverter-defibrillator implant recipients at 3 months and 1 year after implantation. Gen Hosp Psychiatry. 2007 November;29(6):526–536. doi: 10.1016/j.genhosppsych.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Bilge AK, Ozben B, Demircan S, Cinar M, Yilmaz E, Adalet K. Depression and anxiety status of patients with implantable cardioverter defibrillator and precipitating factors. Pacing Clin Electrophysiol. 2006 June;29(6):619–626. doi: 10.1111/j.1540-8159.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 11.Thomas SA, Friedmann E, Kelley FJ. Living with an implantable cardioverter-defibrillator: a review of the current literature related to psychosocial factors. AACN Clin Issues. 2001 February;12(1):156–163. doi: 10.1097/00044067-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Sears SF, Lewis TS, Kuhl EA, Conti JB. Predictors of quality of life in patients with implantable cardioverter defibrillators. Psychosomatics. 2005 September;46(5):451–457. doi: 10.1176/appi.psy.46.5.451. [DOI] [PubMed] [Google Scholar]
- 13.Godemann F, Butter C, Lampe F, Linden M, Werner S, Behrens S. Determinants of the quality of life (QoL) in patients with an implantable cardioverter/defibrillator (ICD) Qual Life Res. 2004 March;13(2):411–416. doi: 10.1023/B:QURE.0000018493.32844.56. [DOI] [PubMed] [Google Scholar]
- 14.Ladwig KH, Deisenhofer I, Simon H, Schmitt C, Baumert JJ. Characteristics associated with low treatment satisfaction in patients with implanted cardioverter defibrillators: results from the LICAD study. Pacing Clin Electrophysiol. 2005 June;28(6):506–513. doi: 10.1111/j.1540-8159.2005.09509.x. [DOI] [PubMed] [Google Scholar]
- 15.Baumert J, Schmitt C, Ladwig KH. Psychophysiologic and affective parameters associated with pain intensity of cardiac cardioverter defibrillator shock discharges. Psychosom Med. 2006 July;68(4):591–597. doi: 10.1097/01.psy.0000221379.17371.47. [DOI] [PubMed] [Google Scholar]
- 16.Dunbar SB, Kimble LP, Jenkins LS, Hawthorne M, Dudley W, Slemmons M, Langberg JJ. Association of mood disturbance and arrhythmia events in patients after cardioverter defibrillator implantation. Depress Anxiety. 1999;9(4):163–168. doi: 10.1002/(sici)1520-6394(1999)9:4<163::aid-da3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Lampert R, Joska T, Burg MM, Batsford WP, McPherson CA, Jain D. Emotional and physical precipitants of ventricular arrhythmia. Circulation. 2002 October 1;106(14):1800–1805. doi: 10.1161/01.cir.0000031733.51374.c1. [DOI] [PubMed] [Google Scholar]
- 18.Whang W, Albert CM, Sears SF, Jr, Lampert R, Conti JB, Wang PJ, Singh JP, Ruskin JN, Muller JE, Mittleman MA. Depression as a predictor for appropriate shocks among patients with implantable cardioverter-defibrillators: results from the Triggers of Ventricular Arrhythmias (TOVA) study. J Am Coll Cardiol. 2005 April 5;45(7):1090–1095. doi: 10.1016/j.jacc.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 19.Carney RM, Blumenthal JA, Freedland KE, Stein PK, Howells WB, Berkman LF, Watkins LL, Czajkowski SM, Hayano J, Domitrovich PP, Jaffe AS. Low heart rate variability and the effect of depression on post-myocardial infarction mortality. Arch Intern Med. 2005 July 11;165(13):1486–1491. doi: 10.1001/archinte.165.13.1486. [DOI] [PubMed] [Google Scholar]
- 20.Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007 February;74(2):224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 21.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998 February 14;351(9101):478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 22.Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation 1997 November 4. 1997 Nov 4;96(9):3224–3232. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- 23.Kleiger RE, Miller JP, Bigger JT, Moss AJ multicenter post-infarction research group. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. American Journal of Cardiology. 1987;59:256–263. doi: 10.1016/0002-9149(87)90795-8. Ref Type: Journal (Full) [DOI] [PubMed] [Google Scholar]
- 24.Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 25.Lombardi F, Porta A, Marzegalli M, Favale S, Santini M, Vincenti A, De RA Participating Investigators of ICD-HRV Italian Study Group. Heart rate variability patterns before ventricular tachycardia onset in patients with an implantable cardioverter defibrillator. Am J Cardiol. 2000 November 1;86(9):959–963. doi: 10.1016/s0002-9149(00)01130-9. [DOI] [PubMed] [Google Scholar]
- 26.Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med. 2005 May;67 Suppl 1:S29–S33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- 27.Horsten M, Ericson M, Perski A, Wamala SP, Schenck-Gustafsson K, Orth-Gomer K. Psychosocial factors and heart rate variability in healthy women. Psychosom Med. 1999 January;61(1):49–57. doi: 10.1097/00006842-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Kim CK, McGorray SP, Bartholomew BA, Marsh M, Dicken T, Wassertheil-Smoller S, Curb JD, Oberman A, Hsia J, Gardin J, Wong ND, Barton B, McMahon RP, Sheps DS. Depressive symptoms and heart rate variability in postmenopausal women. Arch Intern Med. 2005 June 13;165(11):1239–1244. doi: 10.1001/archinte.165.11.1239. [DOI] [PubMed] [Google Scholar]
- 29.Thayer JF, Smith M, Rossy LA, Sollers JJ, Friedman BH. Heart period variability and depressive symptoms: gender differences. Biol Psychiatry. 1998 August 15;44(4):304–306. doi: 10.1016/s0006-3223(98)00008-0. [DOI] [PubMed] [Google Scholar]
- 30.Vaccarino V, Lampert R, Bremner JD, Lee F, Su S, Maisano C, Murrah NV, Jones L, Jawed F, Afzal N, Ashraf A, Goldberg J. Depressive symptoms and heart rate variability: evidence for a shared genetic substrate in a study of twins. Psychosom Med. 2008 July;70(6):628–636. doi: 10.1097/PSY.0b013e31817bcc9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gehi A, Mangano D, Pipkin S, Browner WS, Whooley MA. Depression and heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry. 2005 June;62(6):661–666. doi: 10.1001/archpsyc.62.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeragani VK, Pohl R, Balon R, Ramesh C, Glitz D, Jung I, Sherwood P. Heart rate variability in patients with major depression. Psychiatry Res. 1991 April;37(1):35–46. doi: 10.1016/0165-1781(91)90104-w. [DOI] [PubMed] [Google Scholar]
- 33.Yeragani VK, Pohl R, Berger R, Balon R, Ramesh C, Glitz D, Srinivasan K, Weinberg P. Decreased heart rate variability in panic disorder patients: a study of power-spectral analysis of heart rate. Psychiatry Res. 1993 January;46(1):89–103. doi: 10.1016/0165-1781(93)90011-5. [DOI] [PubMed] [Google Scholar]
- 34.Cohen H, Kotler M, Matar MA, Kaplan Z, Miodownik H, Cassuto Y. Power spectral analysis of heart rate variability in posttraumatic stress disorder patients. Biol Psychiatry. 1997 March 1;41(5):627–629. doi: 10.1016/s0006-3223(96)00525-2. [DOI] [PubMed] [Google Scholar]
- 35.Watkins LL, Grossman P, Krishnan R, Sherwood A. Anxiety and vagal control of heart rate. Psychosom Med. 1998 July;60(4):498–502. doi: 10.1097/00006842-199807000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Kawachi I, Sparrow D, Vokonas PS, Weiss ST. Decreased heart rate variability in men with phobic anxiety (data from the Normative Aging Study) Am J Cardiol. 1995 May 1;75(14):882–885. doi: 10.1016/s0002-9149(99)80680-8. [DOI] [PubMed] [Google Scholar]
- 37.Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biol Psychiatry. 1996 February 15;39(4):255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 38.Lavoie KL, Fleet RP, Laurin C, Arsenault A, Miller SB, Bacon SL. Heart rate variability in coronary artery disease patients with and without panic disorder. Psychiatry Res. 2004 October 30;128(3):289–299. doi: 10.1016/j.psychres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. In: Pachot P, editor. Modern problems in psychopharmacology. Basel: Karger; 1974. pp. 151–169. [DOI] [PubMed] [Google Scholar]
- 40.Beck AT, Steer RA. Beck depression inventory manual. San Antonio, TX: The Psychological Corp., Harcourt Brace Jovanovich Inc; 1987. [Google Scholar]
- 41.Frasure-Smith N, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91(4):999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- 42.Strik JJ, Honig A, Lousberg R, Denollet J. Sensitivity and specificity of observer and self-report questionnaires in major and minor depression following myocardial infarction. Psychosomatics. 2001 September;42(5):423–428. doi: 10.1176/appi.psy.42.5.423. [DOI] [PubMed] [Google Scholar]
- 43.Bendig A. The development of a short form of the manifest anxiety scale. Journal of Consulting Psychology. 1956;20(5):483. doi: 10.1037/h0045580. [DOI] [PubMed] [Google Scholar]
- 44.Terry WS, Burns JS. Anxiety and repression in attention and retention. Journal of General Psychology. 2001;128(4):422–432. doi: 10.1080/00221300109598919. [DOI] [PubMed] [Google Scholar]
- 45.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 46.Pardo Y, Merz CN, Paul-Labrador M, Velasquez I, Gottdiener JS, Kop WJ, Krantz DS, Rozanski A, Klein J, Peter T. Heart rate variability reproducibility and stability using commercially available equipment in coronary artery disease with daily life myocardial ischemia. Am J Cardiol. 1996;78(8):866–870. doi: 10.1016/s0002-9149(96)00458-4. [DOI] [PubMed] [Google Scholar]
- 47.Watkins LL, Blumenthal JA, Carney RM. Association of anxiety with reduced baroreflex cardiac control in patients after acute myocardial infarction. Am Heart J. 2002 March;143(3):460–466. doi: 10.1067/mhj.2002.120404. [DOI] [PubMed] [Google Scholar]
- 48.Rottenberg J, Chambers AS, Allen JJ, Manber R. Cardiac vagal control in the severity and course of depression: the importance of symptomatic heterogeneity. J Affect Disord. 2007 November;103(1–3):173–179. doi: 10.1016/j.jad.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pieper S, Brosschot JF, van der LR, Thayer JF. Cardiac effects of momentary assessed worry episodes and stressful events. Psychosom Med. 2007 December;69(9):901–909. doi: 10.1097/PSY.0b013e31815a9230. [DOI] [PubMed] [Google Scholar]
- 50.Penttila J, Helminen A, Jartti T, Kuusela T, Huikuri HV, Tulppo MP, Coffeng R, Scheinin H. Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: effects of various respiratory patterns. Clin Physiol. 2001 May;21(3):365–376. doi: 10.1046/j.1365-2281.2001.00337.x. [DOI] [PubMed] [Google Scholar]
- 51.Kop WJ, Verdino RJ, Gottdiener JS, O'Leary ST, Bairey Merz CN, Krantz DS. Changes in heart rate and heart rate variability before ambulatory ischemic events. J Am Coll Cardiol. 2001 September;38(3):742–749. doi: 10.1016/s0735-1097(01)01451-6. [DOI] [PubMed] [Google Scholar]
- 52.Sears SF, Jr, Conti JB. Quality of life and psychological functioning of icd patients. Heart. 2002 May;87(5):488–493. doi: 10.1136/heart.87.5.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schron EB, Exner DV, Yao Q, Jenkins LS, Steinberg JS, Cook JR, Kutalek SP, Friedman PL, Bubien RS, Page RL, Powell J. Quality of life in the antiarrhythmics versus implantable defibrillators trial: impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation. 2002 February 5;105(5):589–594. doi: 10.1161/hc0502.103330. [DOI] [PubMed] [Google Scholar]
- 54.Davidson KW, Kupfer DJ, Bigger JT, Califf RM, Carney RM, Coyne JC, Czajkowski SM, Frank E, Frasure-Smith N, Freedland KE, Froelicher ES, Glassman AH, Katon WJ, Kaufmann PG, Kessler RC, Kraemer HC, Krishnan KR, Lesperance F, Rieckmann N, Sheps DS, Suls JM. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med. 2006 September;68(5):645–650. doi: 10.1097/01.psy.0000233233.48738.22. [DOI] [PubMed] [Google Scholar]
- 55.Niemela MJ, Airaksinen KE, Huikuri HV. Effect of beta-blockade on heart rate variability in patients with coronary artery disease. J Am Coll Cardiol. 1994 May;23(6):1370–1377. doi: 10.1016/0735-1097(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 56.Hegel MT, Griegel LE, Black C, Goulden L, Ozahowski T. Anxiety and depression in patients receiving implanted cardioverter-defibrillators: a longitudinal investigation. Int J Psychiatry Med. 1997;27(1):57–69. doi: 10.2190/1G9V-EQMD-MTLQ-E0BW. [DOI] [PubMed] [Google Scholar]