Abstract
Objective
Notch, VEGF and components of the Hedgehog (Hh) signaling pathway have been implicated in vascular morphogenesis. The role of Notch in mediating hedgehog control of adult vascular smooth muscle (SMC) growth and survival remains unexplored.
Methods and Results
In cultured SMC, activation of Hh signaling with recombinant rShh (3.5 μg/ml) or plasmid encoded Shh increased Ptc1 expression, enhanced SMC growth and survival and promoted Hairy-related transcription factor (Hrt) expression while concomitantly increasing VEGF-A levels. These effects were significantly reversed following Hh inhibition with cyclopamine. Shh-induced stimulation of Hrt-3 mRNA and SMC growth and survival was attenuated following inhibition of Notch mediated-CBF-1/RBP-Jk dependent signaling with RPMS-1 while siRNA knockdown of Hrt-3 inhibited SMC growth and survival. Recombinant VEGF-A increased Hrt-3 mRNA levels while siRNA knockdown abolished rShh stimulated VEGF-A expression while concomitantly inhibiting Shh-induced increases in Hrt-3 mRNA levels, proliferating cell nuclear antigen (PCNA) and Notch 1 IC expression, respectively. Hedgehog components were expressed within intimal SMC of murine carotid arteries following vascular injury concomitant with a significant increase in mRNA for Ptc1, Gli2, VEGF-A, Notch 1 and Hrt’s.
Conclusion
Hedgehog promotes a coordinate regulation of Notch target genes in adult SMC via VEGF-A.
Keywords: Hedgehog, Notch, apoptosis, proliferation, Vascular smooth muscle, VEGF, Vascular remodeling
INTRODUCTION
Changes in vascular growth and survival play an important role in neointimal formation during the pathogenesis of atherosclerosis and the arterial response to injury 1. As similar changes are also apparent during vascular morphogenesis and modeling of the embryonic vasculature 2 3, we postulated that the control of cell growth and maintenance following vascular injury may share similar signaling patterns.
Hedgehogs (Hh) are a class of 19 kDa morphogens that interact with heparin on the cell surface through an N-terminal basic domain and are tethered to the surface through cholesterol and fatty acyl modification 4. Sonic hedgehog (Shh) is the most widely expressed hedgehog during development where lack of Shh is embryonically lethal 5. Signaling occurs through interaction with the Patched receptors (Ptc1 and 2) that then activate the transcription factors Gli1, Gli2, and Gli3. The downstream targets of the Gli gene products include both Ptc and Gli themselves; thus, Ptc and Gli are both components and targets of the Hh signaling pathway 6. Several recent observations highlight the involvement of Hh in the development of embryonic vascular tissues, 7, 8 9 and in the maintenance of adult coronary vasculature through activation of perivascular cells 10. Shh signaling is also present in adult vascular tissue and can be activated in vivo to induce robust angiogenesis 11 or in vitro to promote vascular SMC growth and survival12.
The discovery of preferential Hh expression in adult adventitial tissue 12 combined with its known morphogenic functions in embryonic vascular development through Notch13 14 and VEGF-A activation 15, 16 suggests that Shh may also coordinate vascular changes in adult tissue following injury. Notch receptors and downstream Hrt target genes are coordinately regulated in vascular SMC following injury, an effect that is mimicked by addition of serum mitogens (PDGF) to cultured cells 17-19 and exposure to cyclic strain in vitro20. Notch signaling is also a critical determinant of SMC survival by modulating the expression of downstream mediators of apoptosis, Bax and in Bcl-XL21 22. More recent studies suggest that a hierarchy exists for Notch and platelet derived growth factor [PDGF] within the vasculature where PDGFR-beta expression is robustly up-regulated by Notch signaling 23.
In this context, the current study examined whether Hh components specifically co-ordinate Notch signaling through VEGF –A activation in SMC in culture and further whether these component pathways are collectively recapitulated in vivo following vascular injury.
Materials and Methods
Materials
All items were of the highest purity commercially available and purchased from Sigma Aldrich Ltd (Poole, Dorset, UK) unless otherwise stated. Recombinant mouse Sonic Hedgehog (rShh, #461-SH) was purchased from R&D Systems (Abingdon, UK). Anti Ptc1 (ab53715), Gli2 (ab53715) and Hrt-3 (ab26138; HeyL) were purchased from Abcam (Cambridge MA). Ant-ptc1 (Sc-4617) was also purchased from Santa Cruz Biotechnology (Heidelberg, Germany).
Cell Culture
Rat vascular smooth muscle cells (SMC, R354-05) and human SMC were purchased from Cell Applications Inc. (CA, USA) and grown in culture as previously described 20 21. The cells stained positive for smooth muscle cell 〈-actin, but weakly for calponin, myosin and smoothelin.
Mouse Carotid Artery Ligation
The carotid artery ligation model of vascular injury and remodeling was essentially as described by 24. All procedures were approved by the University of Rochester Animal Care Committee.
Immunohistochemistry and Histomorphometry
Mice were perfusion fixed with 10% paraformaldehyde in sodium phosphate buffer (pH 7.0), 14 days post ligation. A series of cross-sections (5 μm) were made from the birfucation every 200 μm through 2 mm length of carotid artery and stained with either hematoxylin and eosin, Van Gieson’s stain for elastic laminae, or antibodies against Notch and Hh components, as described previously24 .
Quantitative Real-Time RT-PCR (QRTPCR)
Quantitative real-time RT-PCR was carried out using the Rotor Gene (RG-3000, Corvett Research, Australia) and the SYBR green PCR kit (Qiagen) as described previously 20, 21.
siRNA Transfection
For gene silencing studies, Lipofectamine™ 2000 Reagent (Invitrogen, Groningen, The Netherlands) was used to transiently transfect SMC with gene-specific siRNA duplexes as previously described 12, 20, 25. The siRNA duplexes for VEGF corresponded to position 121-142 in the rat VEGF sequence (Accession Number: NM031836).
For details regarding additional in vitro assays and statistical analysis see the supplemental materials (available online at http://atvb.ahajournals.org).
RESULTS
Hedgehog stimulates Notch target gene (Hrt) expression in SMC in vitro
Treatment of both rat SMC and human SMC with recombinant rShh (3.5 μg/ml) for 24 h resulted in enhanced Ptc1 immunostaining within both SMC cell types [Figure I, supplemental]. In parallel rat SMC cultures, treatment of SMC with rShh (3.5 μg/ml) for 24 h resulted in a significant increase in Hairy-related transcription factor, Hrt protein expression [Figure IIa] concomitant with a significant increase in Hrt mRNA expression [Figure IIb]. Cyclopamine (40 μM), significantly reduced baseline Hrt-1, 2 and 3 mRNA levels concomitant with a reduction in Hrt protein expression in these cells [Figure IIa, c]. The rShh-induced increase in Hrt-3 protein and mRNA expression was attenuated following treatment with cyclopamine [Figure IId].
The Shh-mediated increases in Notch target gene expression compared favourably with Notch IC activation 21 and were further confirmed following ectopic expression of Shh using an expression vector encoding full-length mouse Shh [Figure IIIa, supplemental]. The percentage of cells expressing Shh was maximized after pooling SMC following puromycin selection, as previously described 20, 22. The resultant increase in Shh expression in puromycin-selected cells was confirmed by immunoblot [Figure IIIa]. Ectopic expression of Shh resulted in a significant increase in Hrt-1, 2 and 3 mRNA levels, an effect that was significantly attenuated following inhibition of CBF-1/RBP-Jk-dependent Notch signaling by co-expression with RPMS-1 [Figure IIIa]. RPMS-1 and the dominant negative R218H-RBP-Jκ were equally potent at inhibiting CBF-1/RBP-Jk transactivation in SMC [Figure IIIb] and rShh stimulated Hrt-3 mRNA expression was significantly attenuated following co-expression with the dominant negative, R218H-RBP-Jκ [Figure IIIc].
Hedgehog stimulation of SMC growth is attenuated following Notch inhibition in vitro
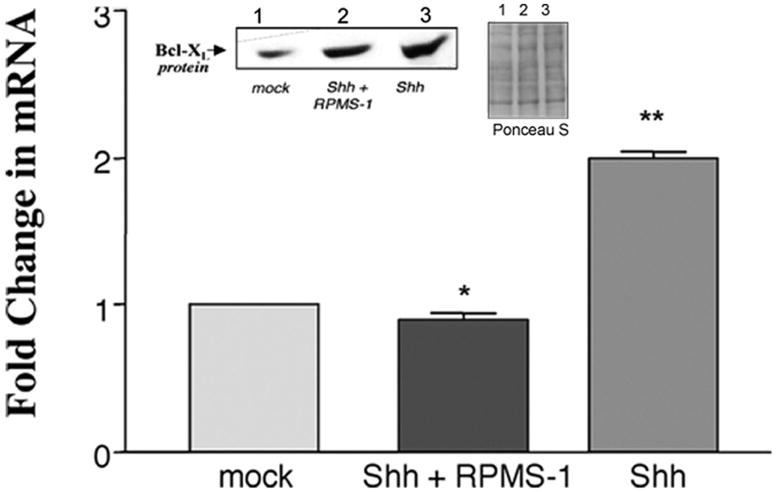
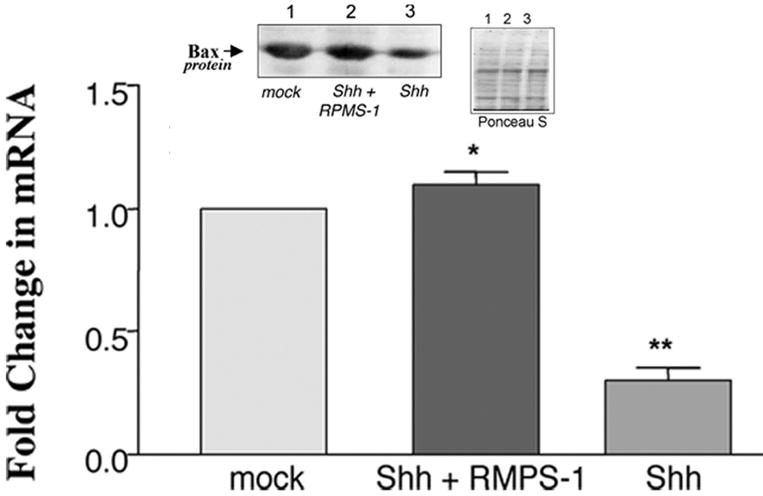
Ectopic expression of Shh in puromycin selected cells resulted in a significant increase in SMC proliferation, concomitant with enhanced proliferating cell nuclear antigen (PCNA) expression when compared to mock controls [Figure 1a], an effect that was significantly attenuated following co-expression with RPMS-1 [Figure 1a]. Ectopic expression of Shh also resulted in a significant increase in Bcl-XL mRNA and protein levels [Figure 1b] while concurrently decreasing Bax mRNA and protein levels [Figure 1c]. The Shh-induced changes in Bax:Bcl-XL protein and mRNA ratios were significantly reversed following inhibition of CBF-1/RBP-Jk-dependent Notch signaling with RPMS-1 [Figure 1b and c].
Figure 1. Notch Inhibition attenuates Shh-stimulated rat SMC growth in vitro.
The effect of enforced ectopic expression of Shh alone or co-transfection with RPMS-1 on (a) serum-stimulated SMC growth and pCNA expression, (b) Bcl-XL and (c) Bax expression. Data are mean ± SEM, n=3. *p≤0.05 vs Shh, **p≤0.05 vs mock control.
Hedgehog stimulates Notch target gene expression through VEGF –A
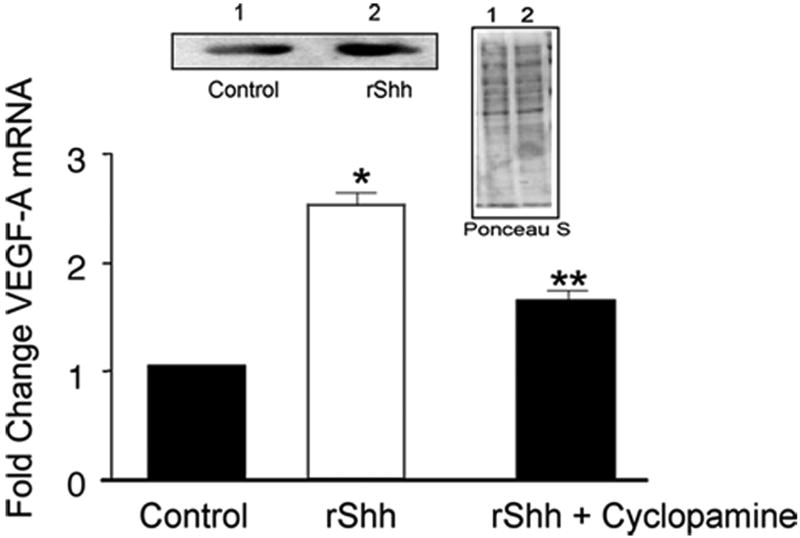
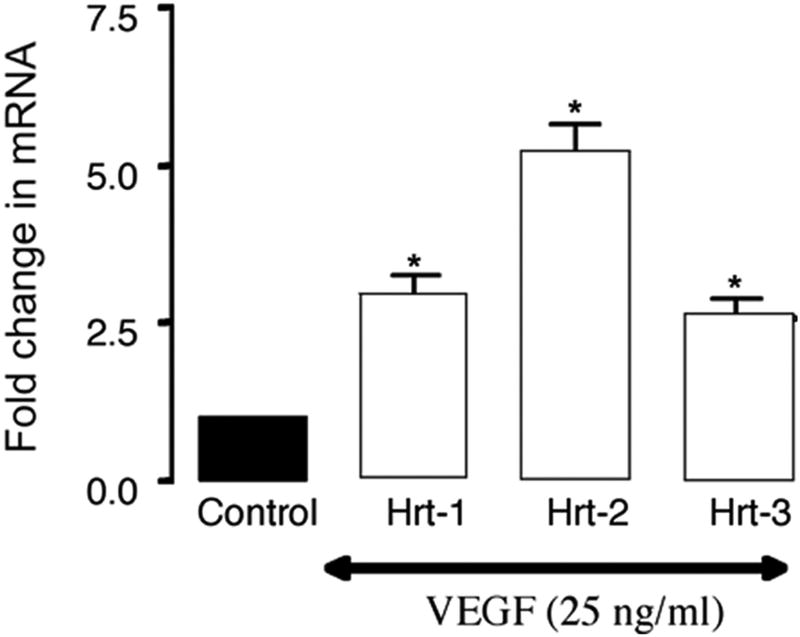
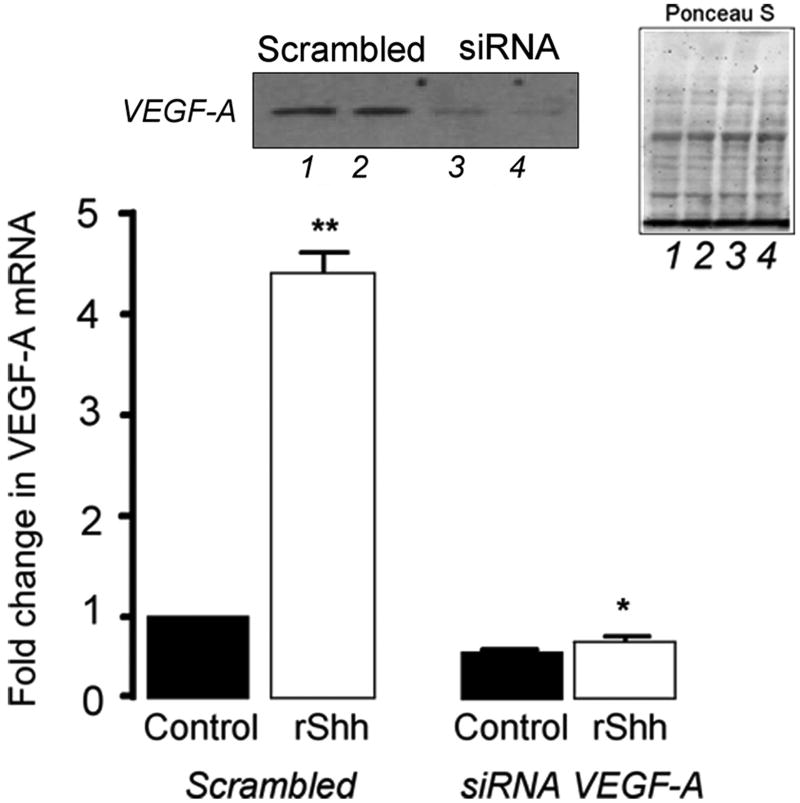
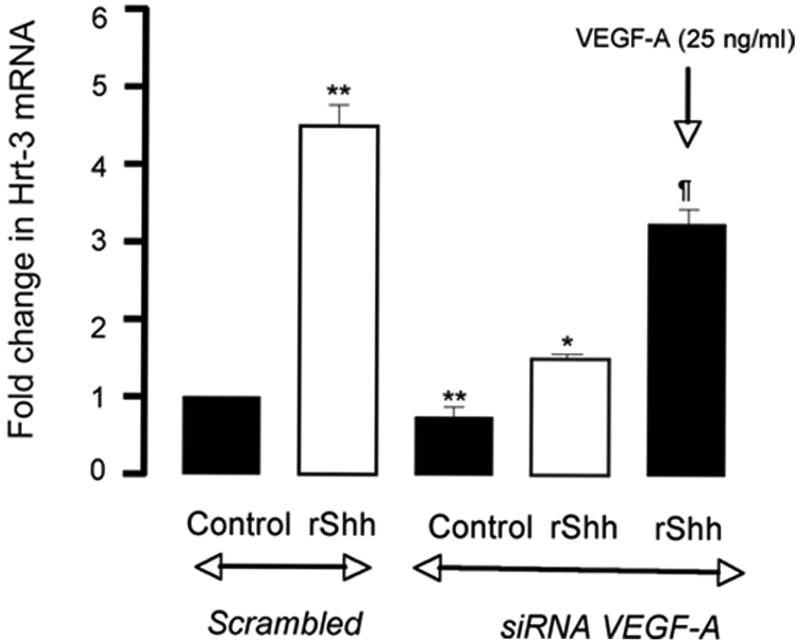
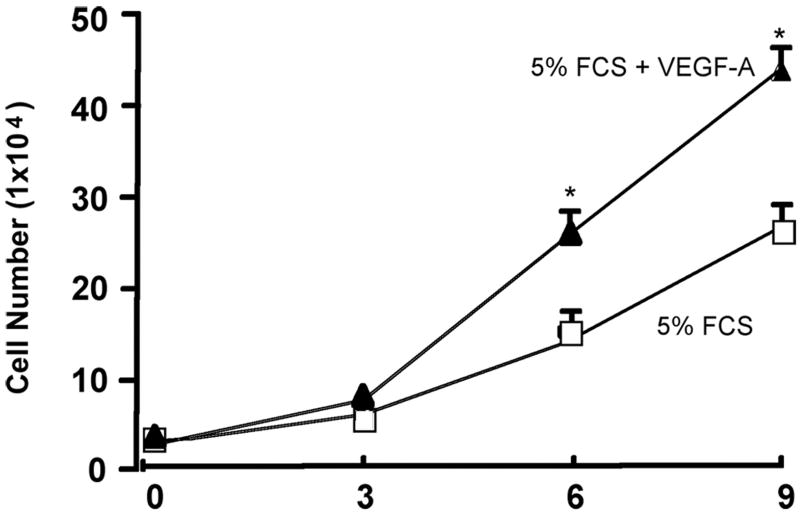
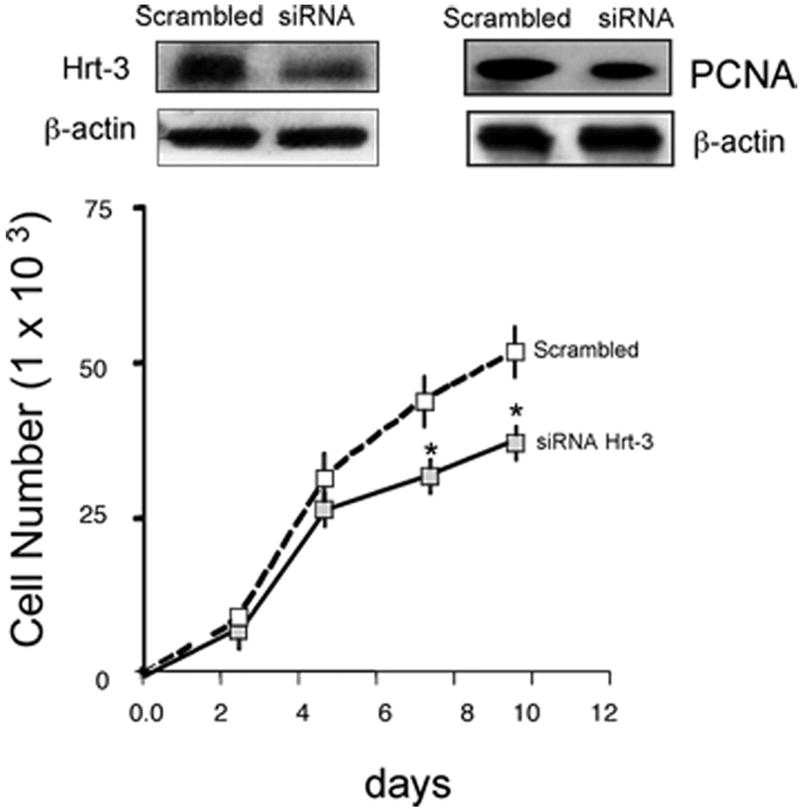
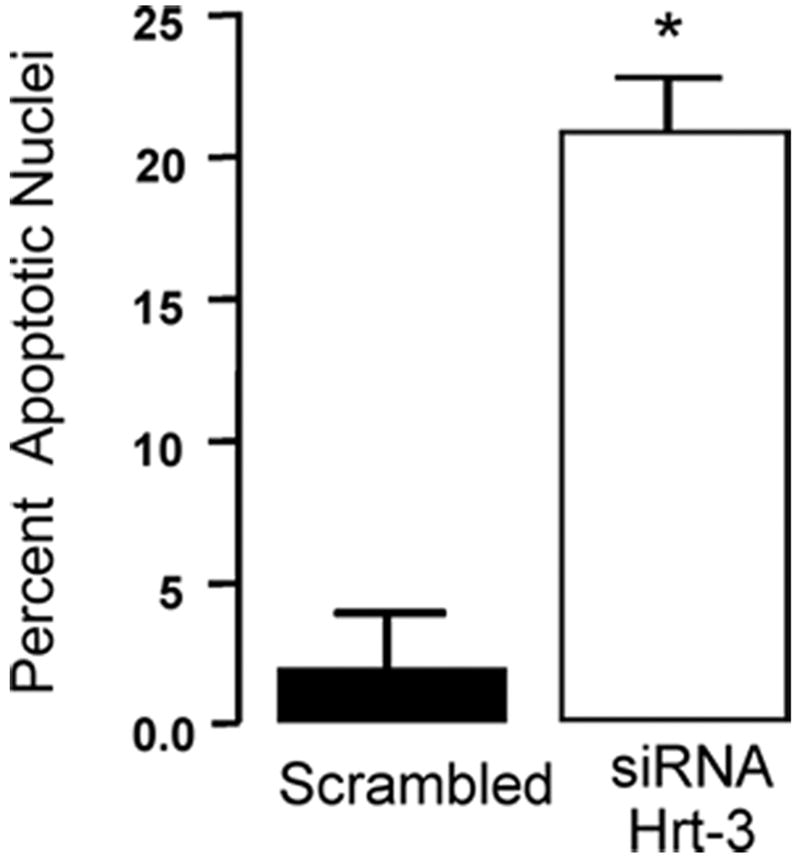
Recombinant Shh significantly increased VEGF-A protein and mRNA expression [Figure 2a] while inhibition of recombinant Hh signaling with cyclopamine (40 ╭M) resulted in a significant decrease in VEGF-A mRNA [Figure 2a]. Treatment of cells with rVEGF-A (25 ng/ml, 24h) increased Hrt-1, 2 and 3 mRNA [Figure 2b]. Selective knockdown of VEGF-A with a targeted siRNA was confirmed at the protein level and mRNA level [Figure 2c] and abolished rShh stimulated VEGF-A expression when compared to scrambled controls [Figure 2c], while concomitantly inhibiting Shh-induced increases in Hrt-3 mRNA expression [Figure 2d], PCNA and Notch 1 IC [Figure 3b] expression, respectively. The Shh- stimulated Hrt-3 mRNA levels were significantly recovered in VEGF-A siRNA treated cells following addition of exogenous recombinant rVEGF-A (25ng/ml) [Figure 2d]. Recombinant VEGF-A alone promoted SMC growth [Figure 3a], PCNA and Notch 1 IC expression [Figure 3b] in the presence of serum. A targeted siRNA knockdown of Hrt-3 inhibited serum stimulated SMC growth and increased the number of apoptotic nuclei, when compared to scrambled controls [Figure 3c and d].
Figure 2. VEGF mediates Shh activation of Notch target gene expression.
(a) The effect of Hh activation and inhibition on VEGF-A protein and mRNA expression (b) VEGF-A stimulation of Hrt-1, 2 and 3 mRNA levels (c) Shh stimulated VEGF-A mRNA levels following VEGF-A knockdown and (d) recovery following re-addition rVEGF-A treatment.
Figure 3. VEGF mediates Shh activation of SMC growth.
(a) The effect of rVEGF-A (25 ng/ml) on serum stimulated SMC proliferation (b) Shh stimulated PCNA and Notch 1 IC expression following VEGF-A knockdown (c) Hrt-3 protein expression and SMC growth (PCNA, cell counts) following Hrt-3 knockdown.
Hedgehog Components, Notch and VEGF-A are Up-regulated in Vivo following Vascular Injury
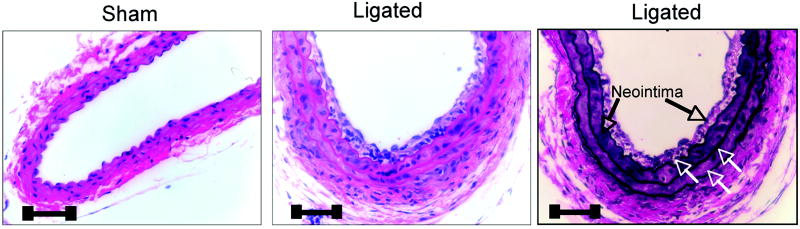
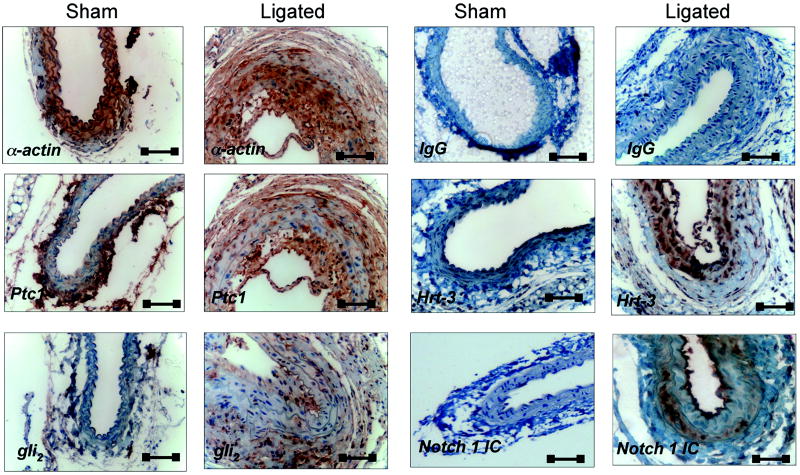
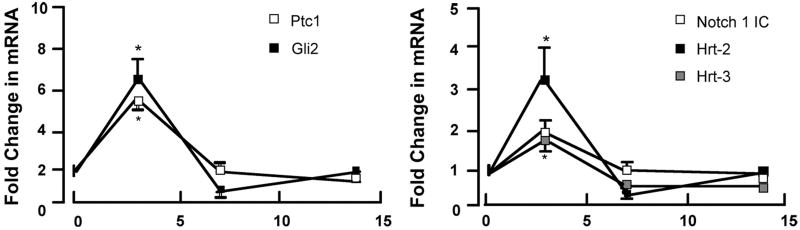
Ligation of the carotid artery of mice induced reproducible vascular remodeling with neointima formation and medial thickening, when compared to sham controls [Figure 4a, 5a]. Immunohistochemical examination of fixed tissue sections of carotids from sham-operated animals confirmed the presence of Hh target gene, Ptc1 and the transcription factor, Gli2 within the SMC medial adventitial boundary and adventitial layer of these vessels [Figure 4b], with little or no Notch 1 IC or Hrt-3 staining present. However, following injury, staining for Ptc1, Gli2, Notch 1 IC and Hrt-3 was predominately in the intima [Figure 4b].
Figure 4. Hedgehog components in murine SMC in vivo.
(a) Hemotoxylin and eosin staining of a carotid artery (CA) from C57Bl6/J mice 14 d post ligation and (b) photomicrographs of immunohistochemical staining for SMC α-actin, Notch 1 IC, Ptc1, Hrt-3 and Gli2 in carotid arteries 14 d post ligation. Magnification 40x. Scale bars=50 μm.
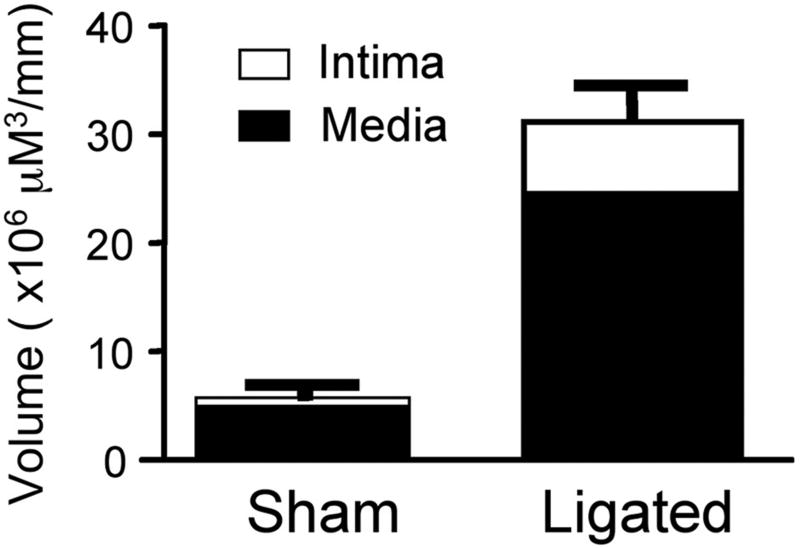
Figure 5. Regulation of Hedgehog and Notch component expression following injury in vivo.
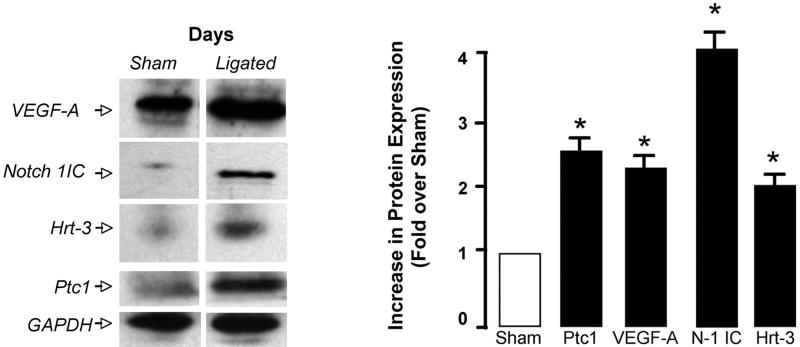
(a) Intimal and medial volumes in histological sections from sham and ligated carotid arteries after 14 d. (b and c) Hedgehog, Notch and VEGF-A mRNA levels in the CA following ligation and (d) Notch 1 IC, Hrt-3, VEGF-A, and Ptc1 protein expression 14 d post ligation.
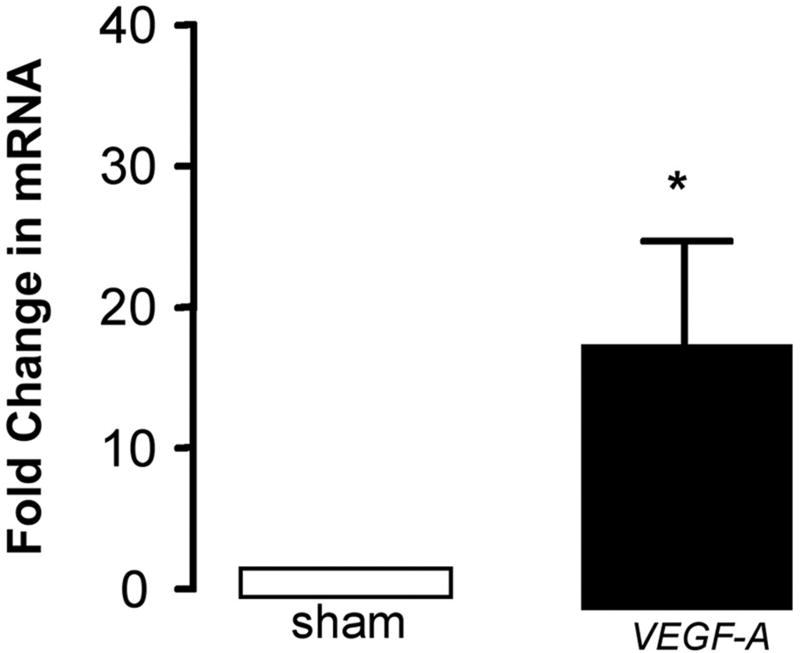
Quantitative analysis of mRNA expression for both Notch and Hh components revealed a temporal response to injury with significant increases in mRNA levels for these components evident within the first 3 d but falling off thereafter [Figure 5b]. VEGF-A mRNA levels were elevated after 3 d when compared to sham [Figure 5c]. The level of Notch 1 IC, Hrt-3, VEGF-A and Ptc1 protein expression was significantly elevated 14 d post-ligation, when compared to sham [Figure 5d].
Discussion
Sonic hedgehog (Shh) is an established morphogen critical to the development of the vascular system 15, 16 and is known to cooperate with vascular-specific growth factors during arterial differentiation 8 9. In adult blood vessels, Shh is angiogenic in the ischemic hindlimb 9, stimulates the production of angiogenic factors, including VEGF-A and angiopoietin-1 by interstitial fibroblasts 9 and promotes endothelial cell chemotaxis and tube formation 26. A restricted domain of Shh signaling has recently been localized to the arterial adventitia 27 and may play an important role in cell maintenance within the artery wall following injury. While medial SMC at the adventitial boundary express Ptc1 receptors 9 27, the role of Hh in SMC, particularly after injury, remains unclear. We therefore examined whether recapitulation of Hh components occurred in SMC following injury in vivo and further whether there is a specific functional role for Shh in activating Notch signaling in vitro to control SMC growth and survival.
The current study clearly defines SMC within the intima of injured vessels as a major target for endogenous Hh signaling in vivo, and further validates proliferative intimal vascular SMC, as observed in culture, as a major target for Shh. Initial studies had suggested that SMC may not specifically respond to Shh activation following exogenous Shh administration, despite expressing Ptc1 receptors 27. However, our study shows that following vascular injury, intimal SMC express abundant Ptc1 receptors and respond to Shh activation in vivo since Gli expression (a Hh target gene) is significantly enhanced in these cells. The preferential staining of Hh components within intimal cells without extensive staining of Notch 1 IC may suggest that relatively little Notch IC is required for robust stimulation of intimal Hrt expression within these lesions. Because the adventitia lies between the vessel wall and surrounding tissues, progenitor cells within the adventitia could, in principle, contribute to remodeling, repair, or disease processes 28 in the vessel wall. The maintenance of the coronary vasculature involves ptc1 activation in perivascular SMC 10, suggesting that Shh signaling may be critically involved in the regeneration of SMC and the maintenance of various vascular beds (aortic vs carotid/coronary) in vivo. In this context, the presence of stem cell antigen-1 (Sca1)-positive cells with a potential to differentiate into SMC were found in the aortic adventitia of adult ApoE −/− mice (AdvSca1 cells) 29. While the original hypothesis that cells of an atherosclerotic lesion originate directly from local vascular SMC of the tunica media has been recently validated 30 31, it remains unclear if lesions generated following carotid ligation follow a similar pattern. Further studies will be required to determine if the abundance of Ptc1/Gli positive cells within the intima of injured vessels are derived directly from the adventitial layer or medial layer or alternatively from circulating SMC progenitors.
Recombinant VEGF-A promotes Notch target gene expression in endothelial cells32. Notch target gene expression also serves as a convergent signaling node within early retinal progenitor cells to integrate various cell-extrinsic cues, including VEGF and Shh, in order to control cell proliferation and neuronal specification 33. In this context, we examined whether Shh promoted changes in the expression of VEGF-A in adult SMC in vitro and whether these changes were in turn responsible for downstream regulation of Notch signaling. Selective knockdown of VEGF-A resulted in inhibition of Shh-induced Notch target gene expression, and is consistent with a regulatory cascade for changes in Notch signaling that begins with Hh promoting VEGF-A expression, which in turn promotes Notch signaling in these cells. This is further supported by data that demonstrate recombinant VEGF-A recovers the Shh-induced increase in Notch target gene expression following selective endogenous VEGF-A knockdown. This hierarchy confirms previous work performed in zebrafish, where exogenous VEGF can restore normal arteriogenesis in the absence of Shh, but not in the absence of Notch function, and addition of Notch can compensate for the loss of VEGF activity 15. Notch also compensates for the loss of Shh signaling in SMC following cyclic strain 12.
We, and others have previously established a functional coupling between Notch signaling and SMC proliferation, differentiation and apoptosis in vitro 21, 34, 35 and in vivo 17, 36. Both Bax and Bcl-xL are downstream targets of CBF-1/RBP-Jk-dependent Notch signaling in SMC and govern, in part, the apoptotic profile of these cells following enforced regulation of Notch 20. It is also clear that Bcl-mediated apoptosis plays a critical role in vascular remodeling events in vivo 37. Selective Hrt-3 knockdown resulted in a significant change in the Bax:Bcl-xL expression ratio 20 and an increase in the number of apoptotic nuclei in these cells thereby underlying the importance of Hrt-3 signaling in controlling the expression of the Bcl-2 family of proteins and SMC survival. The potential molecules that may act upstream of the Notch pathway to induce changes in SMC growth and survival in vitro have not to date been addressed. It was therefore of interest to determine if Shh activation played a role in regulating vascular SMC growth and survival via Notch dependent Hrt-3 signaling. In the current study, we demonstrate that Hh activation resulted in a significant increase in CBF-1/RBP-Jk-dependent Notch target gene expression concomitant with a significant increase in SMC proliferation and survival. The Shh induced increases in SMC growth and survival were attenuated following inhibition of CBF-1/RBP-Jk-dependent Notch target gene expression with RPMS-1 and following VEGF-A knockdown implicating both Notch and VEGF-A in mediating Shh control of SMC growth and survival. The Shh-induced increase in Bcl-xL expression with a concurrent decrease in Bax expression is also consistent with a Hh-induced anti-apoptotic pro-survival phenotype for these cells 12, an effect that was attenuated following inhibition of Hh signaling with cyclopamine or inhibition of Notch target gene expression with RPMS-1. Hedgehog activation also stimulated Notch 1 IC and Hrt-3 expression, an effect that was attenuated following VEGF-A knockdown. The effects of VEGF-A knockdown on Shh-induced Hrt-3 expression was also reversed following recovery with exogenous addition of recombinant VEGF-A. Taken together, it is clear that Shh is an important regulatory molecule upstream of VEGF-A and Notch in adult SMC that governs SMC growth and survival.
The signaling pathway(s) by which Hh up-regulates VEGF-A expression and VEGF engages Notch remain(s) to be determined. In endothelial cells, Shh caused a rapid activation of c-Fes/PI3-kinase pathways 26 an effect similar to that described for VEGF 38 suggesting a possible common mechanism for Hh and VEGF activity. In addition, Hh can induce a Gli-independent pathway that activates the orphan nuclear receptor, COUPTFII 39, 40. Other studies suggest that Notch decreases VEGF receptor expression 41 confirming that these two pathways are closely interlinked. Furthermore, the induction of Notch 1 and Notch ligand (DII4) by VEGF-A in endothelial cells may explain a similar paradigm in SMC where VEGF-A induced Notch ligands increase the activity of Notch target gene expression in these cells 32. This possibility amongst others is currently under investigation. Collectively, our data confirm a central role for VEGF-A in transducing Shh control of Notch signaling in vascular SMC in vitro through induction of Notch target genes in these cells.
In conclusion, we have shown for the first time that Hh signaling is upregulated within intimal SMC of vascular lesions concomitant with increased Notch gene expression and VEGF-A in vivo. Shh controls Notch signaling in cultured vascular SMC that resemble intimal cells in vitro via VEGF-mediated activation of Notch target gene expression. Given that VEGF-A is expressed in human coronary atherosclerotic lesions 42 to promote intimal SMC proliferation in vivo43, it is tempting to speculate that a similar novel Hh/VEGF/Notch axis may represent a potential therapeutic target for disease states in which changes in vascular growth occur unabated in vivo.
Supplementary Material
Acknowledgments
Sources of Funding: This research was supported by grants from Science Foundation Ireland, the Wellcome Trust, Higher Education Authority of Ireland (HEA, PRTLI Cycle III), and the Health Research Board of Ireland (PAC) and by grants from the National Institutes of Health, HL59696 and AA-12610 (EMR) and K99 HL095650-01 (DM).
Acknowledgements: N/A
Footnotes
Disclosure: No conflicts of interest to disclose
References
- 1.Schwartz RS, Henry TD. Pathophysiology of coronary artery restenosis. Rev Cardiovasc Med. 2002;3(Suppl 5):S4–9. [PubMed] [Google Scholar]
- 2.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 3.Fisher SA, Langille BL, Srivastava D. Apoptosis during cardiovascular development. Circulation Research. 2000;87:856–864. doi: 10.1161/01.res.87.10.856. [DOI] [PubMed] [Google Scholar]
- 4.Von Ohlen T, Lessing D, Nusse R, Hooper JE. Hedgehog signaling regulates transcription through cubitus interruptus, a sequence-specific DNA binding protein. Proc Natl Acad Sci U S A. 1997;94:2404–2409. doi: 10.1073/pnas.94.6.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 6.Kalderon D. The mechanism of hedgehog signal transduction. Biochem Soc Trans. 2005;33:1509–1512. doi: 10.1042/BST0331509. [DOI] [PubMed] [Google Scholar]
- 7.Rowitch DH, B SJ, Lee SM, Flax JD, Snyder EY, McMahon AP. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J Neurosci. 1999;19:8954–8965. doi: 10.1523/JNEUROSCI.19-20-08954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- 9.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 10.Lavine KJ, Kovacs A, Ornitz DM. Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. J Clin Invest. 2008;118:2404–2414. doi: 10.1172/JCI34561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pola R, Ling LE, Aprahamian TR, Barban E, Bosch-Marce M, Curry C, Corbley M, Kearney M, Isner JM, Losordo DW. Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation. 2003;108:479–485. doi: 10.1161/01.CIR.0000080338.60981.FA. [DOI] [PubMed] [Google Scholar]
- 12.Morrow D, Sweeney C, Birney YA, Guha S, Collins N, Cummins PM, Murphy R, Walls D, Redmond EM, Cahill PA. Biomechanical regulation of hedgehog signaling in vascular smooth muscle cells in vitro and in vivo. Am J Physiol Cell Physiol. 2007;292:C488–496. doi: 10.1152/ajpcell.00337.2005. [DOI] [PubMed] [Google Scholar]
- 13.Iso T, Hamamori Y, Kedes L. Notch signaling in vascular development. Arterioscler Thromb Vasc Biol. 2003;23:543–553. doi: 10.1161/01.ATV.0000060892.81529.8F. [DOI] [PubMed] [Google Scholar]
- 14.Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- 15.Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the notch pathway during arterial endothelial differentiation. Developmental Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 16.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 17.Campos AH, Wang W, Pollman MJ, Gibbons GH. Determinants of Notch-3 receptor expression and signaling in vascular smooth muscle cells: implications in cell-cycle regulation. Circ Res. 2002;91:999–1006. doi: 10.1161/01.res.0000044944.99984.25. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Campos AH, Prince CZ, Mou Y, Pollman MJ. Coordinate Notch3-hairy-related transcription factor pathway regulation in response to arterial injury. Mediator role of platelet-derived growth factor and ERK. J Biol Chem. 2002;277:23165–23171. doi: 10.1074/jbc.M201409200. [DOI] [PubMed] [Google Scholar]
- 19.Lindner V, Booth C, Prudovsky I, Small D, Maciag T, Liaw L. Members of the Jagged/Notch gene families are expressed in injured arteries and regulate cell phenotype via alterations in cell matrix and cell-cell interaction. Am J Pathol. 2001;159:875–883. doi: 10.1016/S0002-9440(10)61763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrow D, Sweeney C, Birney YA, Cummins PM, Walls D, Redmond EM, Cahill PA. Cyclic strain inhibits Notch receptor signaling in vascular smooth muscle cells in vitro. Circ Res. 2005;96:567–575. doi: 10.1161/01.RES.0000159182.98874.43. [DOI] [PubMed] [Google Scholar]
- 21.Sweeney C, Morrow D, Birney YA, Coyle S, Hennessy C, Scheller A, Cummins PM, Walls D, Redmond EM, Cahill PA. Notch 1 and 3 receptor signaling modulates vascular smooth muscle cell growth, apoptosis, and migration via a CBF-1/RBP-Jk dependent pathway. FASEB J. 2004;18:1421–1423. doi: 10.1096/fj.04-1700fje. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Prince CZ, Hu X, Pollman MJ. HRT1 modulates vascular smooth muscle cell proliferation and apoptosis. Biochem Biophys Res Commun. 2003;308:596–601. doi: 10.1016/s0006-291x(03)01453-0. [DOI] [PubMed] [Google Scholar]
- 23.Jin S, Hansson EM, Tikka S, Lanner F, Sahlgren C, Farnebo F, Baumann M, Kalimo H, Lendahl U. Notch signaling regulates platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Circ Res. 2008;102:1483–1491. doi: 10.1161/CIRCRESAHA.107.167965. [DOI] [PubMed] [Google Scholar]
- 24.Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2003;23:2185–2191. doi: 10.1161/01.ATV.0000103120.06092.14. [DOI] [PubMed] [Google Scholar]
- 25.Morrow D, Cullen JP, Cahill PA, Redmond EM. Cyclic strain regulates the Notch/CBF-1 signaling pathway in endothelial cells: role in angiogenic activity. Arterioscler Thromb Vasc Biol. 2007;27:1289–1296. doi: 10.1161/ATVBAHA.107.142778. [DOI] [PubMed] [Google Scholar]
- 26.Kanda S, Mochizuki Y, Suematsu T, Miyata Y, Nomata K, Kanetake H. Sonic hedgehog induces capillary morphogenesis by endothelial cells through phosphoinositide 3-kinase. J Biol Chem. 2003;278:8244–8249. doi: 10.1074/jbc.M210635200. [DOI] [PubMed] [Google Scholar]
- 27.Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci U S A. 2008;105:9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89:1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 29.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bentzon JF, Weile C, Sondergaard CS, Hindkjaer J, Kassem M, Falk E. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:2696–2702. doi: 10.1161/01.ATV.0000247243.48542.9d. [DOI] [PubMed] [Google Scholar]
- 31.Hoofnagle MH, Thomas JA, Wamhoff BR, Owens GK. Origin of neointimal smooth muscle: we’ve come full circle. Arterioscler Thromb Vasc Biol. 2006;26:2579–2581. doi: 10.1161/01.ATV.0000249623.79871.bc. [DOI] [PubMed] [Google Scholar]
- 32.Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashimoto T, Zhang XM, Chen BY, Yang XJ. VEGF activates divergent intracellular signaling components to regulate retinal progenitor cell proliferation and neuronal differentiation. Development. 2006;133:2201–2210. doi: 10.1242/dev.02385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrow D, Scheller A, Birney YA, Sweeney C, Guha S, Cummins PM, Murphy R, Walls D, Redmond EM, Cahill PA. Notch-mediated CBF-1/RBP-J{kappa}-dependent regulation of human vascular smooth muscle cell phenotype in vitro. Am J Physiol Cell Physiol. 2005;289:C1188–1196. doi: 10.1152/ajpcell.00198.2005. [DOI] [PubMed] [Google Scholar]
- 35.Wang WL, Prince CYZ, Hu X, Pollman MJ. HRT1 modulates vascular smooth muscle cell proliferation and apoptosis. Biochemical and Biophysical Research Communications. 2003;308:596–601. doi: 10.1016/s0006-291x(03)01453-0. [DOI] [PubMed] [Google Scholar]
- 36.Anderson LM, Gibbons GH. Notch: a mastermind of vascular morphogenesis. J Clin Invest. 2007;117:299–302. doi: 10.1172/JCI31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollman MJ, Hall JL, Mann MJ, Zhang L, Gibbons GH. Inhibition of neointimal cell bcl-x expression induces apoptosis and regression of vascular disease. Nat Med. 1998;4:222–227. doi: 10.1038/nm0298-222. [DOI] [PubMed] [Google Scholar]
- 38.Kanda S, Mochizuki Y, Miyata Y, Kanetake H. The role of c-Fes in vascular endothelial growth factor-A-mediated signaling by endothelial cells. Biochem Biophys Res Commun. 2003;306:1056–1063. doi: 10.1016/s0006-291x(03)01106-9. [DOI] [PubMed] [Google Scholar]
- 39.Krishnan V, Pereira FA, Qiu Y, Chen CH, Beachy PA, Tsai SY, Tsai MJ. Mediation of Sonic hedgehog-induced expression of COUP-TFII by a protein phosphatase. Science. 1997;278:1947–1950. doi: 10.1126/science.278.5345.1947. [DOI] [PubMed] [Google Scholar]
- 40.Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–1049. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson AM, Wang SJ, Taylor AC, Aitkenhead M, Hughes CC. The basic helix-loop-helix transcription factor HESR1 regulates endothelial cell tube formation. J Biol Chem. 2001;276:6169–6176. doi: 10.1074/jbc.M008506200. [DOI] [PubMed] [Google Scholar]
- 42.Belgore F, Blann A, Neil D, Ahmed AS, Lip GY. Localisation of members of the vascular endothelial growth factor (VEGF) family and their receptors in human atherosclerotic arteries. J Clin Pathol. 2004;57:266–272. doi: 10.1136/jcp.2003.012419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lazarous DF, Shou M, Scheinowitz M, Hodge E, Thirumurti V, Kitsiou AN, Stiber JA, Lobo AD, Hunsberger S, Guetta E, Epstein SE, Unger EF. Comparative effects of basic fibroblast growth factor and vascular endothelial growth factor on coronary collateral development and the arterial response to injury. Circulation. 1996;94:1074–1082. doi: 10.1161/01.cir.94.5.1074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.