Abstract
Background and Aims
Convective gas flow in helophytes (emergent aquatic plants) is thought to be an important adaptation for the ability to colonize deep water. In this study, the maximum depths achieved by seven helophytes were compared in 17 lakes differing in nutrient enrichment, light attenuation, shoreline exposure and sediment characteristics to establish the importance of convective flow for their ability to form the deepest helophyte vegetation in different environments.
Methods
Convective gas flow development was compared amongst the seven species, and species were allocated to ‘flow absent’, ‘low flow’ and ‘high flow’ categories. Regression tree analysis and quantile regression analysis were used to determine the roles of flow category, lake water quality, light attenuation and shoreline exposure on maximum helophyte depths.
Key Results
Two ‘flow absent’ species were restricted to very shallow water in all lakes and their depths were not affected by any environmental parameters. Three ‘low flow’ and two ‘high flow’ species had wide depth ranges, but ‘high flow’ species formed the deepest vegetation far more frequently than ‘low flow’ species. The ‘low flow’ species formed the deepest vegetation most commonly in oligotrophic lakes where oxygen demands in sediments were low, especially on exposed shorelines. The ‘high flow’ species were almost always those forming the deepest vegetation in eutrophic lakes, with Eleocharis sphacelata predominant when light attenuation was low, and Typha orientalis when light attenuation was high. Depths achieved by all five species with convective flow were limited by shoreline exposure, but T. orientalis was the least exposure-sensitive species.
Conclusions
Development of convective flow appears to be essential for dominance of helophyte species in >0·5 m depth, especially under eutrophic conditions. Exposure, sediment characteristics and light attenuation frequently constrain them to a shallower depth than their flow capacity permits.
Key words: Aeration, convective flow, exposure, helophytes, lakes, lakeshore vegetation, light attenuation, redox, regression tree, sediment motion, trophic state, waves
INTRODUCTION
Convective gas flow is the generation of bulk internal gas movement in plants due to humidity- and temperature-induced pressurization of shoot aerenchyma (Armstrong and Armstrong, 1990; Afreen et al., 2007). Initially documented in water lilies (Dacey, 1980), it is now recognized as a widespread flooding tolerance adaptation in both floating-leaved and helophyte species (Brix et al., 1992; Grosse, 1996; Colmer, 2003). Internal oxygen fluxes can be orders of magnitude greater when convective gas flow is operating than when diffusion is the sole mechanism of gas transport (Armstrong et al., 1996). This leads to higher internal oxygen concentrations and rates of root oxygen release (Armstrong and Armstrong, 1990), greater rhizosphere oxidation (Armstrong et al., 1992, 1996; Aldridge and Ganf, 2003) and less development of tissue anaerobiosis (Rolletschek et al., 1999).
Most studies of convective gas flow have focused on the physiology of flow generation, the gas fluxes achieved and anoxia avoidance (Armstrong and Armstrong, 1990; Bendix et al., 1994; Afreen et al., 2007). Although the very high oxygen fluxes delivered by convective flow confer ecological advantages for below-ground productivity and depth penetration in growth experiments (White and Ganf, 1998; Vretare and Weisner, 2000), very few studies have attempted to probe its ecological significance for water depth penetration in the field. In Eleocharis sphacelata, convective flow in the day-time flushes stagnant oxygen-depleted gas that accumulates internally at night, and the time required for flushing increases with depth (Sorrell and Tanner, 2000). In the only study so far comparing species distributions in situ, Vretare Strand (2002) found that species with convective flow generally occurred more deeply in lakes than species without, and inferred that species with convective flow probably do have a competitive advantage in deeper water than species lacking flow.
A major obstacle in determining the importance of gas transport mechanisms for depth penetration is that maximum depths colonized by helophytes are dependent on a number of factors that may be either independent of, or interact with, gas flow development. Other factors affecting the maximum depths of helophytes in lakes are competition, wave exposure, light attenuation and sediment characteristics (Keddy, 1983; Coops et al., 1999). Competition is most important in shallow water, with species diversity decreasing with depth as less flood-tolerant taxa are excluded by their lack of aquatic specialization (Keddy, 1983). The deepest-growing emergent vegetation therefore rarely comprises more than one or two highly flood-tolerant species (Weisner, 1991), which are usually clonal helophytes. Their depth penetration and zonation have most often been explained by abiotic influences such as sediment texture and mechanical resistance to wave action (Coops et al., 1994), water level fluctuations (Blanch et al., 2000) or oxygen stress imposed by anaerobic substrates (Weisner, 1991), but competition is also occasionally implicated (Grace, 1988).
For these reasons, it is likely that convective flow and internal tissue aeration would define the maximum depth limit of a species when not confounded by these other factors. This would be expected on sheltered shores under oligotrophic conditions, where there would be little wave exposure, low competitive intensity, low light attenuation in the water column, and low organic matter accumulation and hence low oxygen demand in the sediment. Elsewhere, relationships between convective flow development and maximum depth of colonization are likely to be complex, not least because all these other factors interact to some extent. Shoreline exposure has direct mechanical effects on plants due to wave damage and uprooting, but also disturbs sediments, affecting organic matter accumulation and oxygen demand (Keddy, 1983; Coops et al., 1991). Light attenuation is likely to be stronger in more eutrophic lakes (Van Duin et al., 2001), which are also likely to have more reducing sediments (den Heyer and Kalff, 1998).
We hypothesize therefore that the degree of convective flow development defines the potential maximum depth of helophytes, allowing the greatest depth colonization in sheltered, oligotrophic sites with low sediment oxygen demands. As exposure, light attenuation and sediment oxygen demand increase, it is predicted that these will become increasingly important in restricting depth of colonization.
To test our hypothesis, the maximum depths achieved by the deepest-growing helophytes were investigated in 17 lakes differing in size and trophic state throughout New Zealand. Then maximum depth of colonization was related to convective flow development, shoreline exposure and sediment characteristics. The following questions were asked: (a) do the species forming the deepest helophyte vegetation differ in their convective flow development; (b) if so, do species differing in convective flow development have different maximum depths in lakes; and (c) how are these maximum depths affected by lake water quality, shoreline exposure and sediment characteristics?
MATERIALS AND METHODS
Comparison and classification of gas flow capacities
The internal pressurization and gas flow capacities of the seven helophyte species found on the lake shores (Apodasmia similis, Baumea arthrophylla, B. articulata, Eleocharis acuta, E. sphacelata, Schoenoplectus tabernaemontani, Typha orientalis) were compared using plants in outdoor cultures (five replicate plants of each species). These were vegetatively propagated from rhizome tips planted in commercially available topsoil in polythene-lined boxes (0·3 × 0·5 m, 0·5 m height), and fertilized (Yates Magamp and Thrive, Yates NZ Ltd) to a final soil concentration of N = 2·3 g kg−1 and P = 0·9 g kg−1. The boxes were permanently flooded to the soil surface in concrete raceways with a continuous through-flow of local river water throughout the growth period and during measurements. Pressures and flows were measured after 4 weeks, once plants had produced approx. five culms or leaves, and hence were large enough to allow through-flow convection to occur.
As the aim of this experiment was to compare convective flow capacity between species as a species trait, an effort was made to minimize any mechanical damage which could affect development of through-flow convection in whole plants, and to maximize sensitivity of readings from whole plants. Hence, for the cylindrical-stemmed species, pressures and flows were measured on the oldest shoot by excising senescent tissue and thereby ensuring it was an efflux shoot whilst the other shoots remained intact and were influx shoots. These efflux shoots were connected to the gas flow instrumentation described by Sorrell and Tanner (2000), allowing continuous recording of pressures and flows. For T. orientalis, plants had produced only one leafy shoot at this stage and this was connected to the instruments via the rhizome base. Readings were made over 4 d and logged, along with ambient humidity and air temperature, for 30-min periods for each replicate plant. Comparisons of pressures and flows were made at similar high vapour pressure deficits (1300–1500 Pa) to ensure comparability under conditions where high pressurization and flow would be expected. Although internal pressurization and flow have previously been compared in some of these species (Brix et al., 1992), the present comparison allowed all seven species to be classified based on data recorded under similar conditions for all species. From pressure and flow data, the species were classified into the ‘flow absent’, ‘low flow’ and ‘high flow’ gas flow categories previously described by Brix et al. (1992) and Vretare Strand (2002).
Field studies
The 17 lakes chosen for field investigations provided a range of sizes, morphologies and environmental characteristics (Table 1). They are situated between 34°57's and 46°39's and all have largely natural water level fluctuations, without major human regulation. Differences in shoreline development and size allowed plant depths to be measured on shoreline points that varied widely in exposure. The distribution of helophytes on shorelines was mapped during the austral summer of 2003–2004, recording the maximum depth penetration as the water depth adjacent to the lakeward edge of the vegetation (Weisner, 1991) at each of 3–17 points per lake, depending on lake size and extent of vegetation development, giving 135 depth records in total. All depth records included the species forming the outer fringe and were fixed by GPS for later exposure modelling.
Table 1.
Location, morphological parameters, water quality, and number of helophyte depth points recorded for the 17 New Zealand lakes in the study (listed in latitudinal order from north to south)
| Lake | Latitude | Longitude | Area (km2) | Shoreline development (DL)* | Maximum fetch (km) | Kd (m−1) | TLI value and lake type† | No. of depth points |
|---|---|---|---|---|---|---|---|---|
| Waiparera | 34°56·6′ | 173°10·9′ | 1·15 | 1·25 | 1·45 | 0·82 | 4·45 (E) | 13 |
| Ngatu | 35°1·8′ | 173°11·9′ | 0·61 | 1·49 | 1·32 | 0·59 | 3·26 (M) | 12 |
| Waikere | 35°47·9′ | 173°37·8′ | 0·35 | 1·69 | 0·83 | 0·38 | 2·52 (O) | 13 |
| Taharoa | 35°48·4′ | 173°38·8′ | 2·1 | 1·54 | 2·21 | 0·19 | 1·45 (Mc) | 11 |
| Okareka | 38°10·3′ | 176°21·7′ | 3·46 | 1·62 | 2·79 | 0·39 | 3·44 (M) | 17 |
| Tikitapu | 38°11·7′ | 176°19·8′ | 1·4 | 1·19 | 1·70 | 0·15 | 3·15 (M) | 12 |
| Rerewhakaaitu | 38°17·7′ | 176°30·1′ | 7·47 | 2·76 | 3·65 | 0·54 | 3·48 (M) | 6 |
| Okaro | 38°18·0′ | 176°23·6′ | 0·28 | 1·11 | 0·71 | 1·30 | 6·03 (H) | 6 |
| Ngahewa | 38°18·9′ | 176°22·4′ | 0·11 | 1·38 | 0·51 | 1·52 | 5·26 (S) | 18 |
| Lady | 42°35·9′ | 171°34·3′ | 1·8 | 1·51 | 1·94 | 1·53 | 3·33 (D) | 13 |
| Poerua | 42°42·4′ | 171°29·5′ | 2·15 | 2·32 | 2·80 | 1·06 | 3·08 (M) | 5 |
| Mahinapua | 42°47·6′ | 170°55·0′ | 3·4 | 1·78 | 3·17 | 1·73 | 3·36 (D) | 17 |
| Ianthe | 43°3·3′ | 170°37·3′ | 4·42 | 1·34 | 3·48 | 0·52 | 2·83 (O) | 19 |
| Waihola | 46°1·4′ | 170°3·8′ | 6·1 | 2·41 | 5·70 | 3·87 | 4·33 (E) | 9 |
| George | 46°21·4′ | 167°51·4′ | 1·5 | 1·10 | 1·30 | 1·91 | 4·31 (E) | 3 |
| Wilkie | 46°34·8′ | 169°26·3′ | 0·01 | 1·35 | 0·17 | 3·87 | 4·43 (D) | 4 |
| Vincent | 46°35·5′ | 168°49·0′ | 0·17 | 3·13 | 1·01 | 1·57 | 3·50 (M) | 12 |
* Shoreline development calculated as DL = L/2√(πA) where L = shoreline length and A = lake area.
† TLI (trophic level index) values calculated and lakes classified following Burns et al. (1999). D = dystrophic, Mc = microtrophic, O = oligotrophic, M = mesotrophic, E = eutrophic, S = supertrophic, H = hypertrophic.
Lake trophic levels were calculated numerically as the trophic level index (TLI), a composite index based on total nitrogen (TN), total phosphorus (TP), Secchi depth and chlorophyll a data for each lake (Burns et al., 1999), using water quality data from local regulatory authorities. In this categorization, low numbers indicate oligotrophy and high numbers increasing eutrophy. The attenuation coefficient for downwelling irradiance (Kd) at each lake was measured with a Li-Cor cosine-corrected underwater PAR (photosynthetically available radiation) sensor and calculated by log-linear regression of depth versus downwelling irradiance. TLI and Kd are only weakly correlated in Table 1 (r = 0·49, P = 0·07), due to the high suspended sediment and dissolved organic matter concentrations in many lakes. Hence, TLI and Kd were treated as separate factors in the data analysis.
Sediment samples (100 cm3) from the superficial 0–0·2 m layer of the rooting depth were collected by SCUBA divers from up to five sites in each lake, representing the range from exposed to sheltered sites immediately outside the deepest emergent vegetation. The samples were sealed in air-tight containers, kept in an insulated box at 4 °C, and redox potential measured within 12 h with a platinum wire electrode against a Ag/AgCl reference electrode, with the reference potential of +199 mV added for correction to electrical redox potential (Eh) values. Subsamples were then dried at 105 °C, weighed and analysed for loss on ignition (LOI) as loss of dry mass on combustion at 400 °C. Further subsamples were analysed for TP and TN by standard methods (Viner, 1989) and for particle size distribution on a CIS-100 particle size analyser (Galai WCIS 100, Galai Production Ltd, Israel), after removing macrophyte detrital material. Apart from this material, all samples had particle sizes <2·5 mm and lacked gravel, boulders and cobbles. The median particle size for each sample was used in subsequent data analyses. It was not possible for logistical reasons to collect sediment samples from all 135 depth points in the study.
Characterization of exposure
The maximum depth of sediment motion (DSM) was estimated at each depth record location with a computer-generated wave hindcast model based on lake shape, bathymetry and local wind records (NARFET wave generator model; Smith, 1991). DSM from the NARFET model was chosen as the most relevant wave exposure variable, as erosion and uprooting are likely to be the depth-limiting processes for rhizomatous helophytes, but DSM was in any case strongly correlated with other parameters such as wave height and amplitude.
Data analysis
Analysis of variance (ANOVA) was used to identify differences in internal pressurization and gas flow rates for classification of species into gas flow categories. Data were log-transformed (log10 n + 1) in order to satisfy assumptions of heterogeneity of variances according to Cochran's C test. Gas flow categories were allocated based on post-hoc Tukey identification of groups of means.
The importance of flow category, lake trophic level, light attenuation and shoreline exposure in controlling depths were then explored with regression tree analysis (De'ath and Fabricius, 2000). Regression tree analysis is a particularly robust method where there are nonlinear relationships, combinations of categorical and continuous independent variables, unequal sample sizes, and high-order interactions, all of which were features of the data. The regression tree model was selected by cross-validation, by running a series of 50 10-fold cross validations and then choosing the smallest tree with an estimated error rate within one standard error of the minimum (Breiman et al., 1984). Maximum plant depth was the dependent variable and the independent variables were flow category, lake trophic level (TLI), Kd and DSM. ANOVA and post hoc Tukey tests on log-transformed data were then used to compare sediment characteristics between the environmental classes produced in the regression tree analysis.
The relationships between colonization depth and exposure were tested either by linear regression or by calculating Kendall's T, the rank correlation coefficient, depending on which gave the greatest statistical significance. Maximum possible depths versus exposure were further examined using quantile regression analysis, which delineates maximal responses to a given variable in data where multiple limiting variables are in operation (Cade et al., 1999; Cade and Guo, 2000).
RESULTS
Gas flow categories of the deepest-growing helophytes and maximum depths
Gas flow capacity clearly discriminated three gas flow groups amongst the seven species that formed the deepest vegetation in the lakes (Table 2). Apodasmia similis and E. acuta had no detectable internal pressurization or flow, and hence were allocated to the ‘flow absent’ category. The ‘low flow’ category, characterized by static pressures that were significantly greater than zero but <200 Pa, efflux flow rates <1·0 cm3 min−1 shoot−1 and area-normalized flow rates <0·5 cm3 m−2 s−1, included the two Baumea species and S. tabernaemontani. Typha orientalis and E. sphacelata generated high pressures and flow rates per shoot and per unit area; these were allocated to the ‘high flow’ category.
Table 2.
Mean (±s.d., n = 5 plants per species) of static efflux pressures and gas flow rate from efflux shoots for the seven species
| Species | Influx shoot area (m2) | Static pressure (Pa) | Efflux flow (cm3 min−1) | Flow per influx shoot area (cm3 m−2 s−1) | Flow category |
|---|---|---|---|---|---|
| A. similis | n.d. | <10a | <0·01a | – | Absent |
| E. acuta | n.d. | <10a | <0·01a | – | Absent |
| B. arthrophylla | 0·047 ± 0·006 | 29 ± 9b | 0·24 ± 0·11b | 0·09 ± 0·01a | Low |
| B. articulata | 0·038 ± 0·009 | 51 ± 19c | 0·31 ± 0·13b | 0·14 ± 0·04a | Low |
| S. tabernaemontani | 0·063 ± 0·010 | 53 ± 8c | 0·64 ± 0·16c | 0·17 ± 0·03a | Low |
| E. sphacelata | 0·022 ± 0·006 | 135 ± 28d | 5·35 ± 1·43d | 4·05 ± 0·10b | High |
| T. orientalis | 0·020 ± 0·009 | 305 ± 58e | 5·55 ± 0·96d | 4·60 ± 0·27b | High |
Flow categories (absent, low and high) classified as in Brix et al. (1992) and Vretare Strand (2002). All measurements made at vapour pressure deficits of 1300–1500 Pa (23–27 °C, 52–58 %RH), n.d. = not determined.
One-way ANOVA identified significant differences in static pressure (F = 820, P < 0·001), efflux flow (F = 2627, P < 0·001) and area-normalized flow (F = 305, P < 0·001) between species. Superscript letters within columns identify significantly different means.
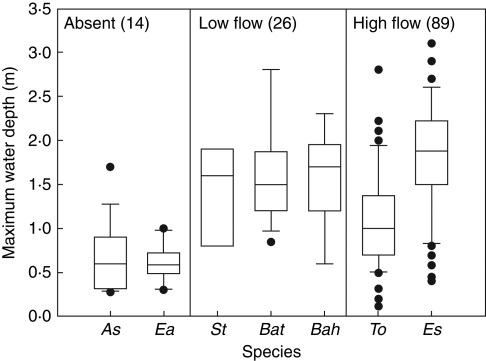
The range of water depths achieved in the field by the seven species is shown in Fig. 1. In all cases, the deepest points were found on extensive bands of monospecific, clonal growth of single species. The two species without flow were generally restricted to <1·0 m depth, whereas there was a much greater range of water depths achieved by the other five species. Maximum water depth data for the three ‘low flow’ species all had medians approx. 1·5 m and similar ranges, although B. articulata did occur more deeply than the other two species. The ‘high flow’ species E. sphacelata was the deepest-growing of the seven species, but its maximum water depth was highly variable and shallow populations of this species were common. The other high-flow species, T. orientalis, had the greatest depth range of all the species, occasionally extending as deep as 1·8 m, but in general its depth limits were considerably less than would be expected from its gas flow capacity. Of the total 135 depth records, 12 % were from ‘flow absent’ species, 19 % from ‘low flow’ species, and the great majority from the two ‘high flow’ species (33 % T. orientalis, 36 % E. sphacelata).
Fig. 1.
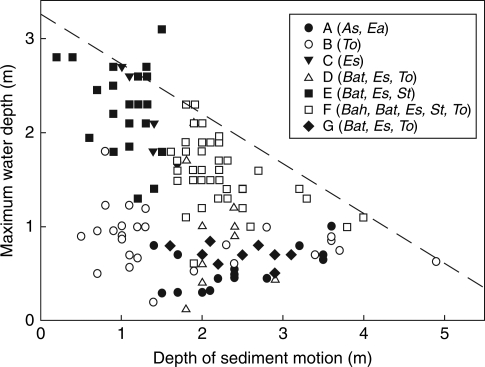
Maximum water depths colonized by helophytes in the three gas flow categories (flow absent, ‘low flow’ and ‘high flow’) in 17 New Zealand lakes. Box and whisker plots show median values with quartiles (boxes), 95 % ranges (whiskers) and >95 % outliers (dots). Numbers in parenthesis are the numbers of records in each category. Species abbreviations: As = Apodasmia similis, Ea = Eleocharis acuta, St = Schoenoplectus tabernaemontani, Bat = Baumea articulata, Bah = Baumea arthrophylla, To = Typha orientalis, Es = Eleocharis sphacelata.
Physiological and environmental controls of maximum depths: regression tree analysis
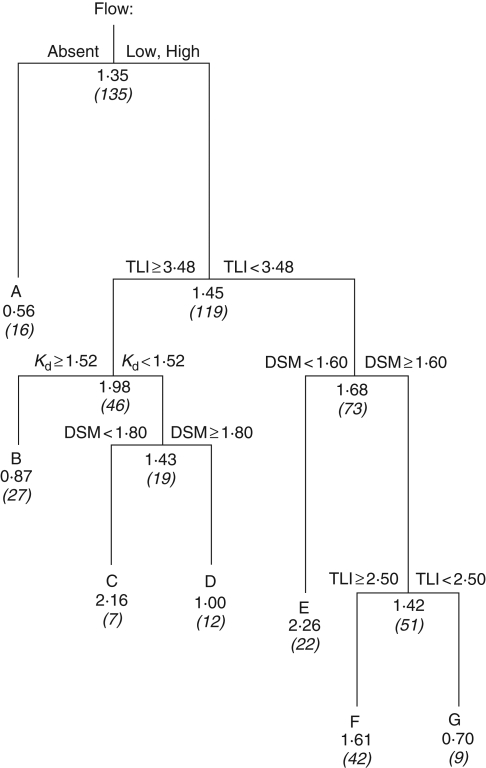
Regression tree analysis relating the maximum water depths achieved by the helophytes to the gas flow capacity and environmental parameters is shown in Fig. 2. Cross-validation using the 1-s.e. rule selected a seven-leaf tree, with the selected tree explaining 72·2 % of the total sum of squares. Gas flow category, TLI, Kd and DSM were all selected as significant drivers of maximum depths. The seven classes delineate significantly different groups of species-depth records defined by particular combinations of gas flow capacity and environmental conditions. These are described in Table 3.
Fig. 2.
Regression tree explaining maximum depths of the helophyte vegetation in terms of gas flow category (absent, ‘low flow’, ‘high flow’), trophic level index (TLI) of the lakes, light attenuation (Kd, m−1) and exposure (DSM, m). The regression has selected a seven-leaf tree by the 1-s.e. cross-validation rule. Each of the six splits is labelled with the variable and its values that determine the split. Each node is labelled with the mean depth value and the number of observations in each class (italic, in parenthesis). The tree explained 72·2 % of the total sum of squares, and the vertical length of each split is proportional to the variance explained. The seven classes (A–G) formed at the nodes are groups of depth records that differ significantly in plant gas flow development and environmental depth limitation, and are described in Table 3.
Table 3.
Least-squares means (± s.e.) of the maximum helophyte depths achieved in the seven classes generated by the regression tree analysis (see Fig. 2), with descriptions of the classes and their constituent species
| Class | Max depth | Class description | Species |
|---|---|---|---|
| A | 0·56 ± 0·04 | Flow absent species | All As and Ea records |
| B | 0·87 ± 0·06 | Eutrophic, very low water clarity | Exclusively To |
| C | 2·16 ± 0·14 | Eutrophic, clearer water, sheltered | Exclusively Es |
| D | 1·00 ± 0·22 | Eutrophic, clearer water, exposed | Bat, Es, To |
| E | 2·26 ± 0·10 | Oligotrophic, sheltered | Bat, Es, St |
| F | 1·61 ± 0·15 | Oligotrophic, exposed | All five species with flow |
| G | 0·70 ± 0·05 | Microtrophic Lake Taharoa | Bat, Es, To |
Species codes: As = Apodasmia similis, Bat = Baumea articulata, Ea = Eleocharis acuta, Es = Eleocharis sphacelata, St = Schoenoplectus tabernaemontani, To = Typha orientalis.
The two ‘flow absent’ species were restricted to shallow depths irrespective of environmental conditions (Class A). In the more eutrophic lakes (TLI > 3·48), the deepest vegetation was restricted to very shallow depths when light attenuation was high (Kd >1·52, Class B), even though these were almost all sheltered sites (DSM ranging from 0·2 m to 1·8 m). In eutrophic lakes with clearer water, vegetation was able to grow deeply in sheltered sites (Class C), but was much shallower in exposed sites (Class D). Exposure was an even more important factor in the more oligotrophic lakes (TLI < 3·48), allowing very deep vegetation in sheltered sites (Class E) and moderately deep vegetation in exposed sites (Class G). Another small, very shallow class (Class F) comprised all the records from the most nutrient-poor lake, microtrophic Lake Taharoa. The regression tree analysis demonstrates that light attenuation was an important factor limiting depths in the more eutrophic lakes, but not in the more oligotrophic lakes, and that sheltered and exposed sites both supported deeper vegetation in oligotrophic lakes than in eutrophic lakes.
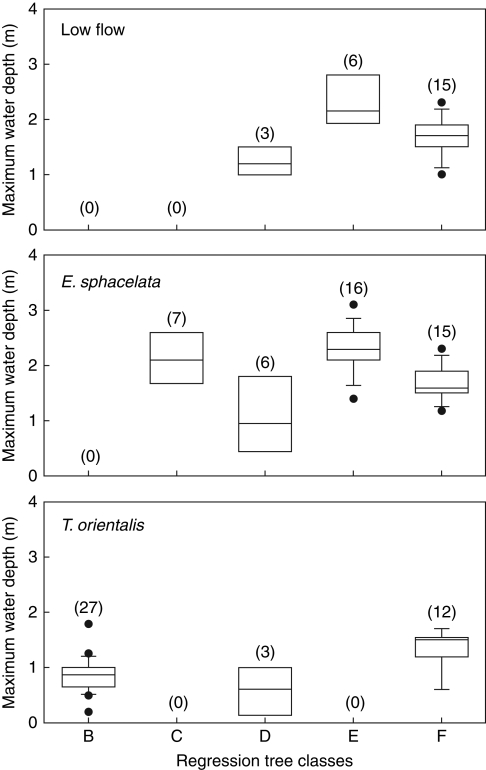
Species differing in gas flow capacity clearly differed in frequency and depth within these classes. Table 3 and Fig. 3 show that the ‘low flow’ species rarely ever formed the deepest vegetation in the more eutrophic lakes. Only B. articulata did so, but only when water clarity was high, and it was always shallow in eutrophic conditions (Class D in Fig. 3). However, all three ‘low flow’ species were frequently the deepest species in oligotrophic lakes, and they were disproportionately common in the oligotrophic exposed Class G (58 % of all ‘low flow’ records in the data set occur in Class G).
Fig. 3.
Comparison of maximum water depths of the ‘low-flow’ species (pooled data for B. articulata, B. arthrophylla, S. tabernaemontani), E. sphacelata and T. orientalis in the five main regression tree classes (see Table 3 for class descriptions). Box and whisker plots show median values with quartiles (boxes), 95 % ranges (whiskers) and >95 % outliers (dots). Numbers in parenthesis refer to numbers of records in each category.
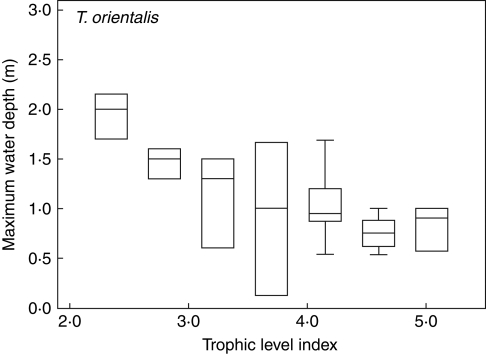
Typha orientalis, in contrast, was the only species in the eutrophic, low-clarity Class B, despite having a very shallow depth range in this class (Table 3). Its maximum depths decreased with lake trophic level (Fig. 4). Note that the median values of the depth records decrease curvilinearily with TLI in Fig. 4, and that the maximum depths are always deep in oligotrophic lakes, highly variable in mesotrophic lakes, but constrained to shallow depths in eutrophic lakes. This species never formed the deepest vegetation in the oligotrophic, sheltered Class E (Fig. 3), and its deep records in Fig. 4 were from relatively exposed oligotrophic sites (DSM = 1·7–3·7 m).
Fig. 4.
Ranges of maximum depth values for T. orientalis in lakes differing in trophic level. Box and whisker plots show median values with quartiles (boxes) and 95 % ranges (whiskers). Data shown for all lakes with more than two T. orientalis observations; microtrophic Lake Taharoa is excluded.
Eleocharis sphacelata, while never forming the deepest vegetation in eutrophic lakes with low water clarity, was the most common species to do so in eutrophic lakes with higher water clarity, and the only species to do so in sheltered sites in these lakes (Table 3, Class C). It was also very common as the deepest species in oligotropic lakes, irrespective of exposure, despite being restricted to shallower water in more exposed sites (Class E cf. Class G in Fig. 3).
Effect of exposure on maximum depths
The relationship between DSM and maximum depth penetration for all species is shown in Fig. 5. Depth penetration was always restricted at exposed sites, whereas a wide range of depths was recorded at sheltered sites. Despite this, there was a highly significant negative correlation between maximum depths and DSM (T = –0·293, P < 0·01), and particularly when modelled with quantile regression analysis (Table 4). The 95 % quantile regression line is the change in depth of the 95th percentage quantile of the maximum depth data versus DSM, and indexes the greatest depth at a given exposure. It was highly significant (P < 0·01) with a depth vs exposure slope of –0·53 m (depth) m−1 (DSM), and a maximum potential depth limit for the helophyte vegetation of 3·26 m when DSM = 0. The scatter of data in Fig. 5 shows that helophyte vegetation in these lakes frequently fails to reach the maximum depth possible at any given degree of exposure, because of the other effects identified with the regression tree analysis.
Fig. 5.
Maximum water depths of vegetation in the seven regression tree classes in relation to depth of sediment motion calculated from the NARFET wave model in 17 New Zealand lakes. The dashed line fitted by 95 % quantile regression indicates the potential depth of emergent vegetation versus exposure (vegetation depth = 3·26 – 0·53DSM, P < 0·01). Classes A–G are as described in Table 3, with constituent species in parenthesis; the species abbreviations are as in Fig. 1.
Table 4.
Quantile regression analysis for maximum water depth penetration relative to depth of sediment motion for all data in Fig. 5 and for T. orientalis alone, including estimates of intercept and slope and P-values testing for HO: slope = 0
| Quantile | Intercept | Slope | 90 % CI for slope | P |
|---|---|---|---|---|
| All data | ||||
| 25th | 0·63 | −0·05 | −0·22 – 0·04 | 0·18 |
| 50th | 2·18 | −0·38 | −0·60 – –0·28 | 0·06 |
| 75th | 2·80 | −0·50 | −0·56 – –0·41 | 0·05 |
| 90th | 3·21 | −0·56 | −0·60 – –0·47 | 0·04 |
| 95th | 3·26 | −0·53 | −0·60 – –0·51 | 0·01 |
| T. orientalis only | ||||
| 25th | 0·47 | 0·03 | −0·31 – 0·07 | 0·35 |
| 50th | 1·03 | −0·04 | −0·10 – –0·02 | 0·19 |
| 75th | 1·28 | −0·04 | −0·22 – –0·29 | 0·12 |
| 90th | 1·98 | −0·22 | −0·31 – –0·50 | 0·06 |
| 95th | 1·91 | −0·14 | −0·30 – –0·55 | 0·05 |
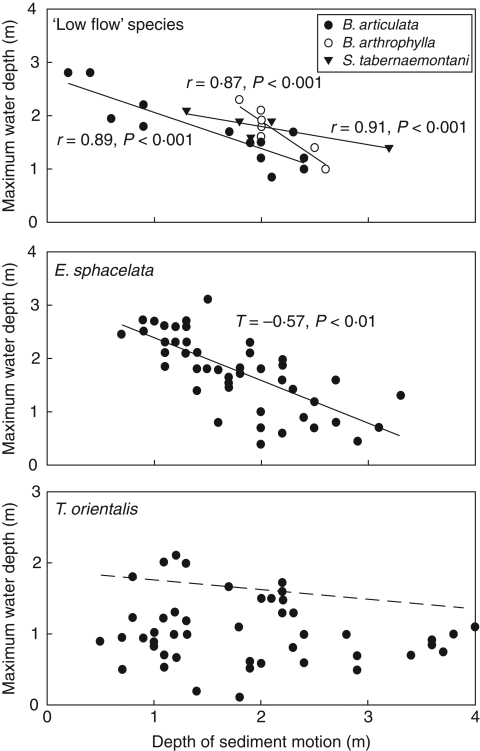
Interpretation of Fig. 5 in terms of the regression tree analysis highlights the limited depth penetration (<1·0 m) of the two flow-absent species (Class A) and plants in Lake Taharoa (Class G) irrespective of exposure, and the restriction of vegetation to shallow depths in the more eutrophic classes (especially Class B). The species also differ in their responses to exposure (Fig. 6). There were highly significant relationships between DSM and maximum depths for the three ‘low flow’ species and E. sphacelata, but not for T. orientalis. However, the 95 % quantile regression slope was significant for depth versus exposure in T. orientalis (Table 4), consistent with the finding of the regression tree analysis that trophic state and light attenuation frequently restricted this species to shallower water than would be expected from DSM alone.
Fig. 6.
The relationship between water depth penetration of helophytes and depth of sediment motion calculated from the NARFET wave model, for the low-flow species and E. sphacelata and T. orientalis. Linear regressions are shown for the low-flow species. Kendall's T (rank correlation co-efficient) is shown for E. sphacelata. There was no significant linear correlation for T. orientalis (T = –0·03, P = 0·34, n.s); the dashed line is the significant 95 % quantile regression formula, which is depth = 1·91 – 0·14DSM (P < 0·005).
Sediment characteristics
Table 5 compares sediment characteristics between sites from the six environmental classes supporting the ‘low flow’ and ‘high flow’ species. Microtrophic Lake Taharoa was characterized by a highly oxidizing mineral substrate with very low organic matter content, high Eh and very low nutrient concentrations, with nitrogen content below the level of detection. Amongst the other classes, several generalities were evident. Sheltered sites had higher organic matter and nitrogen content, smaller particle sizes, lower total solids, and lower Eh than exposed sites. For any given exposure, particle sizes were significantly larger in the oligotrophic classes than the eutrophic classes. In sheltered sites, Eh was significantly lower and organic matter and TN content higher in eutrophic sites than in oligotrophic ones. In contrast, there was no significant difference in these three parameters between oligotrophic and eutrophic exposed sites. Phosphorus concentrations were highly variable and showed few consistent patterns related to either exposure or trophic state.
Table 5.
Sediment characteristics from sites in all six regression tree classes supporting ‘low flow’ and ‘high flow’ species
| Class B | Class C | Class D | Class E | Class F | Class G | ||
|---|---|---|---|---|---|---|---|
| Eutrophic, Kd ≥ 1·52 m−1 | Eutrophic, Kd < 1·52 m−1 | Eutrophic, Kd < 1·52 m−1 | Oligotrophic | Oligotropic | Lake Taharoa | ||
| Parameter | Sheltered | Exposed | Sheltered | Exposed | F (P) | ||
| n | 10 | 4 | 6 | 10 | 17 | 3 | |
| LOI ( %) | 14·8 ± 3·8a | 16·3 ± 13·2b | 2·9 ± 2·1a | 20·0 ± 13·9b | 3·9 ± 3·1a | 0·5 ± 0·0c | 13·4 (<0·001) |
| TS ( %) | 4·8 ± 18·3a | 5·8 ± 0·7b | 63·1 ± 5·9a | 28·5 ± 18·4c | 56·1 ± 17·4a | 79·0 ± 4a | 7·4 (<0·005) |
| TN ( %) | 0·65 ± 0·14a | 0·60 ± 0·4a | 0·11 ± 0·10b | 0·69 ± 0·47b | 0·16 ± 0·15b | <0·02c | 11·9 (<0·001) |
| TP (mg kg−1) | 495 ± 355a | 405 ± 487a | 195 ± 110b | 757 ± 501a | 376 ± 489a | 71 ± 47c | 7·1 (<0·005) |
| Particle size (μm) | 245 ± 205a | 203 ± 172a | 399 ± 358b | 391 ± 171b | 551 ± 315c | 348 ± 15b | 12·3 (<0·01) |
| Eh (mV) | –75 ± 44a | –57 ± 45a | 67 ± 44b | 88 ± 45b | 187 ± 34d | 220 ± 17c | 16·6 (<0·001) |
LOI = loss on ignition, TS = total solids, TN = total nitrogen, TP = total phosphorus, Eh = redox potential. Parameters with different superscript letters (within rows) differ significantly according to post hoc least-squares tests.
* ANOVA, d.f. = 5.
DISCUSSION
This study confirms that environmental variables interact with the physiological depth tolerance conferred by convective flow development to set the maximum depths of helophytes. The inability of species lacking convective flow to penetrate beyond approx. 1 m water depth irrespective of environmental conditions was the first factor to be recognized in the analysis of this multi-site data set. However, a clear competitive advantage for species capable of generating high convective flow rates over those capable of lower flow rates was less evident from depth ranges alone. The ‘low flow’ species B. articulata had a deeper range than the ‘high flow’ species T. orientalis, although less deep than the ‘high flow’ E. sphacelata. All five of the species with some convective flow had wide ranges in maximum depth, regardless of convection capacity, as factors other than aeration played major roles in determining maximum depths at specific sites. At the outset we hypothesized that unfavourable exposure, light attenuation and sediment oxygen demand could prevent plants reaching their physiological depth potential, and the regression tree analysis confirmed that all of these were important, and explained most of the variation in the data set.
The similarity of depth ranges occupied by ‘low flow’ and ‘high flow’ species is consistent with mathematical modelling and experimental data on the relationship between flow rate and internal oxygen concentration, which is hyperbolic (Armstrong et al., 1992; Sorrell et al., 1997). Hence, even a small amount of convection greatly raises internal oxygen concentrations compared with that sustained by diffusion alone when gas transport is occurring over long distances. Vretare Strand (2002) also found that species with low or even no flow could grow as deeply as ‘high flow’ species in her study of species distributions along transects, although ‘high flow’ species had higher internal oxygen concentrations than the others. Both her study and the present one suggest that high rates of convective flow are not essential for colonizing deep water under conditions that are benign for aeration, but that the greater fluxes of oxygen delivered in ‘high flow’ species become important when additional factors apart from depth are lowering internal oxygen concentrations.
The competitive advantage conferred by high flow rates is evident, therefore, in the two ‘high flow’ species forming the deepest vegetation considerably more often than the ‘low flow’ species, and in more anaerobic habitats. In particular, they formed the deepest vegetation almost exclusively in the more eutrophic lakes, T. orientalis when light attenuation was high and E. sphacelata when light attenuation was low. Eutrophic conditions are associated with increased oxygen demands in sediments due to high decomposition rates (den Heyer and Kalff, 1998; Table 5 of the present study) and this is known to cause anaerobic stress in helophytes (Lenssen et al., 1999). In these conditions, the high rates of oxygen supply delivered in ‘high-flow’ species become more critical for growth and survival. The importance of high flow rates in the deep-growing plants is also evident from morphological adjustments such as wider stems and increased lacunal area that decrease internal resistances and increase flow in both E. sphacelata and Typha domingensis as depth increases (Sorrell and Tanner, 2000; White et al., 2007).
Light attenuation may affect helophytes by reducing photosynthesis in the underwater tissues, which is important for maintaining internal oxygen concentrations and carbon balances via re-fixing of respiratory carbon dioxide (Singer et al., 1994). Eleocharis sphacelata has numerous cortical and epidermal chloroplasts in its underwater tissue (Sorrell et al., 1997). Its failure ever to form the deepest vegetation in eutrophic lakes when Kd was high despite commonly doing so when Kd was low suggests that its underwater photosynthesis is an important component of its aeration, along with its convection. Provided there was sufficient underwater light for it to exploit, it always out-competed T. orientalis in deep eutrophic water. Eleocharis sphacelata appears also to be the species with the broadest tolerance, forming the deepest vegetation in all environments except eutrophic lakes with low water clarity.
The most flood-tolerant of the seven species is therefore T. orientalis, given its persistence in the most inimical habitats from which even E. sphacelata was excluded. The many shallow records for this species reflect its ability to persevere in the most turbid eutrophic waters where deep growth is never possible, and in which no other species can achieve even the modest depths it colonizes. Low maximum depths for this species are therefore best interpreted as an ability to cope with extremely high oxygen demands in sediment and water and light attenuation that is lacking in other species. Its greater anoxia tolerance than the other species can be linked to the high rates of flow, oxygen flux into roots, and root oxygen release rates in Typha species (Matsui and Tsuchiya, 2006), and possibly also to photosynthesis in the underwater tissues being sustained by leaf gas films (Colmer and Pedersen, 2008). Like most Typha species, T. orientalis is a vigorous competitive species favoured under high nutrient regimes, and nutrient enrichment often occurs in association with anaerobic conditions that are generally tolerated well, albeit to differing degrees, by different Typha species (Grace, 1988; Tornbjerg et al., 1994; Zedler and Kercher, 2004).
The prevalence of the ‘low flow’ species on exposed shorelines of oligotrophic lakes is consistent with the low oxygen stress experienced at these sites. Wave action on exposed shorelines removes fine organic matter from sediments, as seen in larger particle sizes and lower organic matter content (Keddy, 1983; Coops et al., 1994). The present sediment data show how redox potentials were higher in exposed sites than sheltered sites, and helophytes are known to be able to grow more deeply in water when substrates have higher redox potentials (Weisner, 1991; Vretare Strand and Weisner, 2002). Hence, although exposure reduced the depth colonization of all five species with convective flow, it improved conditions for ‘low flow’ species because of greater sediment aeration. This resulted in the exposed oligotrophic sites having the highest diversity of helophyte species (all five species with convective flow). For a given level of exposure, redox potentials were also higher in oligotrophic than eutrophic lakes, most likely because organic matter accumulation in eutrophic lakes leads to higher oxygen demands and more reducing conditions than the more mineral sediments in oligotrophic lakes (Lenssen et al., 1999). More oligotrophic conditions clearly favour deeper helophyte growth, unless a lake is so infertile that nutrient availability limits growth and depth colonization (e.g. Lake Taharoa).
Exposure to wave action emerged as an important variable, with depth of extension greatest in sheltered waters, regardless of trophic state. Wave action affected every species, but in different ways. Sheltered conditions favoured the cylindrical-stemmed Cyperaceae, especially E. sphacelata and Baumea species, which gave way to the broad-leafed T. orientalis as the plant most likely to form the deepest vegetation as wave energy increased. Cylindrical Cyperaceae have previously been shown to be susceptible to high wave energy (Coops et al., 1994), and E. sphacelata has particularly porous, soft stems that are likely to be vulnerable to mechanical disturbance (Sorrell et al., 1997, 2002). Physical disturbance of plants depends on the interaction between waves, buoyancy, anchoring and form drag of individuals and clonal colonies, and wave height and orbital velocities are key factors restricting growth (Coops et al., 1991). Architecture of leaf and root/rhizome structure is likely to play an important role in determining resistance to wave action, and the present data suggest that T. orientalis may be the most wave-tolerant of the common New Zealand emergent species. This contrasts with the smaller, narrower-leaved T. angustifolia in Europe, which is restricted to sheltered sites (Coops et al., 1991).
The results presented here confirm that the depth extension of emergent vegetation is determined by a complex of interacting external and internal variables, as suggested in earlier studies (Coops et al., 1999). The ability to generate convective gas flow appears to be an absolute requirement for dominance beyond 0·5–1·0 m depth. Two species capable of generating high flows, T. orientalis and E. sphacelata, tended to extend deeper than those generating low flows. Trophic state affected depth extension and dominance patterns, with lesser depths and greater dominance by T. orientalis in nutrient-rich lakes with reducing sediments, and deeper extension, particularly of E. sphacelata, in oligotrophic lakes. Wave action compounded this complexity, with an overall reduction in depth extension with increasing wave energy and again a shift in species dominance from cylindrical-stemmed sedges towards T. orientalis.
ACKNOWLEDGEMENTS
We thank Donna Sutherland, Greg Kelly, Agate Ponder-Sutton and Ron Ovendon for assistance with field work and data analysis, Marc Schallenberg for additional lake water quality data, and Hans Brix and Tenna Riis for critical reviews of the manuscript. This study was funded by the New Zealand Foundation for Research, Science and Technology (Effects-based Protection and Management of Aquatic Ecosystems, Contract CO1X0307).
LITERATURE CITED
- Afreen F, Zobayed SMA, Armstrong J, Armstrong W. Pressure gradients along whole culms and leaf sheaths, and other aspects of humidity-induced gas transport in Phragmites australis. Journal of Experimental Botany. 2007;58:1651–1662. doi: 10.1093/jxb/erm017. [DOI] [PubMed] [Google Scholar]
- Aldridge KT, Ganf GG. Modification of sediment redox potential by three contrasting macrophytes: implications for phosphorus adsorption/desorption. Marine and Freshwater Research. 2003;54:87–94. [Google Scholar]
- Armstrong J, Armstrong W. Light-enhanced convective throughflow increases oxygenation in rhizomes and rhizosphere of Phragmites australis (Cav.) Trin. ex Steud. New Phytologist. 1990;114:121–128. doi: 10.1111/j.1469-8137.1990.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Armstrong J, Armstrong W, Beckett PM. Phragmites australis: venturi- and humidity-induced pressure flows enhance rhizome aeration and rhizosphere oxidation. New Phytologist. 1992;120:197–207. [Google Scholar]
- Armstrong J, Armstrong W, Beckett PM, et al. Pressurized ventilation in wetland macrophytes: the mechanism and mathematical modelling of humidity-induced convection. Aquatic Botany. 1996;54:177–198. [Google Scholar]
- Bendix M, Tornbjerg T, Brix H. Internal gas transport in Typha latifolia L. and Typha angustifolia L. 1. Humidity-induced pressurization and convective throughflow. Aquatic Botany. 1994;49:75–89. [Google Scholar]
- Blanch SJ, Walker KF, Ganf GG. Water regimes and littoral plants in four weir pools of the River Murray, Australia. Regulated Rivers: Research and Management. 2000;16:445–456. [Google Scholar]
- Breiman L, Friedman JH, Olshen RA, Stone CG. Classification and regression trees. Belmont, CA: Wadsworth International Group; 1984. [Google Scholar]
- Brix H, Sorrell BK, Orr PT. Internal pressurization and convective gas flow in some emergent freshwater macrophytes. Limnology and Oceanography. 1992;37:1420–1433. [Google Scholar]
- Burns NM, Rutherford JC, Clayton JS. A monitoring and classification system for New Zealand lakes and reservoirs. Journal of Lake and Reservoir Management. 1999;15:255–271. [Google Scholar]
- Cade BS, Guo Q. Estimating effects of constraints on plant performance with regression quantiles. Oikos. 2000;91:245–254. [Google Scholar]
- Cade BS, Terrell JW, Schroeder RL. Estimating effects of limiting factors with regression quantiles. Ecology. 1999;80:311–323. [Google Scholar]
- Colmer TD. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell & Environment. 2003;26:17–36. [Google Scholar]
- Colmer TD, Pedersen O. Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange. New Phytologist. 2008;177:918–926. doi: 10.1111/j.1469-8137.2007.02318.x. [DOI] [PubMed] [Google Scholar]
- Coops H, Boeters R, Smit H. Direct and indirect effects of wave attack on helophytes. Aquatic Botany. 1991;41:333–352. [Google Scholar]
- Coops H, Geilen N, van der Velde G. Distribution and growth of the helophyte species Phragmites australis and Scirpus lacustris in water depth gradient in relation to wave exposure. Aquatic Botany. 1994;48:273–284. [Google Scholar]
- Coops H, Geilen N, van Der Velde G. Helophyte zonation in two regulated estuarine areas in the Netherlands: vegetation analysis and relationships with hydrological factors. Estuaries. 1999;22:657–668. [Google Scholar]
- Dacey JWH. Internal winds in water lilies: an adaptation for life in anaerobic sediments. Science. 1980;210:1017–1019. doi: 10.1126/science.210.4473.1017. [DOI] [PubMed] [Google Scholar]
- De'ath G, Fabricius KE. Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology. 2000;81:3178–3192. [Google Scholar]
- den Heyer C., Kalff J. Organic matter mineralization rates in sediments: a within- and among-lake study. Limnology and Oceanography. 1998;43:695–705. [Google Scholar]
- Grace JB. The effects of nutrient additions on mixtures of Typha latifolia L. and Typha domingensis Pers. along a water-depth gradient. Aquatic Botany. 1988;31:83–92. [Google Scholar]
- Grosse W. Pressurised ventilation in floating-leaved aquatic macrophytes. Aquatic Botany. 1996;54:137–150. [Google Scholar]
- Keddy PA. Shoreline vegetation in Axe Lake, Ontario: effects of exposure on zonation patterns. Ecology. 1983;64:331–344. [Google Scholar]
- Lenssen JPM, Menting FBJ, van der Putten WH, Blom CWPM. Effects of sediment type and water level on biomass production of wetland plant species. Aquatic Botany. 1999;64:151–165. [Google Scholar]
- Matsui T, Tsuchiya T. Root aerobic respiration and growth characteristics of three Typha species in response to hypoxia. Ecological Research. 2006;21:470–475. [Google Scholar]
- Rolletschek H, Hartzendorf T, Rolletschek A, Kohl J-G. Biometric variation in Phragmites australis affecting ventilation and amino acid metabolism. Aquatic Botany. 1999;64:281–302. [Google Scholar]
- Singer A, Eshel A, Agami M, Beer S. The contribution of aerenchymal CO2 to the photosynthesis of emergent and submerged culms of Scirpus lacustris and Cyperus papyrus. Aquatic Botany. 1994;49:107–116. [Google Scholar]
- Smith JM. Wind-wave generation on restricted fetches. Vicksburg, USA: Coastal Engineering Research Center; 1991. Miscellaneous Paper, CERC-91-92. [Google Scholar]
- Sorrell BK, Tanner CC. Convective gas flow and internal aeration in Eleocharis sphacelata in relation to water depth. Journal of Ecology. 2000;88:778–789. [Google Scholar]
- Sorrell BK, Brix H, Orr PT. Eleocharis sphacelata: internal gas transport pathways and modelling of aeration by pressurized flow and diffusion. New Phytologist. 1997;136:433–442. doi: 10.1046/j.1469-8137.1997.00769.x. [DOI] [PubMed] [Google Scholar]
- Sorrell BK, Tanner CC, Sukias JPS. Effects of water depth and substrate on growth and morphology of Eleocharis sphacelata: implications for culm support and internal gas transport. Aquatic Botany. 2002;73:93–106. [Google Scholar]
- Tornbjerg T, Bendix M, Brix H. Internal gas transport in Typha latifolia L. and Typha angustifolia L. 2. Convective throughflow pathways and ecological significance. Aquatic Botany. 1994;49:91–105. [Google Scholar]
- Van Duin EHS, Blom G, Los FJ, et al. Modeling underwater light climate in relation to sedimentation, resuspension, water quality and autotrophic growth. Hydrobiologia. 2001;444:25–42. [Google Scholar]
- Viner AB. Distribution of carbon, nitrogen, and phosphorus in Lake Taupo surface sediment. New Zealand Journal of Marine and Freshwater Research. 1989;23:393–399. [Google Scholar]
- Vretare V, Weisner SEB. Influence of pressurized ventilation on performance of an emergent macrophyte (Phragmites australis) Journal of Ecology. 2000;88:978–987. [Google Scholar]
- Vretare Strand V. The influence of ventilation systems on water depth penetration of emergent macrophytes. Freshwater Biology. 2002;47:1097–1105. [Google Scholar]
- Vretare Strand V, Weisner SEB. Interactive effects of pressurized ventilation, water depth and substrate conditions on Phragmites australis. Oecologia. 2002;131:490–497. doi: 10.1007/s00442-002-0915-7. [DOI] [PubMed] [Google Scholar]
- Weisner SEB. Within-lake patterns in depth penetration of emergent vegetation. Freshwater Biology. 1991;26:133–142. [Google Scholar]
- White SD, Ganf GG. The influence of convective flow on rhizome length in Typha domingensis over a water depth gradient. Aquatic Botany. 1998;62:57–70. [Google Scholar]
- White SD, Deegan BM, Ganf GG. The influence of water level fluctuations on the potential for convective flow in the emergent macrophytes Typha domingensis and Phragmites australis. Aquatic Botany. 2007;86:369–376. [Google Scholar]
- Zedler JB, Kercher S. Causes and consequences of invasive plants in wetlands: opportunities, opportunists, and outcomes. Critical Reviews in Plant Sciences. 2004;23:431–452. [Google Scholar]