Abstract
Background
According to the Intergovernmental Panel on Climate Change (IPCC) 2007, natural wetlands contribute 20–39 % to the global emission of methane. The range in the estimated percentage of the contribution of these systems to the total release of this greenhouse gas is large due to differences in the nature of the emitting vegetation including the soil microbiota that interfere with the production and consumption of methane.
Scope
Methane is a dominant end-product of anaerobic mineralization processes. When all electron acceptors except carbon dioxide are used by the microbial community, methanogenesis is the ultimate pathway to mineralize organic carbon compounds. Emergent wetland plants play an important role in the emission of methane to the atmosphere. They produce the carbon necessary for the production of methane, but also facilitate the release of methane by the possession of a system of interconnected internal gas lacunas. Aquatic macrophytes are commonly adapted to oxygen-limited conditions as they prevail in flooded or waterlogged soils. By this system, oxygen is transported to the underground parts of the plants. Part of the oxygen transported downwards is released in the root zone, where it sustains a number of beneficial oxidation processes. Through the pores from which oxygen escapes from the plant into the root zone, methane can enter the plant aerenchyma system and subsequently be emitted into the atmosphere. Part of the oxygen released into the root zone can be used to oxidize methane before it enters the atmosphere. However, the oxygen can also be used to regenerate alternative electron acceptors. The continuous supply of alternative electron acceptors will diminish the role of methanogenesis in the anaerobic mineralization processes in the root zone and therefore repress the production and emission of methane. The role of alternative element cycles in the inhibition of methanogenesis is discussed.
Conclusions
The role of the nitrogen cycle in repression of methane production is probably low. In contrast to wetlands particularly created for the purification of nitrogen-rich waste waters, concentrations of inorganic nitrogen compounds are low in the root zones in the growing season due to the nitrogen-consuming behaviour of the plant. Therefore, nitrate hardly competes with other electron acceptors for reduced organic compounds, and repression of methane oxidation by the presence of higher levels of ammonium will not be the case. The role of the iron cycle is likely to be important with respect to the repression of methane production and oxidation. Iron-reducing and iron-oxidizing bacteria are ubiquitous in the rhizosphere of wetland plants. The cycling of iron will be largely dependent on the size of the oxygen release in the root zone, which is likely to be different between different wetland plant species. The role of the sulfur cycle in repression of methane production is important in marine, sulfate-rich ecosystems, but might also play a role in freshwater systems where sufficient sulfate is available. Sulfate-reducing bacteria are omnipresent in freshwater ecosystems, but do not always react immediately to the supply of fresh sulfate. Hence, their role in the repression of methanogenesis is still to be proven in freshwater marshes.
Keywords: Methane emission, methane oxidation, wetlands, emergent macrophytes, nitrogen cycling, sulfur cycling, iron cycling
INTRODUCTION
According to the 2007 report of the Intergovernmental Panel on Climate Change (IPCC; Denman et al., 2007), natural wetlands emit yearly 100–231 Tg of methane to the atmosphere, which represents 20–39 % of global methane emission. For natural wetlands especially, a large variation in emission data is presented by the Panel. Most of the data collected by IPCC 2007 are based on a top-down approach, in which inverse modelling is used that relies on spatially distributed, temporally continuous observations of methane concentrations in the atmosphere. In such an approach it is difficult to account for different concentrations of methane emitted by distinctive plant types on a local or regional scale.
Ding et al. (2004d) determined the annual methane emission rates for different wetland ecosystems in China. They identified four different wetland types, i.e. peatland (approx. 42 000 km2; 45 % of the total wetland area), freshwater marsh (approx. 25 000 km2; 26 %), salt marsh (approx. 24 000 km2; 26 %) and mangrove swamp (approx. 2500 km2; 3 %). In 2001 and 2002, the peatlands and freshwater marshes contributed 33·4 and 65·6 %, respectively, to the total methane emission from Chinese wetlands. The estimated total contributions of mangrove swamps and salt marshes was only 0·1 and 0 %, respectively. Hence freshwater wetlands were most important, while the methane emission from the salt marshes and mangrove swamps was negligible, at least based on area. It should however be noted that the negligibility of mangrove swamps with respect to methane emission is based on the total area occupied by mangrove trees. Purvaja et al. (2004) determined annual methane fluxes by vegetation of the mangrove tree Avicennia marina, which were comparable with emissions measured from freshwater wetlands on a square metre basis. Compared with other coastal wetland ecosystems in the state of Tamil Nadu in south-eastern India, the yearly methane flux from mangroves is not negligible (Purvaja and Ramesh, 2001).
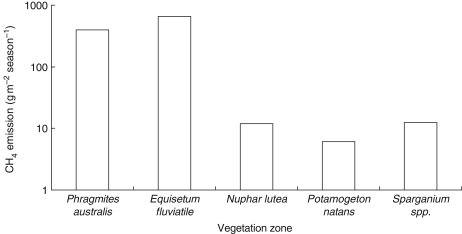
In a study on the role of vegetated littoral area in the efflux of methane in an extensive southern boreal landscape of 1600 km2 in Finland, Bergström et al. (2007) estimated the total methane emission by the different vegetation types during the growing season. The natural open ombrogenous bogs and minerogenous fens in the study region covered a 2·5-fold larger area than the littoral zones with emergent macrophytes, but their emissions were estimated to be only 78 % of the emissions by Phragmites australis and Equisetum fluviatile, the two most dominant species of all zone-forming macrophytes. The zone-forming floating-leaved species Nuphar lutea, Potamogeton natans and Sparganium spp. covered 44 % of all vegetated littoral areas, but their contribution to the efflux of methane was approximated to be 2 % of the dominant emergent macrophytes, indicating that the zone with emergent macrophytes was the most important natural source of methane in this wetland area (Fig. 1).
Fig. 1.
Estimated total seasonal methane emission by different vegetation zones in a southern boreal landscape in Finland (calculated after Bergström et al., 2007).
Also based on area, emergent macrophytes were by far the most important emitters of methane in a typical alpine wetland on the Qinghai-Tibetan Plateau, China, during the growing season of the plants (Hirota et al., 2004; Duan et al., 2005). Although the submerged plant species Potamogeton pectinatus covered approx. 74 % of the total wetland area, the methane emission from this underwater vegetation type was only 0,5, 0·9 and 9·2 % of the values measured for less extensive zones with the emergent wetland plants Hippuris vulgaris, Carex allivescers and Scirpus distigmatus, respectively, again all based on area.
The supremacy of emergent macrophytes compared with floating-leaved or submerged plant species with respect to methane emission was also observed in a study on methane fluxes from wetlands in an arid lake area in western China by Duan et al. (2005). Although the differences in methane emission rates were affected by season, the zones of emergent vegetation (i.e. P. australis, Typha latifolia and Scirpus acutus) always emitted more methane than the zones with the submerged P. pectinatus. It has been suggested that methane emitted into the water phase by submerged species such as P. pectinatus may be oxidized by communities of epiphytic methane-oxidizing bacteria (Heilman and Carlton, 2001). Since surface waters are normally oxic, as much as 90 % of the emitted methane can be oxidized before reaching the atmosphere (King, 1990).
Hence it is clear that emergent macrophytes are superior over floating-leaved and submerged macrophytes with respect to methane emission. This superiority has to do with their continuous access to the atmosphere in combination with their morphological adaptations to a life under partly oxygen-limited conditions.
Compared with the 20–39 % of the global methane flux emitted by natural wetlands, rice or paddy fields also contribute considerably to the annual methane emission. According to the IPCC (Denman et al., 2007), rice fields emit yearly 54–112 Tg of methane to the atmosphere, which is on average almost 50 % of the methane emission by natural wetlands. Many studies have been performed in paddy fields in relation to methane emission as well as to the production and oxidation by the micro-organisms in the root zones. With respect to this topic, the reader is referred to the recent review by Conrad (2007).
FACILITATED METHANE EMISSION BY EMERGENT PLANT SPECIES
Like most aquatic macrophytes, emergent wetland plants are adapted to a life in an oxygen-limited environment such as flooded soils. They possess an internal system for gas transport to provide their underground parts with oxygen. Sorrell and Boon (1994) investigated the importance of lacunar gas transport for the release of methane from a zone with Eleocharis sphacelata in a freshwater wetland in south-eastern Australia. The measured rates of methane efflux from the E. sphacelata site represented 1–15 times the rate of methane release by ebullition of methane bubbles from the non-vegetated sediment. The interstitial methane concentration in sediments from the E. sphacelata zone was approx. 0·6 times that of adjacent non-vegetated sediment, highlighting the role of E. sphacelata in accelerating methane release from sediments.
Van der Nat and Middelburg (1998a) studied the effects of two common macrophytes on methane dynamics in experimental wetlands containing freshwater sediments. During the growing season, methane fluxes in the S. lacustris and P. australis systems were dominantly (i.e. >90 %) plant mediated, but ebullition was also significant in the P. australis system before and after the growing season. Methane fluxes in the P. australis and non-plant systems were rather similar and higher than those in the S. lacustris system. Methane was also almost exclusively emitted by plant-mediated transport in outdoor experimental wetlands vegetated with mature P. australis or S. lacustris, whereas in non-vegetated wetlands with otherwise identical sediment, methane was emitted almost exclusively by ebullition (Van der Nat et al., 1998). In their study on the effect of the presence of P. australis on methane emission, Grünfeld and Brix (1999) observed a reduction of 62 % in the emission of methane after cutting of the plants. The same treatment with shoots of the cotton sedge Eriophorum vaginatum reduced the release of methane by 56 % compared with that of plots with intact plants (Greenup et al., 2000).
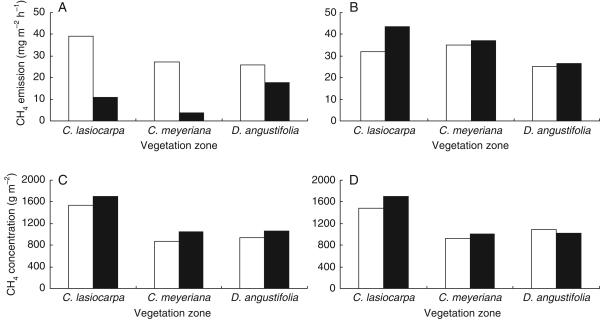
Ding and co-workers established in a series of experiments the contribution of the emergent plant species Carex lasiocarpa, Carex meyeriana and Deyeuxia angustifolia to the total methane emission from vegetated zones in freshwater marshes of the Sanjiang Plain in north-eastern China. Standing water depth governed the distribution of these plant species, with D. angustifolia in the shallowest water, C. lasiocarpa in the deepest water and C. meyeriana in between. The amounts of biomass produced by these species were not significantly different (Ding et al., 2003, 2005), but the amount of methane released from the vegetation was significantly higher in the zone with C. lasiocarpa than in the zone with D. angustifolia (Ding et al., 2003, 2004b). The amount of methane emitted from the zone with C. meyeriana was in between the quantities produced by the zones with the other two plant species. Clipping of the shoots below the water surface led to decreases in the amounts of emitted methane of 72, 86 and 31 % for C. lasiocarpa, C. meyeriana and D. angustifolia, respectively (Fig. 2A). The differences as observed for the effect of clipping below the water surface on methane emission between the three species indicate differences in the supply of methane or dissimilarities in gas flow capacities between the plant species. The flow of methane through the stem was indeed significantly higher in both Carex species compared with D. angustifolia, with the highest flows through C. meyeriana (Ding et al., 2003, 2005). Congruent with the observed differences in gas flow capacities are the measured pore water concentrations of methane, which were significantly lower at the zone with C. meyeriana compared with the zone with C. lasiocarpa (Fig. 2C). The lower gas flow-through rate in C. lasiocarpa compared with C. meyeriana, however, was compensated by the significantly higher stem density produced by the first wetland species, leading to non-significant differences in methane emission rates between these two Carex species (Ding et al., 2005).
Fig. 2.
Methane emission rates (A, B) and soil methane concentration (B, D) measured at different vegetation zones on the Sanjiang plain, China. Open bars indicate direct measurements; black bars represent data collected after clipping the plant shoots below (A, B) or above (C, D) the water surface (calculated after Ding et al., 2005).
In a study by Duan et al. (2005), where methane emission rates had been measured in different zones of emergent wetland plants, lower emission rates were observed in the zones with S. acutus compared with the zones with P. australis and T. latifolia. The difference was also explained by differences in the mechanism of internal gas transport. In contrast, the relatively low efflux rates of methane observed by Hirota et al. (2004) with the emergent S. distigmatus in comparison with H. vulgaris and C. allivescers was explained by the shallower habitat of the Scirpus species with more oxidized sediments in the root and rhizome layers than in the other vegetated zones.
In a study by Holzapfel-Pschon et al. (1986) it appeared that the methane emission rates in the vegetation with weeds were one order of magnitude larger than in the zone with T. latifolia. The vegetation with weed plants consisted of different, mainly monocotyl, species, e.g. Echinogloa crus-galli, Cyperus iria, Scirpus mucronatus and Alisma plantago. The inhibition of methane release by cutting the shoots below the water surface was also more pronounced in the zones with weeds.
In a study by Grünfeld and Brix (1999) on the effect of the water table on methane emissions in the presence of P. australis, the water table also affected significantly the efflux of methane. Lowering the water table 22 cm below the surface decreased the efflux by 60 %. Surprisingly, lowering of the water table to only 8 cm below the surface did not affect the emission rate compared with inundated soils. This was explained by the capillary forces of the organic sediment active under intermediately drained conditions.
GAS TRANSPORT WITHIN EMERGENT MACROPHYTES
In contrast to submerged macrophytes, which meet their below-ground oxygen requirements from photosynthesis in the shoot (Sorrell and Dromgoole, 1987; Pedersen et al., 2004, 2006; Sand-Jensen et al., 2005), emergent macrophytes have an additional source of oxygen in the atmosphere. Most vascular macrophytes rooting in oxygen-limited soils have developed a lacunar system of intercellular airspaces that supply the roots with oxygen to enable the continuation of aerobic metabolic processes in an otherwise reduced environment e.g. (Armstrong, 1979, 1982; Bendix et al., 1994; Tornbjerg et al., 1994). Consumption of oxygen in the underground parts of the plants leads to a diffusion flow of this gas from the shoots to the roots and rhizomes, whereas the metabolically produced carbon dioxide follows the opposite route of diffusion (Armstrong, 1979; Brix, 1988, 1989). In a number of emergent plant species, as well as in some floating-leaved species, this diffusive gas flow might become intensified by humidity- and Venturi-induced convection flows (Dacey, 1980; Armstrong and Armstrong, 1990, 1991; Armstrong et al., 1992). Since oxygen concentrations in the rhizome of a species such as P. australis never exceed the concentration in the atmosphere, it is unlikely that photosynthesis plays more than a minor role in the convection process (Armstrong and Armstrong, 1990; Armstrong et al., 1992). Humidity-induced convection is mainly induced in the light and is dependent upon the continued existence of a difference in humidity between the drier air outside the plant and the humid gases in the plant's aeration system. Oxygen diffuses in the light through the open stomata of living leaves and culms, and is then vented to the atmosphere via old, persistent, dead culms of previous years, especially those which have been damaged and have a lower resistance to through-flow (W. Armstrong et al., 1996). In other plants inflows and outflows of gases occur principally via the living shoots or leaves (Brix et al., 1992; Sorrell and Boon, 1994; Tornbjerg et al., 1994). Humidity-induced convection, which can occur under isothermal conditions and with a negative temperature differential between inside and outside of the plant, has been demonstrated in a number of emergent wetland species, i.e. Baumea articulate, Cyperus involucratus, E. sphacelata, Juncus ingens, P. australis, Schoenoplectus validus, Typha angustifolia, Typha domingensis, T. latifolia and Typha orientalis (Armstrong and Armstrong, 1990, 1991; Brix et al., 1992; Bendix et al., 1994; Sorrell and Boon, 1994; Tornbjerg et al., 1994). Convective flows have never been found so far in the well-known emergent wetland species Glyceria maxima (Vretare Strand, 2002).
In P. australis, Venturi-induced convection is initiated by wind blowing across the tops of tall dead culms, this being especially effective where the tops have been snapped off (J. Armstrong et al., 1992, 1996). The wind creates suction, which draws gases from these culms and the rhizome system. These sucked-up gases are then replaced by air entering the rhizomes via the stubble which is sheltered from wind flow. Venturi-induced flows will be influenced by resistance in the reed bed. As stated by Armstrong and co-workers (J. Armstrong et al., 1996), Venturi-induced convection could be particularly important in the winter, in wet and humid conditions and during the night in the growing season, when humidity-induced convection is slow or absent. The superior aerating effects of convective flows as opposed to diffusive ventilation within the plant and their influence on the rhizosphere have been demonstrated experimentally and by mathematical modelling (J. Armstrong et al., 1992, 1996). As suggested by Armstrong et al. (1992), the raising of the rhizome oxygen concentration by convection could also be important for the spread of P. australis since the growing root tips do not lie on the through-flow path and, being blind branches of the gas space system unless they become emergent, they will rely on the diffusive gas exchange with the nearest junction on the path.
The size of internal pressurization and convective gas flows is different among the different emergent plant species. In a comparison made by Brix and colleagues among 14 emergent wetland species from south-eastern Australia, P. australis, T. domingensis and T. orientalis were superior over successively J. ingens, E. sphacelata, C. involucratus, B. articulate and S. validus, while a species such as Bolboschoenus mediatus did not show a convective gas flow at all (Brix et al., 1992). In a study on two cattail species, the ventilation capacity of T. angustifolia turned out to be about twice as high as that of T. latifolia under the same environmental conditions, indicating that root aeration of the former may be more efficient (Bendix et al., 1994). Sorrell and Brix (2003) measured relatively high values of internal pressurization and convective flows in P. australis and T. orientalis in comparison with B. articulate. Wetland species with a high potential for internal pressurization and a low resistance to convective flow may have competitive advantage over species relying exclusively on diffusive gas transport, allowing them to ventilate their underground tissues and grow in deeper waters (Brix et al., 1992; Sorrell et al., 2000; Vretare Strand and Weisner, 2002). However, two species with low or non-detectable flow rates (Schoenoplectus lacustris and E. fluviatile, respectively) were found unexpectedly in deep water (Vretare Strand, 2002). Thus, pressurized ventilation is not a prerequisite for growth in deep water.
A special situation is encountered with mangrove trees, which rely on the oxidation of their root zone by the so-called pneumatophores. These aerenchyma-containing air roots connect the below-ground parts of the plant with the atmosphere and hence also facilitate the efflux of methane from the vegetation. In vegetation of the mangrove tree A. marina, Purvaja et al. (2004) observed a control of methane emission by the water level, which operated via the pneumatophores. In a tidal area this will create diurnal variations in emission rates, but also a strong seasonal variation in methane emission was observed that could largely be attributed to the seasonal changes in the number of pneumatophores.
METHANE OXIDATION IN THE RHIZOSPHERE
Not all oxygen transported by the plant is kept within the underground systems of roots and rhizomes. A substantial part is released to the rhizosphere. There is evidence that the release of oxygen to the rhizosphere offers protection against phytotoxins that are commonly found in reduced, waterlogged soils, such as sulfides, reduced iron and manganese, as well as volatile fatty acids (Mendelssohn and Postek, 1982; Lee, 1999; Pedersen et al., 2004). Conversion of these toxic compounds to less toxic forms is mediated by the activity of aerobic micro-organisms or by chemical oxidations in the oxidized rhizosphere.
In wetland plants, oxygen loss to the rhizosphere is typically highest in the apical, elongating regions of the roots and declines basipetally (Conlin and Crowder, 1989; Armstrong et al., 2000). As measured by methylene blue oxidation, oxygen release from underground parts of P. australis was most rapid from young adventitious and secondary roots and particularly from basal tufts of fine laterals (Armstrong and Armstrong, 1988). Radial oxygen loss is often undetectable further than a few centimetres behind the root tip (Armstrong and Armstrong, 1988, 1990; Armstrong et al., 2000). For the vertical and horizontal rhizomes of P. australis, Carex rostrata and G. maxima, it has been suggested that oxygen diffuses from the stomata of abaxial-exposed surfaces of scale leaves, from spaces between scale leaves and via apical pores or buds (Armstrong et al., 2006). However, differences with respect to phyllosphere redox potentials were reported for these macrophytes. Carex rostrata was less able than the other two species to maintain a positive redox potential after the transfer of the plants to a nitrogen atmosphere.
A part of the oxygen released into the rhizosphere will be consumed by methane-oxidizing bacteria. For example, methane reservoirs in non-planted controls of experimental wetlands were much larger than in the vegetated systems, and maximum concentrations were reached at more shallow depths in the absence of plants (Van der Nat and Middelburg, 1998a). Methane consumption by methane-oxidizing bacteria had apparently decreased the amount of methane that could potentially be emitted by the vegetation. The relative importance of oxygen for root-associated methane oxidation was also examined by using sediment-free, intact freshwater marsh plants (Pontederia cordata and Sparganium eurycarpum) incubated in split chambers (Calhoun and King, 1997). When the root medium was oxic, methane oxidation accounted for 88 and 63 % of the total methane depletion for S. eurycarpum and P. cordata, respectively (Table 1). Under suboxic conditions, methane oxidation was not detectable for S. eurycarpum but still accounted for 68 % of total methane depletion for P. cordata. The differences between plant species were consistent with their relative ability to oxygenate their rhizospheres. During suboxic incubation, dissolved oxygen decreased by 19 % in S. eurycarpum chambers but increased by 232 % for P. cordata. An in situ comparison also revealed greater methane-oxidizing activity for P. cordata than for S. eurycarpum.
Table 1.
Percentages of methane oxidized in the wetland vegetation before entering the atmosphere
| Vegetation | Percentage oxidized | Method | Reference |
|---|---|---|---|
| Carex rostrata | 20–40* | 14C-labelled acetate in monoliths | Ström et al. (2005) |
| Equisetum fluviatile | 40 | Darkened/nitrogen atmosphere | Kankaala and Bergström (2004) |
| Eriophorum vaginatum | 0† | Changes in methane concentration in bottles containing different plant parts | Frenzel and Rudolf (1998) |
| Eriophorum vaginatum | >90* | 14C-labelled acetate in monoliths | Ström et al. (2005) |
| Juncus effusus | >90* | 14C-labelled acetate in monoliths | Ström et al. (2005) |
| Phragmites australis | 16 | Methylfluoride/nitrogen atmosphere | Van der Nat and Middelburg (1998b) |
| Pontederia cordata | 63 | Soil-free, split chambers | Calhoun and King (1997) |
| Scirpus lacustris | 35 | Methylfluoride/nitrogen atmosphere | Van der Nat and Middelburg (1998b) |
| Sparganium eurycarpum | 88 | Soil-free, split chambers | Calhoun and King (1997) |
| Mixed freshwater vegetation | 43‡ | Nitrogen atmosphere | Roslev and King (1996) |
| Mixed weeds | 95 | Acetylene inhibition/nitrogen atmosphere | Holzapfel-Pschorn et al. (1986) |
* In the root zone.
† In the presence of isolated below-ground plant parts.
‡ Annual average; range 15–76 %.
Weeds did not stimulate the rates of methanogenesis in laboratory experiments with plants on submerged soil (Holzapfel-Pschorn et al., 1986). The lack of stimulation by the weed plants may have been due to their lacking root exudates, but more probably it was due to inhibition of methanogenesis by oxygen transport into the rhizosphere. The latter assumption is supported by the relatively high redox potentials observed in weed-vegetated soil and by the relatively strong stimulation of methane emission after incubation of weed plants under a nitrogen atmosphere. Addition of 5 % acetylene, an inhibitor of methane-oxidizing bacteria, to an oxic atmosphere increased the methane emission by the weeds 4-fold, but pre-incubation for 1 d under an atmosphere of nitrogen stimulated the methane emission rate 25-fold (Table 1).
Treatment with acetylene also yielded higher methane emission rates in zones with sedge vegetations of C. lasiocarpa and C. meyeriana (Ding et al., 2004c). Covering the plant shoots with a black cloth for 72 h led to an increase in methane emission in zones with C. lasiocarpa, C. meyeriana and D. angustifolia. Increases were comparable between both Carex species (Ding et al., 2004c). However, sites with C. lasiocarpa showed a more pronounced increase than sites with D. angustifolia (Ding et al., 2004a). The results of darkening and acetylene treatments indicate the repression of methane oxidation, leading to more emission. In a seasonal study of the effects of water table fluctuations and anoxia on methane emission and oxidation, aerobic methane oxidation rates varied between 15 and 76 % of the potential diffusive methane flux (Roslev and King, 1996). On an annual basis, approx. 43 % of the methane diffusing into the oxic zone was oxidized before reaching the atmosphere (Table 1). Although methane emission was generally not observed during the winter, stems of soft rush (Juncus effusus) emitted methane when the marsh was ice covered.
In an effort to determine the amount of methane oxidation in the root zones of P. australis and S. lacustris, Van der Nat and Middelburg (1998b) applied gaseous methylfluoride overnight to closed systems in large indoor experimental wetlands vegetated with one of these emergent wetland plants. The inhibitor of methane oxidation was transported to the root system by the aerenchyma of the plants themselves. For comparison nitrogen gas was also applied in parallel to closed systems containing plants and sediments. Both methods gave the same amount of inhibition. A significant difference between the plant species was observed with respect to methane oxidation rates. Notwithstanding the various studies that have reported on the effects of light on rhizospheric oxygen concentrations, through its control of photosynthetic activity and oxygen transport (Armstrong and Armstrong, 1991), light or dark conditions had no significant effect on rhizospheric methane oxidation in the study by Van der Nat and Middelburg (1998b). When averaged over the growing season, methane oxidation in the rhizosphere of S. lacustris and P. australis reduced the potential methane efflux from the vegetation by 34·7 and 16·1 %, respectively (Table 1). The highest methane oxidation rates were noted early in the plant growth cycle, with >55 % of the generated methane being oxidized in the S. lacustris system. Methane oxidation rates were lowest after plants matured. During the active growth phase of E. fluviatile the proportion of methane oxidized in the rhizosphere was observed to be 40 ± 10 % (Kankaala and Bergstrom, 2004), which is comparable with the amounts measured by Van der Nat and Middelburg (1998b) with S. lacustris, but higher than in P. australis (Table 1).
Clipping the shoots of emergent wetland plants just above the water surface will interrupt the plant-mediated flows of oxygen and methane; especially so when internal gas flows are governed by humidity- or Venturi-induced convection. Diffusive gas flows will largely be maintained after clipping just above the water level. Clipping the shoots of C. lasiocarpa, C. meyeriana and D. angustifolia, which do not employ convective gas flows, increased the emission rate of methane (Ding et al., 2005). For C. lasiocarpa this increase was even significant at the level of 5 % (Fig. 2B). Also with C. lasiocarpa, the pore water methane concentrations as well as the amounts of total soil methane increased after clipping of the shoot above the water surface (Fig. 2D). It has been concluded by Ding and co-workers (2005) that in the case of C. lasiocarpa, the contribution of the plant to methane oxidation was apparently larger than its role in the production of this gas. For the other two species in the study, i.e. C. meyeriana and D. angustifolia, the contribution of the plants to the oxidation of methane in the root zone was less pronounced.
Clipping of the shoots of E. vaginatum above the water surface also increased the efflux of methane from a Sphagnum-dominated ombrotrophic peatland at Roudsea Moss, UK (Greenup et al., 2000). Like the sedges mentioned in the last paragraph, E. vaginatum does not use convective gas flows. Eriophorum vaginatum apparently contributed more to the oxidation of methane in its root zone than to its production, however, at a lower level than observed with C. lasiocarpa in the study of Ding et al. (2005).
The mire plant E. vaginatum shows strong point sources of methane in arctic tundra (e.g. Christensen, 1993; Schimel, 1995). Frenzel and Rudolph (1998) observed no methane oxidation associated with different below-ground parts of this plant species or of Eriophorum angustifolia from a bog in southern Estonia. These authors suggested that allelopathic compounds such as plant-derived monoterpenes and soil-borne phenolic substances may be important in the repression of methane-oxidizing bacteria. With E. vaginatum from a peat-forming wetland in southern Sweden, Ström et al. (2005) observed an almost complete recapturing of 14C-labelled carbon dioxide after application of [14C]acetate to monoliths dominated by this plant. It is not clear from this labelling experiment whether acetate is directly oxidized to carbon dioxide by aerobic bacteria or whether aceticlastic methanogens split the acetate in carbon dioxide and methane, which was subsequently oxidized to carbon dioxide by methane-oxidizing bacteria. Hines et al. (2001) demonstrated that methanogens in northern wetlands generally do not consume acetate. However, labelling experiments with peat monoliths containing vascular plants from the Zackenberg valley in north-eastern Greenland suggested acetate to be of importance to the methanogens (Strom et al., 2003). In the labelling experiments of Ström et al. (2005), J. effusus behaved similarly to E. vaginatum, i.e. almost complete recapturing of [14C]carbon dioxide, but monoliths dominated by C. rostrata showed a substantial production of 14C-labelled methane, which must be due to the activity of aceticlastic methanogens. Hence, the fate of acetate and also of methane is not clear in northern wetlands, but again it was demonstrated that plant species composition may have dramatic effects on ecosystem functioning.
EMERGENT MACROPHYTES AS SUPPLIERS OF CARBON
In addition to the governing role of emergent wetland plants in the emission of methane by their aerenchyma system as well as in the oxidation of a part of the methane in the root zone by the release of oxygen, these macrophytes also contribute to the production of methane by the production of organic carbon. Whereas studies related to paddy fields showed that substrates derived from living plants may contribute up to 90 % of the total methane emission (cited by Ding et al., 2005), Megonigal et al. (1996) and Juutinen et al. (2003) found that recent products of photosynthesis of wetland plants made a very limited contribution to methane production, and labile organic carbon for methane production was mainly derived from plant litter (Ding et al., 2002). In pot experiments with E. vaginatum labelled with [14C]carbon dioxide, only a minor fraction (0·2 %) was incorporated in exudates (Saarnio et al., 2004). New carbon was primarily fixed in the metabolically important carbohydrates as well as acid anions that comprised the main compounds of fresh exudates, but microbes seemed to metabolize the exudates rapidly into other substances such as acetate, which could then serve for aceticlastic methanogenesis. Acetate has been shown to be a precursor of methane in wet arctic tundra ecosystems in the Zackenberg Valley in north-east Greenland, which was dominated by the sedges Eriophorum scheuchzeri, Carex subspathacea and Dupontia psilosantha (Strom et al., 2003). The formation of acetate in the root zone was related to the rate of photosynthesis and was also significantly lower in E. scheuchzeri under shaded conditions compared with the controls in the light, indicating that higher photosynthetic rates in control plots lead to higher allocation of carbon to the root zone. A significant negative effect of shading on the methane flux during the growing season had been observed before in the tundra of the Zackenberg Valley with E. scheuchzeri, C. subspathacea and D. psilosantha as dominant vascular plant species (Joabsson and Christensen, 2001).
In their study on the effects of two common macrophytes on methane dynamics in freshwater sediments, Van der Nat and Middelburg (1998a) also determined the contribution of methanogenesis to the gross anaerobic mineralization of organic matter. The relative contribution of methanogenesis was much lower in the vegetation with S. lacustris (i.e. 6–19 %) compared with the zones with P. australis (i.e. 24–62 %) or without plants (i.e. 80 %). The vegetated systems exhibited the highest methane production rates when plants were mature.
In order to determine to what extent plant-mediated methane emissions from E. fluviatile stands are due to variation in temperature, sediment quality and rhizospheric methane oxidation, Kankaala and Bergström (2004) studied these processes in detail in mesocosms with two homogenous bottom types. Equisetum fluviatile stands were established on the sediment of the original growth site, organic silt sediment from the littoral zone of Lake Pääjärvi, Finland, and on sand originally very poor in organic matter. As observed before by Grünfeld and Brix (1999) in their study of the effect of substrate type on methane emissions, Kankaala and Bergstrom (2004) found the highest emission rates on the organic silt sediment. In the sand mesocosms the variation of net methane emission was better correlated with the shoot biomass than with sediment temperature variation during the growing season, indicating that methanogens were severely limited by substrate availability and were probably dependent on substrates produced by E. fluviatile. The proportion of the potential methane emission which was oxidized did not differ significantly between the bottom types in summer. The net methane emission during the growing season as a proportion of the seasonal maximum of the shoot biomass was significantly higher in the organic sediment mesocosms (6·5 %) than in sand (1·7 %). The high methane emissions observed from dense well-established E. fluviatile stands in the field appear to be more related to temperature-regulated turnover of detritus in the anaerobic sediment and less to methane oxidation and seasonal variation in plant growth dynamics. From these and other studies involving common emergent macrophytes in boreal mesoeutrophic lakes, Kankaala et al. (2005) suggested that methane production by methanogenic bacteria is more limited by temperature than by substrate in vegetation stands with high methane emission rates. At sites with low methane efflux rates and low productivity, the methanogens are more dependent on the substrates produced by plants during the same growing season. The highest methane efflux rates relative to the standing plant biomass had been observed at ‘hotspots’ where detritus from external sources accumulated. However, the role played by ancient carbon stores as a substrate for methanogens is unknown, but may be marginal in the presence of large amounts of fresh litter and root-derived substrates (Kankaala et al., 2004). As discussed by Kankaala et al. (2005), future changes in hydrology due to climatic warming leading to attenuations in water level alterations may lead to more detritus accumulation and subsequently methane formation under anoxic conditions in boreal lakes.
INTERFERING MICROBIAL PROCESSES IN THE RHIZOSPHERE
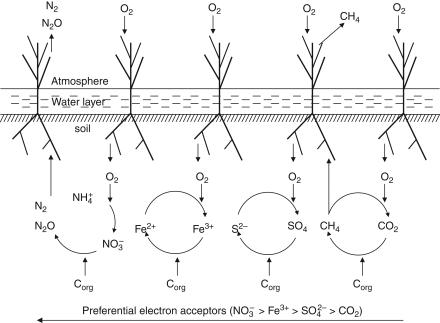
Although oxygen leakage in the rhizosphere is important with respect to the oxidation of methane leading to reduced methane emission rates, oxygen also indirectly plays a role in the production of methane. Plant litter and root-derived substrates induce decomposition processes. In oxygen-limited environments such as flooded soils, alternative electron acceptors are employed by the microbial community for the decomposition of organic matter, as demonstrated in Fig. 3. Largely determined by differences in free energy yields, alternative electron acceptors are used in a fixed order of succession: nitrate > Mn4+ > Fe3+ > sulfate > carbon dioxide (e.g. Ponnamperuma, 1984; Laanbroek, 1990). So, carbon dioxide is usually reduced to methane by methanogenic bacteria only when the other electron acceptors have been exhausted. The exhaustion of alternative electron acceptors is retarded, and therefore the production of methane repressed, when the products of the alternative, anaerobic oxidation pathways, i.e. ammonium, Mn2+, Fe2+ and hydrogen sulfide, are reoxidized to their original oxidized status (Fig. 3). The size of the internal cycling of alternative electron acceptors will be dependent on the size of their respective pools and on the availability of oxygen.
Fig. 3.
Schematic presentation of the element cycles in the root zone of emergent wetland plants. The arrow at the bottom indicates the preferential use of alternative electron acceptors at limiting availability of oxygen. Oxygen is transported from the atmosphere to the root zone by the plants, whereas methane, nitrous oxide and dinitrogen gas flow in the opposite direction.
Nitrogen cycling
The availability of nitrate as an alternative electron acceptor for oxidative mineralization processes in flooded, oxygen-limited soils will be largely dependent on the nitrate supply from the overlaying water. During the growing season, concentrations of ammonium are extremely low in the root zone of emergent wetlands plants such as G. maxima, P. australis and S. lacustris (Bodelier et al., 1996; Van der Nat et al., 1997), which excludes ammonium as a precursor of nitrate in the rhizosphere with a large degree of certainty. Potential nitrification rates in the rhizosphere are repressed in the growing season of G. maxima (Bodelier et al., 1996). Ammonium itself is a well-known inhibitor of methane oxidation in many ecosystems, including littoral zones (Bosse et al., 1993; Nold et al., 1999; Carini et al., 2003; Bodelier and Laanbroek, 2004). Although inhibition of methane oxidation might also occur in sediments vegetated with emergent wetland plants, its relevance is probably restricted to the surface layers, where the concentrations of ammonium are sufficiently high to exceed the concentrations of methane by at least the 30-fold necessary to inhibit methane oxidation (Van der Nat et al., 1997).
In sediments of healthy and degrading P. australis stands, denitrification was the main nitrate-reducing process (Nijburg and Laanbroek, 1997a). Nitrate reduction to ammonium amounted to only a few per cent of the quantity of nitrate reduced to gaseous end-products. This means that the nitrogen cycle in anoxic root zones is probably interrupted between nitrate and ammonium. In accordance with the dominant role of denitrifying bacteria in the rhizosphere of emergent wetland plants, the majority of the nitrate-reducing microbial community in the rhizosphere of T. angustifolia consisted of oxidative denitrifying species and only a small part belonged to fermentative ammonium-producing bacteria (Brunel et al., 1992). Whereas oxidative, denitrifying bacteria also dominated the nitrate-reducing bacterial community in the sediment from a littoral zone of a shallow lake, the composition of the nitrate-reducing microbial community of the rhizosphere of G. maxima was largely determined by the availability of nitrate (Nijburg et al., 1997; Nijburg and Laanbroek, 1997b). In the root zone of G. maxima, ammonium-producing, nitrate-reducing bacteria dominated only under severely nitrate-limiting conditions. Hence, neither nitrogen cycling nor ammonium oxidation will be a major factor repressing or retarding methane production or oxidation in the rhizosphere of emergent wetland plants.
An exception with respect to the occurrence of nitrogen conversions in the root zone of emergent wetland plants might be found in wetlands constructed for the removal of nitrogen-rich waste water. The vegetation of these systems gave rise to increased nitrification in the root zones and concomitant nitrous oxide release (Wang et al., 2008a). This latter study revealed that Zizania latifolia made a larger contribution to nitrous oxide emission than P. australis and T. latifolia. Strikingly, the highest methane efflux rates were also obtained in the Z. latifolia systems and higher emissions were found with higher influent load (Wang et al., 2008b).
Iron cycling
Ferric oxides are assumed to be one of the most abundant electron acceptors in soils as well as in sediments (Ponnamperuma, 1972; Gotoh and Patrick, 1974). High potential iron reduction rates in the presence of washed, excised roots of the freshwater macrophytes P. cordata, S. eurycarpum and T. latifolia revealed active iron cycling in the rhizoplane of these plants (King and Garey, 1999). Lower potential rates were measured in the rhizoplane of the salt marsh cordgrass Spartina alterniflora and the seagrass Zostera marina. It has been suggested by the authors that iron-reducing bacteria in the rhizoplane of aquatic macrophytes might limit the organic carbon availability to other heterotrophic micro-organisms including methanogens in the rhizosphere. In a comparison between more and less productive sites with S. alterniflora in Louisiana, approx. 50 times more iron was found on streamside roots than on roots from inland plants, showing the better developed oxidized rhizosphere associated with more productive streamside S. alterniflora (Mendelssohn and Postek, 1982). The presence of iron-oxidizing bacteria in the rhizosphere of four different species of wetland plants (i.e. J. effuses, L. oryzoides, Sagittaria australis and an unknown sedge) also indicated the presence of active iron cycling in a diverse wetland environment (Emerson et al., 1999). This latter study also presented the first evidence for culturable Fe-oxidizing bacteria associated with Fe plaque in the rhizosphere of emergent wetland plants. In a survey of 13 wetland and aquatic habitats in Virginia, Maryland and West Virginia, iron-oxidizing bacteria were present in the rhizosphere of 92 % of the plant specimens collected, representing 25 plant species (Weiss et al., 2003). It has been concluded that these bacteria are ubiquitous and abundant in wetland ecosystems, and that iron-reducing bacteria are dominant members of the rhizosphere microbial community. These observations provided a strong rationale for the combined role of iron-oxidizing and -reducing bacteria in a rhizosphere iron cycle. Weiss et al. (2004) compared the relative mineralogy and the reduction potential of pools of oxidized iron in the rhizosphere of T. angustifolia and of T. latifolia, and in non-rhizosphere soils of different marshlands in Maryland and West Virginia. The iron mineralogy contributed strongly to the differences in the iron reduction potential between the two pools, which has important biogeochemical implications such as suppression of methane production (Van der Nat and Middelburg, 1998a; Megonigal et al., 2003; Roden and Wetzel, 2003). Hence, the iron cycle acts as an intermediate shuttle between oxygen released by the plant and the anaerobic oxidation of organic carbon. Recently, neutrophilic iron-oxidizing bacteria have been isolated from the rhizosphere of J. effuses, Schoenoplectus (Scirpus) americanus and T. latifolia (Weiss et al., 2007) and of G. maxima (Wang et al., 2009).
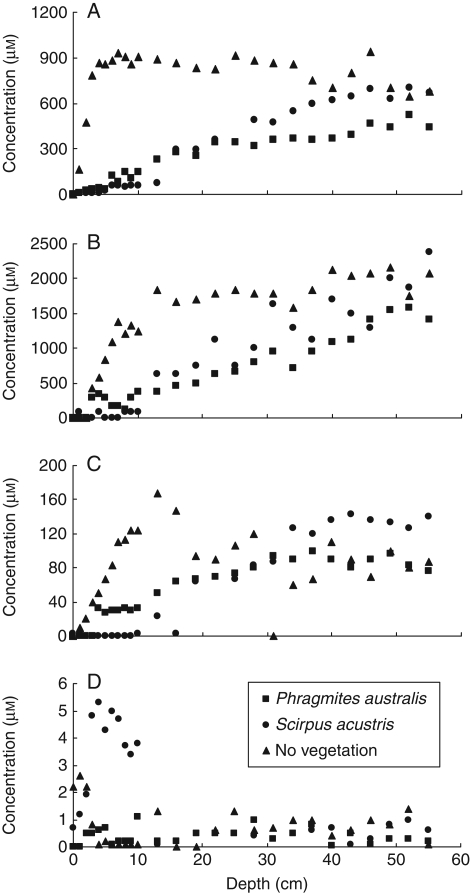
Although grown on sediments from the same origin, experimental wetlands with S. lacustris contained comparatively smaller reservoirs of dissolved iron than experimental wetlands with P. australis (Van der Nat and Middelburg, 1998a). Dissolved iron concentrations increased with depth as a consequence of solid-phase Fe3+ reduction. Dissolved iron concentrations in the non-plant system reached an asymptote at a relatively shallow depth and did not show variability over time. In the vegetated systems, however, maxima of dissolved iron were lower and were reached deeper in the sediment. The absence of reduced iron in the more shallow layers of the vegetated systems was most pronounced in the wetlands with S. lacustris. In a more detailed study on the oxidation in the rhizospheres of P. australis and S. lacustris, Van der Nat (2000) determined the distribution of methane as well as of different elements with depth in indoor experimental wetlands. The concentrations of methane in pore waters of non-vegetated mesocosms increased to maximum concentrations within the first centimetres of the sediment (Fig. 4A). In contrast, pore water methane concentrations in the vegetated mesocosms were repressed in the shallow sediment layers and the final concentration remained lower than in the non-vegetated control. The concentrations of dissolved reduced iron were lower in the more shallow sediments planted with S. lacustris compared with sediments containing P. australis (Fig. 4B). The same was also true for dissolved reduced manganese (Fig. 4C). However, the opposite was observed for dissolved uranium. With this element increased concentrations of oxidized uranium were observed in the shallow sediment layers with S. lacustris (Fig. 4D). Unlike iron and manganese, uranium is removed from pore water solutions in anoxic environments (Klinkhamer and Palmer, 1991). Hence, increased concentrations of this element indicate more oxidized conditions in the rhizosphere of S. lacustris compared with the root zone of P. australis or with the non-vegetated sediments. Concentrations of dissolved organic carbon were relatively low in the more shallow sediment layers of the vegetated systems (Van der Nat and Middelburg, 2000). Potential anaerobic carbon dioxide production rates were higher in the wetlands with P. australis than in the wetlands with S. lacustris and in the non-vegetated controls, indicating the presence of more labile organic carbon compounds in the systems with common reed (Van der Nat and Middelburg, 1998a). The presence of more oxidized electron acceptors in the systems with S. lacustris may explain the relatively low contribution of methanogenesis to gross anaerobic mineralization in the presence of this wetland plant species.
Fig. 4.
Depth distribution of methane (A), reduced iron (B), reduced manganese (C) and oxidized uranium (D) in experimental freshwater wetlands containing Phragmites australis, Scirpus lacustris or no vegetation, as indicated (after Van der Nat, 2000).
Sulfur cycling
The negligible rate of methane emission from salt marshes (Ding et al., 2004d) indicates that methanogenesis in marine systems is likely to be repressed by the excess of alternative electron acceptors in relation to the supply of organic carbon. High concentrations of sulfate in marine or brackish environments will contribute to the excess of alternative electron acceptors. For example, the biogeochemistry of North Atlantic salt marshes is characterized by the interplay between the salt marsh cordgrass S. alterniflora and sulfate-reducing bacteria, which mineralize the diverse carbon substrates provided by the plants (see Klepac-Ceraj et al., 2004). Sulfate reduction appears to be the major form of respiration in the sediments of S. alterniflora salt marshes in Georgia as well as in Massachusetts (Howarth and Teal, 1979; Howarth and Giblin, 1983), although in some marsh sediments oxidized iron has been suggested to be important for respiration (e.g. Koch and Mendelssohn, 1989; Lowe et al., 2000). Relatively high methane emission rates by the pneumatophores of the mangrove A. marina in marine sediments can be explained by the large amount of degradable carbon, which is sufficient to fuel both sulfate reduction and methane production (Purvaja and Ramesh, 2001).
Compared with marine sediments, relatively little is known about sulfur cycling in freshwater soils or sediments. Sulfate-reducing bacterial genera are omnipresent in freshwater environments (Miletto et al., 2008). Degradation of organic sulfur from plant residue may result in increased sulfate concentration in pore water (Wind and Conrad, 1995). Increased levels of sulfate in the soil might also originate from sulfate-polluted river water (Lamers et al., 1998). In situ measurements comparing vertical sulfate profiles in vegetated and non-vegetated sediments of a freshwater marsh located below the confluence of the Kyoungan stream and the Han River in South Korea showed that sulfate concentrations in vegetated sediments increased significantly at the beginning of the growing season and then gradually decreased during the rest of the growing season (Choi et al., 2006). Throughout the growing season, sulfate concentrations remained higher in the vegetated sediments than in the sediments without plants. Since the total pool of solid-phase sulfide is relatively large compared with the mass of sulfate in the sediments, it was suggested by Choi and co-workers that the gradual decrease of sulfate concentrations in the rhizosphere may result from limitation of the solid-phase sulfide that is in direct contact with or very close to the roots and rhizomes.
In an experiment in brackish marsh mesocosms using plants and soils from the Jiuduansha salt marsh in the Yangtze River estuary, methane fluxes from invasive S. alterniflora and native P. australis have been compared (Cheng et al., 2007). Methane emission rates were comparable between both plant species, indicating that S. alterniflora by itself is able to emit methane when the conditions in the soil permit. Unfortunately, Cheng and co-workers did not supply information on sulfate concentrations in their mesocosms. Clipping of the plant shoots below the water surface decreased the methane fluxes by 12–50 % depending on the season, but independent of the plant species. Drainage of the soils for 24 h increased the methane emission rate with both species, but was more pronounced with S. alterniflora. Two possible explanations for the increase in methane emissions in response to non-submergence were presented. First, water might have acted as a diffusion barrier that suppresses methane emissions under submerged conditions. Secondly, the soils were not dried out to support significant methane oxidation in the non-submerged, but still water-saturated soils.
EMERGENT MACROPHYTES IN CONSTRUCTED WETLANDS
It is to be expected that constructed wetlands behave in a similar way to natural wetlands, which means stimulation of methane emission by aerenchymous emergent macrophytes. As in natural wetlands, large differences in relation to methane effluxes have been observed between different emergent wetland plants in a constructed wetland (Johansson et al., 2004). The median methane emission rate from vegetation with Phalaris arundinacea was 3–4 times larger than the median emission rates from zones with G. maxima and T. latifolia. However, in a study on methane fluxes in two created, experimental marshes in the Midwestern USA, no apparent relationship was observed between emergent vegetation and methane flux, as mean flux rates were not significantly different in edge zones where emergent vegetation was removed, compared with edge zones containing emergent vegetation (Altor and Mitsch, 2008). The variability in methane flux from the different treatments, despite similarity in plant composition, implies that herbaceous macrophytes were not a primary driver of methane dynamics. However, it is possible that individual plant species such as T. latifolia or S. eurycarpum did deliver methane to the atmosphere via pressurized ventilation and that this dynamic was not observed because of the presence of diverse mixed vegetation. As discussed by these authors, reducing conditions in wetland soils create an important sink for carbon, as complex and recalcitrant organic matter such as humic acids accumulates under anoxic conditions. This carbon sequestration can outweigh the production of methane in ecosystems such as created and constructed wetlands that are in an early stage of development. In a study of methane fluxes from constructed wetlands treating dairy wastewater, Tanner et al. (1997) found higher fluxes from non-vegetated areas compared with areas with S. validus, which they attributed to a more oxidized sediment surface in the presence of vegetation.
In order to minimize methane emission from constructed as well as restored wetlands more information is needed on the interplay between emergent macrophytes and soil microbial processes in these systems also taking into account their hydrology and mineralogy.
LITERATURE CITED
- Altor AE, Mitsch WJ. Pulsing hydrology, methane emissions and carbon dioxide fluxes in created marshes: a 2-year ecosystem study. Wetlands. 2008;28:423–438. [Google Scholar]
- Armstrong J, Armstrong W. Phragmites australis – a preliminary study of soil-oxidizing sites and internal gas transport pathways. New Phytologist. 1988;108:373–382. [Google Scholar]
- Armstrong J, Armstrong W. Light-enhanced convective throughflow increases oxygenation in rhizomes and rhizosphere of Phragmites australis (Cav.) Trin. ex Steud. New Phytologist. 1990;114:121–128. doi: 10.1111/j.1469-8137.1990.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Armstrong J, Armstrong W. A convective through-flow of gases in Phragmites australis (Cav.) Trin. ex Steud. Aquatic Botany. 1991;39:75–88. [Google Scholar]
- Armstrong J, Armstrong W, Beckett PM. Phragmites australis: Venturi- and humidity-induced pressure flows enhance rhizome aeration and rhizosphere oxidation. New Phytologist. 1992;120:197–207. [Google Scholar]
- Armstrong J, Armstrong W, Beckett PM, et al. Pathways of aeration and the mechanisms and beneficial effects of humidity- and Venturi-induced convections in Phragmites australis (Cav) Trin ex Steud. Aquatic Botany. 1996;54:177–197. [Google Scholar]
- Armstrong J, Jones RE, Armstrong W. Rhizome phyllosphere oxygenation in Phragmites and other species in relation to redox potential, convective gas flow, submergence and aeration pathways. New Phytologist. 2006;172:719–731. doi: 10.1111/j.1469-8137.2006.01878.x. [DOI] [PubMed] [Google Scholar]
- Armstrong W. Aeration in higher plants. In: Woolhouse HW, editor. Advances in botanical research. New York: Academic Press; 1979. pp. 226–332. [Google Scholar]
- Armstrong W. Waterlogged soils. In: Etherington JR, editor. Environment and plant ecology. Chichester: Wiley; 1982. pp. 290–332. [Google Scholar]
- Armstrong W, Armstrong J, Beckett PM. Pressurised ventilation in emergent macrophytes: the mechanism and mathematical modelling of humidity-induced convection. Aquatic Botany. 1996;54:121–135. [Google Scholar]
- Armstrong W, Cousins D, Armstrong J, Turner DW, Beckett PM. Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Annals of Botany. 2000;86:687–703. [Google Scholar]
- Bendix M, Tornbjerg T, Brix H. Internal gas transport in Typha latifolia L. and Typha angustifolia L. 1. Humidity-induced pressurization and convective throughflow. Aquatic Botany. 1994;49:75–89. [Google Scholar]
- Bergstrom I, Makela S, Kankaala P, Kortelainen P. Methane efflux from littoral vegetation stands of southern boreal lakes: an upscaled regional estimate. Atmospheric Environment. 2007;41:339–351. [Google Scholar]
- Bodelier PLE, Laanbroek HJ. Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiology Ecology. 2004;47:265–277. doi: 10.1016/S0168-6496(03)00304-0. [DOI] [PubMed] [Google Scholar]
- Bodelier PLE, Libochant JA, Blom CWPM, Laanbroek HJ. Dynamics of nitrification and denitrification in root-oxygenated sediments and adaptation of ammonia-oxidizing bacteria to low-oxygen or anoxic habitats. Applied Environmental Microbiology. 1996;62:4100–4107. doi: 10.1128/aem.62.11.4100-4107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse UFP, Frenzel P, Conrad R. Inhibition of methane oxidation by ammonium in the surface layer of a littoral sediment. FEMS Microbiology Ecology. 1993;13:123–134. [Google Scholar]
- Brix H. Light-dependent variations in the composition of the internal atmosphere of Phragmites australis (Cav.) Trin. ex Steudel. Aquatic Botany. 1988;30:319–329. [Google Scholar]
- Brix H. Gas exchange through dead culms of reed Phragmites australis (Cav.) Trin. ex Steudel. Aquatic Botany. 1989;35:81–98. [Google Scholar]
- Brix H, Sorrell BK, Orr PT. Internal pressurization and convective gas flow in some emergent freshwater macrophytes. Limnology and Oceanography. 1992;37:1420–1433. [Google Scholar]
- Brunel B, Janse JD, Laanbroek HJ, Woldendorp JW. Effect of transient oxic conditions on the composition of the nitrate-reducing community from the rhizosphere of Typha angustifolia. Microbial Ecology. 1992;24:51–61. doi: 10.1007/BF00171970. [DOI] [PubMed] [Google Scholar]
- Calhoun A, King GM. Regulation of root-associated methanotrophy by oxygen availability in the rhizosphere of two aquatic macrophytes. Applied Environmental Microbiology. 1997;63:3051–3058. doi: 10.1128/aem.63.8.3051-3058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carini SA, Orcutt BN, Joye SB. Interactions between methane oxidation and nitrification in coastal sediments. Geomicrobiology Journal. 2003;20:355–374. [Google Scholar]
- Cheng XL, Peng RH, Chen JQ, et al. CH4 and N2O emissions from Spartina alterniflora and Phragmites australis in experimental mesocosms. Chemosphere. 2007;68:420–427. doi: 10.1016/j.chemosphere.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Choi JH, Park SS, Jaffe PR. The effect of emergent macrophytes on the dynamics of sulfur species and trace metals in wetland sediments. Environmental Pollution. 2006;140:286–293. doi: 10.1016/j.envpol.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Christensen TR. Methane emission from Arctic tundra. Biogeochemistry. 1993;21:117–139. [Google Scholar]
- Conlin TSS, Crowder AA. Location of radial oxygen loss and zones of potential iron uptake in a grass and 2 nongrass emergent species. Canadian Journal of Botany-Revue Canadienne de Botanique. 1989;67:717–722. [Google Scholar]
- Conrad R. Microbial ecology of methanogens and methanotrophs. Advances in Agronomy. 2007;96:1–63. [Google Scholar]
- Dacey JWH. Internal winds in water lilies – an adaptation for life in anaerobic sediments. Science. 1980;210:1017–1019. doi: 10.1126/science.210.4473.1017. [DOI] [PubMed] [Google Scholar]
- Denman KL, Brasseur G, Chidthaisong A, et al. Couplings between changes in the climate system and biogeochemistry. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Ding W, Cai Z, Tsuruta H, Li X. Effect of standing water depth on methane emissions from freshwater marshes in northeast China. Atmospheric Environment. 2002;36:5149–5157. [Google Scholar]
- Ding W, Cai Z, Tsuruta H, Li X. Key factors affecting spatial variation of methane emissions from freshwater marshes. Chemosphere. 2003;51:167–173. doi: 10.1016/s0045-6535(02)00804-4. [DOI] [PubMed] [Google Scholar]
- Ding W, Cai Z, Tsuruta H. Diel variation in methane emissions from the stands of Carex lasiocarpa and Deyeuxia angustifolia in a cool temperate freshwater marsh. Atmospheric Environment. 2004;a 38:181–188. [Google Scholar]
- Ding W, Cai Z, Tsuruta H. Methane concentration and emission as affected by methane transport capacity of plants in freshwater marsh. Water, Air, and Soil Pollution. 2004;b 158:99–111. [Google Scholar]
- Ding W, Cai Z, Tsuruta H. Summertime variation of methane oxidation in the rhizosphere of a Carex dominated freshwater marsh. Atmospheric Environment. 2004;c 38:4165–4173. [Google Scholar]
- Ding W, Cai Z, Wang D. Preliminary budget of methane emissions from natural wetlands in China. Atmospheric Environment. 2004;d 38:751–759. [Google Scholar]
- Ding W, Cai Z, Tsuruta H. Plant species effects on methane emissions from freshwater marshes. Atmospheric Environment. 2005;39:3199–3207. [Google Scholar]
- Duan XN, Wang XK, Mu YJ, Ouyang ZY. Seasonal and diurnal variations in methane emissions from Wuliangsu Lake in arid regions of China. Atmospheric Environment. 2005;39:4479–4487. [Google Scholar]
- Emerson D, Weiss JV, Megonigal JP. Iron-oxidizing bacteria are associated with ferric hydroxide precipitates (Fe-plaque) on the roots of wetland plants. Applied Environmental Microbiology. 1999;65:2758–2761. doi: 10.1128/aem.65.6.2758-2761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzel P, Rudolph J. Methane emission from a wetland plant: the role of CH4 oxidation in Eriophorum. Plant and Soil. 1998;202:27–32. [Google Scholar]
- Gotoh S, Patrick WH. Transformation of iron in a waterlogged soil as influenced by redox potential and pH. Soil Science Society of America Proceedings. 1974;38:66–71. [Google Scholar]
- Greenup AL, Bradford MA, McNamara NP, Ineson P, Lee JA. The role of Eriophorum vaginatum in CH4 flux from an ombrotrophic peatland. Plant and Soil. 2000;227:265–272. [Google Scholar]
- Grunfeld S, Brix H. Methanogenesis and methane emissions: effects of water table, substrate type and presence of Phragmites australis. Aquatic Botany. 1999;64:63–75. [Google Scholar]
- Heilman MA, Carlton RG. Methane oxidation associated with submersed vascular macrophytes and its impact on plant diffusive methane flux. Biogeochemistry. 2001;52:207–224. [Google Scholar]
- Hines ME, Duddleston KN, Kiene RP. Carbon flow to acetate and C-1 compounds in northern wetlands. Geophysical Research Letters. 2001;28:4251–4254. [Google Scholar]
- Hirota M, Tang YH, Hu QW, et al. Methane emissions from different vegetation zones in a Qinghai-Tibetan Plateau wetland. Soil Biology and Biochemistry. 2004;36:737–748. [Google Scholar]
- Holzapfel-Pschorn A, Conrad R, Seiler W. Effects of vegetation on the emission of methane from submerged paddy soil. Plant and Soil. 1986;92:223–233. [Google Scholar]
- Howarth RW, Giblin A. Sulfate reduction in the salt marshes at Sapelo Island, Georgia. Limnology and Oceanography. 1983;28:70–82. [Google Scholar]
- Howarth RW, Teal JM. Sulfate reduction in New-England salt-marsh. Limnology and Oceanography. 1979;24:999–1013. [Google Scholar]
- Joabsson A, Christensen TR. Methane emissions from wetlands and their relationship with vascular plants: an Arctic example. Global Change Biology. 2001;7:919–932. [Google Scholar]
- Johansson AE, Gustavsson AM, Oquist MG, Svensson BH. Methane emissions from a constructed wetland treating wastewater – seasonal and spatial distribution and dependence on edaphic factors. Water Research. 2004;38:3960–3970. doi: 10.1016/j.watres.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Juutinen S, Larmola T, Remus R, Mirus E, Merbach W, Silvola J, Augustin J. The contribution of Phragmites australis litter to methane (CH4) emission in planted and non-planted fen microcosms. Biology and Fertility of Soils. 2003;38:10–14. [Google Scholar]
- Kankaala P, Bergstrom I. Emission and oxidation of methane in Equisetum fluviatile stands growing on organic sediment and sand bottoms. Biogeochemistry. 2004;67:21–37. [Google Scholar]
- Kankaala P, Ojala A, Kaki T. Temporal and spatial variation in methane emissions from a flooded transgression shore of a boreal lake. Biogeochemistry. 2004;68:297–311. [Google Scholar]
- Kankaala P, Kaki T, Makela S, Ojala A, Pajunen H, Arvola L. Methane efflux in relation to plant biomass and sediment characteristics in stands of three common emergent macrophytes in boreal mesoeutrophic lakes. Global Change Biology. 2005;11:145–153. [Google Scholar]
- King GM. Dynamics and control of methane oxidation in a Danish wetland sediment. FEMS Microbiology Ecology. 1990;74:309–323. [Google Scholar]
- King GM, Garey MA. Ferric tron reduction by bacteria associated with the roots of freshwater and marine macrophytes. Applied Environmental Microbiology. 1999;65:4393–4398. doi: 10.1128/aem.65.10.4393-4398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepac-Ceraj V, Bahr M, Crump BC, Teske AP, Hobbie JE, Polz MF. High overall diversity and dominance of microdiverse relationships in salt marsh sulphate-reducing bacteria. Environmental Microbiology. 2004;6:686–698. doi: 10.1111/j.1462-2920.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- Klinkhamer GP, Palmer MR. Uranium in the oceans: where it goes and why. Geochimica Cosmochimica Acta. 1991;55:1799–1806. [Google Scholar]
- Koch MS, Mendelssohn IA. Sulfide as a soil phytotoxin – differential responses in 2 marsh species. Journal of Ecology. 1989;77:565–578. [Google Scholar]
- Laanbroek HJ. Bacterial cycling of minerals that affect plant-growth in waterlogged soils – a review. Aquatic Botany. 1990;38:109–125. [Google Scholar]
- Lamers LPM, Tomassen HBM, Roelofs JGM. Sulfate-induced entrophication and phytotoxicity in freshwater wetlands. Environmental Science and Technology. 1998;32:199–205. [Google Scholar]
- Lee RW. Oxidation of sulfide by Spartina alterniflora roots. Limnology and Oceanography. 1999;44:1155–1159. [Google Scholar]
- Lowe KL, Dichristina TJ, Roychoudhury AN, Van Cappellen P. Microbiological and geochemical characterization of microbial Fe(III) reduction in salt marsh sediments. Geomicrobiology Journal. 2000;17:163–176. [Google Scholar]
- Megonigal JP, Whalen SC, Tissue DT, et al. Fourth Symposium on Biogeochemistry of Wetlands. New Orleans, Louisiana, USA: 1996. The use 14CO2 to trace carbon metabolism from photosynthesis through methanogenesis in a wetland plant–soil–atmosphere microcosm. [Google Scholar]
- Megonigal JP, Hines ME, Visscher PT. Anaerobic metabolism: linkages to trace gases and aerobic processes. In: Holland HD, Turekian KK, editors. Treatise on geochemistry. Elsevier; 2003. pp. 317–424. [Google Scholar]
- Mendelssohn IA, Postek MT. Elemental analysis of deposits on the roots of Spartina alterniflora Loisel. American Journal of Botany. 1982;69:904–912. [Google Scholar]
- Miletto M, Loy A, Antheunisse AM, Loeb R, Bodelier PL, Laanbroek HJ. Biogeography of sulfate-reducing prokaryotes in river floodplains. FEMS Microbiology Ecology. 2008;64:395–406. doi: 10.1111/j.1574-6941.2008.00490.x. [DOI] [PubMed] [Google Scholar]
- Nijburg JW, Laanbroek HJ. The fate of N-15-nitrate in healthy and declining Phragmites australis stands. Microbial Ecology. 1997;a 34:254–262. doi: 10.1007/s002489900055. [DOI] [PubMed] [Google Scholar]
- Nijburg JW, Laanbroek HJ. The influence of Glyceria maxima and nitrate input on the composition and nitrate metabolism of the dissimilatory nitrate-reducing bacterial community. FEMS Microbiology Ecology. 1997;b 22:57–63. doi: 10.1128/aem.63.3.931-937.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijburg JW, Coolen MJL, Gerards S, Gunnewiek PJAK, Laanbroek HJ. Effects of nitrate availability and the presence of Glyceria maxima on the composition and activity of the dissimilatory nitrate-reducing bacterial community. Applied Environmental Microbiology. 1997;63:931–937. doi: 10.1128/aem.63.3.931-937.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nold SC, Boschker HTS, Pel R, Laanbroek HJ. Ammonium addition inhibits C-13-methane incorporation into methanotroph membrane lipids in a freshwater sediment. FEMS Microbiology Ecology. 1999;29:81–89. [Google Scholar]
- Pedersen O, Binzer T, Borum J. Sulphide intrusion in eelgrass (Zostera marina L.) Plant, Cell and Environment. 2004;27:595–602. [Google Scholar]
- Pedersen O, Vos H, Colmer TD. Oxygen dynamics during submergence in the halophytic stem succulent Halosarcia pergranulata. Plant, Cell and Environment. 2006;29:1388–1399. doi: 10.1111/j.1365-3040.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Ponnamperuma FN. The chemistry of submerged soils. Advances in Agronomy. 1972;24:29–96. [Google Scholar]
- Ponnamperuma FN. Effects of flooding on soils. Orlando, FL: Academic Press; 1984. [Google Scholar]
- Purvaja R, Ramesh R. Natural and anthropogenic methane emission from coastal wetlands of South India. Environmental Management. 2001;27:547–557. doi: 10.1007/s002670010169. [DOI] [PubMed] [Google Scholar]
- Purvaja R, Ramesh R, Frenzel P. Plant-mediated methane emission from an Indian mangrove. Global Change Biology. 2004;10:1825–1834. [Google Scholar]
- Roden EE, Wetzel RG. Competition between Fe(III)-reducing and methanogenic bacteria for acetate in iron-rich freshwater sediments. Microbial Ecology. 2003;45:252–258. doi: 10.1007/s00248-002-1037-9. [DOI] [PubMed] [Google Scholar]
- Roslev P, King GM. Regulation of methane oxidation in a freshwater wetland by water table changes and anoxia. FEMS Microbiology Ecology. 1996;19:105–115. [Google Scholar]
- Saarnio S, Wittenmayer L, Merbach W. Rhizospheric exudation of Eriophorum vaginatum L. – potential link to methanogenesis. Plant and Soil. 2004;267:343–355. [Google Scholar]
- Sand-Jensen K, Pedersen O, Binzer T, Borum J. Contrasting oxygen dynamics in the freshwater isoetid Lobelia dortmanna and the marine seagrass Zostera marina. Annals of Botany. 2005;96:613–623. doi: 10.1093/aob/mci214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimel JP. Plant-transport and methane production as controls on methane flux from arctic wet meadow tundra. Biogeochemistry. 1995;28:183–200. [Google Scholar]
- Sorrell BK, Boon PI. Convective gas flow in Eleocharis sphacelata R. Br.: methane transport and release from wetlands. Aquatic Botany. 1994;47:197–212. [Google Scholar]
- Sorrell BK, Brix H. Effects of water vapour pressure deficit and stomatal conductance on photosynthesis, internal pressurization and convective flow in three emergent wetland plants. Plant and Soil. 2003;253:71–79. [Google Scholar]
- Sorrell BK, Dromgoole FI. Oxygen-transport in the submerged fresh-water macrophyte Egeria densa Planch. I. Oxygen production, storage and release. Aquatic Botany. 1987;28:63–80. [Google Scholar]
- Sorrell BK, Mendelssohn IA, McKee KL, Woods RA. Ecophysiology of wetland plant roots: a modelling comparison of aeration in relation to species distribution. Annals of Botany. 2000;86:675–685. [Google Scholar]
- Strom L, Ekberg A, Mastepanov M, Christensen TR. The effect of vascular plants on carbon turnover and methane emissions from a tundra wetland. Global Change Biology. 2003;9:1185–1192. [Google Scholar]
- Strom L, Mastepanov M, Christensen TR. Species-specific effects of vascular plants on carbon turnover and methane emissions from wetlands. Biogeochemistry. 2005;75:65–82. [Google Scholar]
- Tanner CC, Adams DD, Downes MT. Methane emissions from constructed wetlands treating agricultural wastewaters. Journal of Environmental Quality. 1997;26:1056–1062. [Google Scholar]
- Tornbjerg T, Bendix M, Brix H. Internal gas transport in Typha latifolia L. and Typha angustifolia L. 2. Convective throughflow pathways and ecological significance. Aquatic Botany. 1994;49:91–105. [Google Scholar]
- Van der Nat F-J. Methane emission from freshwater marshes. Nijmegen: Nijmegen Universty; 2000. [Google Scholar]
- Van der Nat F, Middelburg JJ. Effects of two common macrophytes on methane dynamics in freshwater sediments. Biogeochemistry. 1998;a 43:79–104. [Google Scholar]
- Van der Nat F, Middelburg JJ. Seasonal variation in methane oxidation by the rhizosphere of Phragmites australis and Scirpus lacustris. Aquatic Botany. 1998;b 61:95–110. [Google Scholar]
- Van der Nat FJ, Middelburg JJ. Methane emission from tidal freshwater marshes. Biogeochemistry. 2000;49:103–121. [Google Scholar]
- Van der Nat FJWA, De Brouwer JFC, Middelburg JJ, Laanbroek HJ. Spatial distribution and inhibition by ammonium of methane oxidation in intertidal freshwater marshes. Applied Environmental Microbiology. 1997;63:4734–4740. doi: 10.1128/aem.63.12.4734-4740.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Nat F, Middelburg JJ, Van Meteren D, Wielemakers A. Diel methane emission patterns from Scirpus lacustris and Phragmites australis. Biogeochemistry. 1998;41:1–22. [Google Scholar]
- Vretare Strand V. The influence of ventilation systems on water depth penetration of emergent macrophytes. Freshwater Biology. 2002;47:1097–1105. [Google Scholar]
- Vretare Strand V, Weisner SEB. Interactive effects of pressurized ventilation, water depth and substrate conditions on Phragmites australis. Oecologia. 2002;131:490–497. doi: 10.1007/s00442-002-0915-7. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Muyzer G, Bodelier PLE, Laanbroek HJ. Diversity of iron oxidizers in wetland soils revealed by novel 16S rRNA primers targeting Gallionella-related bacteria. ISME Journal. 2009;3:715–725. doi: 10.1038/ismej.2009.7. [DOI] [PubMed] [Google Scholar]
- Wang YH, Inamori R, Kong H, et al. Nitrous oxide emission from polyculture constructed wetlands: effect of plant species. Environmental Pollution. 2008;a 152:351–360. doi: 10.1016/j.envpol.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Wang YH, Inamori R, Kong HN, et al. Influence of plant species and wastewater strength on constructed wetland methane emissions and associated microbial populations. Ecological Engineering. 2008;b 32:22–29. [Google Scholar]
- Weiss JV, Emerson D, Backer SM, Megonigal JP. Enumeration of Fe(II)-oxidizing and Fe(III)-reducing bacteria in the root zone of wetland plants: implications for a rhizosphere iron cycle. Biogeochemistry. 2003;64:77–96. [Google Scholar]
- Weiss JV, Emerson D, Megonigal JP. Geochemical control of microbial Fe(III) reduction potential in wetlands: comparison of the rhizosphere to non-rhizosphere soil. FEMS Microbiology Ecology. 2004;48:89–100. doi: 10.1016/j.femsec.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Weiss JV, Rentz JA, Plaia T, et al. Characterization of neutrophilic Fe(II)-oxidizing bacteria isolated from the rhizosphere of wetland plants and description of Ferritrophicum radicicola gen. nov sp nov., and Sideroxydans paludicola sp nov. Geomicrobiology Journal. 2007;24:559–570. [Google Scholar]
- Wind T, Conrad R. Sulfur compounds, potential turnover of sulfate and thiosulfate, and numbers of sulfate-reducing bacteria in planted and unplanted paddy soil. FEMS Microbiology Ecology. 1995;18:257–266. [Google Scholar]