Abstract
Background and Aims
Dry fruits remain around the seeds at dispersal in a number of species, especially the Brassicaceae. Explanations for this vary, but usually involve mechanisms of innate dormancy. We speculate that, instead, a persistent fruit may give additional protection through control of dehydration, to species growing in arid or Mediterranean environments where water is sporadic.
Methods
X-rays and weight measurements were used to determine the extent to which Raphanus raphanistrum seeds within mature fruits imbibe water, and germination tests determined the roles of the fruit and seed coat in seed dormancy. Rates of water uptake and desiccation, and seedling emergence were compared with and without the fruit. Finally, germinability of seeds extracted from fruits was determined after various periods of moist conditions followed by a range of dry conditions.
Key Results
Most seeds rapidly take up water within the fruit, but they do not fully imbibe when compared with naked seeds. The seed coat is more important than the dry fruit wall in maintaining seed dormancy. The presence of a dry fruit slows emergence from the soil by up to 6–8 weeks. The fruit slows the rate of desiccation of the seed to a limited extent. The presence of the fruit for a few days during imbibition somehow primes more seeds to germinate than if the fruit is absent; longer moist periods within the pod appear to induce dormancy.
Conclusions
The fruit certainly modifies the seed environment as external conditions change between wet and dry, but not to a great extent. The major role seems to be: (a) the physical restriction of imbibition and germination; and (b) the release and then re-imposition of dormancy within the seed. The ecological significance of the results requires more research under field conditions.
Keywords: Wild radish, Raphanus raphanistrum, imbibition, desiccation, dry fruit wall, germination, dormancy, X-ray
INTRODUCTION
At maturity, most non-fleshy fruits dry to encase the seeds within. The fruit wall then, in time, fractures or an opening develops, releasing the seeds into the environment. In some species, the fruit has adapted so that it remains around the seeds at dispersal. There may be several reasons why this adaptation has evolved: for example, the outside of the fruit may have appendages that aid dispersal by animals; it may help protect against predation; or it may provide further mechanisms of seed dormancy, either physical or chemical, to ensure that seeds are able to survive for several seasons in the soil. In the case of many Brassicaceae, this mechanism occurs in species growing in arid or Mediterranean environments where water is limiting, at least seasonally (e.g. Myagrum, Rapistrum, Neslia, Cakile and Raphanus). There is a ‘point of no return’ for any germinating seed (Evenari et al., 1971), before which dehydration can be tolerated, but after which dehydration will cause seedling death. Perhaps then, by restricting the entry of water, the fruit might prevent seed imbibition when a rainfall event is poor, hence preventing germination that would prove fatal because there is insufficient water in the soil for subsequent growth. Alternatively, the fruit wall might provide an internal environment that keeps the seed moist after a period of moderate rainfall that causes imbibition, thus extending the effective period over which germination can occur, or enabling a germinating seedling to survive until the next rainfall event by slowing desiccation. A similar function may result in those species that form a mucilagenous coat around the seed after wetting (e.g. Brassica tournefortii, Sisymbrium altissimum and some Lepidium spp.), although this is usually interpreted in relation to oxygen uptake (Heydecker and Orphanos, 1968; Werker, 1980).
Raphanus raphanistrum (wild radish) is a native of the Mediterranean region and has since spread throughout most of Europe, South Africa, North America, Mexico, Brazil, New Zealand and Australia (Parsons and Cuthbertson, 1992). Within Australia, R. raphanistrum occurs in the wheat-growing areas with a Mediterranean climate (Dellow et al., 2006). At maturity, its fruit (colloquially called a pod) falls to the ground and later separates into sections, each containing a single seed (Fig. 1); fruits going through a combine harvester may be broken into sections immediately, creating propagules of a similar size to the seeds of several crops. Its seeds can remain viable in the soil for at least 5 years (Roberts and Boddrell, 1983; Code et al., 1987), a duration achieved by other Brassicaceae species that release naked seeds. At maturity, the fruit wall is extremely difficult to remove from the seed; however, over time in or on the soil, it becomes more easily cleaved along the line of the two fruit valves.
Fig. 1.
Single fruit segment of R. raphanistrum, cut open longitudinally to show the seed within.
It has been proposed elsewhere that the fruit wall of R. raphanistrum, rather than the seed coat, is its major dormancy mechanism: an increase in percentage germination has been observed once the seeds are extracted from the fruits (Mekenian and Willemsen, 1975; Cheam, 1986). Conditions likely to cause fruit (as well as seed coat) degradation, such as cultivation and weathering, enhance seedling emergence (Cheam, 1986). Both Mekenian and Willemsen (1975) and Cheam (1986) postulated that the dormancy mechanism provided by the fruit was through physical restriction, inhibiting the germination process, and/or a non-leachable chemical inhibitor associated with the fruit wall. Other possibilities are that the fruit prevents seed imbibition, restricts gas flow or prevents the escape of inhibitors from the seed coat or from the seed itself.
The aim of our study was to better elucidate the ecological role of the fruit wall in species with persistent fruits, such as R. raphanistrum. Specifically, we examined the potential role of the dry fruit as a dormancy mechanism and a regulator of seed moisture, and the overall impact of this on seedling emergence.
MATERIALS AND METHODS
Does the fruit wall prevent imbibition?
Two approaches were used to determine the effect of the fruit wall on imbibition. First, X-ray images were used to determine whether seed expansion occurs when fruits are immersed in water and, if so, whether the gap between the seed and the inner surface of the fruit closes, physically restricting germination. Secondly, imbibition was measured by weighing seeds and fruits over time under moist conditions.
X-ray studies.
In Experiment 1, two populations of Raphanus raphanistrum L. from Australia were used: a 2-year-old laboratory-stored accession from Chateau Tahbilk, Victoria, and fresh seed collected from Tooleen, Victoria (for a summary of the source populations used in this study, see Table 1). Ninety-six randomly selected fruit segments from each population were weighed and put into enzyme-linked immunosorbent assay (ELISA) plate cells. Prior to each X-ray, the plate was carefully inverted on to its lid, as the depth of the ELISA plate cells limited the clarity of X-ray images. The dry fruit segments were X-rayed at 20 kV, 15 mA, for 0·5 s at a distance of 60 cm. They were then returned to their respective ELISA plate cells, distilled water was added to each cell and they were stored in a refrigerator at 4 °C, to prevent germination. After 5 d, the water was drained off, and the fruits blotted dry and X-rayed again. Following this, seeds were extracted from the fruits and weighed. Another 96 fruit segments were randomly selected from each population and the seeds were extracted and weighed to obtain an estimate of seed weight pre-soaking. The maximum and minimum seed dimensions were measured from the pre- and post-soaking X-ray images under a dissecting microscope. Pre- and post-soaking maximum and minimum dimensions and seed weight from soaked and dry fruits for each population were compared using t-tests.
Table 1.
Source populations for the Raphanus raphanistrum used in this study
| Population | State | Co-ordinates | Experiment |
|---|---|---|---|
| Chateau Tahbilk | Victoria | 36·83°S, 145·22°E | 1, 2 |
| Tooleen | Victoria | 36·70°S, 144·73°E | 1, 2 |
| Dimboola | Victoria | 36·43°S, 142·07°E | 2 |
| Yuna | Western Australia | 28·31°S, 115·03°E | 3 |
| Curyo | Victoria | 36·00°S, 143·27°E | 3, 4 |
| Hopetoun | Victoria | 35·70°S, 142·10°E | 5, 6 |
| Bundoora | Victoria | 37·72°S, 145·04°E | 3 |
| Burnley | Victoria | 37·50°S 145·01°E | 7, 8 |
Fruits collected from all populations were regarded as typical of the species in the region and its within-population variation, with no clear visual differences between collections. All fruits were collected at maturity, when they were dry, brown and easily broken into sections.
To examine the time-course of swelling (Experiment 2), six replicates of 20 fruit segments from each of three R. raphanistrum populations were weighed and glued on to Petri dishes using Superglue® to prevent movement. A minimal amount of glue was used, ensuring that the fruit segments had free access to water. The populations were a 2-year-old accession from Chateau Tahbilk, a 1-year-old accession from Dimboola, Victoria, both stored in the laboratory, and fresh seeds collected from Tooleen, Victoria. The dishes were then X-rayed, as in the previous experiment, soaked in distilled water and stored in a refrigerator at 4 °C. The fruit segments were X-rayed daily for 5 d; on each occasion the water was drained off and fruit segments blotted dry. Water was again added after the X-rays were taken. Seed maximum and minimum dimensions were measured from the X-ray images. Preliminary analysis showed no reason to transform the data. For each day, analysis of variance (ANOVA) was conducted on the dimensions of the seeds. Increasing length or width of seeds over time was compared by paired t-tests between each day's measurement. The number of fruit segments that had imbibed was counted from the images, by assessing those where length or width had clearly increased. An ANOVA was then conducted on this variable.
Mass studies.
The X-ray experiments showed how many seeds had swelled when immersed in water: but had they fully imbibed and would they do so when the fruits were merely kept damp? In Experiment 3, samples of 100 R. raphanistrum fruit segments were weighed, put into Petri dishes lined with two Whatman 1001 filter papers, and 10 mL of distilled water was added. The moisture contents of seeds and fruit segments were assessed after 0·25, 0·5, 1, 2, 4, 6, 8 and 10 d. At each time of weighing, the seeds were dissected from the fruits in six replicate Petri dishes. During this process, both fruits and extracted seeds were kept in a glass Petri dish, with a covering lid to limit moisture loss prior to weighing. Only the fruit being cut was out of the dish at any one time. The extracted seeds and the fruit segments were then put into an oven at 40 °C for 48 h for dry weight assessment. Seed extraction was done by hand, taking about 30 min per replicate. The populations were collected from Yuna (Western Australia), Curyo (Victoria) and Bundoora (Victoria). ANOVA was conducted on percentage water content [100(wtwet – wtdry)/wtdry] of the seeds and the fruit segments.
How much does the presence of an intact seed coat and the breakage of the fruit into sections affect the imbibition of the seed?
Using fresh seeds from Curyo (see above), three replicates, with eight imbibition times (as in the previous experiment), and three seed states (seeds extracted from fruits, seeds within fruit segments and seeds within whole fruits) were established (Experiment 4). Four fruits (a total seed number of between 21 and 37 seeds) were randomly allocated to each treatment. For the extracted seed treatment, seeds were carefully removed from the fruit with a razor blade. Where seed coat damage was observed, this was noted. For the fruit segment treatment, a fruit was broken up by hand into single-seeded sections. The seeds, fruit segments and fruits were weighed, put into Petri dishes lined with two Whatman 1001 filter papers and 5 mL of distilled water was added. The measurement and statistical analysis of water uptake by treatments was the same as in the previous experiment, except that mass was measured on an individual seed basis. In the final analysis, data from the isolated seeds whose coats were damaged were omitted from the calculations.
Does the seed coat or the fruit wall impose dormancy?
In Petri dishes in the laboratory, few seedlings emerge from within fruit segments. This could be because of some sort of physiological dormancy imposed by the fruit or because of the high mechanical strength needed to break through the walls of fresh fruits. We therefore examined whether the seed coat causes dormancy and, thus, whether any effect of the fruit is additional to that of the seed coat or whether it is the sole cause of dormancy (Experiment 5). Seeds were collected from Hopetoun (Victoria) and extracted from their fruits using a razor blade. Extracted seeds were then put into three classes of visually assessed damage (1) no seed coat damage; (2) <10 % damage; and (3) 10–25 % damage. One half of the intact seed coat group was then put into a tumbling device lined with 150-grade ‘wet and dry’ sandpaper, and tumbled for 3 h. A further group of seeds was still enclosed in their fruit segments. Fifty seeds of each group were put into Petri dishes and then into an incubator at temperatures of 25/15 °C on a 12 h/12 h light/dark schedule. They were arranged as a randomized complete block design with four replicates. Germination was assessed daily for 21 d; seedlings were removed and further water added as required. After this time, the seed coats of half the remaining seeds from each treatment were removed. The experiment was concluded 9 d after this. Statistical analysis of the germination percentage at day 21 and at the conclusion of the experiment at day 30 was conducted by ANOVA after data were arcsine transformed.
Does the fruit wall affect rate of emergence?
Seeds of two seed states (i.e. hand-extracted seeds and seeds still enclosed by the fruit segment) were buried in May at four depths in a randomized complete block experiment with three replications (Experiment 6). Seeds were obtained from Hopetoun (see above). Burial depths were 10, 20, 40 and 80 mm in 200 mm diameter pots. Each pot had either 300 seeds or fruit segments and 6 kg of the local clay–loam soil. The pots were sunk into the ground at La Trobe University, Bundoora so that the soil level in the pot was similar to that surrounding it. Soil temperatures were measured at each burial depth and recorded by a data-logger every 15 min. Emergence was assessed daily for the first 30 d, then weekly for 21 months. At the conclusion, seeds were recovered from each pot by wet sieving (1 mm sieve). Fruit segments were cut open and seed viability assessed using a 1 % tetrazolium chloride solution (Moore, 1985).
ANOVA was conducted on: cumulative emergence after 12 and 21 months; time to 50 % emergence in the first 12 months; and total number of viable seeds recovered. Emergence data were arcsine transformed prior to analysis.
By how much does the fruit wall delay seed desiccation?
To determine how much the fruit delays desiccation of the seed, dry fruits from the Burnley Campus of the University of Melbourne, Victoria were soaked in deionized water in the laboratory (Experiment 7). After 4 d of soaking, a sample of seeds was removed from the fruits. These bare seeds and the rest of the soaked fruits were placed at a constant temperature of 30 °C and a relative humidity of 33–35 %. For the first 12 h, samples of seeds were extracted every 2 h from the remaining fruits; thereafter sampling was every 8 h until a constant weight was reached. Each sample was weighed as soon as possible after extraction using the procedures described above. An exponential decay regression model was fitted to the relationship between seed weight and duration of drying. Curves were compared using parallel line analysis.
Will the fruit wall affect tolerance of post-imbibition desiccation?
Here we examined the effect of post-imbibition drying on the germination of seeds extracted from their fruits: would a delay in drying caused by the presence of a fruit potentially increase survival of germinating seeds? Fresh mature fruits were collected from plants grown at the Burnley Campus of the University of Melbourne and stored for 2 weeks under laboratory conditions (Experiment 8). Seeds were extracted by hand from their fruits. There were three replicate Petri dishes of six durations of moist conditions (0, 2, 4, 6, 8 and 12 d) and six subsequent durations of dry conditions (0, 2, 4, 6, 8 and 12 d) in a factorial design. Single-seed fruit segments were first immersed in deionized water at 22 °C for different durations. Seeds were then extracted by hand using a razor blade and 25 were placed in each open Petri dish in an incubator at 30 °C (12 h light and 12 h dark) at 25–28 % relative humidity. A small portion (10–20 %) of each seed coat was deliberately damaged in order to ensure maximum germination in the final phase of the experiment. After the period of drying, seeds were put into plastic Petri dishes with a Whatman No. 1 filter paper and 4 mL of deionized water; dishes were sealed with a strip of Parafilm. These were placed into an incubator at alternating temperatures of 25/20 °C on a 12/12 h light/dark schedule. Germination was recorded daily for 10 d. The remaining non-germinated seeds were examined for firmness, usually a good indicator of viability (Young, 2001).
We repeated the experiment to establish whether the reduced germination after long periods of moist conditions (see below) was due to mortality or the induction of secondary dormancy (Experiment 9). After imbibition for 14 d, drying and subsequent incubation on filter paper, the non-germinated seeds were placed in a 1 % tetrazolium chloride solution for 18 h at 25 °C. The seed coat was then removed, and the staining was examined and compared with illustrations of non-viable seeds (Leist et al., 2003).
RESULTS
Does the fruit wall prevent imbibition?
In both the Chateau Tahbilk and Tooleen accessions there was an increase in seed size within the fruit segment after 5 d of immersion in water (Table 2). Where individual seeds were followed through time, >78 % of seeds had imbibed (as indicated by a clear increase in seed length or seed width) after 1 d (Table 3). There was an effect of population on seed imbibition (P < 0·05), with a greater percentage imbibition in the Chateau Tahbilk and Dimboola accessions than Tooleen. However, the overall patterns over time were very similar. The proportion of seeds that had increased either their maximum or minimum dimension was similar from day 2 onwards.
Table 2.
Greatest dimension (mm) of seeds within fruit segments (means) after 5 d of immersion, as determined by X-ray imaging
| Maximum dimension (mm) |
|||
|---|---|---|---|
| Population | Pre-immersion | Post-immersion | t (pre- vs. post-) |
| Chateau Tahbilk | 3·6 | 4·3 | 27·2*** (62 d.f.) |
| Tooleen | 3·7 | 4·1 | 47·4*** (84 d.f.) |
***indicates significant difference (P <0·001).
Table 3.
Imbibition of seeds within fruit segments, as determined from X-rays
| Day |
||||||||
|---|---|---|---|---|---|---|---|---|
| Population | 0 | 1 | 2 | 3 | 4 | 5 | s.e. | |
| % seeds increased in size | Chateau Tahbilk | – | 85·5 | 92·8 | 94·0 | 95·2 | 97·2 | |
| Dimboola | – | 90·8 | 90·8 | 90·8 | 91·3 | 92·7 | ||
| Tooleen | – | 78·2 | 85·7 | 86·8 | 87·0 | 87·3 | ||
| Mean | – | 84·8 | 89·8 | 90·5 | 91·2 | 92·4 | 1·20 | |
| Maximum dimension (mm) | Chateau Tahbilk | 2·41 | 2·73 | 2·78 | 2·82 | 2·83 | 2·85 | |
| Dimboola | 2·56 | 2·82 | 2·89 | 2·90 | 2·90 | 2·91 | ||
| Tooleen | 2·42 | 2·84 | 2·84 | 2·84 | 2·86 | 2·85 | ||
| Mean | 2·47 | 2·80 | 2·84 | 2·85 | 2·86 | 2·87 | 0·24 | |
The s.e. is for the comparison of population means within any one day.
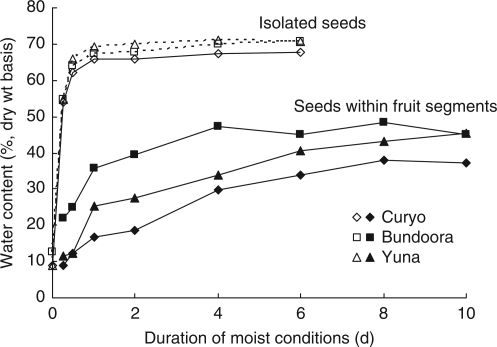
If seeds were enclosed in an entire fruit or fruit segment, their rate of imbibition was considerably reduced (P < 0·001) and the final amount of water taken up was less than for seeds removed from the fruit prior to application of water (Fig. 2 and Table 4). There was no difference in water uptake of seeds within fruit segments and those within whole fruits. The greatest rate of increase in mean seed mass of both extracted and enclosed seeds occurred within the first 24 h, followed by a slower increase thereafter (Fig. 2). The rate of increase in imbibition of enclosed seeds varied between populations, with the Bundoora population reaching equilibrium after about 4 d, whereas the other two populations were still increasing after 10 d. There was also a difference between accessions in the total amount of water taken up by enclosed seeds (Table 5). The final water : dry tissue ratio averaged 0·30 for seeds, compared with 0·61 for fruit material (and around 0·65 for seeds that imbibed without a fruit in an earlier experiment; Fig. 2).
Fig. 2.
Seed imbibition measured by weight increase in three populations. Results for isolated seed and seeds within fruit segments are shown for Curyo, Bundoora and Yuna populations, as indicated. Standard error for comparison of means within durations is 1·6.
Table 4.
Increase in seed mass (dry weight basis) over 10 d of moist conditions
| Water content (%, d. wt basis) |
|||
|---|---|---|---|
| Duration (d) | Extracted seeds | Seeds in fruit segments | Seeds in whole fruits |
| 0 | 10·7 | – | – |
| 0·25 | 45·1 | 11·9 | 10·1 |
| 0·5 | 54·8 | 17·9 | 13·9 |
| 1 | 61·8 | 20·7 | 23·4 |
| 2 | 59·8 | 31·9 | 34·7 |
| 4 | 60·2 | 48·6 | 45·1 |
| 6 | 62·3 | 47·7 | 49·0 |
| 8 | 61·9 | 53·4 | 46·4 |
| 10 | 63·0 | 57·3 | 50·2 |
Seeds were either extracted from the fruits at the start, or left within single-seed fruit segments or within whole fruits. The s.e. for any comparison within a duration is 5·7. Seed accession was from Curyo, Victoria.
Table 5.
Mean dry mass of intact seed and fruit segments (excluding the seed) and water imbibed by each
| Seed |
Fruit segment |
|||
|---|---|---|---|---|
| Population | Mean dry weight (mg) | Water imbibed after 10 d (mg) | Mean dry weight (mg) | Water imbibed after 10 d (mg) |
| Curyo | 2·07 | 0·80 | 6·91 | 12·23 |
| Bundoora | 3·24 | 1·41 | 6·65 | 6·57 |
| Yuna | 3·69 | 1·75 | 9·86 | 21·86 |
| s.e. | 0·04 | 0·18 | 0·14 | 2·55 |
Does the seed coat or the fruit wall impose dormancy?
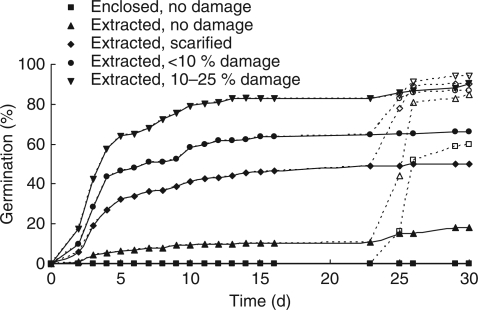
Germination of extracted seeds increased with the level of seed coat damage (Fig. 3) with 10–25 % damage to the seed coat leading to >80 % germination within a week. Seeds that were enclosed within fruit segments or with intact seed coats had 0 and >20 % germination, respectively, after 21 d. The removal of the seed coat from the remaining non-germinated seeds after 21 d resulted in considerable further germination, the greatest germination response occurring in seeds that previously had intact seed coats and in seeds previously enclosed in fruit segments.
Fig. 3.
The effect of varying levels of seed coat damage on germination: fruit enclosed seed with no seed coat damage, extracted seeds with no seed coat damage, extracted seeds with scarified seed coat, extracted seeds with <10 % seed coat damage, and extracted seed with 10–25 % seed coat damage, as indicated. After day 21, the seed coat was removed (dashed lines and open symbols) on half the remaining seeds in each treatment. Points show back-transformed means; error bars have therefore been omitted for clarity. All differences within a date are highly significant (P < 0·001).
Does the fruit affect rate of emergence?
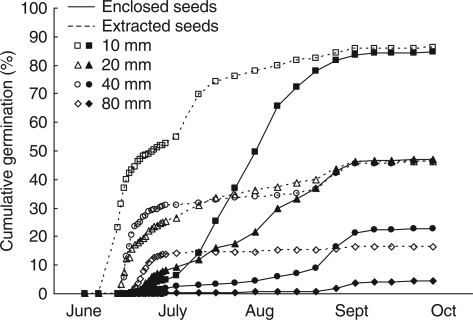
The extraction of seed from the enclosing fruit did not affect (P > 0·05) either total emergence or seed longevity, but it did increase the initial rate of emergence (Fig. 4 and Table 6) within each burial depth. An exception was from seeds buried at 40 mm, where there was greater emergence (P < 0·01) and less survival (P = 0·055) of seeds that had been extracted from fruits. Depth of seed burial did not affect the rate of emergence of seedlings (1/time to 50 % emergence) from seeds that had been extracted from their fruits. However, in seedlings from seeds still enclosed by fruits, the rate of emergence was slower (P < 0·001) from the two greater depths than the shallower depths (Table 6). As depth of seed burial increased, total germination decreased proportionally (Table 6 and Fig. 4). In addition, with increasing depth there was an increase in seed longevity (P < 0·05; Table 6).
Fig. 4.
The effect of depth of burial on emergence of extracted seeds (dashed lines and open symbols) and fruit-enclosed seed (solid lines and filled symbols) at depths of 10 mm, 20 mm, 40 mm and 80 mm, as indicated. Points are back-transformed means, and error bars are therefore not shown. Within any given date, burial depth and fruit removal always had significant effects (P < 0·01) while their interaction was usually significant (P < 0·05).
Table 6.
The effect of the enclosing fruit segment and depth of seed burial on the rate of emergence and seed longevity
| Time to 50 % emergence in year 1 (d) |
Total emergence over two seasons (%) |
Viable seeds recovered after 18 months burial (%) |
||||
|---|---|---|---|---|---|---|
| Depth (mm) | Seeds within fruits | Extracted seeds | Seeds within fruits | Extracted seeds | Seeds within fruits | Extracted seeds |
| 10 | 58·7 | 19·3 | 89·9 (71·5) | 90·1 (71·6) | 4·0 | 1·4 |
| 20 | 64·0 | 27·3 | 53·2 (46·9) | 48·2 (44·0) | 10·2 | 6·3 |
| 40 | 90·0 | 19·0 | 23·5 (29·0) | 48·2 (44·0) | 25·0 | 16·3 |
| 80 | 94·7 | 22·0 | 4·4 (12·5) | 16·4 (23·9) | 33·6 | 36·2 |
| s.e. | 4·76 | (6·47) | 4·20 | |||
The seed accession was from Hopetoun, Victoria, collected 6 months before burial.
Numbers in parentheses are means of transformed data.
By how much does the fruit wall delay seed desiccation?
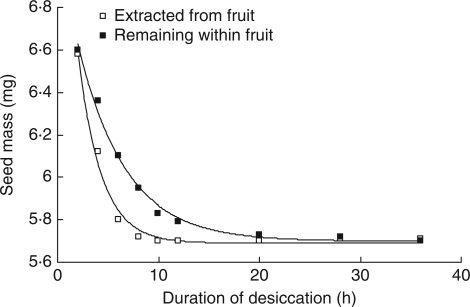
Seeds extracted from their fruits dried faster than those retained in fruits (Fig. 5). This difference was a matter of hours rather than days under the temperature and humidity conditions used in these studies. From the fitted exponential decay curves, it took seeds in fruits 6 h longer to lose 95 % of their water (that was absorbed through the fruit) and 12 h longer to lose 99 % of their water.
Fig. 5.
Rate of reduction in seed mass (mg) when either extracted from fruits or remaining within fruits, as indicated, when kept at 33–35 % relative humidity and 30 °C. Lines represent fitted exponential decay functions. Parallel curve analysis showed that the two lines are significantly different (P < 0·01).
Will the fruit affect tolerance of post-imbibition desiccation?
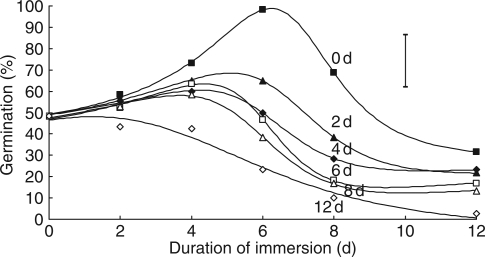
In the absence of post-imbibition drying, the percentage germination of seeds increased to a peak of almost 100 % after 6·2 d of immersion (estimated from fitted equations), then declined rapidly (Fig. 6). Drying after imbibition decreased germination percentage, with a significant drying time × immersion time interaction (P < 0·001). Two days of drying after the optimum immersion time decreased germination by about one-third. Drying after only 2 d of immersion, however, had little effect on later germination other than after 12 d drying. The estimated peak of the immersion time response became earlier as drying time increased, to only 3·7 d if there was 8 d of drying; for 12 d drying there was no longer a peak. However, it should be noted that all estimates for the peaks are close to just two adjacent experimental durations of immersion. The tetrazolium test showed that all the non-germinated seeds were still viable.
Fig. 6.
Effect of different durations of immersion (x-axis) followed by a range of durations of desiccation (0, 2, 4, 6, 8 or 12 d, as indicated) on seed germination of R. raphanistrum. Symbols are observed values. The error bar is the s.e. for comparison of any two observations for a given duration of immersion. Fitted curves are described in the text.
DISCUSSION
There were a number of clear results that emerged from these experiments, relating to the uptake of water and germination of seeds.
(1) Neither the fruit nor the seed coat prevent imbibition of the seed (Tables 2 and 3, and Fig. 2) though the seed coat may slow the uptake for some seeds (Table 4). This was the case whether seeds were fully immersed in water or merely kept moist.
(2) Seeds within fruits do not fully imbibe: more water is taken up if seeds are removed from the fruit (Fig. 2 and Table 4).
(3) The seed coat has a significant role in dormancy, reducing percentage germination unless it is damaged (Fig. 3).
(4) The presence of a fruit wall in freshly shed seeds initially slows emergence from the soil (Fig. 4 and Table 6).
(5) The fruit has the ability to absorb more water per gram of tissue than seeds within fruits (Table 5), but seeds extracted from the fruit can achieve a similar water : tissue ratio.
(6) The fruit slows the rate of desiccation of the seed to some extent (Fig. 5), and this may be sufficient to allow seeds to survive short periods of drying after germination has begun (Fig. 6).
(7) The presence of the fruit for a few days during and immediately after imbibition somehow primes more seeds to germinate than if the fruit is removed prior to imbibition (Fig. 6).
(8) Long periods of moist conditions within the fruit appear to induce dormancy, thus preventing seeds from germinating past the point of no return (Fig. 6).
The ecological significance of these results, however, remains unclear. Water passage through the fruit wall takes place within a few hours. Some seeds do not imbibe, suggesting that the seed coat can exclude water in a proportion of seeds. Most seeds partially imbibe, but then do not germinate in the laboratory: however, they emerge when in soil, albeit at a much slower rate than seedlings from naked seeds. The physical restriction by the fruit may require a breakage force greater than the radicle can apply, at least until the cleavage plane in the fruit weakens over time spent in the soil (resulting in a second wave of emergence; Fig. 4). Witztum et al. (1969) found that mucilage around Blepharis persica swelled when placed in water, forming a barrier to oxygen that may cause dormancy in that species. The composition or anatomy of the fruit wall in R. raphanistrum may provide a barrier to gas exchange, wet or dry.
Our results demonstrated that dormancy of a substantial proportion of R. raphanistrum seeds is caused by the seed coat (confirmed in 37 of 38 accessions from populations around Australia; Young, 2001). This is in contradiction to two other studies, that concluded dormancy was caused by the persistent fruit wall (Mekenian and Willemsen, 1975; Cheam, 1986). The explanation for this may be the method of extracting seeds from the fruit, since mature fresh fruits are difficult to cut open and even a small amount of damage can result in germination. Mekenian and Willemsen (1975) and A.-H. Cheam (Department of Agriculture and Food, South Perth, Australia, pers. comm.) mechanically extracted seeds from the fruit, and damage to the seed coat would have been inevitable. To determine the effect of the fruit on germination, extreme care is required, and even only slightly damaged seeds must be discarded. In another study where seeds were removed with extreme care, the relative effects of the fruit wall and the seed coat varied among three populations (Eslami et al., 2006); however, where plants were grown under extreme water stress, >50 % of seeds germinated even from intact fruits.
At least in the laboratory, the fruit wall appears initially to aid the release of dormancy caused by the seed coat. Far fewer seeds germinated in Petri dishes when extracted immediately from the fruit, in comparison with those whose fruits were first immersed in water for several days and then extracted (uppermost curve in Fig. 6). It is possible that the fruit absorbs an inhibitor from the seed coat as it imbibes; however, the same would be true for a seed without a persistent fruit when surrounded by soil. The relatively rapid increase in seed size in intact fruits indicates that the fruits are quite porous and allow moisture to move freely into the seed. Perhaps, then, inhibitors could also move freely out of the seed coat and into the fruit wall (though this does not occur when bare seeds are placed on filter paper).
Does the fruit wall act as a ‘sponge’, absorbing water which then maintains an internal moist condition as the environment dries? Our studies have found that 83–94 % of the total water absorbed by the combined seed/fruit structure may be due to the fruit, in situations of high water availability. Germinating seeds could thus be protected from complete desiccation during short-term wetting and drying cycles. In our experiments, the fruit wall certainly delayed the rate of drying of the seed, but only by a few hours. This may be sufficient to reduce mortality in some circumstances (Fig. 6), but it is unlikely to be a major influence. Again, a fruit segment in close proximity to soil would be expected to equilibrate quite quickly and would not maintain a high water content for long. Given that our experiments incorporated a single temperature and relative humidity, more research is required over a wider range of conditions and in a soil medium.
We were unable to estimate the ‘point of no return’ from germination in wetting and drying cycles, due to the shapes of the responses to duration of wetting. In addition to the increase in germination through imbibition while within the fruit, germination declined under longer durations of immersion. This could be due to the seeds beginning to rot, although when removed from fruits immersed for 16 d the seeds were still firm. Alternatively, they may be induced into secondary dormancy by either the water or the gaseous environment: the viability test suggested that this was the case, rather than mortality. Regardless of the period of immersion, increasing durations of post-imbibition drying reduced germination of R. raphanistrum. Baskin and Baskin (1998) reported that wetting/drying cycles are required for breakage of dormancy in the mucilage-coated Lesquerella filiformis; they also reviewed other studies in which species responded positively to wet/dry cycles. Clearly, this is worthy of further research and has potential ecological importance in species living in unpredictable soil water conditions during germination (Hunt et al., 2009). For example, a ‘false break’ at the end of summer, when rainfall events occur but the soil then dries out again, may reduce the emergence of crops. The durations and magnitudes of hydration and dehydration can be critical to the germination process (Watt, 1982; Lalonde and Bewley, 1985). Amongst other species, the effects of wetting and drying cycles have been studied in the arid rangeland Krascheninnikovia lanata (Hou et al., 1999), the shallow soil Cyperus inflexus (Baskin and Baskin, 1982), Sonoran desert cacti (Dubrovsky, 1996), various warm season grasses (Frasier et al., 1985), various Sahelian rangeland plants (Elberse and Berman, 1990) and the temperate species Rumex crispus (Vincent and Cavers, 1978) and Triticum aestivum (Hanson, 1973).
One ecological factor that we have not considered here is predation: perhaps the persistent fruit deters or prevents seed predators. This would certainly be worthy of investigation. However, we note that seeds and their coats can be very hard, so many insects, birds and rodents are likely to be able to penetrate a dry fruit just as easily. In the field, we have found small collections of fruits segments that have been broken open and their seeds removed. By effectively making the seed larger, the fruit segment will make it more likely that the seed will be masticated before being swallowed by large animals; or it will increase handling time for animals to remove the seeds, thus reducing the number eaten (see Cousens et al., 2008). A large seed may also make transportation by small seed-eating ants less likely. This could easily be tested by placing extracted seeds and intact fruit segments on the ground under mouse/bird-proof cages and recording losses (e.g. Panetta, 1988).
In our experiments we used a number of source populations (Table 1). Where these were included in the same experiment, trends were consistent, although magnitudes varied. We are therefore confident of the generality of most of our results. Differences between accessions in seed dormancy and fruit morphology may be due to genetic variation or they may be a result of a phenotypic response to their environment (Eslami et al., 2006). In one experiment fruit structure has been shown to vary with different (extreme) watering conditions under which maternal plants were grown (Young, 2001). However, there were no obvious morphological differences between populations; although fruits varied somewhat in shape, the range of variation appeared to be similar in all populations. Given the possibility that R. raphanistrum has been introduced into Australia on multiple occasions and that it occurs over a great range of climates to which it may well have adapted, studies of the quantitative variation in anatomy, its dependence on genotype and environment, and its implications for seed bank dynamics would be an interesting topic for further research.
The results from this study, the first that we know to examine in detail the role of persistent dry fruits, therefore raise as many issues as they answer. The persistent fruit wall has some impact on hydration and dehydration, it physically restricts the expansion of seeds as they imbibe, may have an effect on dormancy release caused by the seed coat and may later induce dormancy. However, it is quite possible that its main role is physical protection of a soft seed, reducing the incidence of predation. There is thus considerable scope for further research on seed ecology in species with persistent fruits. Contrasts between species, especially those from different families, may be informative. Specific areas for research in these and R. raphanistrum include the effects of soil drying on seed/fruit water content, the apparent seed ‘priming’ that occurs when seeds are imbibed within ripe fruits, the interaction between wetting and drying with respect to seed mortality and dormancy, both genetic variation and phenotypic plasticity in seed behaviour and fruit wall morphology, and longer term effects on seeds remaining in the soil for several years. Our results on the effect of the fruit vs. the seed coat on dormancy indicate that (a) care with methodological detail is needed in these experiments and (b) caution is needed when interpreting the ecological significance of seed studies using Petri dishes and filter paper.
ACKNOWLEDGEMENTS
We thank La Trobe University farm staff for assistance with the field experiment and the La Trobe Radiography Clinic for help with X-ray equipment. Thanks also to Gurjeet Gill and Michael Walsh for their comments on an earlier draft. Part of this work was supported by a research grant from the Grains Research and Development Corporation.
LITERATURE CITED
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press; 1998. [Google Scholar]
- Baskin JM, Baskin CC. Effects of wetting and drying cycles on the germination of seeds of Cyperus inflexus. Ecology. 1982;63:248–252. [Google Scholar]
- Cheam AH. Seed production and seed dormancy in wild radish (Raphanus raphanistrum L.) and some possibilities for improving control. Weed Research. 1986;26:405–413. [Google Scholar]
- Code GR, Walsh MJ, Reeves TG. Proceedings of weed seed biology workshop. Orange, Australia: 1987. Effect of depth of burial of wild radish seed on seed viability and seedling emergence; pp. 136–138. Medd RW. ed. [Google Scholar]
- Cousens R, Dytham C, Law R. Dispersal in plants: a population perspective. Oxford: Oxford University Press; 2008. [Google Scholar]
- Dellow JJ, Storrie A, Cheam AH, King WM, Jacob S, Kemp DR. Major brassicaceous weeds in Australia agriculture. In: Cheam AH, editor. Proceedings of the wild radish and other cruciferous weeds symposium; 11–12 July; South Perth. Western Australia: Department of Agriculture and Food; 2006. pp. 1–10. [Google Scholar]
- Dubrovsky JG. Seed hydration memory in Sonoran Desert cacti and its ecological implication. American Journal of Botany. 1996;83:624–632. [Google Scholar]
- Elberse WT, Breman H. Germination and establishment of Sahelian rangeland species. II. Effects of water availability. Oecologia. 1990;85:32–40. doi: 10.1007/BF00317340. [DOI] [PubMed] [Google Scholar]
- Eslami SV, Gill GS, Bellotti B, McDonald G. Pod and seed coat factors in seed dormancy of wild radish (Raphanus raphanistrum L.). In: Preston C, Watts JH, Crossman ND, editors. Papers and Proceedings of the Fifteenth Australian Weeds Conference; 24–28 September; Adelaide. Australia: Weed Management Society of South Australia; 2006. pp. 141–144. [Google Scholar]
- Evenari M, Shanan L, Tadmor N. The Negev, the challenge of a desert. Cambridge, MA: Harvard University Press; 1971. [Google Scholar]
- Frasier GW, Cox JR, Woolhiser DA. Emergence and survival response of seven grasses for six wet–dry sequences. Journal of Range Management. 1985;38:372–377. [Google Scholar]
- Hanson AD. The effects of imbibition–drying treatments on wheat seeds. New Phytologist. 1973;72:1063–1073. [Google Scholar]
- Heydecker W, Orphanos PI. The effect of excess moisture on the germination of Spinacia oleracea L. Planta. 1968;83:237–247. doi: 10.1007/BF00385333. [DOI] [PubMed] [Google Scholar]
- Hou JQ, Romo JT, Bai Y, Booth DT. Responses of winterfat seeds and seedlings to desiccation. Journal of Range Management. 1999;52:387–393. [Google Scholar]
- Hunt JR, Cousens RD, Knights SE. Heliotropium europaeum only germinates following sufficient rainfall to allow reproduction. Journal of Arid Environments. 2009;73:602–610. [Google Scholar]
- Lalonde L, Bewley JD. Desiccation of imbibed and germinating pea axes causes a partial reversal of germination events. Canadian Journal of Botany. 1985;63:2248–2253. [Google Scholar]
- Leist N, Kramer S, Jonitz A, editors. ISTA Working Sheets on tetrazolium testing Volume I, Agricultural, vegetable and hortucultural species. Bassersdorf, Switzerland: International Seed Testing Association; 2003. [Google Scholar]
- Mekenian MR, Willemsen RW. Germination characteristics of Raphanus raphanistrum. 1. Laboratory studies. Bulletin of the Torrey Botanical Club. 1975;102:243–252. [Google Scholar]
- Moore RT. Handbook on tetrazolium testing. Zurich: International Seed Testing Association; 1985. [Google Scholar]
- Panetta FD. Factors determining seed persistence of Chondrilla juncea L. (skeleton weed) in southern Western Australia. Australian Journal of Ecology. 1988;13:211–224. [Google Scholar]
- Parsons WT, Cuthbertson EG. Noxious weeds of Australia. Melbourne: Inkata Press; 1992. [Google Scholar]
- Roberts HA, Boddrell JE. Seed survival and periodicity of seedling emergence in eight species of Cruciferae. Annals of Applied Biology. 1983;103:301–304. [Google Scholar]
- Vincent EM, Cavers PB. The effects of wetting and drying on the subsequent germination of Rumex crispus. Canadian Journal of Botany. 1978;56:2207–2217. [Google Scholar]
- Watt LA. Germination characteristics of several grass species affected by limiting water potential imposed through a cracking black clay soil. Australian Journal of Agricultural Research. 1982;33:223–231. [Google Scholar]
- Werker E. Seed dormancy as explained by the anatomy of embryo envelopes. Israel Journal of Botany. 1980;29:22–44. [Google Scholar]
- Witztum A, Gutterman Y, Evenari M. Integumentary mucilage as an oxygen barrier during germination of Blepharis persica (Burm.) Kuntze. Botanical Gazette. 1969;130:238–241. [Google Scholar]
- Young KD. Australia: The University of Melbourne; 2001. Germination and emergence of wild radish (Raphanus raphanistrum L.) PhD Thesis. [Google Scholar]