Abstract
Background and Aims
Dalbergia nigra is one of the most valuable timber species of its genus, having been traded for over 300 years. Due to over-exploitation it is facing extinction and trade has been banned under CITES Appendix I since 1992. Current methods, primarily comparative wood anatomy, are inadequate for conclusive species identification. This study aims to find a set of anatomical characters that distinguish the wood of D. nigra from other commercially important species of Dalbergia from Latin America.
Methods
Qualitative and quantitative wood anatomy, principal components analysis and naïve Bayes classification were conducted on 43 specimens of Dalbergia, eight D. nigra and 35 from six other Latin American species.
Key Results
Dalbergia cearensis and D. miscolobium can be distinguished from D. nigra on the basis of vessel frequency for the former, and ray frequency for the latter. Principal components analysis was unable to provide any further basis for separating the species. Naïve Bayes classification using the four characters: minimum vessel diameter; frequency of solitary vessels; mean ray width; and frequency of axially fused rays, classified all eight D. nigra correctly with no false negatives, but there was a false positive rate of 36·36 %.
Conclusions
Wood anatomy alone cannot distinguish D. nigra from all other commercially important Dalbergia species likely to be encountered by customs officials, but can be used to reduce the number of specimens that would need further study.
Keywords: Dalbergia nigra, Brazilian rosewood, CITES, wood anatomy, PCA, naïve Bayes analysis
INTRODUCTION
Dalbergia is a pantropical genus belonging to the Leguminosae–Papilionoideae and comprises approx. 250 species (Klitgaard and Lavin, 2005). Of these, around 10–15 species (Record and Hess, 1943; Richter et al., 1996) are known for the beauty and quality of their timber, making them of particular economic importance.
The Brazilian species Dalbergia nigra, commonly known as Brazilian rosewood, has been traded for over 300 years (Record and Hess, 1943). It grows in the Atlantic forest, primarily from southern Bahia to northern Espírito Santo, but also on the coast of São Paulo state and inland in Minas Gerais (Carvalho, 1989, 1997). Specimens in the coastal Atlantic forest may reach approx. 125 ft (38 m) in height (Record and Hess, 1943), whereas inland specimens are shorter (Carvalho, 1989). The heartwood of D. nigra is slow-forming, ranging in colour from brown to purplish-black, and is durable, resisting fungal and insect attack (Flynn and Holder, 2001; Wiedenhoeft and Miller, 2005). Due to the striking colour and grain pattern, early explorers brought the wood back to Spain and Portugal where it fast became a most desirable wood. Its timber has been used in the manufacture of furniture and cabinets and is also highly prized for musical instruments, particularly guitars, as it is believed to have superior acoustic qualities to other woods (Flynn and Holder, 2001).
Because of its popularity, D. nigra has been overexploited and has been listed as ‘vulnerable’ on the Red List of the International Union for the Conservation of Nature (IUCN) since 1998 (Varty, 1998), meaning it is considered at a high risk of extinction in the medium-term if efforts are not made to conserve it. In 1992 it was listed on Appendix I of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). All international trade is prohibited, except for the purposes of scientific research.
CITES legislation makes it necessary to be able to distinguish the wood of D. nigra from species that are not protected by trade laws. Experienced wood anatomists recognize a specimen as belonging to the genus Dalbergia by the presence of short, regularly storied rays, typically large vessels, abundance of diffuse and diffuse-in-aggregate axial parenchyma, and vestured pits. North Carolina State University maintains an online database for the identification of hardwoods, InsideWood (2004 onwards), which uses the International Association of Wood Anatomists (IAWA) List of Microscopic Features for Hardwood Identification (IAWA Committee, 1989) to provide descriptions of the wood anatomy of over 5700 specimens. Of these, the characters ‘all rays storied’, ‘diffuse-in-aggregate axial parenchyma’ and ‘vestured pits’ are listed in only 38 records. Careful comparison with reference material is usually enough to make a distinction to genus level. However, for CITES legislation to be implemented, the identification must be to species level, and this is much more difficult.
The Brazilian species D. spruceana is not protected by trade laws and is often confused with D. nigra (Kribs, 1959; Miller and Wiemann, 2006, and other sources). Although InsideWood (2004 onwards) suggests a possible separation between these two species, the features showing differences are variable or not sufficient to make an accurate separation.
Currently, there are two resources relating to the identification of D. nigra for CITES purposes. The first is the CITES Identification Guide – Tropical Woods (CITES, 2002), which provides a general key to identifying CITES species, together with low magnification photographs of the transverse surface of D. nigra and similar species. This book is aimed primarily at non-experts such as customs inspectors to allow most species to pass through inspection. For Dalbergia species, inspectors need to send wood samples to experts for positive identification. The second is the more recent CITESwoodID CD-ROM (Richter et al., 2008), which includes an interactive key, providing more detail than the printed guide. However, it is still not possible to distinguish D. nigra from D. spruceana because recorded differences between the species are variable; for example, D. spruceana does not have distinct growth rings, but D. nigra may have distinct growth rings, or they may be indistinct or absent. Another potential resource for identification is the InsideWood database (InsideWood 2004 onwards), but whereas it can be readily used to identify Dalbergia, the discriminatory power of the individual character states is insufficient for species identification.
Miller and Wiemann (2006) developed a system for separating heartwood specimens of D. nigra and D. spruceana based on measurements of density and water and ethanol fluorescence of extracts. However, the results only apply if the specimen being examined is definitely one of these two species from Brazil, and it is not always appropriate to make this assumption. Guzman et al. (2008) applied this technique to three Mexican species including D. granadillo and D. stevensonii, but the latter ovelaps with D. nigra in the fluorescence of its ethanol extract. Future studies using non-anatomical features including all commercial Latin American species are planned.
Therefore, it is clear that, if CITES regulations are to be implemented successfully, a more conclusive method for distinguishing D. nigra from other Dalbergia species is needed.
One way to investigate subtle wood anatomical differences between species is to collect quantitative data relating to key characteristics, and to subject these data to multivariate statistical classification. Broadly, such techniques fall into two categories: unsupervised and supervised. Unsupervised classification techniques, such as cluster analysis and principal components analysis (PCA) take the measurements associated with a set of specimens and look for clusters of similarity without taking into account any pre-existing categorization of the specimens. Supervised classification techniques, such as naïve Bayes classification or artificial neural networks make use of categories to which (some of) the specimens belong. These specimens are known as the training set. The aim is to identify features in the measurements of the training set that accurately predict the categories of the training set, so that these features can be used to predict the category of future specimens for which the category is unknown.
Angyalossy-Alfonso and Miller (2002) used average linkage cluster analysis to investigate 51 species and varieties of Swartzia together with eight other genera in the tribe Swartzieae. The species and varieties of Swartzia became four groups, whilst the tribe Swartzieae became seven groups. Canonical discriminant analysis showed that the characters – storied structure, number of tiers per millimetre, axial parenchyma strand length, ray height, intervascular pit size and exclusively uniseriate rays – could be used to discriminate between the groups.
Hellberg and Carcaillet (2003) used PCA to investigate carbonized samples of four Betula species from Western Europe: B. nana, a shrub, and B. pendula, B. pubescens and B. pubescens ssp. tortuosa, which are all trees. Their PCAs placed B. nana in a cluster distinct from the three tree species, with size of vessel groups, mean vessel abundance and ray density being the key distinguishing characters. PCA was not able to fully distinguish the tree species from each other, although a further PCA of only B. pendula and B. pubescens did give separation on the first PC, with the key characters being; vessel diameter, ray density, ray width and ray height.
MacLachlan and Gasson (2010) applied PCA to 21 specimens from seven species in the genus Pterocarpus, with the aim of distinguishing P. santalinus (CITES Appendix II), from closely related taxa. Specimens of P. santalinus formed a cluster that was discrete from the six other species studied, with the key characters being specific gravity, fibre wall thickness, parenchyma band width, and vessel density/mean tangential vessel diameter. Two further species also formed discrete clusters, and the authors concluded that when used in conjunction with comparative anatomy, PCA has the potential to be a useful tool in identifying CITES species.
The use of supervised classification techniques in wood identification has been largely confined to the development of intelligent identification programs, such as that created by Khalid et al. (2008) who used artificial neural networks to train a computer to recognize the wood of 30 tropical species from Malaysia. In addition, Esteban et al. (2009) used artificial neural networks to differentiate between Juniperus cedrus and J. phoenicea with a 92 % probability. As far as we are aware, there is no record of naïve Bayes classification being used for wood identification.
The aim of this study was to use a combination of qualitative and quantitative wood anatomy and statistical analysis to establish one set of characters that could be used to distinguish D. nigra from similar species in the genus. This was approached in three ways: (1) a comparison of qualitative and quantitative wood anatomy; (2) using an unsupervised classification technique, PCA; and (3) with a supervised classification technique, naïve Bayes classification. Ultimately we intend to extend this study to include all the commercial Dalbergia species from the Americas, Africa and Asia.
MATERIALS AND METHODS
In addition to Dalbergia nigra, six Latin American Dalbergia species were studied because they have some degree of commercial importance and are similar enough to D. nigra in terms of overall appearance and/or anatomy, to be mistaken for it.
(1) D. cearensis (Brazilian kingwood) is a component of the caatinga vegetation, occurring from Ceará and southern Piauí to southern Bahia in Brazil. The timber is violet brown to black and is used mostly for decorative purposes, such as inlay work and marquetry (Carvalho, 1989).
(2) D. miscolobium (various common names, all Portuguese: jacarandá-do-cerrado, jacarandá-do-campo, caviúna-do-cerrado) is a typical component of the cerrado vegetation in Brazil. Its timber is similar to D. nigra (Rizzini, 1978 cited by Carvalho, 1989) but the tree has a ‘tortuous trunk’, unique amongst Dalbergia (Carvalho, 1989), limiting its use to small products.
(3) D. spruceana (Amazon rosewood) is from Brazil, Bolivia and Venezuela (Carvalho, 1989, 1997). According to Kuhlmann (1977, cited by Carvalho, 1989) it is found in both evergreen and deciduous Amazonian forests. It has dark brown to black wood (Carvalho, 1989) and is used in cabinet work, as well as for smaller items such as knife and brush handles (Loureiro and Silva, 1968 cited by Carvalho, 1989).
(4) D. granadillo (‘granadillo’) is native to Mexico and traded as cocobolo, a name also applied to D. retusa (from Mexico to Colombia). The timber is deep red to black and is used extensively for the production of cutlery handles as well as other small items, ranging from jewellery boxes to musical instruments (Flynn and Holder, 2001).
(5) D. stevensonii (Honduras rosewood) occurs in Belize, south-east Mexico and Guatemala and is commonly known as Honduras rosewood (Richter et al., 1996). The timber is brown to purplish, often with black markings and is used primarily for the production of percussion bars of xylophones and occasionally for veneers and cabinets (Flynn and Holder, 2001). Guatemalan D. stevensonii is protected by CITES Appendix III.
(6) D. tucurensis (Guatemala rosewood) including D. cubilquitensis is native to Honduras and Guatemala. The wood is a relatively recent addition to the international market and is exported mainly for use in decorative veneers (Richter et al., 1996).
Another commercially important Brazilian species of Dalbergia is D. decipularis (Brazilian tuplipwood). This species was not included in the present study, since it produces a yellow or pinkish wood, which is easily distinguished from the brown to purplish black wood of D. nigra. Dalbergia retusa (from Mexico to Panama) has similar coloured wood to D. nigra but was not included in this study due to a lack of specimens with reliable provenance. However, in the specimens that are available, the pattern of diffuse-in-aggregate parenchyma is very regular, forming an almost reticulate pattern, and this appears to be diagnostic.
Specimens were obtained from the Economic Botany collection at RBG Kew (Kw), the Forest Products Laboratory, Wisconsin, USA (MADw and SJRw), the Institute for Technology Research of the Federal State, Sao Paulo, Brazil (BCTw) and the Federal Research Centre for Forestry and Forest Products, Hamburg, Germany (RBHw). Table 1 shows the identity of each specimen studied, together with its current location and, where available, collection information. Only heartwood specimens were used to increase the validity of comparisons.
Table 1.
Collection details of the specimens examined
| Specimen no. | Species | Xylarium no. | Collector and no. | Country |
|---|---|---|---|---|
| 1 | D. cearensis | BCTw 18817 | A. Mattos Filho, C. T. Rizzini | Brazil |
| 2 | D. cearensis | MADw 31946* | A. Dahlgren | Brazil |
| 3 | D. cearensis | SJRw 44336† | A. Ducke; wood 422 | Brazil |
| 4 | D. cearensis | SJRw 45480 | A. Ducke | Brazil |
| 5 | D. miscolobium | BCTw 1588 | Brazil | |
| 6 | D. miscolobium | BCTw 3659 | Brazil | |
| 7 | D. miscolobium | BCTw 4133 | Brazil | |
| 8 | D. miscolobium | BCTw 15956 | Brazil | |
| 9 | D. miscolobium | RBHw 10922 | E.P. Heringer 3349 | Brazil |
| 10 | D. nigra | Kw 6269 | Brazil | |
| 11 | D. nigra | Kw 6270 | F.H. Pierpont 85 | Brazil |
| 12 | D. nigra | Kw 6272 | Brazil | |
| 13 | D. nigra | Kw 72717 | A. Euponino, F. Souza Santo 414 | Brazil |
| 14 | D. nigra | SJRw 3273 (MADw 10769)† | H.N. Whitford 76 | Brazil |
| 15 | D. nigra | SJRw 4146 (MADw 7017)† | H.M. Curran 6 | Brazil |
| 16 | D. nigra | SJRw 4230 | Brazil | |
| 17 | D. nigra | SJRw 5990 | Brazil | |
| 18 | D. spruceana | BCTw 13349 | Brazil | |
| 19 | D. spruceana | BCTw 14315 | Brazil | |
| 20 | D. spruceana | BCTw 16612 | Brazil | |
| 21 | D. spruceana | Kw 21266 | M. Bastos | Brazil |
| 22 | D. spruceana | MADw 18588‡ | B. A. Krukoff 4921 | Brazil |
| 23 | D. spruceana | RBHw 7118 | Brazil | |
| 24 | D. spruceana | RBHw 13049 | Brazil | |
| 25 | D. spruceana | SJRw 22610 (MADw 31968)† | A. Ducke 150 | Brazil |
| 26 | D. tucurensis | BCTw 12619 | ||
| 27 | D. tucurensis | RBHw 13987 | Honduras | |
| 28 | D. tucurensis | RBHw 18255 | Honduras | |
| 29 | D. tucurensis | RBHw 16326 | ||
| 30 | D. tucurensis | RBHw 18277 | ||
| 31 | D. tucurensis | SJRw 3738 (MADw 31973)† | H. N. Whitford, L. R. Stadtmiller 79 | Guatemala/Honduras |
| 32 | D. tucurensis | SJRw 10729 (MADw 31975)† | C. Galusser 9 | Guatemala |
| 33 | D. tucurensis | SJRw 3721 (MADw 10836)† | H. N. Whitford, L. R. Stadtmiller 61 | Guatemala/Honduras |
| 34 | D. granadillo | BCTw 18439 | ||
| 35 | D. granadillo | RBHw 24160 | Mexico | |
| 36 | D. granadillo | MADw 3781 | L. E. Harthan | Mexico |
| 37 | D. granadillo | SJRw 38303 | Nicaragua | |
| 38 | D. granadillo | SJRw 4410† | J. A. Gamon | Mexico |
| 39 | D. stevensonii | RBHw 13048 | Honduras | |
| 40 | D. stevensonii | RBHw 10840 | Belize | |
| 41 | D. stevensonii | RBHw none | ||
| 42 | D. stevensonii | RBHw none | ||
| 43 | D. stevensonii | Kw 6303 | F. H. Pierpont | Honduras |
Voucher is held by: *the Field Museum in Chicago, †MAD (housed in the University of Wisconsin) or ‡the New York Botanical Garden.
Preparation of slides
Sectioning blocks, approx. 1 cm3 were softened in boiling water or in a 5 % solution of ethylenediamine placed in a vacuum oven at 1000 mb, 60 °C for up to 40 h (MacLachlan and Gasson, 2010, modified from Kukachka, 1977). Transverse, tangential and radial sections were cut (10–20 µm thick) using a Reichert sliding microtome. The sections were stained in Safranin (1 % in 50 % ethanol) for 5 min and Alcian Blue (1 % aqueous) for 2 min. Sections were then rinsed with distilled water, before being dehydrated in a series of alcohols, 3 min each in 50 %, 70 %, 95 % and 100 % (twice). Finally, sections were rinsed in histoclear and mounted in Euparal on a microscope slide.
Choice of characters
All of the species studied have the following characters from the IAWA List (IAWA Committee, 1989), characters not listed are either absent or do not apply:
5. Wood diffuse porous
13. Simple perforation plates
22. Intervessel pits alternate
29. Vestured pits
30. Vessel-ray pits with distinct borders; similar to intervessel pits in size and shape throughout the ray cell
58. Gums and other deposits in heartwood vessels
61. Fibres with simple to minutely bordered pits
66. Non-septate fibres present
76. Axial parenchyma diffuse
77. Axial parenchyma diffuse-in-aggregates
97. Ray width one to three cells
104. All ray cells procumbent
118. All rays storied
120. Axial parenchyma and/or vessel elements storied
136. Prismatic crystals present.
In D. cearensis, D. granadillo and D. stevensonii, growth rings are always distinct (character 1), but in the rest of the species studied, growth boundaries may be distinct, or indistinct/absent (character 2).
Dalbergia cearensis and D. miscolobium have a mean tangential vessel diameter of 50–100 µm (character 41), whereas, D. tucurensis has a mean tangential vessel diameter of ≥ 200 µm (character 43). In the four other species studied, mean tangential vessel diameter is between 100 and 200 µm (character 42).
All of the species studied, except D. cearensis, have ≤5 vessels mm−2 (character 46). Dalbergia cearensis has 20–40 vessels mm−2 (character 48).
Dalbergia cearensis and D. stevensonii lack abundant vasicentric parenchyma (character 79), whereas in D. miscolobium, parenchyma is always associated with the vessels and frequently forms winged-aliform (character 82) or confluent patterns (character 83). In the remaining species, parenchyma pattern is more variable, although there is a tendency towards winged-aliform parenchyma in D. tucurensis.
This illustrates the difficulties of using only the IAWA List to distinguish between members of the same genus; the characters are too broad to allow subtle distinctions to be made.
Therefore, for this study, characters were chosen using the IAWA List as a guide, but with modifications to allow for increased discrimination accuracy and subsequently species level separation.
Quantitative characters:
(1) Mean vessel lumen diameter (μm)
(2) Maximum vessel lumen diameter (μm)
(3) Minimum vessel lumen diameter (μm)
(4) Vessel frequency (1 mm−2)
(5) Solitary vessel frequency (1 mm−2)
(6) Mean ray height (cells)
(7) Maximum ray height (cells)
(8) Minimum ray height (cells)
(9) Mean ray width (cells)
(10) Ray frequency (10 mm−2)
(11) Frequency of axially fused rays (10 mm−2)
(12) Frequency of uniseriate rays (10 mm−2).
Qualitative characters:
(13) Growth ring boundaries distinct or indistinct/absent
(14) Paratracheal axial parenchyma scanty or abundant vasicentric
(15) Axial parenchyma winged aliform present or absent
(16) Axial parenchyma confluent present or absent
Characters 1–3 correspond to characters 40–44 on the IAWA List, but use actual measurements rather than size classes.
Character 4 corresponds to IAWA characters 46–51, and character 5 is equivalent to characters 9–11. Actual frequencies were used instead of frequency classes to increase accuracy, and an area of 10 mm2 was examined, as there were often only 3 or 4 vessels mm−2, which did not give enough variation to separate the species.
Characters 6–8 replace IAWA List characters 102 and 103, with height in cells providing more opportunity for distinction than recording rays taller than 1 mm (in the Dalbergia species studied, the majority of rays were under 200 µm high, and none were >500 µm tall), or of two distinct heights.
Characters 9 and 12 replace IAWA List characters 96 and 97; since all rays were 1–3 cells wide, mean ray width indicates whether more uniseriate (mean ray width close to 1) or tri-seriate rays were present (mean ray width over 2).
Characters 10–12, which measure ray frequency, are not covered by the IAWA List. Rays per 10 mm2 was used instead of the more conventional area of 1 mm2, because axially fused and uniseriate rays were rare in some specimens, and would have been missed altogether using the smaller area.
Character 13 is IAWA List characters 1 and 2. Character 14 is IAWA List characters 78 and 79. Characters 15 and 16 are IAWA characters 82 and 83.
Microscopy and measurements
Qualitative characters were determined by examining transverse sections, approx. 10 mm2, at ×40 magnification, using light microscopy. Presence of a qualitative character was scored as 1 and absence as 0. For each species, the percentage of specimens in which each character was present was calculated.
Quantitative characters were measured using a light microscope with a drawing tube attached. Measurements were superimposed onto grids corresponding to 10 mm2 on the slide to facilitate counting. Vessels were observed using transverse sections at ×40 magnification, whereas rays were viewed using tangential longitudinal sections at ×100 magnification. Rays bisecting the bottom or left-hand side of the grid were included in the count; rays bisecting the top or right-hand side of the grid were excluded. The measurements for vessel frequency were then divided by 10 to give vessels per square millimetre, which is the IAWA List character. For mean vessel diameter, mean ray height and mean ray width, 30 measurements were made at random. The mean and standard deviation were calculated for each character for each species. Maximum and minimum values are the single highest or lowest measurement recorded within a species.
PCA
PCA was conducted according to the methodology of Möller et al. (2007) using the multivariate statistical package R-pack Le Progiciel R.4·0d10 (http://www.bio.umontreal.ca/Casgrain /en/labo/R/v4/progress.html). Since PCA works by finding the directions of maximum variability in the data, and qualitative characters were scored only as 0 or 1, they were not included in the PCAs.
Two PCAs were carried out, one on the four Brazilian species – D. nigra, D. spruceana, D. miscolobium and D. cearensis – and a second on all seven species together.
Naïve Bayes classification
Class prediction was carried out using naïve Bayes classification (Hand and Yu, 2001) on the four characters: minimum vessel diameter; frequency of solitary vessels; mean ray width; and frequency of axially fused rays. Minimum vessel diameter and mean ray width were modelled as normal distributions; the two measurements of frequency were modelled as binomial distributions with pseudocounts of 0·25 included to compensate for under-sampling (Durbin et al., 1998). Equal priors were used for two classes to avoid bias, and marginal posteriors were used to compute the joint posterior in order to ensure equal weight to the four characters. The model was validated using leave-one-out cross-validation (Miller, 1974). The four characters were selected on the basis of: biological relevance; reasonable distinction of the classes in univariate analyses; having low correlation with each other (the largest R2 between selected characters is 0·178); and statistical expediency.
Miller and Wiemann (2006) report that specimen 17, D. nigra SJRw 5990 has been misidentified and this was confirmed by chemical analysis of extracts (Geoffrey Kite, RBG Kew, pers. comm.). This specimen was therefore not included when the quantitative and qualitative wood anatomy data were analysed according to species, and was also excluded from all training sets used in the naïve Bayes classification, but was used as a test specimen. It was left in the PCAs as this test treats each specimen individually.
RESULTS AND DISCUSSION
Qualitative wood anatomy
No one species was consistent in, and unique for, any single discrete character or combination of discrete characters studied. Therefore these characters on their own are not enough to identify these species, or to identify D. nigra.
Dalbergia cearensis and D. stevensonii had identical results and showed the most consistency across the specimens studied, with all specimens from both species showing distinct growth rings and lacking abundant vasicentric, or axial parenchyma in winged-aliform or confluent patterns (see Table 2).
Table 2.
Qualitative and quantitative wood anatomy measurements, summarized by species
| D. cearensis | D. miscolobium | D. nigra | D. spruceana | D. tucurensis | D. granadillo | D. stevensonii | |
|---|---|---|---|---|---|---|---|
| QUALITATIVE CHARACTERS (%) | |||||||
| Growth rings distinct | 100 | 80 | 71 | 50 | 63 | 100 | 100 |
| Parenchyma abundant vasicentric | 0 | 100 | 71 | 38 | 75 | 20 | 0 |
| Axial parenchyma winged aliform | 0 | 20 | 14 | 63 | 75 | 0 | 0 |
| Axial parenchyma confluent | 0 | 40 | 29 | 50 | 38 | 0 | 0 |
| QUANTITATIVE CHARACTERS | |||||||
| Vessel diameter (μm) | |||||||
| Mean ± s.d. | 76·7 ± 41·3 | 98·7 ± 48·3 | 138·2 ± 76·6 | 130·6 ± 77·9 | 205·2 ± 73·1 | 167·4 ± 67·5 | 153·4 ± 53·7 |
| Maximum | 248 | 224 | 656 | 344 | 440 | 299 | 374 |
| Minimum | 16 | 24 | 16 | 16 | 66 | 42 | 50 |
| Vessel frequency (mm−2) | |||||||
| Mean ± s.d. | 22·0 ± 7·9 | 4·5 ± 2·1 | 2·8 ± 1·1 | 3·5 ± 1·2 | 2·8 ± 1·4 | 3·8 ± 2·6 | 3·1 ± 1·0 |
| Maximum | 29 | 8 | 5 | 5 | 6 | 8 | 4 |
| Minimum | 11 | 2 | 2 | 2 | 1 | 1 | 2 |
| Frequency solitary vessels (mm−2) | |||||||
| Mean ± s.d. | 5·1 ± 2·6 | 1·4 ± 0·5 | 1·5 ± 0·6 | 1·0 ± 0·5 | 1·3 ± 0·6 | 1·9 ± 1·5 | 2·0 ± 1·0 |
| Maximum | 9 | 2 | 3 | 2 | 2 | 5 | 4 |
| Minimum | 3 | 1 | 0 | 0 | 0 | 0 | 0 |
| Ray height (cells) | |||||||
| Mean ± s.d. | 6·3 ± 1·7 | 5·2 ± 1·3 | 7·1 ± 1·5 | 7·1 ± 1·7 | 6·1 ± 1·4 | 6·2 ± 1·2 | 6·7 ± 1·3 |
| Maximum | 10 | 8 | 12 | 11 | 9 | 9 | 10 |
| Minimum | 2 | 2 | 3 | 2 | 3 | 3 | 3 |
| Ray width (cells) | |||||||
| Mean ± s.d. | 1·7 ± 0·6 | 1·5 ± 0·6 | 2·0 ± 0·4 | 1·7 ± 0·7 | 2·0 ± 0·5 | 1·5 ± 0·5 | 1·9 ± 0·5 |
| Ray frequency (10 mm−2) | |||||||
| Mean ± s.d. | 64·5 ± 14·5 | 97·6 ± 11·3 | 64·6 ± 15·6 | 56·0 ± 9·9 | 55·6 ± 8·0 | 69·6 ± 8·9 | 57·0 ± 13·3 |
| Maximum | 80 | 113 | 84 | 68 | 67 | 83 | 77 |
| Minimum | 47 | 83 | 42 | 39 | 41 | 59 | 40 |
| Frequency axially fused rays (10 mm−2) | |||||||
| Mean ± s.d. | 1·5 ± 1·9 | 6·4 ± 5·8 | 0·1 ± 0·4 | 0·9 ± 1·1 | 0·5 ± 1·1 | 2·2 ± 2·9 | 0·2 ± 0·4 |
| Maximum | 4 | 14 | 1 | 3 | 3 | 7 | 1 |
| Minimum | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Frequency uniseriate rays (10 mm−2) | |||||||
| Mean ± s.d. | 25·25 ± 19·7 | 52·8 ± 16·7 | 5·9 ± 3·8 | 23·4 ± 13·9 | 7·8 ± 7·0 | 37·0 ± 20·8 | 6·4 ± 4·3 |
| Maximum | 48 | 75 | 11 | 40 | 22 | 61 | 13 |
| Minimum | 6 | 37 | 2 | 4 | 0 | 14 | 2 |
Distinct growth rings were also recorded in all specimens of D. granadillo and in four out of five specimens of D. miscolobium. For D. nigra, D. spruceana and D. tucurensis, the presence or absence of growth ring boundaries was more variable with a maximum of 71 % (D. nigra) of specimens having distinct growth rings. However, the presence or absence of growth rings is most likely to be a reflection of the environment in which the tree grew, may vary in a given tree, and is therefore not a good character for determining species identity.
Abundant vasicentric parenchyma was present in all the specimens of D. miscolobium and in six out of eight specimens of D. tucurensis. However, D. nigra, D. spruceana and D. granadillo showed more variability in this character, with a maximum of 75 % (D. tucurensis) of specimens having parenchyma that was abundant vasicentric. In addition to D. cearensis and D. stevensonii, no specimens of D. granadillo showed a winged-aliform or confluent pattern of axial parenchyma. In the four remaining species, these patterns were sometimes present, but never for all specimens of one species. Patel and Shah (1980) also found inconsistency in the patterns of axial parenchyma present in specimens of Dalbergia latifolia from India. Although a more comprehensive study might show consistent trends that have not been observed here, it appears that parenchyma patterns are inconsistent and are not diagnostic for these Dalbergia species.
Quantitative wood anatomy
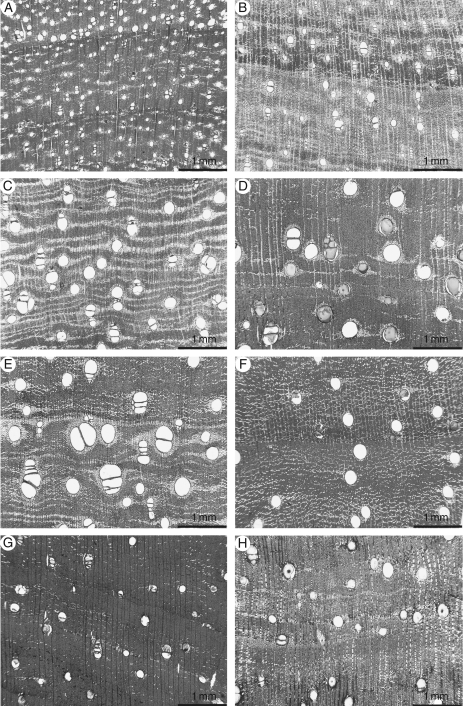
Dalbergia cearensis is one of the most easily identifiable of the Latin American species of Dalbergia due to the presence of numerous small vessels (Kribs, 1959; Miller and Wiemann, 2006). The results of this study show D. cearensis can be separated from the other species by vessel frequency, with all specimens of D. cearensis, and no specimens from other species, having a vessel frequency of over 10 vessels mm−2 (see Fig. 1). Dalbergia miscolobium, had the second highest vessel frequency at 4·48 vessels mm−2 and D. tucurensis had the lowest vessel frequency at 2·75 vessels mm−2 (see Table 2 and Fig. 1).
Fig. 1.
Transverse sections: (A) D. cearensis (MADw 31946); (B) D. miscolobium (BCTw 1588); (C) D. nigra (Kw 6270); (D) D. spruceana (RBHw 7118); (E) D. tucurensis (SJRw 3738); (F) D. granadillo (SJRw 4410); (G) D. stevensonii (RBHw trade specimen); (H) D. nigra (SJRw 5990). Note the differences in vessel diameter.
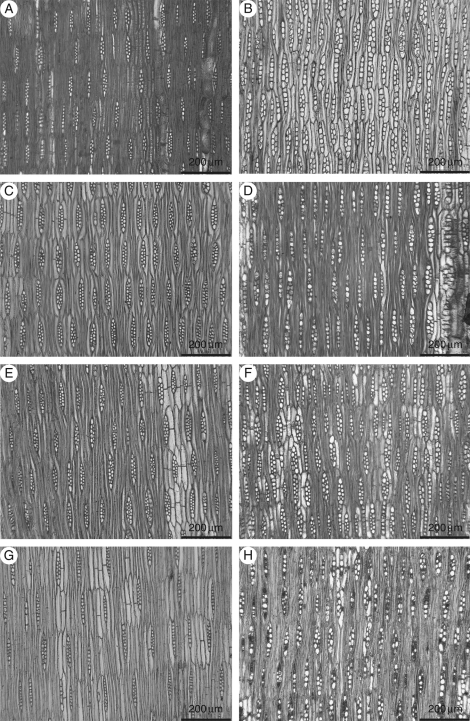
Dalbergia miscolobium also stands out in terms of ray characteristics, with the shortest ray height, greatest ray frequency, highest frequency of uniseriate rays and highest frequency of axially fused rays. A ray frequency of over 90 rays 10 mm−2 distinguishes four out of five of the D. miscolobium specimens from all the other specimens studied (see Fig. 2). This may reflect its twisted habit when compared with the other species which have straighter trunks (Carvalho, 1997). Dalbergia granadillo has the second highest ray frequency at 69·6 rays 10 mm−2. The difference between D. granadillo and the species with the lowest ray frequency, D. tucurensis is just 14 rays 10 mm−2.
Fig. 2.
Tangential longitudinal sections: (A) D. cearensis (MADw 31946); (B) D. miscolobium (BCTw 3659); (C) D. nigra (Kw 6269); (D) D. spruceana (BCTw 14315); (E) D. tucurensis (SJRw 3738); (F) D. granadillo (SJRw 38303); (G) D. stevensonii (Kw 6303); (H) D. nigra (SJRw 5990).
Quantitative characters cannot be used to distinguish D. nigra from D. spruceana, D. tucurensis, D.granadillo or D. stevensonii, as the results for D. nigra fall within the range of these species. In particular, D. nigra and D. spruceana had the most similar mean vessel diameter, with vessels of D. nigra being, on average, just 7·6 µm larger than those of D. spruceana, and identical mean ray heights.
PCA
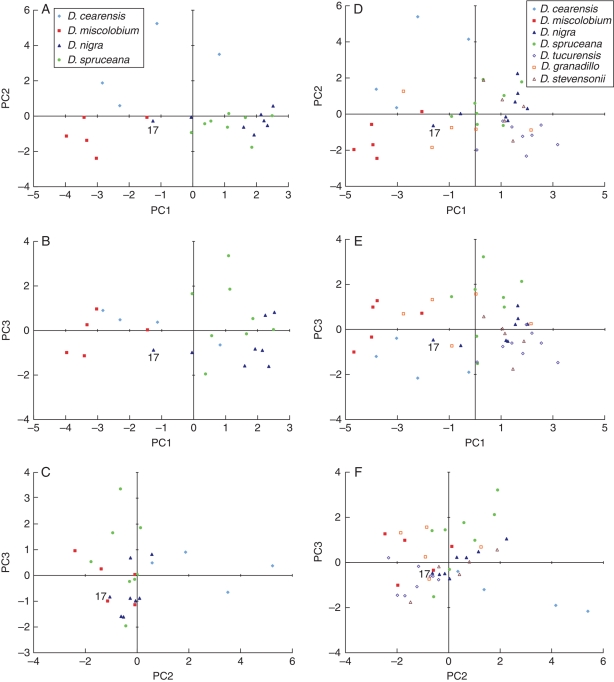
Analysis of the Brazilian species alone and then all seven species together yielded cumulative variance for PC1 and PC2 at 58·74 % for the former and 51·25 % for the latter group. This is considerably less than the cumulative PC1 and PC2 score of 70–90 % considered appropriate by Everitt and Dunn (2001). The outcomes of the PCAs conducted for this study are therefore treated with caution. However, when the cumulative value of PCs 1–3 is considered, 71·23 % for the Brazilian species and 68·73 % for all seven species were achieved, which are higher than the 60 % reported by MacLachlan and Gasson (2010) and thus some discussion of the results produced by these PCAs is appropriate.
When considering the four Brazilian species alone, PC1 accounted for 38·07 % of the variance and was most highly correlated with ray characteristics (see Table 3). Figure 3A shows that PC1 splits the species into two groups, with eight out of nine specimens of D. cearensis and D. miscolobium being located on the negative side of the axis, and 13 out of 15 specimens of D. nigra and D. spruceana on the positive side. PC2, which correlated most strongly with vessel characteristics can then be used to separate D. miscolobium, with all specimens on the negative side, from D. cearensis on the positive side of the axis. However, these distinctions were apparent directly from the raw data and PC1 and PC2 are still unable to split D. nigra from D. spruceana. Figure 3B and C shows PC3 plotted against PC1 and PC2, respectively. PC3 accounted for only 12·40 % of the variance, and the specimens were not separated by this axis.
Table 3.
PCA eigenvectors
| Brazilian species |
All species |
|||||
|---|---|---|---|---|---|---|
| Character | Axis 1 | Axis 2 | Axis 3 | Axis 1 | Axis 2 | Axis 3 |
| Mean vessel diameter | 0·31 | −0·36 | −0·02 | 0·36 | −0·33 | −0·01 |
| Maximum vessel diameter | 0·30 | −0·24 | −0·19 | 0·33 | −0·24 | −0·06 |
| Minimum vessel diameter | 0·22 | −0·16 | 0·49 | 0·27 | −0·26 | −0·11 |
| Vessel frequency | −0·13 | 0·55 | 0·05 | −0·20 | 0·42 | −0·34 |
| Solitary vessel frequency | −0·12 | 0·54 | 0·06 | −0·17 | 0·39 | −0·36 |
| Mean ray height | 0·39 | 0·18 | 0·16 | 0·29 | 0·40 | 0·33 |
| Maximum ray height | 0·37 | 0·17 | 0·28 | 0·24 | 0·42 | 0·34 |
| Minimum ray height | 0·27 | 0·09 | −0·34 | 0·27 | 0·13 | −0·01 |
| Mean ray width | 0·22 | 0·12 | −0·54 | 0·28 | 0·02 | −0·56 |
| Ray frequency | −0·35 | −0·23 | −0·17 | −0·34 | −0·24 | −0·05 |
| Frequency uniseriate rays | −0·28 | −0·07 | −0·24 | −0·28 | −0·11 | −0·15 |
| Frequency axially fused rays | −0·34 | −0·21 | 0·35 | −0·37 | −0·14 | 0·41 |
| Percentage of variation explained | 38·07 | 20·67 | 12·50 | 35·81 | 21·44 | 11·48 |
Fig. 3.
Principal component axes score plots: (A) Brazilian species PC1 vs. PC2; (B) Brazilian species, PC1 vs. PC3; (C) Brazilian species PC2 vs. PC3; (D) all species PC1 vs. PC2; (E) all species PC1 vs. PC3; (F) all species PC2 vs. PC3.
When all seven species were considered together, the same eight characters made the biggest contributions to PC1 and PC2, but the distinction between vessel and ray characters did not exist because D. tucurensis had the largest mean vessel diameter and lowest vessel frequency and D. cearensis had the smallest mean vessel diameter and the highest vessel frequency, thus, when these two species are considered together, the variability in vessel characteristics is increased (see Table 3). However, there is no difference in terms of the ability of the PCs to separate the species. Specimens of D. nigra, D. spruceana, D. tucurensis and D. stevensonii tend to be located on the positive side of PC1, with D. miscolobium, D cearensis and D. granadillo on the negative side, but PC2 is unable to separate the species any further (see Fig. 3D–F). As was the case when only the Brazilian species were considered, PC3 does not separate the species (see Fig. 3E, F).
In all cases, specimen 17, D. nigra SJRw 5990, is the furthest D. nigra outlier on PC1, supporting Geoffrey Kite's chemical analysis which suggests it has been misidentified.
PCA identifies the characters with the maximum variability in the data, and the fact that the PCs are unable to separate the species in this study means that the variation is largely between members of the same species, rather than between members of different species.
Naïve Bayes classification
Table 4 shows the outcome of the naïve Bayes classification, with each specimen classified using the other 41 specimens as a training set. Mean ray width was the most successful character, correctly identifying 27 out of 41 specimens, with six out of seven D. nigra correctly identified. Frequency of axially fused rays was the character least good at classifying the specimens when used alone, correctly identifying only 18 out of 41 specimens, but again six out of seven D. nigra were classified correctly using just this character.
Table 4.
Results of naïve Bayes classification
| Specimen no. | Species | Overall outcome | Minimum vessel diameter | Solitary vessel frequency | Mean ray width | Frequency axially fused rays |
|---|---|---|---|---|---|---|
| 1 | D. cearensis | − | + | − | − | + |
| 2 | D. cearensis | − | + | − | + | − |
| 3 | D. cearensis | − | + | − | − | + |
| 4 | D. cearensis | − | + | − | − | − |
| 5 | D. miscolobium | − | + | + | − | − |
| 6 | D. miscolobium | − | + | − | − | − |
| 7 | D. miscolobium | − | + | − | − | − |
| 8 | D. miscolobium | − | + | − | − | − |
| 9 | D. miscolobium | − | + | − | − | − |
| 10 | D. nigra | + | + | + | + | + |
| 11 | D. nigra | + | + | − | + | − |
| 12 | D. nigra | + | + | + | + | + |
| 13 | D. nigra | + | + | + | + | + |
| 14 | D. nigra | + | + | + | + | + |
| 15 | D. nigra | + | + | + | − | + |
| 16 | D. nigra | + | + | − | + | + |
| 18 | D. spruceana | − | + | − | − | − |
| 19 | D. spruceana | − | + | + | − | + |
| 20 | D. spruceana | − | + | + | − | − |
| 21 | D. spruceana | + | + | + | + | + |
| 22 | D. spruceana | − | + | − | − | + |
| 23 | D. spruceana | − | − | + | − | − |
| 24 | D. spruceana | − | + | − | − | + |
| 25 | D. spruceana | − | + | − | + | + |
| 26 | D. tucurensis | − | − | + | − | + |
| 27 | D. tucurensis | − | − | − | + | + |
| 28 | D. tucurensis | − | − | − | + | + |
| 29 | D. tucurensis | − | − | + | + | + |
| 30 | D. tucurensis | − | − | − | + | + |
| 31 | D. tucurensis | − | − | + | − | + |
| 32 | D. tucurensis | − | − | + | + | − |
| 33 | D. tucurensis | + | − | + | + | + |
| 34 | D. granadillo | − | − | + | − | + |
| 35 | D. granadillo | − | + | − | − | − |
| 36 | D. granadillo | − | − | + | − | + |
| 37 | D. granadillo | − | − | + | − | + |
| 38 | D. granadillo | − | − | + | − | − |
| 39 | D. stevensonii | + | + | + | − | + |
| 40 | D. stevensonii | − | − | − | + | + |
| 41 | D. stevensonii | − | − | + | + | + |
| 42 | D. stevensonii | − | − | + | + | + |
| 43 | D. stevensonii | + | − | + | + | + |
+=‘nigra’; −=‘not nigra’.
Overall, 39 out of 42 specimens were correctly identified, with all seven D. nigra specimens being classified as ‘nigra’. Of the 34 remaining specimens, 30 were classified correctly as ‘not nigra’, with four classified incorrectly as ‘nigra’. This gives a false negative rate of 0 out of 7 and a false positive rate of 4 out of 11, or 36·36 %. When developing the model, the false negative rate was minimized because any unknown specimen being tested under CITES regulations and classified as ‘not-nigra’ can then be confidently assumed to be another species, and thus can be imported or exported legally. Any specimen that is classified as ‘nigra’, which form a minority, would need further investigation.
Specimen 17, D. nigra SJRw 5990, was classified as ‘not nigra’, which is in line with the chemical analysis of Geoffrey Kite and strongly suggests that this specimen has been misidentified.
One limitation of statistical analysis is that the results cannot be generalized to apply to species that were not used in the original analysis. Therefore, the naïve Bayes classification developed for the present study should only be applied when the specimen under examination is strongly believed to belong to one of the seven species used to develop the model.
CONCLUSIONS
This study confirms that D. cearensis can be reliably distinguished from D. nigra and the other species in this study by its small, numerous vessels, with any specimen with a vessel frequency in excess of 10 vessels mm−2 being D. cearensis. Specimens with a high ray frequency, over 100 rays 10 mm−2, and a high number of axially fused rays are likely to be D. miscolobium.
PCA is not able to provide a set of characters that distinguishes D. nigra from other commercially important Latin American members of the genus, because the majority of variation is within members of the same species, not between species.
However, using the four characters – minimum vessel diameter, frequency of solitary vessels, mean ray width, and frequency of axially fused rays in a naïve Bayes classification – unidentified specimens can be determined as ‘not nigra’ with no false negatives. Specimens determined as ‘nigra’ are twice as likely to be genuine D. nigra as not. This suggests that whilst wood anatomy alone is unlikely to provide the level of identification certainty needed by legislation such as CITES, it can be used as a relatively inexpensive and straightforward way of reducing the number of specimens that would need more comprehensive study.
The success of naïve Bayes classification over PCA indicates that such supervised classification techniques could be used more widely to solve problems in wood anatomy. Indeed it is a promising technique for helping to solve other intractable biological identification problems.
ACKNOWLEDGEMENTS
We would like to thank H. G. Richter and G. Koch (Hamburg) and R. Pigozzo (IPT, Sao Paulo) for providing several wood samples. Ian MacLachlan helped with our initial methodology and commented on the manuscript.
LITERATURE CITED
- Angyalossy-Alfonso V, Miller R. Wood anatomy of the Brazilian species of Swartzia and considerations within the tribe Swartzieae. IAWA Journal. 2002;23:359–390. [Google Scholar]
- Carvalho AMV de. UK: University of Reading; 1989. Systematic studies in the genus Dalbergia L. f. in Brazil. PhD Thesis. [Google Scholar]
- Carvalho AMV de. A synopsis of the genus Dalbergia (Fabaceae: Dalbergieae) in Brazil. Brittonia. 1997;49:87–109. [Google Scholar]
- CITES on the World Wide Web. UNEP-WCMC Species Database: CITES-Listed Species. www.cites.org. (7 April 2009) [Google Scholar]
- CITES. CITES identification guide – tropical woods. Guide to the identification of tropical woods controlled under the Convention on International Trade in Endangered Species of wild Fauna and Flora. Canada: Minister of Supply and Services; 2002. Authority of the Minister of Environment. [Google Scholar]
- Durbin R, Eddy S, Krogh A, Mitchison G. Biological sequence analysis. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- Esteban LG, Fernández FG, De Palacios De Palacios P, Romero RM, Navarro Cano N. Artificial neural networks in wood identification: the case of two Juniperus species from the Canary Islands. IAWA Journal. 2009;30(1):87–94. [Google Scholar]
- Everitt BS, Dunn G. Applied multivariate data analysis. 2nd edn. London: Arnold Publishing; 2001. [Google Scholar]
- Flynn JH, Holder CD, editors. A guide to useful woods of the world. 2nd edn. Madison, WI: Forest Products Society; 2001. [Google Scholar]
- Guzman JAS, Richter HG, Rodriguez Anda R, Fuentes Talavera FJ. Wood fluorescence of commercial timbers marketed in Mexico. IAWA Journal. 2008;29:311–322. [Google Scholar]
- Hand DJ, Yu K. Idiot's Bayes – not so stupid after all? International Statistical Review. 2001;69:385–398. [Google Scholar]
- Hellberg E, Carcaillet C. Wood anatomy of west European Betula: quantitative descriptions and applications for routine identification in paleoecological studies. Ecoscience. 2003;10:370–379. [Google Scholar]
- Wheeler EA, Baas P, Gasson P, editors. IAWA Committee. IAWA list of microscopic features for hardwood identification, with an appendix on non-anatomical information. IAWA Bulletin. 1989;10:219–332. [Google Scholar]
- InsideWood. 2004 onwards. Published on the Internet. http://insidewood.lib.ncsu.edu/search . [Google Scholar]
- Khalid M, Lee ELY, Yusof R, Miniappan N. Design of an intelligent wood species recognition system. 2008 IJSSST 9 3 http://www.ijssst.info/Vol-9/No-3/paper2.pdf. (27 April 2009) [Google Scholar]
- Klitgaard BB, Lavin M. The tribe Dalbergieae sens. lat. In: Lewis GP, Schrire B, MacKinder B, Lock M, editors. Legumes of the World. London: Royal Botanic Gardens, Kew; 2005. [Google Scholar]
- Kribs DA. Commercial foreign woods on the American market. Ann Arbor, MI: Edwards Brothers; 1959. [Google Scholar]
- Kukachka BF. Sectioning refractory woods for anatomical studies. Madison: USDA Forest Service Products Laboratory; 1977. USDA Forest Research Note FPL-0236. [Google Scholar]
- MacLachlan IR, Gasson P. PCA of CITES listed Pterocarpus santalinus (Leguminosae) wood. IAWA Journal. 2010 in press. [Google Scholar]
- Miller RB, Wiemann MC. Madison, WI: US. Department of Agriculture, Forest Service, Forest Products Laboratory; 2006. Separation of Dalbergia nigra from Dalbergia spruceana. Research Paper FPL-RP-632. http://www.fpl.fs.fed.us/documnts/fplrp/fpl_rp632.pdf. (27 April 2009) [Google Scholar]
- Miller RG. The Jackknife – a review. Biometrika. 1974;61:1–15. [Google Scholar]
- Möller M, Gao LM, Mill RR, Li DZ, Hollingsworth ML, Gibby M. Morphometric analysis of the Taxus wallichiana complex (Taxaceae) based on herbarium material. Botanical Journal of the Linnean Society. 2007;155:307–335. [Google Scholar]
- Patel JD, Shah JJ. Sapwood and heartwood in Dalbergia latifolia. Phytomorphology. 1980;30:140–149. [Google Scholar]
- Record SJ, Hess RW. Timbers of the New World. New Haven, CT: Yale University Press; 1943. [Google Scholar]
- Richter HG, Krause UJ, Much C. Dalbergia congestiflora Standl.: wood structure and physico-chemical properties compared with other Central American species of Dalbergia. IAWA Journal. 1996;17:327–341. [Google Scholar]
- Richter HG, Gembruch K, Koch G. Berlin: BFH German Federal Agency for Nature Conservation; 2008. Software program: CITESwoodID version 2.0. [Google Scholar]
- Varty N. 1998 Dalbergia nigra. In: IUCN 2008. IUCN Red List of threatened species. www.iucnredlist.org . (7 April 2009) [Google Scholar]
- Wiedenhoeft AC, Miller RB. Structure and function of wood. In: Rowell RM, editor. Handbook of wood chemistry and wood composites. Boca Raton, FL: CRC Press; 2005. [Google Scholar]