Abstract
We investigated the temporal variation of bacterial production, respiration, and growth efficiency in the tropical coastal waters of Peninsular Malaysia. We selected five stations including two estuaries and three coastal water stations. The temperature was relatively stable (averaging around 29.5°C), whereas salinity was more variable in the estuaries. We also measured dissolved organic carbon and nitrogen (DOC and DON, respectively) concentrations. DOC generally ranged from 100 to 900 μM, whereas DON ranged from 0 to 32 μM. Bacterial respiration ranged from 0.5 to 3.2 μM O2 h−1, whereas bacterial production ranged from 0.05 to 0.51 μM C h−1. Bacterial growth efficiency was calculated as bacterial production/(bacterial production + respiration), and ranged from 0.02 to 0.40. Multiple correlation analyses revealed that bacterial production was dependent upon primary production (r2 = 0.169, df = 31, and P < 0.02) whereas bacterial respiration was dependent upon both substrate quality (i.e., DOC/DON ratio) (r2 = 0.137, df = 32, and P = 0.03) and temperature (r2 = 0.113, df = 36, and P = 0.04). Substrate quality was the most important factor (r2 = 0.119, df = 33, and P = 0.04) for the regulation of bacterial growth efficiency. Using bacterial growth efficiency values, the average bacterial carbon demand calculated was from 5.30 to 11.28 μM C h−1. When the bacterial carbon demand was compared with primary productivity, we found that net heterotrophy was established at only two stations. The ratio of bacterial carbon demand to net primary production correlated significantly with bacterial growth efficiency (r2 = 0.341, df = 35, and P < 0.001). From nonlinear regression analysis, we found that net heterotrophy was established when bacterial growth efficiency was <0.08. Our study showed the extent of net heterotrophy in these waters and illustrated the importance of heterotrophic microbial processes in coastal aquatic food webs.
As our understanding of the marine food web evolves, we recognize the importance of microorganisms in aquatic ecosystems. Bacteria are the main respirers and recycle a large pool of dissolved organic matter to higher trophic levels (6, 13). Therefore, bacterial production is a key process in dissolved organic matter flux. However, the transfer of dissolved organic matter to bacteria is more accurately reflected by bacterial carbon demand (BCD) or carbon consumption (23). One way to obtain BCD from bacterial production is through bacterial growth efficiency (BGE) or growth yield. BGE is an important parameter to evaluate the fate of organic carbon inputs and to determine whether bacteria act as a link (recyclers) or sink (mineralizers). Therefore, understanding the patterns of variation in BGE is fundamental for our knowledge of carbon cycling (14).
BGE is essentially the ratio of carbon converted to biomass relative to all the carbon consumed, where carbon consumption is either measured as the sum of bacterial production and respiration (5, 24), dissolved organic matter utilization (3), or both (12). Although bacterial production is frequently measured, bacterial respiration measurements are still scarce (23) and are often derived from production rates assuming constant growth efficiency (7, 30). The use of a constant growth efficiency is, however, not valid in some situations as studies have shown that BGE varies over both time and space (8, 27, 28, 32).
From cross-system compilations (15, 38, 49) and a comprehensive study in a temperate salt marsh estuary (4, 5), we begin to understand the factors that affect bacterial growth efficiency. Although substrate quantity and quality affect growth efficiency (4, 15), temperature is also an important factor (49). However, the effect of temperature differs for both bacterial production and bacterial respiration (5) and is distorted by substrate limitation (38).
Most of the above studies are from temperate regions where there is marked seasonality in temperature. In temperate regions, the effects of temperature are usually more apparent (5, 32) and can sometimes distort the effects of other factors (4). Although temperature plays a major role in controlling heterotrophic activity in temperate regions, it plays a lesser role in the tropics, where temperatures are more stable and relatively higher. Tropical oceans cover about 40% of the global ocean (37), and yet knowledge of the structure and function of this ecosystem remains limited, especially in the region of Southeast Asia (29). Only a few related studies are available, and those are from tropical coastal waters in Goa, India (45), and mangrove and estuarine waters in Peninsular Malaysia (27, 28). Substrate quality is often suggested as a more important factor than temperature (27, 28, 45).
In this paper, we addressed the following question: What is the effect of substrate quality and temperature toward BGE in tropical coastal waters? We measured bacterial respiration, production, and growth efficiency in tropical coastal waters and related their variation to changes in temperature and dissolved organic nutrient concentrations. Here, we show that although both temperature and substrate quality affected respiration, BGE was related to substrate quality only.
MATERIALS AND METHODS
Sampling.
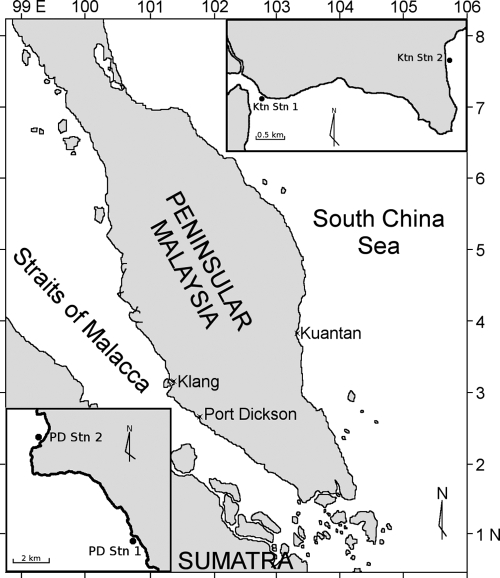
We sampled several near-shore sites both east (i.e., Kuantan) and west (Klang and Port Dickson) of Peninsular Malaysia for about 1 year. Two stations were selected at Kuantan (Ktn Stn 1 and Ktn Stn 2) and Port Dickson (PD Stn 1 and PD Stn 2) (Fig. 1) (Table 1). Due to logistic constraints, we sampled at the east coast of Peninsular Malaysia after completion of the west coast study. Our sampling at the east coast was also interrupted by the northeast monsoon winds from November until February. Surface seawater samples were collected using an acid-cleaned bucket, and in situ measurements of temperature (± 0.1°C) and salinity (± 0.1 ppt) were carried out using a salinometer (YSI-30). Seawater samples were kept in a cooler box for no more than 3 h until processing in the laboratory.
FIG. 1.
Map showing the location of the sampling stations east and west of Peninsular Malaysia. The inset at lower left shows PD Stn 1 and PD Stn 2 at Port Dickson, whereas the inset at upper right shows Ktn Stn 1 and Ktn Stn 2 at Kuantan. (Adapted from reference 29.)
TABLE 1.
Location of sampling stations in this studya
| Station name (description) | Location | Temp (°C) | Salinity (ppt) | NH4 (μM) | NO2 (μM) | NO3 (μM) | PO4 (μM) |
|---|---|---|---|---|---|---|---|
| Klang (estuary) | 03°00.1′N, 101°23.4′E | 30.0 ± 0.8 | 26.4 ± 5.1 | 13.50 ± 10.09 | 2.65 ± 1.84 | 3.08 ± 2.83 | 1.74 ± 1.60 |
| PD Stn 1 (sandy coasts) | 02°29.5′N, 101°50.3′E | 30.0 ± 1.1 | 30.7 ± 1.0 | 4.74 ± 4.74 | 0.38 ± 0.23 | 0.20 ± 0.10 | 0.48 ± 0.22 |
| PD Stn 2 (sandy coasts) | 02°32.7′N, 101°48.0′E | 29.8 ± 0.9 | 30.4 ± 1.9 | 3.15 ± 3.19 | 0.56 ± 0.35 | 0.25 ± 0.12 | 0.50 ± 0.19 |
| Ktn Stn 1 (estuary) | 03°48.4′N, 103°20.6′E | 28.7 ± 1.3 | 23.8 ± 9.7 | 3.53 ± 4.03 | 0.46 ± 0.24 | 0.58 ± 0.23 | 0.53 ± 0.22 |
| Ktn Stn 2 (sandy coasts) | 03°48.7′N, 103°22.4′E | 29.6 ± 0.6 | 30.9 ± 1.4 | 1.24 ± 0.71 | 0.41 ± 0.27 | 0.53 ± 0.27 | 0.57 ± 0.27 |
Values are means (± standard deviations) of surface water temperature, salinity, ammonium (NH4), nitrite (NO2), nitrate (NO3), and phosphorus (PO4).
In the laboratory, seawater samples for dissolved nutrient analyses were filtered through precombusted (450°C for 5 h) Whatman GF/F filters (nominal pore size of 0.7 μm) and stored at −20°C until analysis. Dissolved inorganic nitrogen (DIN; nitrate [NO3], nitrite [NO2], and ammonium [NH4]), and phosphate (PO4) concentrations were measured using a spectrophotometer (43). All nutrient measurements above were carried out in triplicates. Coefficients of variation (CV) were <5% for NH4, NO2, and PO4 analyses and <10% for NO3 analysis.
Dissolved organic nutrients.
Total dissolved nitrogen (TDN) concentrations were analyzed by a high-temperature combustion method using a total organic carbon (TOC) auto analyzer (Shimadzu TOC-VCSH; Japan) with an attached TDN measuring unit (Shimadzu TNM-1; Japan) (39). High-purity synthetic O2 (99.9%) was used as the carrier gas for the analysis. The sample was injected into the analyzer by using an auto sampler (Shimadzu ASI-V; Japan). The volume injected was 80 μl, with three injections made for each sample. TDN concentration was obtained from a five-point potassium nitrate calibration curve. A quality assurance sample of 1.0 mg of urea-N liter−1 was analyzed together with the water samples. Nitrogen recovery rate was >94%. The CV for TDN analysis was <8% (average ± standard deviation [SD], 3.4% ± 2.3%). Dissolved organic nitrogen (DON) was then calculated as follows: DON = TDN − DIN.
Dissolved organic carbon (DOC) concentration was calculated as the difference between total dissolved carbon and dissolved inorganic carbon (22). Total dissolved carbon was determined by the high-temperature catalytic oxidation method (680°C) using a TOC auto analyzer (Shimadzu TOC-VCPH; Japan) (36). High-purity O2 (99.9%) was used as the carrier gas. The volume injected was 50 μl, with three injections being made for each sample by an auto-sampler. Prior to the determination of dissolved inorganic carbon, 50 μl of 17% phosphoric acid was added to the sample. The sample was acidified and sparged, and the dissolved inorganic carbon was determined by the TOC auto analyzer (Shimadzu TOC-VCPH, Japan). Concentrations of total dissolved carbon and dissolved inorganic carbon were obtained from a five-point potassium hydrogen phthalate and sodium carbonate standard curve, respectively. A quality assurance sample of 10 mg liter−1 for both total dissolved carbon and dissolved inorganic carbon was analyzed together with the water samples, and recovery was >96%. All reported values were corrected for the instrument blank, and the CV was <9.0% (average ± SD, 3.0% ± 2.3%).
Bacterial respiration and production.
Seawater samples were filtered through precombusted Whatman GF/F filters to remove particles and bacterial grazers and then siphoned into acid-washed 60-ml dissolved oxygen bottles (32). Although reduction of the number of bacteria was unavoidable in this size-fractionation type of study, we have shown elsewhere that the bacterial growth rates in the GF/F filtrate is not significantly different from that in total seawater (32). These bottles were incubated in the dark at the in situ temperature (± 1°C) for 12 h. The presence of protists was also examined, and the sample was essentially protist free (<60 protist ml−1). The rate of dissolved oxygen decrease or bacterial respiration was measured in sets of five in a four-time-point analysis and analyzed using the least-squares linear regression method (53). Dissolved oxygen concentration was measured by the Winkler method (20). A respiratory quotient of 1.0 was used to convert bacterial respiration into carbon units (43). The bacterial growth rate (μ) was measured in another set of batch cultures carried out simultaneously. Subsamples for bacterial abundance were collected regularly over a 12-h incubation period. The value of μ was then calculated using the least-squares method as the slope of the linear regression analysis (53) of natural logarithmic bacterial abundance. Bacterial production was then calculated as the product μ × initial bacterial abundance. In order to obtain bacterial production in carbon equivalents, bacterial carbon content was used as a constant conversion factor. Bacterial carbon content at each location was different and ranged from 14.4 to 32.8 fg of C bacterium−1 (29). BGE was then calculated as bacterial production/(bacterial production + bacterial respiration).
Microbial abundance.
Bacterial abundance was determined by an epifluorescence microscope (Olympus BX60; Japan) with a U-MWU filter cassette (excitation, 330 to 385 nm; dichroic mirror, 400 nm; barrier, 420 nm). Sample (1 to 2 ml) was filtered onto a black 0.2-μm-pore-size Isopore filter and then stained with 4′6-diamidino-2-phenylindole (final concentration, 1 μg ml−1) for 7 min (25). Slides were kept frozen for <3 days before enumeration. A minimum of 15 microscope fields or 500 cells were counted for bacteria. In order to exclude phototrophs from our counts, we also viewed each field under a U-MWG filter cassette (excitor, 510 to 550 nm; dichroic mirror, 570 nm; barrier, 590 nm) for the autofluorescence of chlorophyll a. In order to exclude the presence of protists in the grazer-free filtrate, 10 ml of sample was filtered onto a black 0.8-μm-pore-size Isopore filter (Millipore) and then stained with the fluorochrome primulin (final concentration, 40 μg ml−1) for 5 min (9). At least 50 microscope fields were observed under the U-MWU filter cassette.
Statistical analyses.
Statistical tests (e.g., Student's t test; correlation, linear regression and nonlinear regression analyses, analysis of variance [ANOVA]; and Tukey's test) were carried out according to Zar (53). All data, unless noted otherwise, are reported as means ± SD.
RESULTS
Environmental conditions.
Table 1 shows the physicochemical variables measured in this study. Average surface seawater temperature was around 29.6°C, whereas the average salinity measured ranged from 28.7 to 30.0 ppt. Salinity fluctuated over a wider range in estuaries (CV, >20%) than in coastal waters (CV, <6%). The average concentration of NH4, NO2, NO3, and PO4 was highest at Klang, and NH4 was the major component (NH4/DIN = 68% ± 19%) of DIN.
Dissolved organic nutrients.
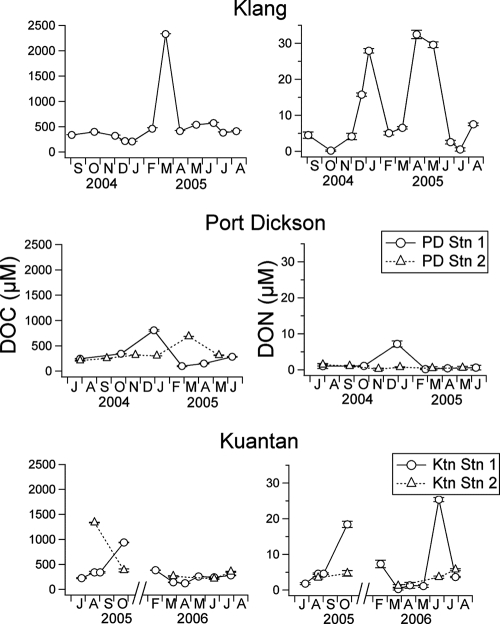
Fig. 2 shows the temporal variation of DOC and DON measured at all the stations. DOC generally ranged from 100 to 900 μM. DOC was outside this range on two occasions: when DOC jumped fourfold to 2,300 μM at Klang in March 2005 and at Ktn Stn 2 in August 2005 (1,300 μM). ANOVA showed that the DOC concentrations were not significantly different and that average DOC concentrations without these two extreme values were 390 ± 120, 320 ± 250, 350 ± 170, 330 ± 280, and 300 ± 80 μM at Klang, PD Stn 1, PD Stn 2, Ktn Stn 1, and Ktn Stn 2, respectively.
FIG. 2.
Temporal variation of DOC (μM) and DON (μM) at Klang, Port Dickson, and Kuantan. Error bars (± SD) are shown except when values are smaller than the symbol.
Unlike DOC, DON was significantly higher (Student's t test: t = 3.21, df = 23, and P = 0.002) in estuarine (Klang and Ktn Stn 1) than in coastal waters (Port Dickson and Ktn Stn 2). Observations at both Klang and Ktn Stn 1 also showed episodic increases of 4- to 22-fold in DON, whereas DON at Port Dickson and Ktn Stn 2 varied over a small range. Average DON was 11.4 ± 11.9, 1.7 ± 2.7, 0.7 ± 0.4, 6.8 ± 8.4, and 3.7 ± 1.7 μM at Klang, PD Stn 1, PD Stn 2, Ktn Stn 1, and Ktn Stn 2, respectively.
Bacterial respiration and production.
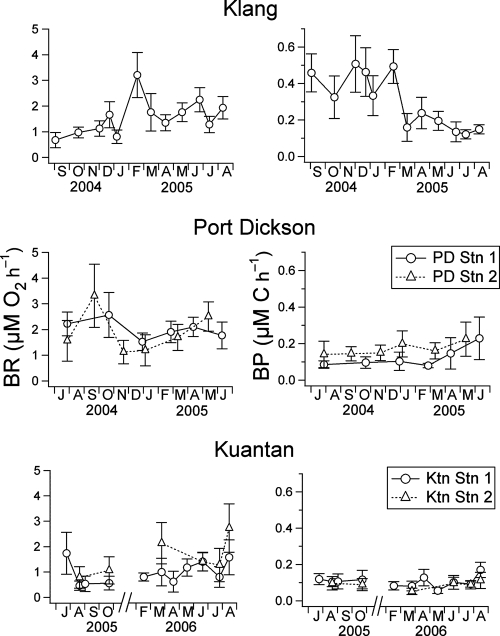
Figure 3 shows the temporal variation of bacterial respiration and production measured at all the stations. Bacterial respiration at Klang fluctuated between 0.7 and 2.2 μM O2 h−1 but increased nearly fourfold to 3.2 μM O2 h−1 in February 2005. Bacterial respiration ranged from 1.1 to 3.3 and 0.5 to 2.7 μM O2 h−1 at Port Dickson and Kuantan, respectively. There was significant variation in the bacterial respiration measured at the five stations (ANOVA: F = 3.58, df = 40, and P = 0.01). Multiple comparisons via Tukey's test showed that bacterial respiration at PD Stn 1 (average, 2.0 ± 0.4 μM O2 h−1) was significantly different from that at Ktn Stn 1 (average, 1.0 ± 0.4 μM O2 h−1) (q = 4.51, df = 36, and P < 0.05) (Fig. 4) .
FIG. 3.
Temporal variation of bacterial respiration ([BR] μM O2 h−1) and bacterial production ([BP] μM C h−1) at Klang, Port Dickson, and Kuantan. Error bars (± SD) are shown except when values are smaller than the symbol.
FIG. 4.
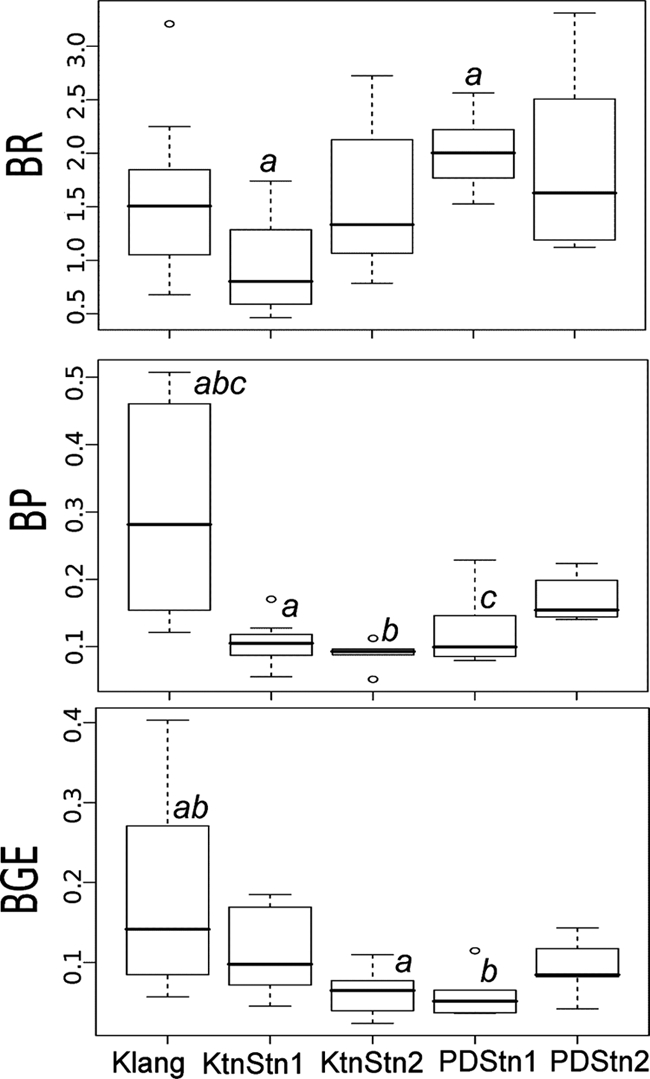
Box-and-whisker plots showing the range and the median of bacterial respiration ([BR] μM O2 h−1), bacterial production ([BP] μM C h−1), and BGE measured in this study. Outliers are also shown as open circles. The same letters of the alphabet are used to indicate values whose means were significantly different.
The temporal variation of bacterial production measured at all the stations was also highly significant (F = 9.41, df = 40, and P < 0.001). At Klang, bacterial production was from 0.12 to 0.51 μM C h−1 and was generally higher in the earlier than later part of the sampling period. At Port Dickson, bacterial production ranged from 0.08 to 0.23 μM C h−1, whereas at Kuantan bacterial production was from 0.05 to 0.17 μM C h−1. Bacterial production at Klang (0.30 ± 0.15 μM C h−1) was significantly different from that at PD Stn 1 (0.12 ± 0.06 μM C h−1; q = 5.38, df = 36, and P < 0.01), Ktn Stn 1 (0.11 ± 0.03 μM C h−1; q = 5.92, df = 36, and P < 0.01), and Ktn Stn 2 (0.09 ± 0.02 μM C h−1; q = 6.43, df = 36, and P < 0.01) (Fig. 4).
BGE ranged from 0.02 to 0.40 and followed the trend exhibited by bacterial production (F = 4.36, df = 40, and P = 0.005). BGE for Klang also decreased in the later part of the sampling period, whereas bacterial growth efficiency at Kuantan and Port Dickson were relatively stable (Fig. 5). Average bacterial growth efficiency was highest at Klang (0.18 ± 0.11) and was significantly different from BGE values calculated at Ktn Stn 2 (0.06 ± 0.03; q = 4.46, df = 36, and P < 0.05) and PD Stn 1 (0.06 ± 0.03; q = 4.61, df = 36, and P < 0.05) (Fig. 4). The average bacterial growth efficiencies at Ktn Stn 1 and PD Stn 2 were 0.11 ± 0.01 and 0.09 ± 0.03, respectively.
FIG. 5.
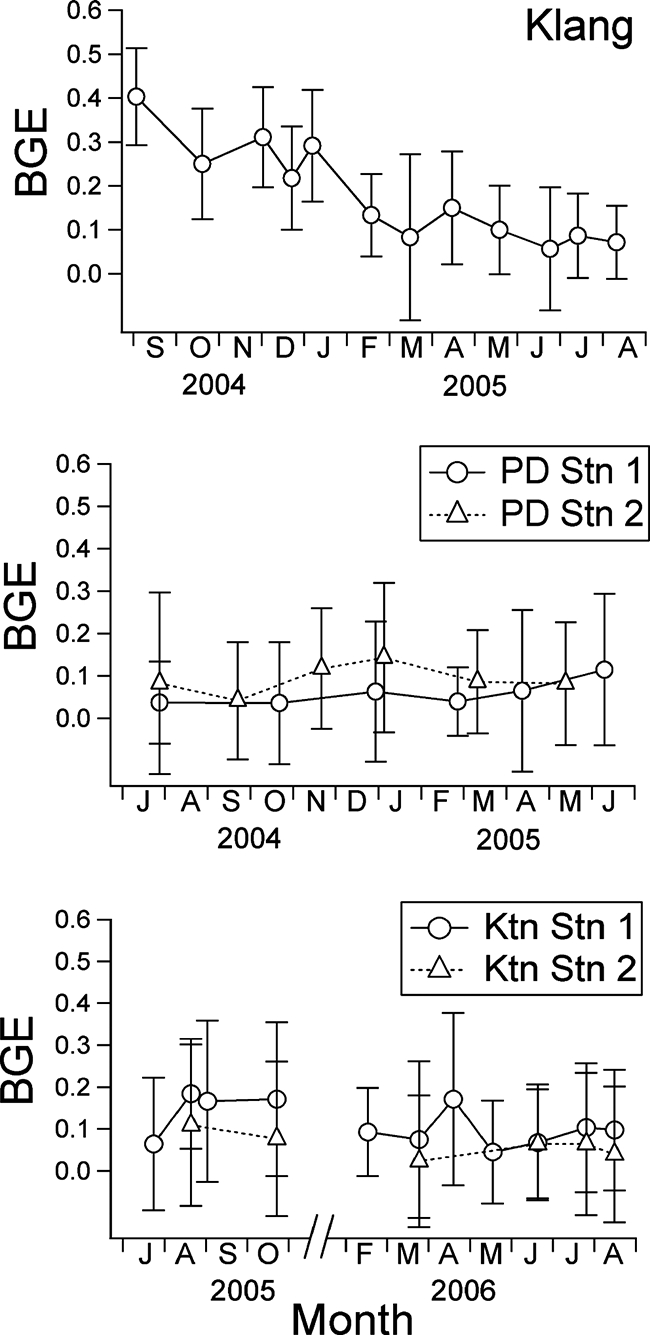
Temporal variation of BGE at Klang, Port Dickson, and Kuantan. Error bars (± 95% CI) are shown.
DISCUSSION
Environmental conditions.
Surface seawater temperatures observed in this study are typical of tropical waters (29, 30). Salinity was generally more variable at the estuaries (Klang and Ktn Stn 1) due to the episodic inputs of fresh river water. Inorganic nutrient concentrations were within the range for both estuarine and coastal waters in Malaysia (30, 41), and Klang had the highest level of eutrophication. The eutrophication at Klang is caused by the rapid development and industrialization taking place upstream (27). In this study, NH4 was the most dominant nitrogen species and probably reflected a reducing environment where NH4 accumulates (2, 16).
Dissolved organic nutrients.
Very few published data for both DOC and DON measurements are available for the Southeast Asia region. Available data from this region generally point toward elevated DOC concentrations, e.g., 420 to 4,200 μM in the rivers of Borneo (22) and up to 2,000 μM in the rivers of East Java (1) and 200 to 500 μM for the mangrove ecosystem in Thailand (26). Our study confirmed this trend. The DOC concentrations measured in this study were also similar to the concentration in a tropical mangrove creek in Brazil (190 to 900 μM) (16) but generally higher than those of temperate waters, e.g., coastal waters off the East China Sea (75 to 120 μM) (21) and the Mediterranean Sea (50 to 200 μM) (54).
The fourfold increase in DOC observed at Klang in March 2005 did not coincide with high primary production (29) and effectively ruled out in situ production as a source. We also ruled out river input as a source for the DOC increase because salinity was 31 ppt and indicated no significant freshwater input. A possible source was the lateral or horizontal transfer from outside this estuary. However, we did not carry out any measurements at other nearby stations to confirm this. Breakdown of organic matter in sediments could also contribute some of the DOC increase (18, 42). In this study, DON concentrations are also in the range reported for tropical mangrove ecosystems (5 to 30 μM) (16, 26). Data from other types of ecosystems within the region were not available, however.
DOC correlated significantly with DON (r2 = 0.256, df = 31, and P = 0.003), and linear regression analysis gave the following equation: DOC = 22(DON) + 270 (F = 10.68, df = 32, and P = 0.003) (Fig. 6A). The high C-to-N ratio (200 ± 190) was untenable and indicated a large pool of DOC that was uncoupled from DON. A possible reason for this is that a substantial amount of organic matter in this region is sourced from leaching, e.g., from forest litter (40), and is usually dominated by refractory humic substances (50). The origin intercept in Fig. 6A represented the fraction of DOC that did not covary with DON and was also the lowest mean value of DOC. This fraction of DOC could represent the refractory pool and was 270 ± 70 μM (± 95% confidence interval [CI]) or 70 to 90% of the DOC measured. The percentage of refractory DOC in this study was slightly higher than the estimation by Druffel et al. (17) based on mass balance calculations and natural δ14C estimates. The slope of the linear regression equation was 22 ± 13 (± 95% CI) and represented the C-to-N ratio without the DOC refractory pool. The adjusted ratio was still higher than Redfield's ratio of phytogenic organic matter, i.e., 6.6 (47), and also higher than the elemental composition of bacterial biomass, i.e., 5 (19). The high C-to-N ratio provided prima facie evidence of nitrogen limitation for bacterial activity.
FIG. 6.
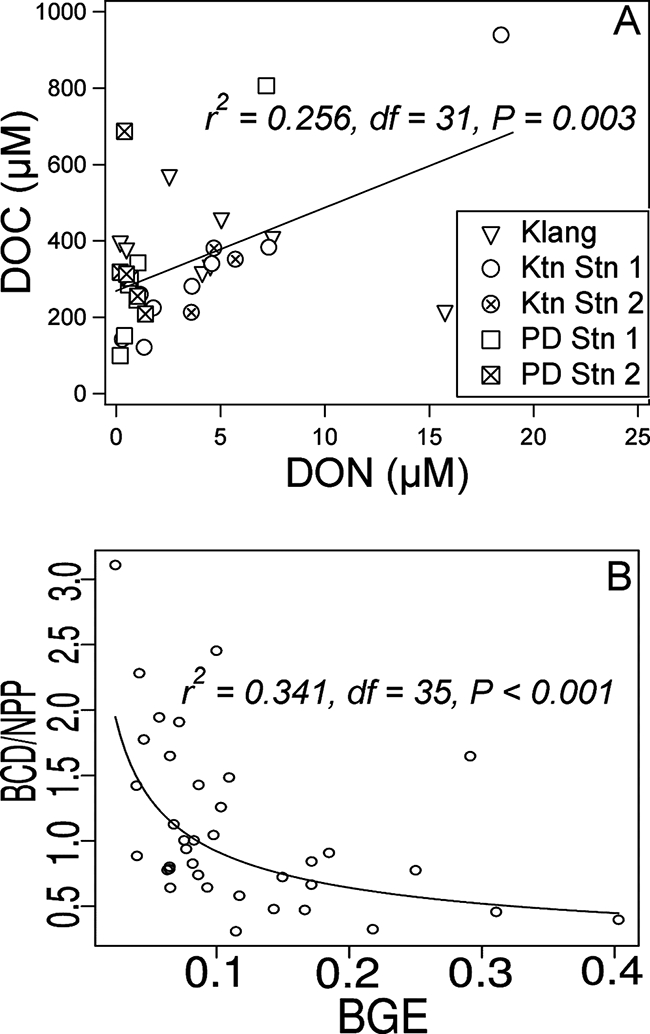
(A) Correlation analysis between DOC (μM) and DON (μM). Outliers (DOC of >1,000 μM and DON of >25 μM) are not shown and are not included in the correlation analysis. (B) Correlation analysis between BCD/NPP and BGE. Regression slopes are also shown.
Factors affecting BGE.
We carried out multiple correlation analysis to determine whether BGE was coupled to any of the physical, chemical, and biological variables available (Table 2). In this study, bacterial production did not correlate with respiration. Although both production and respiration are essentially linked to heterotrophy, they are not always tightly coupled (4), which may lead to variation in growth efficiency. The uncoupling between production and respiration is also due to their different responses to different variables. Therefore, knowledge of the factors that regulate bacterial production and respiration will help us to understand the temporal variability of BGE in natural waters.
TABLE 2.
Correlation matrix of variables measureda
| Variable | BP | BR | BGE | Water temp (°C) | Chl a | NPP | DOC | DON | C/N |
|---|---|---|---|---|---|---|---|---|---|
| BP (μM C h−1) | 1 | ||||||||
| BR (μM O2 h−1) | −0.000 | 1 | |||||||
| BGE | 0.471*** | −0.324*** | 1 | ||||||
| Water temp (°C) | 0.007 | 0.113* | −0.031 | 1 | |||||
| Chl a (μg liter−1)b | −0.055 | −0.070 | 0.027 | −0.025 | 1 | ||||
| NPP (μM C liter −1 h−1)b | 0.169* | 0.066 | 0.082 | 0.005 | −0.033 | 1 | |||
| DOC (μM) | 0.003 | −0.004 | 0.002 | −0.001 | −0.186 | −0.000 | 1 | ||
| DON (μM) | −0.042 | −0.075 | 0.109 | −0.076 | 0.156 | 0.050 | 0.256** | 1 | |
| C/N | 0.001 | 0.138* | −0.119* | 0.018 | 0.134 | −0.015 | −0.054 | −0.310*** | 1 |
Values for the coefficient of determination (r2) are shown. BP, bacterial production; BR, bacterial respiration; Chl a, chlorophyll a. A negative sign denotes an inverse relationship. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Chlorophyll a and NPP data are from Lee and Bong (29).
In this study, bacterial production ranged from 0.05 to 0.51 μM C h−1 and was within the range previously published for tropical coastal waters (29, 30). Average bacterial production at Klang was the highest among all the stations and reflected the eutrophic conditions at Klang (27, 29). Although net primary production (NPP) was not measured in this study, primary production and chlorophyll a data at the time of sampling are available (29). We found that bacterial production correlated significantly with NPP (r2 = 0.169, df = 31, and P < 0.02) but not with chlorophyll a. Chlorophyll a is a product of both top-down and bottom-up controls and is not a good proxy for resource availability as it does not always reflect in situ primary production rates (29). Bacterial production was also not correlated with DOC concentration, and the uncoupling observed here is similar to the results from Apple et al. (5). As a large portion of the DOC measured in this study was refractory and not readily available to bacteria, bacterial production depended more on primary production than ambient DOC.
Similar to Pradeep Ram et al. (45), we did not observe any correlation between bacterial production and temperature. Although numerous studies have shown the temperature dependence of bacterial growth and production, temperature dependence is stronger at lower temperatures (5, 10, 46). In tropical coastal waters where temperatures are relatively high and stable, bacterial production gave a different response and was generally independent of temperature. Moreover, most organisms already function at their temperature optimum (44) in the tropics.
For bacterial respiration, the rates measured in this study ranged from 0.5 to 3.2 μM O2 h−1 and were similar to rates for other tropical coastal waters (27, 30, 45) but more than 1 order higher than rates in temperate waters (4, 32, 34). We have shown earlier that in this respiration setup, the dissolved oxygen decrease in the <0.7-μm fraction is due to biological activity as the <-0.2-μm control fraction did not exhibit any significant dissolved oxygen decrease (30). We attributed the oxygen utilization in the <0.7-μm fraction to bacterial respiration as only bacteria were observed.
Unlike bacterial production, bacterial respiration increased as both the C-to-N ratio (r2 = 0.137, df = 32, and P = 0.03) and temperature (r2 = 0.113, df = 36, and P = 0.04) increased (Table 2). Although Apple et al. (5) reported that bacterial respiration is regulated exclusively by temperature, we found that both substrate quality and temperature regulated bacterial respiration. This is a first report showing the temperature dependence of bacterial respiration in tropical waters although the temperature range was small, i.e., from 28 to 32°C. Studies of temperature dependence of bacterial activity are usually carried out in temperate regions and cover the range of 20 to 30°C (5). We were able to observe a significant correlation between temperature and respiration but not with production. One reason could be that the temperature dependence of bacterial respiration is usually stronger than bacterial production (5, 38).
Other than temperature, bacterial respiration was also correlated with the C-to-N ratio. A higher C-to-N ratio indicates a reduction in organic matter quality (19, 52) or bioreactivity (3) that increases respiration requirements for catabolic processes. In this study, we found that bacterial production was dependent on NPP, whereas bacterial respiration correlated with both temperature and substrate quality. Their dependence on different variables also affected the variation of BGE.
BGE measured here was within the range reported for tropical coastal waters (27, 28, 45). As estuaries were more productive (29), we showed that BGE was higher in estuaries (0.15 ± 0.09) than in coastal waters (0.07 ± 0.03) (Student's t test: t = 3.63, df = 29, and P < 0.001). The trend of higher growth efficiency in estuaries than in coastal waters is similar to the report of Pradeep Ram et al. (45). The spatial and temporal variability of bacterial growth efficiency suggested the importance of measuring growth efficiency. Growth efficiencies assumed in many studies (2, 26, 31) are seldom achieved under natural conditions and would underestimate the role of bacteria in carbon cycling especially in tropical waters. In this study, BGE was correlated with both bacterial production (r2 = 0.471, df = 35, and P < 0.001) and respiration (r2 = −0.324, df = 37, and P < 0.001). From the coefficient of determination, both bacterial production and bacterial respiration played important but contrasting roles in determining the BGE. As different variables affect bacterial respiration and bacterial production, the effect of these variables toward BGE could be shrouded by each other.
In this study, BGE was inversely correlated with substrate quality or the C-to-N ratio (r2 = 0.119, df = 33, and P = 0.04). There are similar reports of BGE dependence on substrate quality (5, 45, 48). The relatively low growth efficiency reflected the low-quality substrate or organic matter available in tropical coastal waters. In contrast to the findings of Apple et al. (5), there was no temperature dependence for BGE. Although the effect of temperature on respiration was significant in this study, the temperature range was probably not enough to influence BGE. López-Urrutia and Moran (38) have mentioned that other environmental conditions, e.g., availability of inorganic nutrients and the quality and quantity of organic matter substrates, can sometimes distort temperature dependence of BGE.
Although seasonal change in bacterial composition has some effect on BGE (48), we did not evaluate this factor due to inadequate bacterial composition data. Although Lee et al. (33) reported on the bacterial composition in these coastal waters, the study was not carried out along a temporal scale. As seasonal change in bacterial composition is related to water temperature (51), a change in bacterial composition should have minimal effect in tropical waters with a stable temperature regime.
Net heterotrophy in tropical coastal waters.
The relatively low BGE reported for tropical coastal waters (27, 28, 45) gives a presumption that net heterotrophy is prevalent in these waters. In this study, the average BGE was from 0.06 to 0.18. BCD ranged from 5.30 to 11.28 μM C h−1 and was highest in Klang (Table 3). When we compared the BCD to NPP, we found that net heterotrophy was established only at Klang and Ktn Stn 2. Primary productivity alone was inadequate to support bacterial heterotrophy at these stations, and an external source of organic matter was required. There was little similarity between these two stations except, perhaps, a relatively higher DOC concentration (>500 μM) than the other stations (<350 μM). The similarity seemed to justify our suggestion that bacterial heterotrophy at these two stations was supported by allochthonous organic matter. Although primary production also contributes to the DOC pool, there was presumptive evidence that a substantial fraction (70 to 90%) of DOC was allochthonous. Moreover, primary productivity at Ktn Stn 2 was the lowest among the stations studied (29).
TABLE 3.
A summary of carbon related measurements available for the stations in this studya
| Station | NPP (μM C h−1)b | BP (μM C h−1)b | BGE | BCD (μM C h−1) | BCD/NPP | DOC (μM) |
|---|---|---|---|---|---|---|
| Klang | 9.72 ± 1.85 | 1.62 ± 0.80 | 0.18 ± 0.06 | 11.28 ± 3.86 | 1.19 ± 0.43 | 548 ± 324 |
| PD Stn 1 | 5.99 ± 0.35 | 0.26 ± 0.06 | 0.06 ± 0.02 | 5.30 ± 2.35 | 0.65 ± 0.25 | 321 ± 203 |
| PD Stn 2 | 6.28 ± 0.94 | 0.46 ± 0.04 | 0.09 ± 0.03 | 5.77 ± 2.15 | 0.98 ± 0.65 | 347 ± 137 |
| Ktn Stn 1 | 6.65 ± 1.31 | 0.61 ± 0.10 | 0.11 ± 0.03 | 6.15 ± 1.38 | 0.96 ± 0.21 | 327 ± 143 |
| Ktn Stn 2 | 5.00 ± 1.46 | 0.38 ± 0.05 | 0.06 ± 0.02 | 7.92 ± 4.29 | 1.57 ± 0.66 | 509 ± 410 |
Values are means ± 95% CI. BP, bacterial production.
Obtained from Lee and Bong (29).
In this study, the ratio of BCD to NPP (BCD/NPP) correlated significantly with BGE (r2 = 0.341, df = 35, and P < 0.001) (Fig. 6B). Nonlinear regression analysis revealed a power relationship between these two variables: BCD/NPP = 0.278BGE−0.52 (F = 18.1, df = 36, and P < 0.001). Therefore, net heterotrophy (or when BCD/NPP is >1.0) occurs when BGE is <0.08. About half of the BGE values measured in this study were <0.08. Our study showed the extent of net heterotrophy in these waters and illustrated the importance of heterotrophic microbial processes in coastal aquatic food webs, which could contribute to the cycling of organic carbon.
There is concern about the effect of the continuous warming of the sea toward tropical waters which already have relatively high temperatures. From our study, increasing seawater temperature will further increase bacterial respiration but not production rates. CO2 release from tropical coastal waters will be exacerbated. The increasing bacterial respiration and excess heterotrophy raise concerns about the occurrence of dissolved oxygen depletion or hypoxia, which could threaten fisheries and biodiversity.
Our study helps fill a gap in current knowledge as data from tropical waters are relatively scarce, and there is still a lack of knowledge of the factors that affect bacterial activity. We showed that bacterial respiration depended upon substrate quality and, to a lesser extent, temperature, whereas bacterial production was closely linked to primary production. Substrate quality was also the most important factor responsible for the regulation of BGE.
Acknowledgments
We are grateful to the Southeast Asia Regional Committee for START or SARCS (94/01/CW and 95/01/CW), National Oceanography Directorate of Malaysia (04-01-03-SF0194), and University of Malaya (FQ009/2007A) for their research grants that supported this work.
Part of this paper was written when C.W.L. was a visiting lecturer at the Graduate School of Earth and Environmental Sciences, Hokkaido University, under the Global COE Program (Establishment of Center for Integrated Field Environmental Science), MEXT, Japan.
We also thank the reviewers for their helpful comments.
Footnotes
Published ahead of print on 9 October 2009.
REFERENCES
- 1.Aldrian, E., C. T. A. Chen, S. Adi, Prihartanto, N. Sudiana, and S. P. Nugroho. 2008. Spatial and seasonal dynamics of riverine carbon fluxes of the Brantas catchment in East Java. J. Geophys. Res. 113:G03029. doi: 10.1029/2007JG000626. [DOI] [Google Scholar]
- 2.Alongi, D. M., V. C. Chong, P. Dixon, A. Sasekumar, and F. Tirendi. 2003. The influence of fish cage aquaculture on pelagic carbon flow and water chemistry in tidally dominated mangrove estuaries of peninsular Malaysia. Mar. Environ. Res. 55:313-333. [DOI] [PubMed] [Google Scholar]
- 3.Amon, R. M. W., and R. Benner. 1996. Bacterial utilization of different size classes of dissolved organic matter. Limnol. Oceanogr. 41:41-51. [Google Scholar]
- 4.Apple, J. K., and P. A. del Giorgio. 2007. Organic substrate quality as the link between bacterioplankton carbon demand and growth efficiency in a temperate salt-marsh estuary. ISME J. 1:729-742. [DOI] [PubMed] [Google Scholar]
- 5.Apple, J. K., P. A. del Giorgio, and W. M. Kemp. 2006. Temperature regulation of bacterial production, respiration and growth efficiency in a temperate salt-marsh estuary. Aquat. Microb. Ecol. 43:243-254. [Google Scholar]
- 6.Azam, F., T. Fenchel, J. E. Field, J. S. Gray, L. A. Meyer-Reil, and F. Thingstad. 1983. The ecological role of water column microbes in the sea. Mar. Ecol. Prog. Ser. 10:257-263. [Google Scholar]
- 7.Bano, N., M. U. Nisa, N. Khan, M. Saleem, P. J. Harrison, S. I. Ahmed, and F. Azam. 1997. Significance of bacteria in the flux of organic matter in the tidal creeks of the mangrove ecosystem of the Indus River delta, Pakistan. Mar. Ecol. Prog. Ser. 157:1-12. [Google Scholar]
- 8.Biddanda, B. A., and J. B. Cotner. 2002. Love handles in aquatic ecosystems: the role of dissolved organic carbon drawdown, resuspended sediments, and terrigenous inputs in the carbon balance of Lake Michigan. Ecosystems 5:431-445. [Google Scholar]
- 9.Bloem, J., M. J. B. Bär-Gilissen, and T. E. Cappenberg. 1986. Fixation, counting, and manipulation of heterotrophic nanoflagellates. Appl. Environ. Microbiol. 52:1266-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carignan, R., D. Planas, and C. Vis. 2000. Planktonic production and respiration in oligotrophic Shield Lakes. Limnol. Oceanogr. 45:189-199. [Google Scholar]
- 11.Reference deleted.
- 12.Cherrier, J., J. E. Bauer, and E. R. M. Druffel. 1996. Utilization and turnover of labile dissolved organic matter by bacterial heterotrophs in eastern North Pacific surface waters. Mar. Ecol. Prog. Ser. 139:267-279. [Google Scholar]
- 13.Cole, J. J., S. Findlay, and M. L. Pace. 1988. Bacterial production in fresh and saltwater ecosystems: a cross-system overview. Mar. Ecol. Prog. Ser. 43:1-10. [Google Scholar]
- 14.del Giorgio, P. A., and J. J. Cole. 1998. Bacterial growth efficiency in natural aquatic systems. Annu. Rev. Ecol. Syst. 29:503-541. [Google Scholar]
- 15.del Giorgio, P. A., J. J. Cole, and A. Cimbleris. 1997. Respiration rates in bacteria exceed phytoplankton production in unproductive aquatic systems. Nature 385:148-151. [Google Scholar]
- 16.Dittmar, T., and R. J. Lara. 2001. Driving forces behind nutrient and organic matter dynamics in a mangrove tidal creek in North Brazil. Estuar. Coast. Shelf Sci. 52:249-259. [Google Scholar]
- 17.Druffel, E. R. M., P. M. Williams, J. E. Bauer, and J. R. Ertel. 1992. Cycling of dissolved and particulate organic matter in the open ocean. J. Geophys. Res. 97:15639-15659. [Google Scholar]
- 18.Fenchel, T. 1998. Bacterial biogeochemistry: the ecophysiology of mineral cycling. Academic Press, San Diego, CA.
- 19.Goldman, J. C., D. A. Caron, and M. R. Dennett. 1987. Regulation of gross growth efficiency and ammonium regeneration in bacteria by substrate C:N ratio. Limnol. Oceanogr. 32:1239-1252. [Google Scholar]
- 20.Grasshoff, K., K. Kremling, and M. Ehrhardt. 1999. Methods of seawater analysis, 3rd ed. Wiley-VCH, Weinheim, Germany.
- 21.Hung, J. J., C. H. Chen, G. C. Gong, D. D. Sheu, and F. K. Shiah. 2003. Distributions, stoichiometric patterns and cross-shelf exports of dissolved organic matter in the East China Sea. Deep Sea Res. II 50:1127-1145. [Google Scholar]
- 22.Ishikawa, T., Trisliana, Yurenfrie, and S. Gumiri. 2006. Dissolved organic carbon concentration of a natural water body and its relationship to water color in Central Kalimantan, Indonesia. Limnology 7:143-146. [Google Scholar]
- 23.Jahnke, R. A., and D. B. Craven. 1995. Quantifying the role of heterotrophic bacteria in the carbon cycle: a need for respiration rate measurements. Limnol. Oceanogr. 40:436-441. [Google Scholar]
- 24.Jimenez-Mercado, A., R. Cajal-Medrano, and H. Maske. 2007. Marine heterotrophic bacteria in continuous culture, the bacterial carbon growth efficiency, and mineralization at excess substrate and different temperatures. Microb. Ecol. 54:56-64. [DOI] [PubMed] [Google Scholar]
- 25.Kepner, R. L., Jr., and J. R. Pratt. 1994. Use of fluorochromes for direct enumeration of total bacteria in environmental samples: past and present. Microbiol. Rev. 58:603-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kristensen, E., and P. Suraswadi. 2002. Carbon, nitrogen and phosphorus dynamics in creek water of a Southeast Asian mangrove forest. Hydrobiologia 474:197-211. [Google Scholar]
- 27.Lee, C. W., and C. W. Bong. 2006. Carbon flux through bacteria in a eutrophic tropical environment: Port Klang waters, p. 329-345. In E. Wolanski (ed.), The environment in Asia Pacific harbours. Springer, Dordrecht, The Netherlands.
- 28.Lee, C. W., and C. W. Bong. 2007. Bacterial respiration, growth efficiency and protist grazing rates in mangrove waters in Cape Rachado, Malaysia. Asian J. Water Environ. Pollut. 4:11-16. [Google Scholar]
- 29.Lee, C. W., and C. W. Bong. 2008. Bacterial abundance and production and their relation to primary production in tropical coastal waters of Peninsular Malaysia. Mar. Freshw. Res. 59:10-21. [Google Scholar]
- 30.Lee, C. W., C. W. Bong, M. A. Mohamed Yusoff, and S. A. Alias. 2005. Bacterial mediated carbon flux in mangrove waters: a Malaysian perspective. Int. J. Ecol. Environ. Sci. 31:203-211. [Google Scholar]
- 31.Lee, C. W., I. Kudo, M. Yanada, and Y. Maita. 2001. Bacterial abundance and production and heterotrophic nanoflagellate abundance in subarctic coastal waters (Western North Pacific Ocean). Aquat. Microb. Ecol. 23:263-271. [Google Scholar]
- 32.Lee, C. W., I. Kudo, T. Yokokawa, M. Yanada, and Y. Maita. 2002. Dynamics of bacterial respiration and related growth efficiency, dissolved nutrients and dissolved oxygen concentration in a subarctic coastal embayment. Mar. Freshw. Res. 53:1-7. [Google Scholar]
- 33.Lee, C. W., A. Y. F. Ng, K. Narayanan, E. U. H. Sim, and C. C. Ng. 2009. Isolation and characterization of culturable bacteria from tropical coastal waters. Cienc. Mar. 35:153-167. [Google Scholar]
- 34.Lemée, R., E. Rochelle-Newall, F. Van Wambeke, M.-D. Pizay, P. Rinaldi, and J.-P. Gattuso. 2002. Seasonal variation of bacterial production, respiration and growth efficiency in the open NW Mediterranean Sea. Aquat. Microb. Ecol. 29:227-237. [Google Scholar]
- 35.Reference deleted.
- 36.Lønborg, C., and M. Søndergaard. 2009. Microbial availability and degradation of dissolved organic carbon and nitrogen in two coastal areas. Estuar. Coast. Shelf Sci. 81:513-520. [Google Scholar]
- 37.Longhurst, A. R., and D. Pauly. 1987. Ecology of tropical oceans. Academic Press, San Diego, CA.
- 38.López-Urrutia, Á., and X. A. G. Morán. 2007. Resource limitation of bacterial production distorts the temperature dependence of oceanic carbon cycling. Ecology 88:817-822. [DOI] [PubMed] [Google Scholar]
- 39.Mahaffey, C., C. R. Benitez-Nelson, R. R. Bidigare, Y. Rii, and D. M. Karl. 2008. Nitrogen dynamics within a wind-driven eddy. Deep Sea Res. II 55:1398-1411. [Google Scholar]
- 40.Möller, A., K. Kaiser, and G. Guggenberger. 2005. Dissolved organic carbon and nitrogen in precipitation, throughfall, soil solution, and stream water of the tropical highlands in northern Thailand. J. Plant Nutr. Soil Sci. 168:649-659. [Google Scholar]
- 41.Nixon, S. W., B. N. Furnas, V. Lee, N. Marshall, J. E. Ong, C. H. Wong, W. K. Gong, and A. Sasekumar. 1984. The role of mangroves in the carbon and nutrient dynamics of Malaysia estuaries, p. 496-513. In E. Soepadmo, A. N. Rao, and D. J. Macintosh (ed.), Proceedings of the Asian Symposium on Mangrove Environment: Research and Management. University of Malaya and UNESCO, Kuala Lumpur, Malaysia.
- 42.Otsuki, A., and T. Hanya. 1972. Production of dissolved organic matter from dead green algal cells. II. Anaerobic microbial decomposition. Limnol. Oceanogr. 17:248-257. [Google Scholar]
- 43.Parsons, T. R., Y. Maita, and C. M. Lalli. 1984. A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford, United Kingdom.
- 44.Pomeroy, L. R., and W. J. Wiebe. 2001. Temperature and substrates as interactive limiting factors for marine heterotrophic bacteria. Aquat. Microb. Ecol. 23:187-204. [Google Scholar]
- 45.Pradeep Ram, A. S., S. Nair, and D. Chandramohan. 2003. Bacterial growth efficiency in the tropical estuarine and coastal waters of Goa, southwest coast of India. Microb. Ecol. 45:88-96. [DOI] [PubMed] [Google Scholar]
- 46.Raymond, P. A., and J. E. Bauer. 2000. Bacterial consumption of DOC during transport through a temperate estuary. Aquat. Microb. Ecol. 22:1-12. [Google Scholar]
- 47.Redfield, A. C., B. H. Ketchum, and F. A. Richards. 1963. The influence of organisms on the composition of sea water, p. 26-77. In M. N. Hill (ed.), The sea, vol. 2. Wiley-Interscience, New York, NY. [Google Scholar]
- 48.Reinthaler, T., C. Winter, and G. J. Herndl. 2005. Relationship between bacterioplankton richness, respiration, and production in the southern North Sea. Appl. Environ. Microbiol. 71:2260-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rivkin, R. B., and L. Legendre. 2001. Biogenic carbon cycling in the upper ocean: effects of microbial respiration. Science 291:2398-2400. [DOI] [PubMed] [Google Scholar]
- 50.Steinberg, C. E. W. 2003. Ecology of humic substances in freshwaters. Determinants from geochemistry to ecological niches. Springer, Berlin, Germany.
- 51.Tomoko, S. 2008. Seasonal and spatial variation of bacterial community structure in river-mouth areas of Gokasho Bay, Japan. Microbes Environ. 23:277-284. [DOI] [PubMed] [Google Scholar]
- 52.Touratier, F., L. Legendre, and A. Vézina. 1996. Model of bacterial growth influenced by substrate C:N ratio and concentration. Aquat. Microb. Ecol. 19:105-118. [Google Scholar]
- 53.Zar, J. H. 1999. Biostatistical analysis, 4th ed. Prentice Hall, Upper Saddle River, NJ.
- 54.Zeri, C., H. Kontoyiannis, and A. Giannakourou. 2009. Distribution, fluxes and bacterial consumption of total organic carbon in a populated Mediterranean Gulf. Cont. Shelf Res. 29:886-895. [Google Scholar]