Abstract
Occupational health symptoms related to bioaerosol exposure have been observed in a variety of working environments. Bioaerosols contain microorganisms and microbial components. The aim of this study was to estimate the total inflammatory potential (TIP) of bioaerosols using an in vitro assay based on granulocyte-like cells. A total of 129 bioaerosol samples were collected in the breathing zone of workers during their daily working routine at 22 biofuel plants. The samples were analyzed by traditional assays for dust, endotoxin, fungal spores, (1→3)-β-d-glucan, total number of bacteria, the enzyme N-acetyl-β-d-glucosaminidase (NAGase; primarily originating from fungi), Aspergillus fumigatus, and mesophilic and thermophilic actinomycetes; the samples were also assayed for TIP. In a multilinear regression four factors were significant for the TIP values obtained: endotoxin (P < 0.0001), fungal spores (P < 0.0001), (1→3)-β-d-glucan (P = 0.0005), and mesophilic actinomycetes (P = 0.0063). Using this model to estimate TIP values on the basis of microbial composition, the correlation to the measured values was r = 0.91. When TIP values obtained in the granulocyte assay were related to the primary working area, we found that bioaerosol samples from personnel working in straw storage facilities showed high TIP values (≈50 times the TIP of unstimulated controls). In contrast, bioaerosol samples from personnel with work functions in offices or laboratories showed low TIP values (≈5 times the TIP of the unstimulated control). This indicates, as expected, that these areas were less contaminated. In conclusion, the granulocyte assay reacts to multiple contaminants in the environmental samples and can be used to obtain a measurement of TIP. Therefore, potential occupational health effects related to inflammation of the airways in a working environment can be estimated using this assay.
For several years reports have related increased prevalence of respiratory symptoms to the exposure to bioaerosols containing, e.g., organic dust particles, actinomycetes, endotoxin, and fungal spores. Occupational health effects of bioaerosols have been reported in swine confinement houses and poultry farms as well as during hay handling. Airway diseases are frequent occupational disorders among farmers in many countries around the world (19, 23). During mechanical handling, biofuels such as straw and wood chips release high amounts of various microbial components such as, e.g., actinomycetes, fungal spores, and endotoxin (11, 25). Studies of personnel exposure to bioaerosols at biofuel plants reveal high concentrations of different microbial components and exposure levels higher than the suggested occupational exposure limits (10).
Exposure limits or suggested exposure limits are usually based on studies where traditional microbial quantification methods have been applied and are normally related to the concentration of a single contaminant (e.g., endotoxin) (17) or to gravimetric measures of dust. Furthermore, it is recognized that exposure to various microbial components may cause inflammation in the airways (4, 5, 15, 16, 20, 34, 35). Consequently, both the quantitative and the qualitative composition of bioaerosols may be of importance. In optimal settings, risk assessment of bioaerosols is based on the presence and concentration of a variety of microbial components. However, such analyses are tedious and costly. Therefore, an assay taking multifactorial contaminations into account may be highly relevant, and ideally this assessment should also correlate to airway inflammation. The objective of this work is to find a rapid and cost-efficient alternative to microbial analyses in characterizing bioaerosols from occupational settings.
Normally, airways and alveoli contain a relatively small number of granulocytes, but the pulmonary vasculature represents the largest reservoir of granulocytes in the human body. From this pool, granulocytes can be rapidly recruited upon microbial challenge (26). This recruitment is crucial for the immune response of the host against pathogens. Consequently, we speculate that the production of reactive oxygen species (ROS) of granulocytes can be used as readout for bioaerosol exposure. However, isolated granulocytes are short-lived, with a half-life of only a few hours (33). For this reason a granulocyte-like cell line can represent a practical alternative to freshly isolated cells.
In 2006 a granulocyte assay based on a cell line was developed mainly to assess microbial contamination in medicines (32). The assay was shown to be very sensitive to a wide range of microorganisms (32) and severalfold more sensitive to fungi than monocyte assays (e.g., the Mono Mac 6 assay) (18, 32). Furthermore, the assay showed sensitivity very similar to that of freshly isolated granulocytes toward a variety of microorganisms and microbial components (our unpublished observations). This indicates that the granulocyte assay is suitable to assess the inflammatory potential of bioaerosols or similar environmental samples.
The granulocyte assay is based on differentiated HL-60 cells. To assess the immunomodulatory effect of a given environmental sample, the production of ROS from the granulocyte-like cells is measured in a luminol-dependent chemiluminometric assay. The induced ROS production is used as a measure of the total inflammatory potential (TIP) of the sample. The term TIP as described herein refers to the inflammatory effects induced in the granulocyte assay by the bioaerosols tested. These effects can be induced by both viable and dead microorganisms as well as related cell wall debris. Furthermore, the term covers inflammatory effects induced by other organic and inorganic components of the bioaerosols, such as pollen, particles, viruses, soot, etc., that are known to have or speculated to have inflammatory effects in the granulocyte assay.
To examine the potential of the granulocyte assay to measure the TIP of an environmental sample, we examined 129 bioaerosol samples collected at 22 biofuel plants. In occupational settings the presence of fungi, Aspergillus fumigatus, β-glucan, endotoxin, actinomycetes, and dust has been related to airway symptoms (4, 5, 15, 16, 20, 34, 35). Therefore, we have quantified these components in the sampled bioaerosols. Furthermore, we have quantified N-acetyl-β-d-glucosaminidase (NAGase) activity because this enzyme is associated with fungi and has been shown to correlate significantly with the total number of fungal spores counted by microscopy (9, 14). Our hypothesis is that the TIP values obtained in the granulocyte assay can be used as a rapid way to evaluate the bioaerosol exposure and thus could indicate where a more extensive characterization of microbial parameters would be necessary.
MATERIALS AND METHODS
Biofuel plants.
The collection of bioaerosols was obtained from 22 Danish biofuel plants located all over Denmark. Between 2 and 50 people were handling biofuels at each plant. Energy was generated mainly by converting straw, but two plants were also converting wood chips. The sampling was performed during early spring, late autumn, and wintertime over two successive working days. One of the main working areas was the straw storage area where farmers or truck drivers arrived with straw. The straw could be the present year's harvest, but it could also be from an earlier harvest season. The biofuel plants could refuse to receive the straw if it had a water content that is too high or if it looked moldy.
Main working areas.
While personal bioaerosol sampling was performed, technicians recorded the duration of occupation for different work areas. Each person was classified as belonging to a certain area in accordance with where they spent the majority of the working day. The areas were the following: transport truck, wood chip storage, straw storage, boiler room, office, laboratory, and workshop. Also included were five workers conducting outdoor welding. Three persons were not placed in any of these groups. At each plant, outdoor reference airborne dust was sampled in the wind toward the plant. The reference measurement was performed using stationary samplers during the same periods that personal dust was sampled.
Personal sampling of inhalable aerosols.
Personal bioaerosol monitoring was conducted using GSP inhalable samplers (CIS by BGI, Inc., Waltham, MA). The samplers were mounted with Teflon filters (pore size, 1 μm) to collect inhalable dust, endotoxin, and NAGase, and polycarbonate filters (pore size, 1 μm) were used for quantification of the total number and number of CFU of bacteria and fungi and for analysis in the granulocyte assay. For the investigation of the association of the microbial factors to TIP, time-weighted averages were used. The average sampling time was 5 h.
Gravimetric analysis.
The mass of the bioaerosol components collected on the Teflon filters (hereafter denoted dust) was determined by weighing the filters before and after sampling. Before being weighed, the filters were equilibrated at constant air temperature and humidity for 20 to 24 h (22°C and 50% ± 5% relative humidity). To establish detection limits, we used three times the standard deviation of 10 blanks and divided by the mean sampled value. The detection limit for dust was 0.03 mg/m3.
Extraction of bioaerosols.
The bioaerosols sampled on Teflon filters were extracted in 10.0 ml of pyrogen-free water with 0.05% Tween 20 by orbital shaking (300 rpm) at room temperature for 60 min and subsequent centrifugation (1,000 × g) for 15 min. The supernatant was used for the endotoxin and NAGase assay. The bioaerosols sampled on polycarbonate filters were extracted in 10.0 ml of sterile 0.05% Tween 80 and 0.85% NaCl solution at 16 h postsampling by shaking for 15 min (500 rpm) at room temperature.
Quantification of endotoxin and (1→3)-β-d-glucan.
The supernatant was analyzed in duplicate for endotoxin using a chromogenic kinetic Limulus amebocyte lysate test (Kinetic-QCL endotoxin kit; BioWhittaker, Walkersville, MD) with β-glucan blocker. A standard curve obtained from an Escherichia coli O55:B5 reference endotoxin was used to determine the concentration in terms of endotoxin units (EU) (12.0 EU ≈ 1.0 ng). The detection limit for endotoxin was 0.05 EU/m3. Dust suspensions from the polycarbonate filters were used for quantification of (1→3)-β-d-glucan in duplicate using a kinetic Fungitic G Test (Seikagaku Co., Tokyo, Japan) with a detection limit of 8 pg/m3. The triple-helix structure of the β-glucan was made water soluble using 0.3 M NaOH (the extraction time was 10 min).
Quantification of microorganisms.
Microorganisms were quantified using a modified CAMNEA method (22). The number of fungi cultivable on dichloran glycerol agar (DG 18 agar; Oxoid, Basingstoke, England) at 25°C was counted after 3 and 7 days of incubation. In addition, agar plates were incubated at 45°C to quantify cultivable Aspergillus fumigatus. Mesophilic actinomycetes and thermophilic actinomycetes (55°C) were after 3 and 7 days of incubation quantified on 10% and 100% nutrient agar (Oxoid, Basingstoke, England) with actidione (cycloheximide; 50 mg/liter), respectively. The detection limit was between 10 and 20 CFU/m3 air, and for measures below the detection limit, the value equal to 50% of the detection limit was used.
The total numbers of bacteria and fungal spores were determined after staining in 20 ppm acridine orange (Merck) in acetate buffer for 30 s, with subsequent filtration through a polycarbonate filter (0.4 μm; Nuclepore, Cambridge, MA). Fungi and bacteria were counted at a magnification of ×1,250 using epifluorescence microscopy (Orthoplan; Leitz Wetzlar). The numbers of microorganisms were determined in 40 randomly chosen fields or until at least 400 cells were counted.
Quantification of NAGase.
We have measured exposure to NAGase, which is an enzyme mainly produced by fungi (9). To quantify the activity of NAGase (EC 3.2.1.30), the release of p-nitrophenol from the substrate p-nitrophenol-N-acetyl-β-d-glucosaminide (Sigma Chemical Co.) was estimated (12). Appropriate controls without either the enzyme or the substrate were run simultaneously. The tests and standards were incubated at 50°C for 16 h. Reactions were terminated, and the samples appeared yellow following the addition of 50 μl of 0.4 M Na2CO3 to each well. Absorbance was measured at 405 nm. One unit of enzyme activity is defined as the amount of enzyme which releases 1 μmol of p-nitrophenol/ml of dust suspension/min. The detection limit was 0.06 pmol/sec/m3.
Sample activity in the granulocyte assay.
The surfactant Tween, which was used for the extraction of dust from the filters, interferes with the granulocyte assay. Therefore, the samples were ultrafiltrated prior to analysis (36). The individual filter extracts were placed on an ultrafilter with a 20-kDa cutoff (Ultrasart D 20; Sartorius, Germany) and washed three times with Hanks balanced salt solution (HBSS). The samples (retained on the filter) were then reconstituted in HBSS in their original volume and tested in the granulocyte assay.
Granulocyte-like cells were obtained by differentiating the HL-60 cell line, originally acquired from ATCC (CCL-240) with all-trans retinoic-acid (ATRA). The cell line was cultured in RPMI 1640 medium (Biological Industries, Israel) supplemented with 10% heat-inactivated fetal bovine serum (Biological Industries, Israel), 1% glutamax (Gibco), 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich). The cells were maintained in a humidified atmosphere (5% CO2-95% air) at 37°C, and the culture medium was renewed twice a week. Cells were kept below 1 × 106 cells/ml at all times. To induce differentiation, cells were collected from the continuously growing cell culture and seeded in supplemented RPMI 1640 medium with 1 μM ATRA (Fluka/Sigma-Aldrich) at a concentration of 3 × 105 cells/ml. Cells were then allowed to differentiate for 7 days without further renewal of culture medium or cell passage.
To analyze the bioaerosol samples, the differentiated HL-60 cell culture was centrifuged (125 × g for 10 min), and the pellet was washed once in preheated (37°C) HBSS. The cells were resuspended in preheated HBSS and set to 1 × 107 cells/ml using a Bürker-Türk chamber. The cell suspension was then plated on a white polystyrene LumiNuncTM 96-well plate (Nunc, Denmark) at a concentration of 5 × 105 cells/well, with 283 μM luminol (Across Organics) and 2.5% human plasma (obtained from two healthy volunteers). The cells were then allowed to equilibrate for 15 min at 37°C prior to the addition of either test, standard, or control solutions.
Chemiluminescent measurements were initiated immediately after the addition of the test or standard (1 s/well every 120 s from 0 to 180 min) using a thermostated (37°C) ORION II Microplate luminometer (Berthold Detection Systems, Germany). Light intensity from the reaction was quantified as relative light units per second (RLU/s). The area under the curve (AUC) was calculated by integrating the responses (RLU/s in the range of 0 to 180 min). TIP values were obtained by correcting the AUC values for the time-weighted average of the volume (m3) of sampled air.
All samples were tested in duplicates, and each plate assayed contained a reference endotoxin (3.8 EU ≈ 1.0 ng from E. coli O55:B5; Cambrex Bioscience) standard curve in the range of 0.1 EU/ml to 200 EU/ml, as described earlier (31). A correlation coefficient greater than 0.98 was considered acceptable for the endotoxin standard curve. Also, a standard of glucan (zymosan A from Saccharomyces cerevisiae [Sigma Chemical Co.]) was included at a concentration of 1 μg/ml to validate reactivity toward nonendotoxins.
Statistics.
Log transforms of both the TIP values and the sample compositions were used to meet the linearity and normality assumptions of the model. To examine the relationship between sample compositions and corresponding TIP values, we used multiple linear regression with stepwise exclusion of the least significant factor. We considered a P value of <0.05 significant. The effects of sample composition on TIP values are displayed as estimates of regression coefficients together with standard errors (SE) of the estimates. Relations between TIP values and working area are shown as mean ± standard error of the mean (SEM). Data were analyzed using SAS, version 9.1.
RESULTS
A total of 129 personal bioaerosol samples were collected during working hours of 81 persons at 22 biofuel plants. The samples were subjected to gravimetric analysis and quantified for the microbial components listed in Table 1. The exposure to endotoxin, bacteria, fungal spores, β-glucan, NAGase, and dust were above the detection limits for all persons. For mesophilic actinomycetes, thermophilic actinomycetes, and A. fumigatus, 117, 112, and 103, respectively, of 129 samples were above the detection limit.
TABLE 1.
Personnel exposure and TIP of bioaerosols (n = 129)
| Factor | Unit | Personnel exposure (unit per m3 of air)a |
||
|---|---|---|---|---|
| Average | Median | Range | ||
| Endotoxin | EU | 5.1 × 102 | 1.2 × 102 | 3.0 × 10−2-8.0 × 103 |
| Bacteria | No. | 2.6 × 106 | 8.9 × 105 | 1.0 × 104-5.4 × 107 |
| Mesophilic actinomycetes | CFU | 1.6 × 104 | 1.9 × 103 | BD to 3.4 × 105 |
| Thermophilic actinomycetes | CFU | 1.3 × 104 | 1.1 × 103 | BD to 3.5 × 105 |
| Fungal spores | No. | 8.4 × 105 | 3.7 × 105 | 8.1 × 102-1.3 × 107 |
| A. fumigatus | CFU | 3.3 × 103 | 7.7 × 102 | BD to 4.4 × 104 |
| (1→3)-β-d-glucan | ng | 3.5 × 104 | 1.0 × 104 | 3.4 × 102-6.8 × 105 |
| NAGase | pmol/s | 4.2 × 10−1 | 3.3 × 10−1 | 5.0 × 10−2-2.5 |
| Dust | mg | 7.8 × 10−1 | 3.9 × 10−1 | BD to 9.4 |
| TIP | AUC | 1.2 × 107 | 8.1 × 106 | 3.7 × 105-6.1 × 107 |
BD, below detection limit. Background (outdoor air) median levels were as follows: endotoxin, 1.5 EU/m3; bacteria, 3,200/m3; fungal spores, 3,100/m3; (1→3)-β-d-glucan, 2 ng/m3; NAGase, 0.16 pmol/s/m3. In outdoor air, levels of mesophilic actinomycetes, thermophilic actinomycetes, A. fumigatus, and dust were below the limit of detection.
The ability of the samples to induce ROS production in the granulocyte assay was examined, and the results were quantified as TIP values. All samples gave TIP values above the limit of detection. Both the sample composition and the TIP values followed a log-normal distribution.
The granulocyte assay.
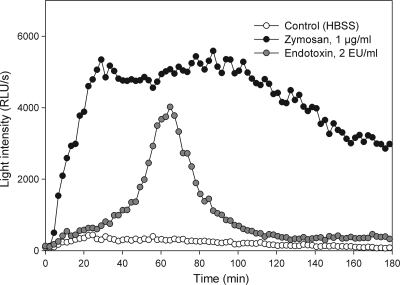
The ROS production profile in the granulocyte assay is stimulus dependent. Figure 1 shows the ROS production profiles induced by 1.0 μg/ml zymosan, 2.0 EU/ml endotoxin, and a control (HBSS). The granulocytes have a fast onset of ROS production in response to the zymosan challenge, within the first 2 to 6 min, whereas the response to endotoxin is initiated much later and does not peak until 60 min or later. Dose dependency was observed and was verified in each assay with the included endotoxin standards in the range of 0.1 to 200 EU/ml (data not shown).
FIG. 1.
ROS production profiles in the granulocyte assay of responses to stimuli. The granulocyte-like cells were stimulated at time zero with control solution (HBSS), zymosan (1 μg/ml), or endotoxin (2 EU/ml). Light intensity (in RLU/s) was measured every second minute for 180 min.
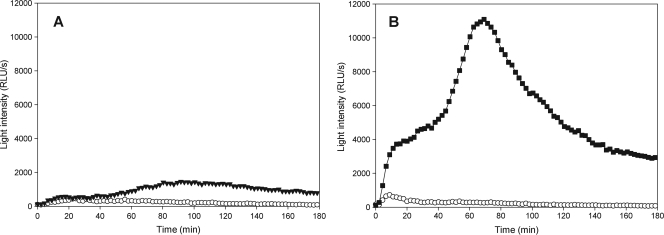
All collected bioaerosol samples activated the cells in the granulocyte assay to produce ROS. The reaction profiles and onset of ROS production differed between the tested samples. Figure 2 shows the ROS release in the granulocyte assay when cells were challenged with two different bioaerosol samples. Figure 2A shows a sample with a large amount of dust containing only a small amount of microbial contaminant. The resulting ROS release in the granulocyte assay shows a very late activation of the cells, with only minor or no initial activation. Figure 2B shows the ROS release caused by a sample contaminated with a variety of microbial components. The sample activates the cells to have both large initial and subsequently sustained ROS production.
FIG. 2.
ROS production profiles of the granulocyte assay to different bioaerosol samples. The granulocyte-like cells are stimulated at time zero with either control solution (HBSS; open symbols) or bioaerosol samples (filled symbols). Light intensity (in RLU/s) was measured every second minute for 180 min. Graphs show the responses of the granulocytes when cells are challenged with a bioaerosol sample where the majority of the contamination can be related to dust containing only a small amount of microbial contaminants (A) and with a bioaerosol sample containing a variety of microbial components (B).
Correlation of TIP to bioaerosol components.
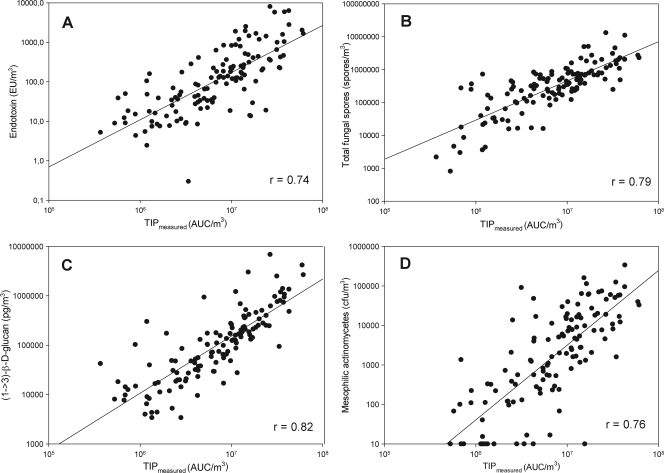
Individual correlation of the TIP values and the measured bioaerosol components gave relatively high and significant correlation coefficients (r) in the range of an r of 0.65 for dust/m3 to an r of 0.82 for (1→3)-β-d-glucan (Fig. 3). However, the values for dust, fungal spores, (1→3)-β-d-glucan, total number of bacteria, NAGase, A. fumigatus, and mesophilic and thermophilic actinomycetes also correlated significantly interdependently (r = 0.47 to 0.86; P ≤ 0.0001).
FIG. 3.
TIP correlations to the four factors significant for the descriptive model.
To assess which components of the bioaerosols had the strongest association to the measured TIP values, we created a model describing the TIP as a function of all nine factors. A multiple linear regression with mean structure (y1 = a + a1x1 + a2x2 + … + a9x9) was used, where y1 is the estimated log TIP value and x1 to x9 are the logarithmic contents per m3 of air for endotoxin, dust, fungal spores, (1→3)-β-d-glucan, total number of bacteria, NAGase, A. fumigatus, and mesophilic and thermophilic actinomycetes, respectively. In the model a1 to a9 are the regression coefficients, and a is the intercept.
The analysis showed that the effects of some factors could be explained by other factors (e.g., the total number of fungal spores could also be described by NAGase activity). We therefore conducted iterative removal of the least significant factor until only significant factors remained. We used a P value of <0.05 as the acceptance criterion for the data evaluation. The factors, their estimates, SEs, and P values are given in Table 2. To explain the TIP values, the combination of four factors was relevant for the model (Table 2).
TABLE 2.
Estimate, SE, and P values of the nine examined factors analyzed together and the four factors significant for the TIP value in the final model
| Factor | Value for all factors together |
Value for factors significant for the TIP |
Partial r2 | ||||
|---|---|---|---|---|---|---|---|
| Estimate | SE | P | Estimate | SE | P | ||
| Endotoxin | 0.18 | 0.041 | <0.0001 | 0.20 | 0.037 | <0.0001 | 0.192 |
| Bacteria | −0.06 | 0.054 | 0.26 | ||||
| Mesophilic actinomycetes | 0.052 | 0.039 | 0.18 | 0.07 | 0.026 | 0.0063 | 0.060 |
| Thermophilic actinomycetes | 0.027 | 0.033 | 0.41 | ||||
| Fungal spores | 0.22 | 0.063 | 0.0007 | 0.22 | 0.043 | <0.0001 | 0.178 |
| A. fumigatus | 0.0001 | 0.029 | 0.99 | ||||
| (1→3)-β-d-glucan | 0.16 | 0.051 | 0.0028 | 0.17 | 0.048 | 0.0005 | 0.096 |
| NAGase | 0.12 | 0.12 | 0.31 | ||||
| Dust | 0.07 | 0.059 | 0.26 | ||||
The resulting model had the following estimated mean structure: log TIPestimated = 4.14 + (0.20 × log endotoxin) + (0.22 × log fungal spores) + [0.17 × log (1→3)-β-d-glucan] + (0.07 × log mesophilic actinomycetes).
The estimate of the residual variance was 0.047.
The same four factors were also shown to be significant when the predicted residual sum of squares statistic was used.
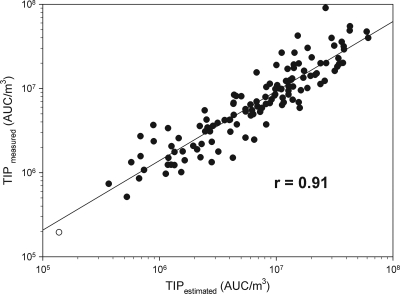
Using the stepwise multiple regression model, the estimated TIP values based on the microbial data were plotted against the TIP obtained in the granulocyte assay (Fig. 4). The estimated TIP values correlate to the actual values measured, with a correlation coefficient, r, of 0.91. This indicates that the four factors in the final model explain a large amount of the total variation of the observed TIP values. Figure 4 shows that the data vary around the line with no clear signs of curvature, thus giving us no reason to question the linearity assumption of the model. Also the variation seems to be the same for both small and large TIP values, giving us no reason to question the assumption of constant variance in the measured area.
FIG. 4.
Measured TIP values (TIPmeasured) plotted against estimated TIP values (TIPestimated). Open circle, control (HBSS); filled circles, bioaerosol samples (n = 129). The straight line represents the corresponding linear regression.
TIP in relation to working area.
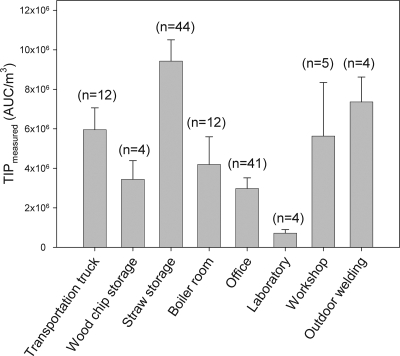
The bioaerosol samples were divided into groups representative of where each worker had spent the most time on the day of sampling. Figure 5 shows the mean measured TIP response (± SEM) from the bioaerosol samples in respect to the main working area.
FIG. 5.
Measured TIP values (AUC/m3) in relation to the main working areas of 126 persons. Results are shown as means ± SEMs (n = 4 to 44).
The majority of workers at the biofuel plants could be related to work functions in either straw storage or office areas. In general, the samples from straw storage workers tended to have high TIP values, and the samples from workers mainly situated in the office environment tended to have TIP values below average as assessed by the granulocyte assay.
Personnel related to driving transport trucks, welding, and workshops were exposed to bioaerosols with high average TIP values. The lowest values obtained were related to bioaerosol samples collected by the laboratory personnel.
DISCUSSION
All 129 bioaerosol samples collected at the biofuel plants contained substantial amounts of microorganisms, microbial cell wall components, and NAGase. All samples activated the granulocyte-like HL-60 cells to produce ROS above baseline release levels. When we tested different reference substances in this granulocyte assay, we observed that the kinetics of the activation profiles varied (Fig. 1). This is in accordance with previous observations showing that different microorganisms and microbial cell wall components show different activation patterns, as exemplified by zymosan (a Saccharomyces cerevisiae cell wall component), which induces fast production of ROS, and endotoxin, which induces a later onset of ROS production (32). The collected bioaerosol samples are therefore expected to induce different activation patterns in the granulocyte assay since these samples also vary in microbial composition. In fact, it may even be possible to make an assessment as to the composition of a sample by using the activation profiles of the granulocyte assay. Without further reference to this observation, the TIP values were determined.
Responses in cell-based assays are generally associated with day-to-day variation. One common way to account for this is to include internal standards. In these experiments we have included an endotoxin standard curve in each experiment to verify dose dependency and to have a valid internal standard. However, it is problematic to use endotoxin as an internal standard for bioaerosol samples since the responses are shown to be the result of several microbial components. Normalization of the total response to one standard, therefore, may not represent the best way to account for variations. In fact, normalizing the responses of the granulocyte assay in this study to the 2 EU/ml of the endotoxin standard gives reduced correlations to the individual components and reduced P values for the final descriptive model. This indicates that if one chooses to normalize data to account for day-to-day variations, this should be done with care and perhaps by using a standard comprised of multiple microbial components.
High and significant correlation coefficients were obtained when measured TIP values were correlated to the concentrations of dust, endotoxin, fungal spores, (1→3)-β-d-glucan, total number of bacteria, NAGase, A. fumigatus, mesophilic actinomycetes and thermophilic actinomycetes. However, since microbial components also correlated well interdependently, this may provide a false coherence since these samples to some extent have proportional concentrations of contaminants. By using multiple linear regression, we have determined which of the nine microbial components included here were determinative of the observed TIP values. By running the model first with all nine factors and then subsequently excluding the least descriptive factor one by one until all remaining factors were significant (P < 0.05), the four descriptive factors for the model were identified as endotoxin, fungal spores, (1→3)-β-d-glucan, and mesophilic actinomycetes. However, we found that some of the nine chosen factors could be descriptive for each other. Therefore, removal of one factor from the model resulted in another related factor's becoming descriptive instead. For example, NAGase becomes significant for the descriptive model (P = 0.032) if (1→3)-β-d-glucan is initially removed from the model. This indicates that the exact substance tested may be of less importance than the origin of the substances. Using this assumption, the descriptive factors in the granulocyte assay seems to be a combination of fungal [(1→3)-β-d-glucan and fungal spores] and bacterial (endotoxin and actinomycetes) contaminations. When the model described here is used to predict the TIP values, the results correlate closely to the actually obtained values (r = 0.91). Using this model, the correlation to multifactor contamination represents a better estimate than any one contaminant alone. This indicates that the granulocyte assay responds to the multifactorial composition of the samples and thus provides a measure of multifactorial contamination or the TIP of a given sample.
The TIP values obtained in the granulocyte assay were related to main working areas (Fig. 5). Workers situated at the straw storage facility provided dust samples with the highest TIP values. This is in accordance with earlier studies showing high exposures to microbial components in straw storage facilities (10). The TIP value of bioaerosols collected during work in the straw storage areas were higher than of that of bioaerosols collected during work in the boiler rooms. This is in accordance with both a higher exposure to microbial components in straw storage areas than in boiler rooms (10) and with a higher inflammatory potential of dust from a straw storage facility than from a boiler room (13). Personnel related to driving transport trucks also displayed high TIP values. This is also as expected because the truck drivers take part in the loading and unloading of the truck, during which straw is intensively handled. One interesting fact is that the welders were high responders. This is surprising since they were generally situated outdoors for the majority of the workday. The reason for these high responses is yet unknown, but we found that the samples collected showed remarkably high concentrations of endotoxin. This would explain the activation observed in the granulocyte assay, but the origin of the endotoxin remains unknown. Generally speaking, the bioaerosols from persons mainly working at the offices and laboratories showed lower TIP values than those from persons related to other working areas. Considering that office spaces were physically separate from the contaminated areas such as straw storage, the dust samples from some offices show surprisingly high average TIP values. This is in accordance with an earlier study showing that an office at a biofuel plant had a higher concentration of airborne microbial components than outdoor reference measurements even though it was much lower than that in most areas at biofuel plants in general (10). Overall, these results show that the TIP values obtained in the granulocyte assay are related to the working areas of the personnel. The fact that there were high responders among the office workers may be speculated to relate either to poor indoor ventilation or lack of maintaining closed physical barriers between the office facilities and the more contaminated workspaces.
Others have used cell assays based on lung epithelial cells, alveolar macrophages, or monocytic cells to evaluate occupational health effects of dust (1, 2, 8, 21). These assays are based on cellular cytokine production after dust exposure. It is evident that the choice of cell system is highly relevant for the sensitivity toward bioaerosol components and that some systems are almost insensitive toward single components such as endotoxin (1).
We have shown that the response of the granulocyte assay is related to the multifactorial composition of a given sample and that the factors that best describe the TIP in these experiments are (1→3)-β-d-glucan, endotoxin, fungal spores, and mesophilic actinomycetes. Due to the fact that ATRA-differentiated HL-60 cells resemble neutrophilic granulocytes, it should be evident that the assay has relevance in describing immunomodulatory effects of a given bioaerosol sample. When we consulted the literature, we found that the factors that describe the TIP in the granulocyte assay are all known to exert adverse effects in relation to occupational health. Previously, β-glucan has been used as an indicator of fungal exposure, but studies have also shown that it can elicit respiratory inflammation (3, 24, 28, 30), and it has a definite proinflammatory potential in monocytes (27). These observations are in accordance with the data presented here that bioaerosol samples containing (1→3)-β-d-glucan activate granulocyte-like HL-60 cells and that (1→3)-β-d-glucan is a determinative factor in our explanatory model for the resulting ROS production from the cells. In concordance with our findings, the exposure to extensive amounts of endotoxin have also been shown to have proinflammatory effects and have been implicated in work-related respiratory symptoms (6, 7). Exposure to fungal spores (5) and actinomycetes (29) has also been implicated in work-related respiratory symptoms. Altogether, these results support the relevance of the observed effect of these microbial components on the TIP values obtained in the granulocyte assay.
In conclusion, these results indicate that the granulocyte assay described here, based on the ROS production of differentiated HL-60 cells, can provide measurements of the TIP of bioaerosols in a working environment.
Acknowledgments
The sampling of bioaerosols was supported by a grant from PSO-ELTRA (grant 5786).
We especially thank Signe H. Nielsen and Tina T. Olsen at the National Research Centre for the Working Environment and Janne M. Colding and Betina Schøler at the University of Copenhagen for specialist technical assistance.
Footnotes
Published ahead of print on 16 October 2009.
REFERENCES
- 1.Allermann, L., and O. M. Poulsen. 2000. Inflammatory potential of dust from waste handling facilities measured as IL-8 secretion from lung epithelial cells in vitro. Ann. Occup. Hyg. 44:259-269. [PubMed] [Google Scholar]
- 2.Allermann, L., and O. M. Poulsen. 2002. Interleukin-8 secretion from monocytic cell lines for evaluation of the inflammatory potential of organic dust. Environ. Res. 88:188-198. [DOI] [PubMed] [Google Scholar]
- 3.Bonlokke, J. H., G. Stridh, T. Sigsgaard, S. K. Kjaergaard, H. Lofsted, K. Andersson, E. C. Bonefeld-Jorgensen, M. N. Jayatissa, L. Bodin, J. E. Juto, and L. Molhave. 2006. Upper-airway inflammation in relation to dust spiked with aldehydes or glucan. Scand. J. Work Environ. Health 32:374-382. [DOI] [PubMed] [Google Scholar]
- 4.Douwes, J., P. Thorne, N. Pearce, and D. Heederik. 2003. Bioaerosol health effects and exposure assessment: progress and prospects. Ann. Occup. Hyg. 47:187-200. [DOI] [PubMed] [Google Scholar]
- 5.Eduard, W., J. Douwes, R. Mehl, D. Heederik, and E. Melbostad. 2001. Short term exposure to airborne microbial agents during farm work: exposure-response relations with eye and respiratory symptoms. Occup. Environ. Med. 58:113-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gladding, T., J. Thorn, and D. Stott. 2003. Organic dust exposure and work-related effects among recycling workers. Am. J. Ind Med. 43:584-591. [DOI] [PubMed] [Google Scholar]
- 7.Heldal, K. K., A. S. Halstensen, J. Thorn, P. Djupesland, I. Wouters, W. Eduard, and T. S. Halstensen. 2003. Upper airway inflammation in waste handlers exposed to bioaerosols. Occup. Environ. Med. 60:444-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirvonen, M. R., K. Huttunen, and M. Roponen. 2005. Bacterial strains from moldy buildings are highly potent inducers of inflammatory and cytotoxic effects. Indoor Air 15(Suppl. 9):65-70. [DOI] [PubMed] [Google Scholar]
- 9.Madsen, A. M. 2003. NAGase activity in airborne biomass dust and relationship between NAGase concentrations and fungal spores. Aerobiologia 19:97-105. [Google Scholar]
- 10.Madsen, A. M. 2006. Exposure to airborne microbial components in autumn and spring during work at Danish biofuel plants. Ann. Occup Hyg. 50:821-831. [DOI] [PubMed] [Google Scholar]
- 11.Madsen, A. M., L. Mårtensson, T. Schneider, and L. Larsson. 2004. Microbial dustiness and particle release of different biofuels. Ann. Occup. Hyg. 48:327-338. [DOI] [PubMed] [Google Scholar]
- 12.Madsen, A. M., and E. de Neergaard. 1999. Interactions between Pythium oligandrum and sclerotia of the plant pathogen Sclerotinia sclerotiorum. Eur. J. Plant Pathol. 105:761-768. [Google Scholar]
- 13.Madsen, A. M., A. T. Saber, P. Nordly, A. K. Sharma, H. Wallin, and U. Vogel. 2008. Inflammation but no DNA (deoxyribonucleic acid) damage in mice exposed to airborne dust from a biofuel plant. Scand. J. Work Environ. Health 34:278-287. [DOI] [PubMed] [Google Scholar]
- 14.Madsen, A. M., and H. Würtz. 2005. Fungal enzymes in indoor dust, p. 384-391. In Johanning Eckardt (ed.), Bioaerosols, fungi, bacteria, mycotoxins and human health. Fungal Research Group Foundation, Inc., Albany, NY.
- 15.Malmberg, P., A. Rask-Andersen, S. Höglund, B. Kolmodin-Hedman, and J. R. Guernsey. 1988. Incidence of organic dust toxic syndrome and allergic alveolitis in Swedish farmers. Int. Arch. Allergy Appl. Immunol. 87:47-54. [DOI] [PubMed] [Google Scholar]
- 16.Mandryk, J., K. U. Alwis, and A. D. Hocking. 2000. Effects of personal exposures on pulmonary function and work-related symptoms among sawmill workers. Ann. Occup. Hyg. 44:281-289. [PubMed] [Google Scholar]
- 17.Michel, O., A. M. Nagy, M. Schroeven, J. Duchateau, J. Neve, P. Fondu, and R. Sergysels. 1997. Dose-response relationship to inhaled endotoxin in normal subjects. Am. J. Respir. Crit. Care Med. 156:1157-1164. [DOI] [PubMed] [Google Scholar]
- 18.Moesby, L., S. Jensen, E. W. Hansen, and J. D. Christensen. 1999. A comparative study of Mono Mac 6 cells, isolated mononuclear cells and Limulus amoebocyte lysate assay in pyrogen testing. Int. J. Pharm. 191:141-149. [DOI] [PubMed] [Google Scholar]
- 19.Monso, E., R. Magarolas, K. Radon, B. Danuser, M. Iversen, C. Weber, U. Opravil, K. J. Donham, and D. Nowak. 2000. Respiratory symptoms of obstructive lung disease in European crop farmers. Am. J. Respir. Crit. Care Med. 162:1246-1250. [DOI] [PubMed] [Google Scholar]
- 20.Moore, J. E., J. Xu, B. C. Millar, J. S. Elborn, and J. R. Rao. 2008. Identification of an organism associated with mushroom worker's lung. Compost Sci. Util. 12:192-195. [Google Scholar]
- 21.Palmberg, L., B. M. Larsson, P. Malmberg, and K. Larsson. 1998. Induction of IL-8 production in human alveolar macrophages and human bronchial epithelial cells in vitro by swine dust. Thorax 53:260-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmgren, U., G. Ström, G. Blomquist, and P. Malmberg. 1986. Collection of airborne micro-organisms on Nuclepore filters, estimation and analysis—CAMNEA method. J. Appl. Bacteriol. 61:401-406. [DOI] [PubMed] [Google Scholar]
- 23.Radon, K., E. Monso, C. Weber, B. Danuser, M. Iversen, U. Opravil, K. Donham, J. Hartung, S. Pedersen, S. Garz, D. Blainey, U. Rabe, and D. Nowak. 2002. Prevalence and risk factors for airway diseases in farmers—summary of results of the European Farmers’ Project. Ann. Agric. Environ. Med. 9:207-213. [PubMed] [Google Scholar]
- 24.Rylander, R. 1999. Indoor air-related effects and airborne (1 → 3)-beta-d-glucan. Environ. Health Perspect. 107(Suppl. 3):501-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sebastian, A., A. M. Madsen, L. Mårtensson, D. Pomorska, and L. Larsson. 2006. Assessment of microbial exposure risks from handling of biofuel wood chips and straw—effect of outdoor storage. Ann. Agric. Environ. Med. 13:139-145. [PubMed] [Google Scholar]
- 26.Sibille, Y., and F. X. Marchandise. 1993. Pulmonary immune cells in health and disease: polymorphonuclear neutrophils. Eur. Respir. J. 6:1529-1543. [PubMed] [Google Scholar]
- 27.Sigsgaard, T., E. C. Bonefeld-Jorgensen, H. J. Hoffmann, J. Bonlokke, and T. Kruger. 2005. Microbial cell wall agents as an occupational hazard. Toxicol. Appl. Pharmacol. 207:310-319. [DOI] [PubMed] [Google Scholar]
- 28.Sigsgaard, T., E. C. Bonefeld-Jorgensen, S. K. Kjaergaard, S. Mamas, and O. F. Pedersen. 2000. Cytokine release from the nasal mucosa and whole blood after experimental exposures to organic dusts. Eur. Respir. J. 16:140-145. [DOI] [PubMed] [Google Scholar]
- 29.Skorska, C., B. Mackiewicz, J. Dutkiewicz, E. Krysinska-Traczyk, J. Milanowski, H. Feltovich, J. Lange, and P. S. Thorne. 1998. Effects of exposure to grain dust in polish farmers: work-related symptoms and immunologic response to microbial antigens associated with dust. Ann. Agric. Environ. Med. 5:147-153. [PubMed] [Google Scholar]
- 30.Thorn, J., L. Beijer, and R. Rylander. 2001. Effects after inhalation of (1 → 3)-beta-d-glucan in healthy humans. Mediators Inflamm. 10:173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timm, M., S. Bartelt, L. Moesby, and E. W. Hansen. 2009. Cryopreservation of differentiated HL-60 cells for pyrogen testing. J. Immunol. Methods. doi: 10.1016/j.jim.2008.09.005. [DOI] [PubMed]
- 32.Timm, M., E. W. Hansen, L. Moesby, and J. D. Christensen. 2006. Utilization of the human cell line HL-60 for chemiluminescence based detection of microorganisms and related substances. Eur. J. Pharm. Sci. 27:252-258. [DOI] [PubMed] [Google Scholar]
- 33.von Vietinghoff, S., and K. Ley. 2008. Homeostatic regulation of blood neutrophil counts. J. Immunol. 181:5183-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams, L. K., D. R. Ownby, M. J. Maliarik, and C. C. Johnson. 2005. The role of endotoxin and its receptors in allergic disease. Ann. Allergy Asthma Immunol. 94:323-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yocum, M. W., A. R. Saltzman, D. M. Strong, J. C. Donaldson, G. W. Ward, Jr., F. M. Walsh, O. M. Cobb, Jr., and R. C. Elliott. 1976. Extrinsic allergic alveolitis after Aspergillus fumigatus inhalation. Evidence of a type IV immunologic pathogenesis. Am. J. Med. 61:939-945. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann, G., R. Kiltz, and D. Krüger. 1985. Entfernung störender Substanzen durch Einweg-Ultrafilter bei der Endotoxinbestimmung mittels Limulustest. Pharm. Ind. 47:647-651. [Google Scholar]