Abstract
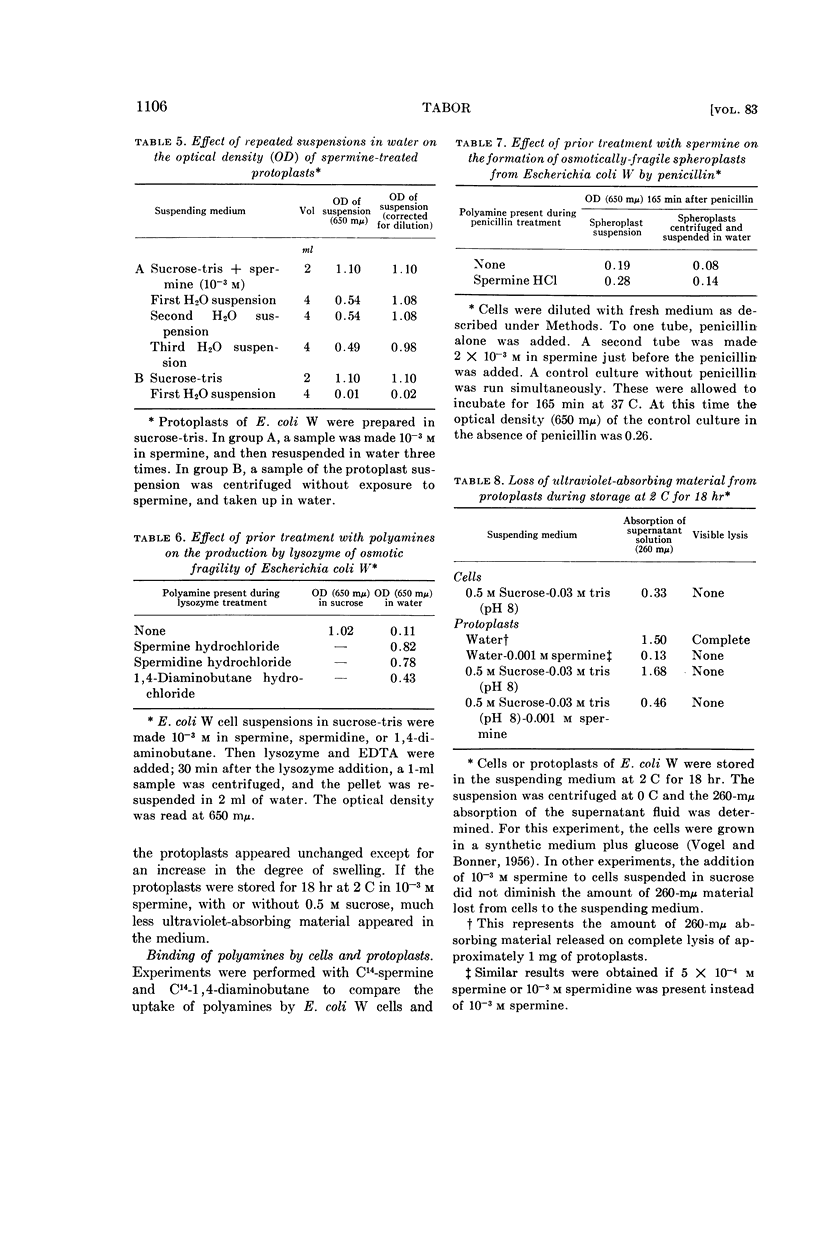
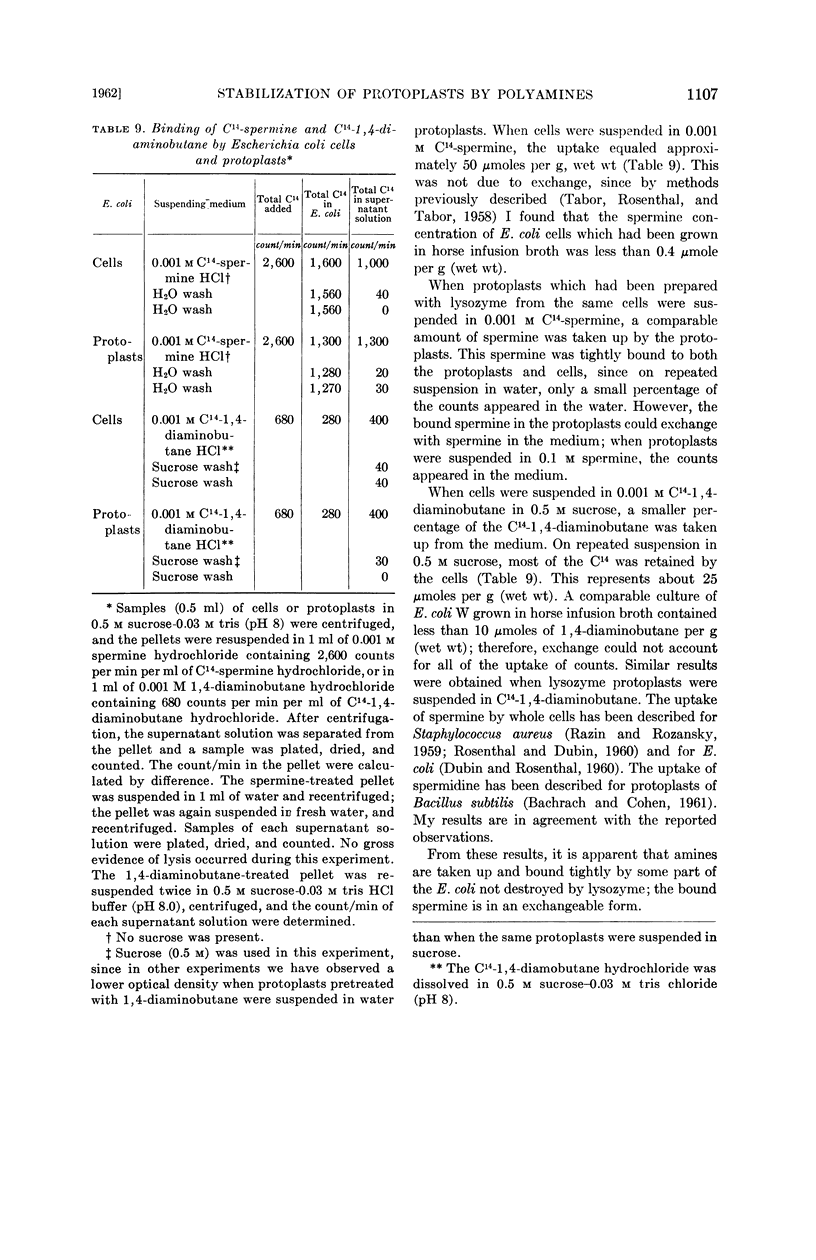
Tabor, Celia W. (National Institute of Arthritis and Metabolic Diseases, Bethesda, Md.). Stabilization of protoplasts and spheroplasts by spermine and other polyamines. J. Bacteriol. 83:1101–1111. 1962.—Spermine (10−3m) or spermidine prevents lysis of lysozyme-produced protoplasts of Escherichia coli W, E. coli B, and Micrococcus lysodeikticus in hypotonic media. Spheroplasts prepared by the action of penicillin are also stabilized by these concentrations of spermine and spermidine, but the protection is not as complete. Streptomycin, polylysine, and Ca++ are also effective or partially effective stabilizers, but 1,4-diaminobutane, 1,5-diaminopentane, ornithine, Mg++, and monovalent cations have no protective action at 10−3m concentration, and only a slight effect at higher concentrations. The osmotic stability conferred on protoplasts by spermine is irreversible. However, the protective effect of polyamines against lysis is not accompanied by restoration of viability to lysozyme protoplasts. There is a marked reduction in the loss of ultra-violet-absorbing material from the protoplasts to the medium when 10−3m spermine is present.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACHRACH U., COHEN I. Spermidine in the bacterial cell. J Gen Microbiol. 1961 Sep;26:1–9. doi: 10.1099/00221287-26-1-1. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr Submerged culture of Micrococcus lysodeikticus for large-scale production of cells. Science. 1955 Nov 25;122(3178):1016–1016. doi: 10.1126/science.122.3178.1016. [DOI] [PubMed] [Google Scholar]
- COHEN S. S., LICHTENSTEIN J. Polyamines and ribosome structure. J Biol Chem. 1960 Jul;235:2112–2116. [PubMed] [Google Scholar]
- COLBOURN J. L., WITHERSPOON B. H., HERBST E. J. Effect of intracellular spermine on ribosomes of Escherichia coli. Biochim Biophys Acta. 1961 May 13;49:422–424. doi: 10.1016/0006-3002(61)90155-x. [DOI] [PubMed] [Google Scholar]
- DUBIN D. T., ROSENTHAL S. M. The acetylation of polyamines in Escherichia coli. J Biol Chem. 1960 Mar;235:776–782. [PubMed] [Google Scholar]
- HANNON J. P. Tissue energy metabolism in the cold-acclimatized rat. Fed Proc. 1960 Dec;19(Suppl 5):139–144. [PubMed] [Google Scholar]
- HERBST E. J., DOCTOR B. P. Inhibition of ribonucleic acid degradation in bacteria by spermine. J Biol Chem. 1959 Jun;234(6):1497–1500. [PubMed] [Google Scholar]
- HERSHKO A., AMOZ S., MAGER J. Effect of polyamines and divalent metals on in vitro incorporation of amino acids into ribonucleoprotein particles. Biochem Biophys Res Commun. 1961 May 15;5:46–51. doi: 10.1016/0006-291x(61)90078-x. [DOI] [PubMed] [Google Scholar]
- Lederberg J. BACTERIAL PROTOPLASTS INDUCED BY PENICILLIN. Proc Natl Acad Sci U S A. 1956 Sep;42(9):574–577. doi: 10.1073/pnas.42.9.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGER J. Influence of osmotic pressure on the polyamine requirement of Neisseria perflava and Pasteurella tularensis for growth in defined media. Nature. 1955 Nov 12;176(4489):933–934. doi: 10.1038/176933a0. [DOI] [PubMed] [Google Scholar]
- MAGER J. Spermine as a protective agent against osmotic lysis. Nature. 1959 Jun 27;183:1827–1828. doi: 10.1038/1831827a0. [DOI] [PubMed] [Google Scholar]
- MAGER J. The stabilizing effect of spermine and related polyamines and bacterial protoplasts. Biochim Biophys Acta. 1959 Dec;36:529–531. doi: 10.1016/0006-3002(59)90195-7. [DOI] [PubMed] [Google Scholar]
- MAHLER H. R., MEHROTRA B. D., SHARP C. W. Effects of diamines on the thermal transition of DNA. Biochem Biophys Res Commun. 1961 Jan 25;4:79–82. doi: 10.1016/0006-291x(61)90260-1. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- PARK J. T., STROMINGER J. L. Mode of action of penicillin. Science. 1957 Jan 18;125(3238):99–101. doi: 10.1126/science.125.3238.99. [DOI] [PubMed] [Google Scholar]
- PRIMOSIGH J., PELZER H., MAASS D., WEIDEL W. Chemical characterization of mucopeptides released from the E. coli B cell wall by enzymic action. Biochim Biophys Acta. 1961 Jan 1;46:68–80. doi: 10.1016/0006-3002(61)90647-3. [DOI] [PubMed] [Google Scholar]
- RAZIN S., ROZANSKY R. Mechanism of the antibacterial action of spermine. Arch Biochem Biophys. 1959 Mar;81(1):36–54. doi: 10.1016/0003-9861(59)90173-0. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative organisms and the role of versene. Biochim Biophys Acta. 1958 Nov;30(2):225–232. doi: 10.1016/0006-3002(58)90044-1. [DOI] [PubMed] [Google Scholar]
- TABOR H., ROSENTHAL S. M., TABOR C. W. The biosynthesis of spermidine and spermine from putrescine and methionine. J Biol Chem. 1958 Oct;233(4):907–914. [PubMed] [Google Scholar]
- TABOR H. Stabilization of bacteriophage T5 by spermine and related polyamines. Biochem Biophys Res Commun. 1960 Oct;3:382–385. doi: 10.1016/0006-291x(60)90049-8. [DOI] [PubMed] [Google Scholar]
- TABOR H. The stabilization of Bacillus subtilis transforming principle by spermine. Biochem Biophys Res Commun. 1961 Mar 10;4:228–231. doi: 10.1016/0006-291x(61)90276-5. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WEIBULL C. Bacterial protoplasts. Annu Rev Microbiol. 1958;12:1–26. doi: 10.1146/annurev.mi.12.100158.000245. [DOI] [PubMed] [Google Scholar]


