Abstract
Matrix metalloproteinases (MMPs) are a group of proteases that belong to the metazincin family. These proteins consist of similar structures featuring a signaling peptide, a propeptide domain, a catalytic domain where the notable zinc ion binding site is found and a hinge region that binds to the C-terminal hemoplexin domain. MMPs can be produced by numerous cell types through secretion or localization to the cell membrane. While certain chemical compounds have been known to generally inhibit MMPs, naturally occurring proteins known as tissue inhibitors of metalloproteinases (TIMPs) effectively interact with MMPs to modify their biological roles. MMPs are very important enzymes that actively participate in remodeling the extracellular matrix by degrading certain constituents, along with promoting cell proliferation, migration, differentiation, apoptosis and angiogenesis. In normal adult tissue, they are almost undetectable; however, when perturbed through injury, disease or pregnancy, they have elevated expression. The goal of this review is to identify new experimental findings that have provided further insight into the role of MMPs in skeletal muscle, nerve and dermal tissue, as well as in the liver, heart and kidneys. Increased expression of MMPs can improve the regeneration potential of wounds; however, an imbalance between MMP and TIMP expression can prove to be destructive for afflicted tissues.
Matrix metalloproteinases (MMPs) belong to a superfamily of enzymes known as metazincins, which encompass a number of other endopeptidases including serralysins, asatacins, adamalysins, leishmanolysins, snapalysins and pappalysins [1,2]. Currently, there are 23 known human MMPs, with other species having slightly variable structures, all of which share similar characteristics (e.g., a zinc ion binding site) and are inhibited by tissue inhibitors of metalloproteinases (TIMPs) [3–5]. Furthermore, these enzymes have similar structures, including a signaling peptide, a propeptide domain, a catalytic domain where the zinc ion binding site resides and a hinge region that binds to the C-terminal hemoplexin domain [4,6]. The enzymes can be classified by small differences in structure, such as insertions of vitronectin, cysteine array, fibronectin domains, IgG-like domains and distinct types of transmembrane domains or the deletion of the hemoplexin domain. Based on their structural elements, MMPs are categorized into several groups: collagenases, gelatinases, matrilysins, membrane-type (MT) MMPs, metalloelastases, stromelysins and other various types (Table 1). A majority of MMPs are secreted in a latent form known as a pro-MMP and can only become active when the bond between the free thiol of a conserved cysteine residue on the propeptide domain and the zinc ion on the catalytic domain is broken or through complete cleavage of the propeptide domain through the use of other MMPs [7,8]. Other MMPs are activated intracellularly by furin before they are secreted or incorporated into the cell membrane.
Table 1.
Different types of matrix metalloproteinases, their location within tissues and source of activation from a pro-form.
| Enzyme | MMP | Location | Activation |
|---|---|---|---|
| Collagenases | MMP1 | Secreted | MMP3 |
| MMP8 | Secreted | MMP3 | |
| MMP13 | Secreted | MMP14/TIMP2/MMP3 | |
| MMP18* | Secreted | Unknown | |
| Gelatinases | MMP2 | Secreted | MMP14/TIMP2 |
| MMP9 | Secreted | MMP3/MMP13 | |
| Matrilysin | MMP7 | Secreted | MMP3 |
| MMP26 | Secreted | Unknown | |
| Membrane-type MMPs | MMP14 | Membrane | Furin |
| MMP15 | Membrane | Furin | |
| MMP16 | Membrane | Furin | |
| MMP17 | Membrane | Furin | |
| MMP24 | Membrane | Furin | |
| MMP25 | Membrane | Furin | |
| Metalloelastase | MMP12 | Secreted | Unknown |
| Stromelysin | MMP3 | Secreted | MMP14/TIMP2 |
| MMP10 | Secreted | Unknown | |
| MMP11 | Secreted | Furin | |
| Others | MMP19 | Secreted | Unknown |
| MMP20 | Secreted | Unknown | |
| MMP21 | Secreted | Unknown | |
| MMP23 | Secreted | Furin | |
| MMP27 | Secreted | Unknown | |
| MMP28 | Secreted | Furin | |
MMP18 is only found in Xenopus.
MMP: Matrix metalloproteinase; TIMP: Tissue inhibitor of matrix metalloproteinase.
Despite differences in their biomolecular structure, all MMPs are known for their involvement in a number of biological tasks. Generally, they participate to a moderate extent in embryogenic development and are almost undetectable in normal adult resting tissues; however, they become clearly activated when perturbed through injury, disease and during pregnancy [9–13]. While some MMPs are known primarily for their ability to degrade certain components of the extracellular matrix (ECM), they are not solely limited to this physiological task [14,15]. When stimulated, MMPs interact with various cytokines and chemokines to become engaged in different roles such as cell proliferation, migration, differentiation, apoptosis and angiogenesis [16–22]. Overall, these processes occur to facilitate tissue or organ regeneration by actively remodeling it when damaged.
Complementary to MMPs are four inhibitors known as TIMPs (Table 2), which serve the purpose of inhibiting MMPs, in addition to closely related members of the adamalysin group, a disintegrin and a metalloproteinase (ADAMs) [23–25]. Like most MMPs, TIMPs are secreted proteins that regulate degradation of ECM constituents and tissue remodeling through interaction with MMPs. They can limit the extent of MMP participation in the regenerative process by restricting cellular functions such as proliferation and migration [26]. The typical shape of TIMPs is wedge-like, containing both an N- and C-terminal domain with a molecular weight ranging from 21 to 29 kDa [4,6]. Both terminals consist of six conserved cysteine residues forming three disulfide bonds; however, only the N terminal is responsible for inhibiting MMP activity [6,26].
Table 2.
Biological matrix metalloproteinase inhibitors.
| Inhibitor | Expression | Tissue distribution | Location | Specificity/function |
|---|---|---|---|---|
| TIMP1 | Inducible | Wide distribution | Secreted | Inhibits most MMPs well, except MMP14, 15, 16, 19 and 24; ADAM10 |
| TIMP2 | Constituted | Wide distribution | Secreted/cell membrane | Inhibits most MMPs; ADAM12; activates MMP2, 3 and 13 |
| TIMP3 | Inducible | Wide distribution | Extracellular matrix | Inhibits all MMPs; ADAM10, 12 and 17; ADAMST2, 4 and 5 |
| TIMP4 | Highly regulated and restricted |
Heart, brain, ovary, kidney, testes, fat, pancreas and colon |
Secreted/cell membrane | Inhibits all MMPs |
ADAM: A disintegrin and a metalloproteinase; MMP: Matrix metalloproteinase; TIMP: Tissue inhibitor of matrix metalloproteinase.
With the range of influence that MMPs have on developmental biology and regenerative medicine, one of the best methods to examine them is using MMP gene-knockout mice. Over a dozen MMP and TIMP mutant types have been generated and mouse lines surviving to birth were inspected for their faint phenotypic modifications. Some of the well-studied MMP or TIMP null mice were deficient for MMP2, MMP3, MMP9, MMP12 and MT1 MMP (MMP14) or TIMP1 and TIMP3, which caused underdevelopment or impaired tissue regeneration [27–33].
Regeneration
One of the key mysteries in biology is limb regeneration. It is commonly known that, for adult mammals, amputated limbs do not grow back. However, adult amphibians such as newts and salamanders can regenerate fully functional arms and legs over the course of 70 days, a process in which MMPs play a vital role [34,35]. Regeneration of amputated digits has been documented in higher-order mammals in the embryonic and neonatal stages, however, this was only permissible if the amputation was restricted to the phalangeal bones [36]. In other instances, fingertip regeneration has been observed in children and, if managed properly, could restore the finger’s contour, fingerprint and function with minimal scarring; however, full lengthening of the amputated digit is not always entirely possible [37,38]. The unique ability of amphibians to regenerate whole limbs demonstrates a greater capacity for regeneration and healing when compared with wound healing in mammals.
Despite the dissimilarity in both species’ regenerative ability, the events that follow immediately after injury are comparable. Both processes of wound healing in mammals and limb regeneration in newts use MMPs to guide the remodeling of damaged tissue. The first stage of mammalian wound healing initiated after incidental epithelial damage is inflammation [39]. During this period, cytokines and growth factors are released by a combination of cells including platelets, macrophages and neutrophils, as well as epithelial and stromal cells [35,40]. At the same time, MMPs are synthesized by many similar cell types, including macrophages, epithelial, stromal and inflammatory cells, in order to promote leukocyte migration, as well as to degrade collagen of the damaged tissue. In the days following inflammation, MMPs play a more active part in re-epithelialization of the wound [41]. By degrading the ECM surrounding the wounded area, they stimulate migration of the cells encompassing the region to the site of injury. Upon arrival, these cells proliferate and work in conjunction to direct differentiation and angiogenesis to prevent further fluid loss or bacterial infection by closing the ruptured tissue barrier [40]. In the final stages of wound healing, collagen synthesis is observed. For very severe injuries, excessive collagen synthesis occurs due to the overproduction of TGF-β, which invariably restricts the recovery of muscle function [42].
In contrast to wound healing in mammals, limb regeneration follows a different course for amphibians, yet MMP activity is still present. Almost instantly after a limb amputation of a newt, MMP activity becomes highly elevated in order to rapidly produce an apical epithelial cap to cover the stump, minimizing additional tissue damage, contamination or inflammatory response [35,43]. Following AEC formation, peripheral cells at the amputation site undergo dedifferentiation, whereby they are converted into a multipotent state [44]. This phenomenon results in the formation of a blastema from migrated fibroblasts. Several MMPs have been identified with blastema formation and limb regeneration in newts. One study found sequence homology between MMPs MMP3/10a and MMP3/10b expressed in newts and humans only for regenerating limbs and not in normal tissue [43]. Both MMPs aided in tissue remodeling by digesting gelatin, casein and collagen I and IV. Other studies provided evidence that MMP9 contributed to limb regeneration by digesting gelatin along with collagen I and IV, thereby removing damaged cartilage elements [45,46]. A study by Vinarsky et al. demonstrated that MMP9, MMP3/10a, MMP3/10b and nCol (a Notophthalmus collagenase gene) were notably upregulated immediately following limb amputation, based on proteolytic analysis of regenerating limb extracts, and remained elevated up to 20 days [45]. Furthermore, the administration of an MMP inhibitor, GM6001, demonstrated severely dwarfed and dysfunctional limbs or regeneration impeded at an early stage, resulting in a stump covered by uncharacteristic acellular scar-like tissue.
Under normal circumstances, once the blastema is generated, it continues to grow distally, producing an entire limb, remarkably even when it is grafted to different locations on the body [47]. It should be noted that axolotls or newts are not the only creatures known to form a blastema after tissue trauma. Blastema formation in response to MMPs has been identified in several other species including Drosophila, mouse and zebrafish [48–50]. In Drosophila, two genes, the regeneration (rgn) gene and MMP1 gene, were found to actively take part in leg disc regeneration, but not development. It is believed that they become activated in the blastema by wingless signaling and that mutations directed toward rgn or MMP1 will affect the formation of a blastema [48]. Likewise, Murphy–Roth–Large (MRL) mice, which have a greater capacity for regeneration, demonstrated regrowth of lost cartilage and complete closure of through-and-through ear hole punches, with no scar formation in comparison to normal adult mice [51]. Consequently, MRL mice have a greater MMP to TIMP ratio of MMP2 and MMP9 for the purpose of breaking down the ECM and basement membrane prior to blastema formation, while normal B6 mice never display blastema formation [49]. Moreover, zebrafish expressed MMP2, in addition to MMP14 and TIMP2, during the formation of the blastema, as with MRL mice [50]. Additionally, GM6001 was found to produce a negative effect on blastema formation, thus inhibiting caudal fin regeneration [50]. It should be noted that MMP2 may play a specific role in regeneration of connective tissue, as confirmed through studies of MRL mice and zebrafish. In the following article sections, updated research on MMPs within various tissues is reviewed.
Skeletal muscle tissue
Muscle injuries can occur by a number of misfortunes such as lacerations, strains, ischemia or neurological dysfunctions. After countless years of study, researchers have defined a timeline for muscle recovery in three stages [52]:
Muscle degeneration and inflammation
Regeneration
Scar tissue formation (fibrosis).
The main limitation of muscle regeneration is the period of fibrosis, occurring between the second and third week after the muscle injury [52,53]. As previously mentioned, increased TGF-β1 production will trigger secretion of collagen type I and III by myofibroblasts in order to generate fibrotic scar tissue [42,54,55]. The administration of several remedies, such as basic-fibroblast growth factor (bFGF), insulin growth factor type 1 (IGF-1), suramin, decorin, IFN-γ and angiotensin II receptor blocker were used to successfully improve muscle healing by either promoting myoblast proliferation or blocking TGF-β1 expression [42,52–59]. Unfortunately, these treatments of stimulating myoblast proliferation/migration or inhibiting TGF-β1 are ineffective in regenerating muscle with pre-existing fibrotic tissue [18,42,52].
To resolve this dilemma, researchers have begun examining MMPs as a prospect for muscle regeneration. Recent studies performed both in vitro and in vivo have shown the beneficial impact of MMP1 administration on muscle healing [18,60,61]. In vitro experiments illustrated cellular increases in mobility by increased expression of four migration proteins, N-cadherin, β-catenin, pre-MMP2 and TIMP1 [18]. Differentiation into myotubes was greater for cells treated with MMP1 compared with nontreated cells, based on quantity and the increased expression of myogenin, a check-point protein involved in myofiber formation. In vivo evidence showed that direct injection of C2C12 myoblasts in combination with MMP1 improved cell migration and increased myofiber differentiation from the point of injection in both gastrocnemius and tibialis anterior muscles of mice with musclular dystrophy (MDX) [18]. Another study illustrated that the injection of MMP1 alone to a site of injury created in mice TA muscles improved muscle healing by reducing fibrotic tissue and increasing myofiber formation [60]. Complementary to these experiments, one investigation provided further evidence demonstrating the involvement of MMPs in skeletal muscle cell migration. Using two MMP inhibitors at moderate concentrations, MMP inhibitor II (inhibits MMP1, MMP3, MMP7 and MMP9; from Calbiochem) and GM6001 (inhibits MMP1, MMP2, MMP3, MMP8 and MMP9) suppressed C2C12 cell migration [62]. In another experiment, MMP3 was targeted using a siRNA because it plays an important role in activating pro-MMP1, MMP7, MMP8 and MMP9 [62]. These experimental results also demonstrated reduced cell migration, providing speculation that, by inhibiting MMP3, MMP1 could not become activated to increase the expression of certain migratory proteins.
Despite progress in reducing fibrotic scar tissue using MMPs, one hindrance of simply administering therapeutic proteins is their reduced enzymatic stability and natural clearance [61,63]. In this regard, multiple inoculations would be necessary, contrary to the patient’s inclination for comfort. As such, research has been conducted to develop synthetic and biodegradable scaffolds incorporated with various cytokines and growth factors to promote tissue and organ remodeling by mimicking the natural healing processes [64]. These efforts to integrate bio-molecules with biocompatible polymers are designed to facilitate cell migration and proliferation within the designated environment, to assist in the natural stages of tissue formation. In several studies, researchers have constructed 3D networks using MMPs crosslinked with linear oligopeptide substrates and functionalized them to poly(ethylene glycol) (PEG) macromers [65,66]. The development of these synthetic materials is designed to assist in tissue regeneration by triggering cells to remodel the ECM by secreting signaling proteins and promoting differentiation. Subsequently, the same researchers who administered native MMP1 to fibrotic muscle tissue also tested PEGylated active MMP1 as a method to overcome the limitation of protein clearance [61]. Attaching PEG to MMP1 rendered the protein less immunogenic, prevented proteolysis and reduced its clearance. Unfortunately, in an effort to improve degradation of fibrotic tissue, it failed to reduce collagen content in lacerated muscles. This caused speculation that the collagen was impeded from reaching the catalytic site of modified MMP1 when bound to PEG [61].
Other MMPs explored recently for their involvement in muscle regeneration are the gelatinases MMP2 and MMP9. The regulation of these two proteins has had a profound impact in the status of MDX mice, an X-linked genetic disease where muscles lack the dystrophin gene, causing an increased rate of degeneration [67]. In a study using MDX mice, scientists administered l-arginine to decrease the expression of MMP2 and MMP9, which in turn destabilized satellite cell adhesion and myoblast fusion [68]. Their principle result was that both MMPs work conjointly to maintain the integrity of the muscle membrane while l-arginine reduces inflammation. A similar study determined that elevated MMP9 activity mutually increases the number of stem cell antigen 1-positive cells (a stem cell marker for muscle-derived stem cells or muscle satellite cells) to favor cell migration and activation of myogenic precursors [69]. An increase in MMP2, on the other hand, corresponded to an increase in the number of neural cell-adhesion molecule (NCAM)-positive cells aiding in regeneration. However, confounding results indicate that their activity may be linked to persistent inflammation and membrane fragility in spite of their regenerative myofiber capacity [69]. Another team of investigators decided to look at the variable expression of MMP2 and MMP9 in two different muscle types. In Soleus, slow-twitch muscle, MMP9 was upregulated for 14 days postinjury, compared with 7 days in extensor digitorum longus (EDL), fast-twitch muscle [70]. MMP2 activity was higher for fast-twitch (EDL) muscle than for the Soleus muscle and may be responsible for ECM remodeling during the reconstruction phase, by preventing accumulation of ECM components [70]. The extensive fibrosis resulting in the Soleus muscle could be contributed to insufficient degradation of ECM components. Furthermore, MMP2 was found to play a crucial role in cell fusion, whereas MMP9 was involved in all stages of myoblast differentiation (including an active part in cell proliferation). These researchers concluded that the differences in the activity of both MMPs modifying myoblast migration and fusion are the root of the difference in the regenerative response of the two muscle types.
Additional studies have confirmed the role of MMP9 in muscle cell differentiation and MMP2 in myoblast elongation/fusion, working in conjunction to remodel the ECM [71,72]. In a study using canine X-linked muscular dystrophy in Japan (CXMDJ), increases in MMP2, MMP9 and MMP14, and TIMP1 and TIMP2 expression were found in comparison to normal wild-type canines. Increased MMP9 expression was found to correspond with degradation of the basal lamina in necrotic tissue of CXMDJ and the promotion of inflammatory cell migration to the site of interest. Following the process of muscle healing, muscle fibers were regenerated based on the expression of MMP2 [71]. The researchers hinted that TIMP1 upregulation most likely followed peak MMP9 levels, while TIMP2 and MMP14 expression might be involved with MMP2 as they are known activators of pro-MMP2. Although some of these results are speculative, they suggested that additional experiments using cardiotoxin injury could clarify whether TIMP1 and 2 activity is dependent on the stage of muscle healing. Another investigation evinced a more defined function of MMP14 in muscle tissue by providing a number of intriguing results. First, MMP14 serves as a checkpoint for the three stages of morphological differentiation (proliferation, elongation and fusion) and also engages in degrading fibronectin [72]. Myotube formation decreased when MMP14 activity was blocked using short hairpin RNA and upon observation in MMP14-deficient mice. Inhibiting MMP14 during the proliferative and elongation stages of skeletal muscle development prevents proper activation of MMP2, which in turn blocks myotube fusion. Furthermore, while TIMP2 has been known to work in combination with MMP14 to activate MMP2, an overexpression of TIMP2 and 1 will have a distinct inhibitory effect on MMP14 and drastically reduce myotube formation [72]. Ultimately, these findings demonstrate that an absence of MMP14 causes fibronectin to act as a negative regulator of myogenic differentiation. As fibronectin is degraded during the elongation phase of skeletal muscle development by MMP14, myotube formation will continue with the aid of MMP2. Leading into the discussion of MMP involvement with regenerating nerve tissue, one study found that overexpressing osteoactivin in skeletal muscle cells increased their expression of MMP3 and MMP9 to repress denervation-mediated fibrosis [73].
Nervous tissue
Spinal cord injuries (SCIs) occur with a traumatic hit that fractures or dislocates vertebrae. Most injuries do not sever the spine, but instead crush the axons. The initial damage and location of the SCI determines the severity of disruption to signaling between the brain and certain parts of the body [74]. When this happens, the axon terminals of the neuron retract from their normal synaptic targets, leading to apoptosis [75]. Axon regeneration is difficult following SCI; however, the prospect of regaining lost nerve function is noticeably better for the PNS [76]. A barrier to axon regeneration is the formation of glial scars ensuing injury to the CNS as well as the PNS [77].
Numerous studies have found that MMPs in nerve tissue have similar tasks as in skeletal muscle. Based on their known biological roles, they are upregulated during an inflammatory response to promote regeneration of damaged nerve cells and allow penetration of the blood–brain barrier (BBB) in the CNS [75,78]. A number of studies have been performed using several types of nerve cells including astrocytes, dorsal root ganglia (DRG) neurons, oligodendrocytes and Schwann cells to assess which traits are modified when they interact with MMPs [79–82]. Two studies by the same research team shed light on the specific involvement of MMP2 and MMP9 in wound healing of mice subjected to a moderate spinal cord contusion [79]. Their results indicated MMP2 was preferentially expressed in astrocytes bordering the lesion epicenter during wound healing, between 7 and 14 days after SCI. Furthermore, the presence of MMP2 was notably beneficial in the sparing of white brain matter, reducing axon plasticity and maintaining locomotor recovery. In MMP2-null mice, larger glial scar formation was observed with an increase in chondroitin sulfate proteoglycans (CSPGs), which are known to arrest the regrowth of injured axons across a lesion site [79,83]. The concluding find of this study was that MMP2-null mice with a SCI overexpress MMP9, which prompted a second investigation. This follow-up examination concluded that MMP9 expression from astrocytes and leukocytes did not facilitate glial scar formation, but promoted greater CSPG deposition when compared with MMP9-null and wild type mice [27]. Abnormalities observed in the actin cytoskeletal organization and function reduced astrocyte migration of MMP9-null mice or wild-type mice when MMP9 activity was blocked with a pharmacologic inhibitor; however, MMP is not the sole modulator of cell migration. The researchers suggest that, while MMP9 secreted by astrocytes is able to cleave CSPGs, its absence truly reduced the complexity of the glial scar by preventing the migration of macrophages across the BBB to deposit CSPGs. Neither MMP9 nor MMP2 had any impact on astrocyte proliferation, adhesion or viability.
Alternative studies have continued to indicate similar findings in terms of MMP2 and MMP9 expression with regard to axon regeneration. Examining the relationship between DRG neurons and Schwann cells, a recent study determined that MMP2 is initially upregulated, then subsequently downregulated during myelination. There is a negative correlation between MMP2 expression and the number of myelinated nodes and inhibiting MMP2 activity accounts for incomplete and aberrant myelin formation [80]. Another investigation by Chattopadhya et al. looked at the role of MMP9 on Schwann cell proliferation by analyzing its effect on several signaling pathways [82]. They concluded that administering recombinant MMP9 to Schwann cells suppresses their growth and mitogenic activity. More specifically, MMP9 produced a dose-dependent activation of ERK1/2, which is counteracted when inhibitors of IGF-1, ErbB and platelet-derived growth factor receptors are present. It was also implicated in different effects on axonal regeneration when comparing blood–nerve barrier disruption in the CNS and PNS. In a study by Liu et al., MMP9 expression increased dramatically after an injury to the sciatic nerve (PNS injury) until day 7, while MMP9 expression was delayed until day 7 and lasted for 28 days following an injury to the optic nerve (CNS injury) [76]. The increased expression of MMP9 in the optic nerve was observed along with a decreased expression of neurofilament. This correlation between MMP9 and neurofilament suggests that MMP9 may take part in axonal degeneration in the CNS; whereas, in the PNS, MMP9 is responsible for the clearance of myelin debris and other various components of damaged cells to improve axonal regeneration and remyelination.
Although MMP2 and MMP9 hold most of the spotlight when it comes to nerve regeneration, several other MMPs have been suggested to play influential roles. Recent work by Werner et al. examined the connection between MMP28 and myelination [84]. They found that, in the early development of embryos of Xenopus and mice, expression was greatest in the nerve tissues, including peripheral nerves and some components of the CNS such as the spinal cord. Using ascorbic acid, researchers were able to induce myelination, which in turn reduced MMP28 activity. Upon deducing that MMP28 took no part in stimulating migration of nerves into peripheral tissues, the new challenge was determining whether a change in the expression of MMP28 established an extracellular environment needed for myelination or simply inhibited the ability of Schwann cells to remyelinate axons. Further studies indicated that when MMP28 was added to in vitro cultures of rat DRG, myelination was attenuated and MAPK, ErbB2 and ErbB3 phosphorylation were enhanced [81]. When MMP28 was activated in conjunction with two inhibitory antibodies (pAb180 and pAb183) targeting its activity, there was a 2.6- and 4.8-fold enhancement in myelination, respectively. In vivo studies verified this point by detecting increases in MMP28 expression within demyelinated lesions of experimental autoimmune encephalitis (EAE) and human multiple sclerosis (MS) nervous system tissue [81].
In addition, MT MMPs have been investigated using EAE mice as a model for human MS. Previous research has discovered that MMP15, 16, 17 and 24 are downregulated in the spinal cord of mice with myelin basic protein-induced adoptively transferred EAE, while MMP14 and 25 have moderately increased activity [85]. However, five genes, MMP8, MMP10, MMP12, ADAM12 and TIMP1, showed significant increase in expression compared with the control. More recently, the same researchers examined the activity of these MT MMPs with regards to microglia, often referred to as resident CNS macrophages, which are highly mobile and serve as the main immune defense of the CNS by monitoring damaged neurons, as well as potential infectious agents [86]. As such, recent studies revealed only a minor difference in the activity of these MT MMPs. Instead, significant downregulation was only observed for MMP15, MMP17 and MMP25; MMP14 and MMP16 expression did not vary and MMP24 was not expressed at all [87]. Unfortunately, it still remains to be determined how or if MT MMPs affect neuroinflammation as they did not impact the expression of the proinflammatory cytokines TNF-α, IL-1β and IFN-γ. If MT MMPs cannot be considered mediators during neuroinflammation, they may have no other purpose than to simply enable infiltrating cells to navigate across the BBB or support nerve regeneration.
Dermal tissue
Adult skin contains two tissue layers: a keratinized stratified epidermis and an underlying layer of collagen-rich dermal connective tissue. Skin wound healing, as mentioned previously, initiates immediately after injury and consists of inflammatory, proliferative and maturation phases, which are complex processes requiring the collaborative efforts of multiple types of tissues and cells [35,40]. Various factors, including growth factors, necrotic cell/tissue and matrix components, trigger macrophages, inflammatory cells, keratinocytes and fibroblasts to accumulate at the site of injury [88]. These active cells at the wound margin begin to proliferate, become invasive and generate new ECM.
The composition and re-organization of the ECM during skin wound healing is regulated by MMPs and the cells producing them. Fibroblasts produce the majority of collagens, proteoglycans and proteins constituting the ECM at the wound edge, whereas macrophages, endothelial cells, keratinocytes and some fibroblasts secrete a conglomeration of MMPs [88,89]. As in other tissues, they directly regulate skin wound healing by modifying macrophage function, cell migration and proliferation, angiogenesis and matrix remodeling. Different MMPs usually serve different functions during healing; for example, in cutaneous wound healing; MMP9 is responsible for degrading the basal lamina of type IV collagen, anchoring fibril collagen type VII and for releasing keratinocytes from their tethers to the basal lamina [90]. Studies involving MMP1 suggest its expression is controlled by cell–collagen interactions and upregulated only when keratinocytes have migrated beyond the free edge of the basal lamina [88,91]. It has been observed that keratinocytes have enhanced MMP1 production when they migrate on type I collagen, the most abundant structural component of the dermis [92]. Presumably, MMP1 aids migration of keratinocytes and other cells by degrading both type I and III collagen at sites of focal adhesion attachment to the dermal substratum [88]. MMP10 has been known to be upregulated by keratinocytes at the edge of the wounds, yet has broader substrate specificity than MMP1, with its ability to degrade multiple ECM components, such as proteoglycans, laminin, fibronectin, type IV and IX collagen and even the globular domains of procollagens I and III [88,93]. MMP13, which is undetected in keratinocytes and found exclusively in fibroblasts deep within the ulcer bed of chronic skin wounds, is indicated to function in matrix remodeling [89].
Generally, MMPs are shown to have positive roles in promoting acute skin wound healing. However, expression of their adversaries, MMP inhibitors, TIMP1, 2 and 3 can negatively affect the healing outcome [94]. Studies have shown that the expression of MMPs and TIMPs together carefully regulates wound healing and that the balance between them is critical for proper skin repair. It is understood that different pathological states can result in aberrant function of these regulatory mechanisms. For miscellaneous and harmful injuries, such as ischemia, repeated trauma, bacterial/viral infection or physical/chemical burden, the inflammatory phase is sometimes impeded, leading to chronicity. One of the key components of chronic wounds is an elevated level of MMPs [89,95–97]. Compared with acute skin wounds, the expression of certain MMPs (MMP2, MMP9, MMP10 and MMP12) is largely increased during situations of impaired healing, while the expression of TIMPs is dramatically reduced [89,93,95–98]. Again, it is recognized that a common miscommunication of signals between MMPs and TIMPs is the root of erroneous regeneration for skin, as in other biological tissues.
It has been suggested that the ratio of MMP to TIMPs is a critical hindrance in the healing of damaged skin; it has therefore been speculated that a higher ratio of MMP to TIMP expression could be associated with scarless repair, as is the case in newts [99,100]. In contrast to the healing of adult cutaneous wounds, which re-establishes the dermal integrity at the expense of scar formation, early fetal wounds heal without fibrotic scar tissue. One study reported that the wound healing of skin in fetal rat transitions from scarless to scar-forming between days 16.5 (E16) and 18.5 (E18) of gestation [100]. This investigation showed that E16 scarless wounds had a greater expression of MMP1, MMP2 and MMP14 relative to TIMP1 and TIMP2 expression than E19 scarring wounds. This evidence demonstrated improved ECM turnover, reduced collagen accumulation, increased migration of fetal cells and, most importantly, increased scarless repair [100]. In the model of skin healing and limb regeneration using axolotls, MMP9 is shown to be an early, nerve-independent marker of the regenerating epidermis, observed throughout all the cells of the multilayered epithelium that covered the wound [101]. MMP9 was also revealed to be necessary for the re-epithelialization and formation of a blastema during limb regeneration. In another study using athymic nude mice, which are known for their exceptional ability among mature mammals to undergo skin healing without scar formation, MMP9 expression was upregulated and proposed as a major contributor to scar-free healing [102]. Similar studies examining human skin have suggested that the intrinsic nature of skin is transformed during fetal development by altering the gene and protein expression of MMPs and TIMPs, which may be accountable for the phenotypic metamorphosis from scarless to scar formation of injured skin during repair [103].
In addition to TIMPs, the involvement of MMP activity in skin wound healing can be connected with other proteins including various growth factors. Recent studies revealed the positive effect of bFGF and IL-6 in promoting skin repair by stimulating MMP expression [104–106]. One in vitro study found that using bFGF upregulated MMP1, as well as fibronectin, in human skin fibroblasts, aiding in reduced collagen deposition and suppressed scar formation [104]. Similarly, another study found that bFGF stimulated MMP2 in mouse adipocytes, ameliorating the survival of fat grafts; however, the beneficial effect was attenuated with the administration of GM6001 [105]. For normal skin fibroblasts, IL-6 stimulation promoted an increase in the expression of MMP1 and 3, while there was no change in MMP expression of fibroblasts isolated from hypertrophic scars [106]. These data indicate that perhaps some pathways or mechanisms become perturbed during thermal injury, resulting in skin deformity by an excessive production of collagen and downregulated MMP expression. Other factors have been shown to affect MMP production in opposite manners. For instance, TGF-β stimulates collagen and MMP2 expression while inhibiting MMP1 in dermal fibroblasts, suggesting that MMPs attempt to repair skin damage during an inflammatory response even when it is negatively impacted [107]. Ultimately, an elevated level of MMPs during skin injury will prove most beneficial, regardless of autocrine signaling, self administration or stimulation by other factors, but the challenge is determining the most effective method.
Other tissues
Liver
As a vital organ, the liver provides some of the most essential bodily functions, but its most unique trait is its ability to regenerate. This has been demonstrated in a number of studies, with healthy and cirrhotic livers regenerating almost entirely after a partial hepatectomy [108,109]. Unfortunately, this quality may become debilitated in seriously diseased or damaged livers in a condition known as irreversible fibrotic scar tissue, where an accumulation of fibrillar collagens type I and III are secreted by hepatic stellate cells (HSCs) [110–112].
Liver regeneration research has prominently documented the important, yet perplexing roles of MMPs in the process of liver regeneration. To date, increased expression of MMP2, MMP3, MMP9, MMP10, MMP13 and MMP14 was observed after liver injury in both human tissue and animal model studies, with the peak expression coinciding with inflammatory cytokine upregulation [110,111,113–116]. As with most of the tissues during regeneration, MMP2 and MMP9 have outstanding connections. Several studies have indicated that both MMP2 and MMP9 exist at very low levels in normal liver, with increasingly higher expression in more severe disease states, such as rejected human liver transplant tissue or chemically induced liver fibrosis using carbon tetrachloride in animal studies [114–116]. With similar results found for partial hepatectomies, one study identified a delayed hepatic regenerative response for MMP9-deficient mice [117]. Furthermore, MMP9-deficient mice, in comparison with wild-type mice, showed less expression of VEGF, HGF and TNF-α, constituents associated with liver regeneration. They also demonstrated reduced apoptosis, which correlated with a slower rate of hepatocyte proliferation. Another study found that the MMP inhibitor Marimastat® could prevent liver regeneration in hepatectomized mouse livers by downregulating MMP2 and MMP9 [118]. In spite of the reduced MMP expression, increased levels of IL-6 were observed, promoting MMP expression, as comparable with hypertrophic skin scars. Some studies have pointed out the crucial role of the membrane protein MMP14 [113]. It has been observed that when MMP2 or MMP9 are secreted in a proenzyme form by HSCs or hepatic myofibroblasts, they become activated through association with MMP14 [113,116,119]. An MMP activation cascade was proposed where MMP14 from HSCs activates a pro-enzyme form of MMP13, which in turns activates pro-MMP9. The activated MMP9 provides a positive-feedback loop whereby it continues to upregulate and activate MMP13 [113]. Both of these MMPs, MMP9 and MMP13, are key components that help degrade collagens in the ECM matrix of liver fibrogenesis, and their activation in the cascade of events can be inhibited by TIMP1 or 2, respectively.
Despite the ability of MMPs to repair damaged livers, their efforts are unfortunately undermined by TIMPs. Research has demonstrated an elevated level of TIMP activity is paralleled to increases in MMP activity for severely diseased livers [116,120]. While a variety of studies have hinted that only certain TIMPs inhibit specific MMPs in certain tissues, ultimately, they all are culprits in promoting a profibrotic environment by protecting newly synthesized collagens from degradation. Based on this outlook, investigations were performed to aid liver regeneration by inhibiting TIMP activity with gene therapy. Using siRNA to target TIMP2 in rat fibrotic livers helped to increase ECM degradation by reducing activated HSCs, which enhanced hepatocyte proliferation [121]. A corresponding study, producing similar results, administered different proteolytic inactive MMP9 mutants to mice with hepatic fibrosis, which bound to and inhibited the function of TIMP1 [120]. A different study further supported these results by illustrating that TIMP1 serves as a negative regulator of HGF signaling by reducing hepatocyte proliferation in partially hepatectomized mice [122]. In both cases, where TIMP1 and TIMP2 were inhibited, liver regeneration improved; however, this is not always true as they can regulate cytokines other than MMPs to influence ECM remodeling. One investigation showed that TIMP3 played a significant part in liver regeneration by inhibiting TNF-α-converting enzyme to reduce elevated levels of inflammation and TNF-mediated cell death [123]. Not all liver gene therapy solely inhibited TIMPs; one animal study observed the affects of transfecting rats suffering from hepatic fibrosis with a recombinant adenovirus containing human pro-MMP1 cDNA [110]. The results demonstrated that MMP1 decreased the number of activated HSCs, thereby reducing collagen I and II content and promoting hepatocyte proliferation.
Heart
Heart disease is the leading cause of death in the USA. With a number of clinical and animal studies aiming to improve impaired cardiac function, the use of MMPs has also been considered in this area of research. A popular animal model used to examine heart disease is induced myocardial infarction (MI) [124,125]. During a MI, blood supply to a portion of the heart is interrupted, causing collagen deposition and, when excessive, the surrounding myocardium becomes stiffer, which adversely affects the myocardial viscoelasticity, promoting ventricular diastolic and systolic dysfunction and potential heart failure (HF) [126].
Various studies have shown the diverse number of tasks MMPs are involved in during valve and wall remodeling. In one study, while MMP9 expression was increased in late scarring from MI, MMP2 was the predominant gelatinase that increased in decompensated left ventricular (LV) myocardium by cardiomyocytes for continued LV remodeling [127]. An increase in MMP7 activity was observed in scar myocardium, suggesting involvement in proteoglycan degradation through secretion by local fibroblasts. MMP7 was also suggested to have a possible role in postinfarction angiogenesis, as demonstrated by plasminogen activation by increased secretion of urokinase-type plasminogen activator, which is known to degrade basement proteins and activate growth factors involved in angiogenesis [127,128]. Furthermore, deviations in TIMP1 (increased expression) and TIMP4 (decreased expression) activity in mice suffering from HF may represent distinct variations in regional MMP activity regulation or matrix affinity. Alternatively, a study by Lu et al. noticed elevated levels of TIMP1 mRNA for MI and inflamed pericardium [129]. They also identified activity of MMP1 in combination with adhesion molecule, ICAM1 and chemoattractant cytokine, MCP1, which facilitated homing, chemotaxis and migration of local cells to reform the encompassing vascular network by reducing scar formation. Scar formation was also attenuated by MMP3, as found in a study by Mukherjee et al., which demonstrated that MMP3-deficient mice had greater scar volumes because fewer lymphocytes and macrophages were seen at the scar border [130]. Another study discovered the role of MMP8 in degrading cardiac scaffolds made from bovine collagen type I in mice and replacing them with a vascularized ECM instead of scar tissue, without stimulating a foreign body reaction on injured myocardium [131].
After gaining perspective into how MMP stimulation impacts remodeling of a damaged heart, MMP inhibitors were investigated for therapeutic effect. Numerous studies found that using MMP inhibitors to target specific MMPs affected heart remodeling in a positive manner, which contradicted evidence that MMPs are purely beneficial [124,125,132–135]. Various studies have used general MMP inhibitors to examine the outcome on heart function. A study by Moe et al. used PGE-7113313, an MMP1-sparing-MMP inhibitor, in canines subjected to simultaneous atrioventricular pacing; the findings illustrated differences in atrial myocyte cross-sectional areas as well as collagen-deposition areas, leading investigators to believe that it prevented detrimental atrial structure remodeling and evolving HF from atrial fibrillation [132]. In comparison, PD166793, a MMP inhibitor of MMP2, MMP3 and MMP13, was administered to mice subjected to MI by ligation of left anterior descending artery and was found to improve LV function, particularly in mice lacking osteopontin [124]. These results exhibited reduced MMP2 and MMP9 activity and decreased LV end-diastolic and end-systolic diameters, in addition to the percent fractional shortening. Other studies, such as by the Okada et al. group, used angiotensin-converting enzyme inhibitors to attenuate MMP2 and MMP9, thus preventing the development of monocrotaline-induced hypertrophy in the right ventricles of rats [133]. Some studies have targeted inflammation in heart disorders, which ultimately minimized MMP expression. One specific study injected recombinant IL-10 in mice subjected to MI [134]. They believed that IL-10 treatment subdued infiltrating inflammatory cells and cytokines to infarcted myocardium, allowing for improved LV function by diminishing fibrosis through reduced HuR (a cytokine mRNA-stabilizing protein) and MMP9 expression. By contrast, antibiotics were tested against MMP activity. Mice given clarithromycin in combination with transplanted heart allografts displayed lower rejection, which was attributed to lessened MMP expression and inflammatory infiltration [135]. Contrary to clarithromycin administration, evidence from using doxycycline did not suggest a positive outcome. Instead, investigators found that the tetracycline antibiotic impaired angiogenesis and compensatory LV hypertrophy with reductions in MMP2 and MMP9 activity [125]. Given their observations of early healing after MI, they cautioned against aggressive and nonselective inhibition of MMPs.
These experiments using synthetic MMP inhibitors produced similar results to studies investigating how TIMPs affect remodeling of the heart structure after injury. Research using TIMP1-deficient mice has demonstrated its importance in remodeling the structure of the heart in mice [32,136]. In a study by Roten et al., TIMP1-knockout mice were found to have an increased end-diastolic (ED) volume and LV ED wall stress than wild-type mice [136]. Comparatively, MI induced in TIMP1-deficient mice, by surgical ligation of the coronary artery, revealed exacerbation of LV remodeling afterward [32]. Furthermore, increases in LV ED volume were observed, as well as increases in the LV ED pressure, weight and cross-sectional area, which were indicative of a hypertrophic response. Both studies concluded that the nonexistent TIMP1 expression was the cause of decreased fibrillar collagen content. Another study found that developing TIMP3-deficient mice produced comparable results for remodeling the heart structure [33]. The loss of TIMP3 in developing mice triggered spontaneous LV dilation, cardiomyocyte hypertrophy and contractile dysfunction at 21 months. Furthermore, its absence caused elevated MMP9 expression, a leading culprit of significant reduction in fiber connectivity of the collagen network, and activated TNF-α, a hallmark of human myocardial remodeling. While both MMPs and their inhibitors have demonstrated positive outcomes, there is ultimately a balance between MMPs and TIMPs that is crucial to restoring proper function of damaged heart tissue.
Kidney
Matrix metalloproteases have been indicated to have a regulatory role in the collagen–integrin interactions that drive the proteolytic processes that are vital to remodeling the renal basement membrane in developing and diseased-state kidneys [137]. With multiple kidney disorders affecting individuals, such as diabetic nephropathy, focal segmental glomeralosclerosis and tubulointerstitial fibrosis, the unknown implications of MMPs in these diseases are currently under investigation [138–140]. As with other biological tissues, research aiming to correct renal problems found some promising results through altered MMP expression. In a study using mycophenolate mofetil (MMF) in combination with benazapril on nephrotectomized rats, reduced proteinuria, improved renal function and ameliorated tubulointerstitial fibrosis were observed [140]. The beneficial effects of administering MMF/benazapril were attributed to the effects of downregulating TIMP1 while upregulating MMP9 in renal tissue. These findings implied that some sort of imbalance between matrix generation and degradation was corrected for MMP9/TIMP1 expression to promote regeneration of injured kidneys. A previous study found similar results in children with pyelonephritis where urinary TIMP1 was greater than MMP9 and associated with renal scarring [141].
Unfortunately, various investigations have found mixed results pointing at MMPs as the perturbers of healthy renal function. Conflicting evidence found that rosiglitazone protects against renal injury from proteinuria in rats by decreasing the MMP9 expression [142]. Similarly, other studies found adverse effects of MMP2 in renal function. A study by Munkert et al. showed that an increase in the activity of MMP2 corresponded with an increase in MMP14, causing proliferation, an inflammatory response and an enhanced deposition of ECM in the kidneys [139]. These responses were consistent with features of glomerular inflammatory disease processes, developing sclerosis and eventual loss of renal function. An equivalent study produced a similar finding, where MMP2 upregulation was attributed to vascular dysfunction and remodeling during renal hypertension, but could be prevented by antioxidant approaches [143].
Future perspective
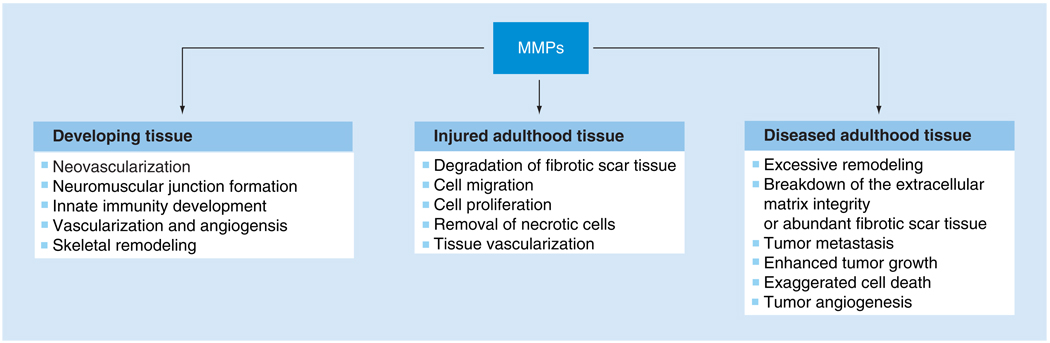
As science progresses, an innumerable amount of literature is generated, detailing the precise roles MMPs play in maintaining the structural integrity of the human anatomy. Modifications to the MMP or TIMP expression level can positively or negatively alter the stability of the tissue state. One of the key points in MMP research is determining which MMPs or TIMPs have an integral role in a specific disease scenario. For instance, in terms of regenerating damaged skeletal muscle, researchers may want to look at methods of stimulating MMP expression to degrade fibrotic tissue and then promote cell migration, proliferation and differentiation [18,60,62,69]. On the other hand, increased MMP expression has been known to have detrimental effects not only in injured tissue, but in diseased tissue. Some studies have indicated that increased expression of MMPs is the cause of enhanced tumor proliferation and metastasis or excessive ECM remodeling in diseased tissues [138,144–146]. Over the next decade, scientists will need to dedicate their time to appropriately identifying whether to promote or inhibit the activity of selected MMPs or TIMPs based on how their interaction with other ECM molecules (i.e., collagen, fibronectin and laminin) or cellular proteins (i.e., actin, neurofilament and myogenin) influences tissue remodeling. These parameters would need to be further assessed given the specific health complication and status of the tissue type (Figure 1). The outcome of this process could be to beneficially restore proper function to the damaged tissue.
Figure 1. Biological roles of MMPs with various tissue states.
MMP: Matrix metalloproteinase.
Challenges to this work include determination of adequate dosing and sustained delivery. As shown in numerous investigations, an imbalance between MMP and TIMP expression is the likely reason for problems resulting in persistent injury to damaged or diseased tissue. Administering a proper dose of MMP or TIMP must either restore the balance of the MMP/TIMP ratio (diseased tissue) or only alter it slightly to maximize regeneration (injured tissue). Several tactics of prolonged delivery have been suggested, such as continuous intravenous injection, gene therapy or degradable polymers. While attaching functionalized MMP to PEG for the purpose of reducing its systemic clearance was successful, it failed to degrade pre-existing fibrotic scar tissue. Future studies will engineer similar biomaterials to maintain the reduced protein-clearance quality, but also guarantee a satisfactory therapeutic effect of the MMP or TIMP when delivered to the site of injury. Similarly, such approaches must prove beneficial when used in combination with chemical drugs or antibiotics to preserve the tissue integrity or improve it. These methods, under rigorous scrutiny, can generate a favorable regenerative response without any adverse consequences.
Using such techniques, investigators must focus on transitioning from scar healing to scarless healing. As previously mentioned, the most likely factor in this barrier to complete regeneration is the MMP/TIMP ratio. Often, the limitation of perfect regeneration is the over-compensation of a particular MMP or TIMP, highly expressed during a response to injury or disease. Understanding how to manage the relationship between MMPs and TIMPs may be the way forward for producing antiscarring therapies. Ultimately, our goal as scientists is to mimic the natural and scarless healing mechanisms of embryonic and fetal tissues with untimely afflicted adult tissue.
Executive summary
Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) play active roles in tissue remodeling by regulating cell proliferation, migration, differentiation, apoptosis and angiogenesis.
Using growth factors, proteins or nontoxic chemical drugs can prove beneficial in tissue remodeling by upregulating MMP expression.
Similarly, using components such as siRNA, drugs or antibiotics may prevent the damaging effects of MMP-induced injury.
MMPs are useful in degrading fibrotic scar tissue; however, they are limited by their ability to be retained in vivo.
Understanding the explicit relationship between MMPs and TIMPs during tissue regeneration may be the key to unlocking the mystery behind scarless healing.
Glossary
- Matrix metalloproteinases
Group of protein-degrading proteinases that aid in tissue remodeling
- Angiogenesis
Physiological process involving the growth of new blood vessels during development, wound healing and the repair of diseased tissue
- TGF-β
Plays crucial roles in tissue regeneration, cell differentiation, embryonic development, fibrosis formation and regulation of the immune system
- Blastema
A mass of undifferentiated cells capable of regenerating organs or limbs in newts
- Fibrosis
Development of excess fibrous connective tissue in an organ or tissue resulting in scar tissue and loss of function
- Muscular dystrophy
X-linked genetic disease where muscles lack the dystrophin gene, causing an increased rate of muscle degeneration
- Dorsal root ganglia
Nodule on a dorsal root that contains cell bodies of neurons in afferent spinal nerves, sometimes referred to as spinal ganglion
- Experimental autoimmune encephalitis
Animal model of autoimmune diseases such as multiple sclerosis
- Focal segmental glomerulosclerosis
Cause of nephrotic syndrome in children and adolescents, which can lead to kidney failure. It can be characterized by edema, hypoalbuminemia, hyperlipidemia and hypertension
- Tubulointerstitial fibrosis
Disease that can lead to end-stage renal failure. It is characterized by infiltration of inflammatory cells, proliferation of mesenchymal cells and excessive accumulation of extracellular matrix components in the renal interstitium
- Pyelonephritis
Ascending urinary tract infection that has reached the pyelum (pelvis) of the kidney
Footnotes
Financial & competing interests disclosure
The authors gratefully acknowledge financial support from the Department of Defense and NIH grants to Yong Li. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Huxley-Jones J, Clarke TK, Beck C, Toubaris G, Robertson DL, Boot-Handford RP. The evolution of the vertebrate metzincins; insights from Ciona intestinalis and Danio rerio. BMC Evol. Biol. 2007;7:63. doi: 10.1186/1471-2148-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomis-Ruth FX. Structural aspects of the metzincin clan of metalloendopeptidases. Mol. Biotechnol. 2003;24:157–202. doi: 10.1385/MB:24:2:157. [DOI] [PubMed] [Google Scholar]
- 3.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parsons SL, Watson SA, Brown PD, Collins HM, Steele RJ. Matrix metalloproteinases. Br. J. Surg. 1997;84:160–166. [PubMed] [Google Scholar]
- 5.Somerville RP, Oblander SA, Apte SS. Matrix metalloproteinases: old dogs with new tricks. Genome Biol. 2003;4:216. doi: 10.1186/gb-2003-4-6-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 7.Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caron NJ, Asselin I, Morel G, Tremblay JP. Increased myogenic potential and fusion of matrilysin-expressing myoblasts transplanted in mice. Cell Transplant. 1999;8:465–476. doi: 10.1177/096368979900800502. [DOI] [PubMed] [Google Scholar]
- 9.Yu F, Kamada H, Niizuma K, Endo H, Chan PH. Induction of MMP-9 expression and endothelial injury by oxidative stress after spinal cord injury. J. Neurotrauma. 2008;25:184–195. doi: 10.1089/neu.2007.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akhavani MA, Madden L, Buysschaert I, Sivakumar B, Kang N, Paleolog EM. Hypoxia upregulates angiogenesis and synovial cell migration in rheumatoid arthritis. Arthritis Res. Ther. 2009;11:R64. doi: 10.1186/ar2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul S, Sharma AV, Mahapatra PD, et al. Role of melatonin in regulating matrix metalloproteinase-9 via tissue inhibitors of metalloproteinase-1 during protection against endometriosis. J. Pineal Res. 2008;44:439–449. doi: 10.1111/j.1600-079X.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 12.Vu TD, Yun F, Placido J, Reznik SE. Placental matrix metalloproteinase-1 expression is increased in labor. Reprod. Sci. 2008;15:420–424. doi: 10.1177/1933719108314625. [DOI] [PubMed] [Google Scholar]
- 13.Choi SJ, Jung KL, Oh SY, Kim JH, Roh CR. Cervicovaginal matrix metalloproteinase-9 and cervical ripening in human term parturition. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;142:43–47. doi: 10.1016/j.ejogrb.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 14.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr. Opin. Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 15.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 16.Yang P, Liu Z, Wang H, et al. Enhanced activity of very low density lipoprotein receptor II promotes SGC7901 cell proliferation and migration. Life Sci. 2009;84:402–408. doi: 10.1016/j.lfs.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 17.McCawley LJ, Wright J, LaFleur BJ, Crawford HC, Matrisian LM. Keratinocyte expression of MMP3 enhances differentiation and prevents tumor establishment. Am. J. Pathol. 2008;173:1528–1539. doi: 10.2353/ajpath.2008.080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang W, Pan H, Murray K, Jefferson BS, Li Y. Matrix metalloproteinase-1 promotes muscle cell migration and differentiation. Am. J. Pathol. 2009;174:541–549. doi: 10.2353/ajpath.2009.080509. •• Describes for the first time how MMP1 influences muscle cell migration and differentiation to improve muscle regeneration.
- 19.Pereira AM, Strasberg-Rieber M, Rieber M. Invasion-associated MMP-2 and MMP-9 are up-regulated intracellularly in concert with apoptosis linked to melanoma cell detachment. Clin. Exp. Metastasis. 2005;22:285–295. doi: 10.1007/s10585-005-8672-8. [DOI] [PubMed] [Google Scholar]
- 20.Menon B, Singh M, Singh K. Matrix metalloproteinases mediate β-adrenergic receptor-stimulated apoptosis in adult rat ventricular myocytes. Am. J. Physiol. Cell Physiol. 2005;289:C168–C176. doi: 10.1152/ajpcell.00606.2004. [DOI] [PubMed] [Google Scholar]
- 21.Zeng ZZ, Yao H, Staszewski ED, et al. α(5) (1) integrin ligand PHSRN induces invasion and α5 mRNA in endothelial cells to stimulate angiogenesis. Transl. Oncol. 2009;2:8–20. doi: 10.1593/tlo.08187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shan B, Morris CA, Zhuo Y, Shelby BD, Levy DR, Lasky JA. Activation of proMMP-2 and Src by HHV8 vGPCR in human pulmonary arterial endothelial cells. J. Mol. Cell Cardiol. 2007;42:517–525. doi: 10.1016/j.yjmcc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Melendez-Zajgla J, Del Pozo L, Ceballos G, Maldonado V. Tissue inhibitor of metalloproteinases-4. The road less traveled. Mol. Cancer. 2008;7:85. doi: 10.1186/1476-4598-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J. Cell Sci. 2002;115:3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 25.Jacobsen J, Visse R, Sorensen HP, et al. Catalytic properties of ADAM12 and its domain deletion mutants. Biochemistry. 2008;47:537–547. doi: 10.1021/bi701629c. [DOI] [PubMed] [Google Scholar]
- 26.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci. Signal. 2008;1:6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu JY, Bourguignon LY, Adams CM, et al. Matrix metalloproteinase-9 facilitates glial scar formation in the injured spinal cord. J. Neurosci. 2008;28:13467–13477. doi: 10.1523/JNEUROSCI.2287-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato T, Kure T, Chang JH, et al. Diminished corneal angiogenesis in gelatinase A-deficient mice. FEBS Lett. 2001;508:187–190. doi: 10.1016/s0014-5793(01)02897-6. [DOI] [PubMed] [Google Scholar]
- 29.VanSaun M, Herrera AA, Werle MJ. Structural alterations at the neuromuscular junctions of matrix metalloproteinase 3 null mutant mice. J. Neurocytol. 2003;32:1129–1142. doi: 10.1023/B:NEUR.0000021907.68461.9c. [DOI] [PubMed] [Google Scholar]
- 30.Wells JE, Rice TK, Nuttall RK, et al. An adverse role for matrix metalloproteinase 12 after spinal cord injury in mice. J. Neurosci. 2003;23:10107–10115. doi: 10.1523/JNEUROSCI.23-31-10107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Z, Apte SS, Soininen R, et al. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl Acad. Sci. USA. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creemers EE, Davis JN, Parkhurst AM, et al. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H364–H371. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- 33.Fedak PW, Smookler DS, Kassiri Z, et al. TIMP-3 deficiency leads to dilated cardiomyopathy. Circulation. 2004;110:2401–2409. doi: 10.1161/01.CIR.0000134959.83967.2D. [DOI] [PubMed] [Google Scholar]
- 34.Brockes JP. Amphibian limb regeneration: rebuilding a complex structure. Science. 1997;276:81–87. doi: 10.1126/science.276.5309.81. [DOI] [PubMed] [Google Scholar]
- 35. Yokoyama H. Initiation of limb regeneration: the critical steps for regenerative capacity. Dev. Growth Differ. 2008;50:13–22. doi: 10.1111/j.1440-169X.2007.00973.x. • Provides details comparing limb regeneration and wound healing.
- 36.Han M, Yang X, Lee J, Allan CH, Muneoka K. Development and regeneration of the neonatal digit tip in mice. Dev. Biol. 2008;315:125–135. doi: 10.1016/j.ydbio.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vidal P, Dickson MG. Regeneration of the distal phalanx. A case report. J. Hand Surg. [Br.] 1993;18:230–233. doi: 10.1016/0266-7681(93)90116-w. [DOI] [PubMed] [Google Scholar]
- 38.Alagoz MS, Uysal CA, Kerem M, Sensoz O. Reverse homodigital artery flap coverage for bone and nailbed grafts in fingertip amputations. Ann. Plast. Surg. 2006;56:279–283. doi: 10.1097/01.sap.0000200086.24613.da. [DOI] [PubMed] [Google Scholar]
- 39.Shen W, Li Y, Zhu J, Schwendener R, Huard J. Interaction between macrophages, TGF-β1, and the COX-2 pathway during the inflammatory phase of skeletal muscle healing after injury. J. Cell Physiol. 2008;214:405–412. doi: 10.1002/jcp.21212. [DOI] [PubMed] [Google Scholar]
- 40.Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int. J. Biochem. Cell Biol. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell LJ, Crews CM. Wound epidermis formation and function in urodele amphibian limb regeneration. Cell Mol. Life Sci. 2008;65:73–79. doi: 10.1007/s00018-007-7433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li Y, Foster W, Deasy BM, et al. Transforming growth factor-β1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am. J. Pathol. 2004;164:1007–1019. doi: 10.1016/s0002-9440(10)63188-4. • Provides evidence to demonstrating that TGF-β is a key protein in promoting fibrosis in skeletal muscle.
- 43.Miyazaki K, Uchiyama K, Imokawa Y, Yoshizato K. Cloning and characterization of cDNAs for matrix metalloproteinases of regenerating newt limbs. Proc. Natl Acad. Sci. USA. 1996;93:6819–6824. doi: 10.1073/pnas.93.13.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satoh A, Bryant SV, Gardiner DM. Regulation of dermal fibroblast dedifferentiation and redifferentiation during wound healing and limb regeneration in the Axolotl. Dev. Growth Differ. 2008;50:743–754. doi: 10.1111/j.1440-169X.2008.01072.x. [DOI] [PubMed] [Google Scholar]
- 45. Vinarsky V, Atkinson DL, Stevenson TJ, Keating MT, Odelberg SJ. Normal newt limb regeneration requires matrix metalloproteinase function. Dev. Biol. 2005;279:86–98. doi: 10.1016/j.ydbio.2004.12.003. •• Illustrates the importance of MMPs in newt limb regeneration and when they are inhibited, regeneration is severely impaired.
- 46.Yang EV, Gardiner DM, Carlson MR, Nugas CA, Bryant SV. Expression of Mmp-9 and related matrix metalloproteinase genes during axolotl limb regeneration. Dev. Dyn. 1999;216:2–9. doi: 10.1002/(SICI)1097-0177(199909)216:1<2::AID-DVDY2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 47.Crawford K, Stocum DL. Retinoic acid coordinately proximalizes regenerate pattern and blastema differential affinity in axolotl limbs. Development. 1988;102:687–698. doi: 10.1242/dev.102.4.687. [DOI] [PubMed] [Google Scholar]
- 48.McClure KD, Sustar A, Schubiger G. Three genes control the timing, the site and the size of blastema formation in Drosophila. Dev. Biol. 2008;319:68–77. doi: 10.1016/j.ydbio.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gourevitch D, Clark L, Chen P, Seitz A, Samulewicz SJ, Heber-Katz E. Matrix metalloproteinase activity correlates with blastema formation in the regenerating MRL mouse ear hole model. Dev. Dyn. 2003;226:377–387. doi: 10.1002/dvdy.10243. [DOI] [PubMed] [Google Scholar]
- 50.Bai S, Thummel R, Godwin AR, et al. Matrix metalloproteinase expression and function during fin regeneration in zebrafish: analysis of MT1-MMP, MMP2 and TIMP2. Matrix Biol. 2005;24:247–260. doi: 10.1016/j.matbio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Fitzgerald J, Rich C, Burkhardt D, Allen J, Herzka AS, Little CB. Evidence for articular cartilage regeneration in MRL/MpJ mice. Osteoarthritis Cartilage. 2008;16:1319–1326. doi: 10.1016/j.joca.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 52.Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J. Bone Joint Surg. Am. 2002;84:822–832. [PubMed] [Google Scholar]
- 53.Nozaki M, Li Y, Zhu J, et al. Improved muscle healing after contusion injury by the inhibitory effect of suramin on myostatin, a negative regulator of muscle growth. Am. J. Sports Med. 2008;36:2354–2362. doi: 10.1177/0363546508322886. [DOI] [PubMed] [Google Scholar]
- 54.Chan YS, Li Y, Foster W, Fu FH, Huard J. The use of suramin, an antifibrotic agent, to improve muscle recovery after strain injury. Am. J. Sports Med. 2005;33:43–51. doi: 10.1177/0363546504265190. [DOI] [PubMed] [Google Scholar]
- 55.Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am. J. Physiol. Cell Physiol. 2007;293:C661–C669. doi: 10.1152/ajpcell.00061.2007. [DOI] [PubMed] [Google Scholar]
- 56.Sato K, Li Y, Foster W, et al. Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle Nerve. 2003;28:365–372. doi: 10.1002/mus.10436. [DOI] [PubMed] [Google Scholar]
- 57.Foster W, Li Y, Usas A, Somogyi G, Huard J. γ-interferon as an antifibrosis agent in skeletal muscle. J. Orthop. Res. 2003;21:798–804. doi: 10.1016/S0736-0266(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Li J, Zhu J, et al. Decorin gene transfer promotes muscle cell differentiation and muscle regeneration. Mol. Ther. 2007;15:1616–1622. doi: 10.1038/sj.mt.6300250. [DOI] [PubMed] [Google Scholar]
- 59.Bedair HS, Karthikeyan T, Quintero A, Li Y, Huard J. Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Am. J. Sports Med. 2008;36:1548–1554. doi: 10.1177/0363546508315470. [DOI] [PubMed] [Google Scholar]
- 60. Bedair H, Liu TT, Kaar JL, et al. Matrix metalloproteinase-1 therapy improves muscle healing. J. Appl. Physiol. 2007;102:2338–2345. doi: 10.1152/japplphysiol.00670.2006. •• MMP1 was demonstrated to improve muscle healing degrading fibrotic scar tissue as shown in this article.
- 61.Kaar JL, Li Y, Blair HC, et al. Matrix metalloproteinase-1 treatment of muscle fibrosis. Acta Biomater. 2008;4:1411–1420. doi: 10.1016/j.actbio.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 62.Nishimura T, Nakamura K, Kishioka Y, Kato-Mori Y, Wakamatsu J, Hattori A. Inhibition of matrix metalloproteinases suppresses the migration of skeletal muscle cells. J. Muscle Res. Cell Motil. 2008;29:37–44. doi: 10.1007/s10974-008-9140-2. [DOI] [PubMed] [Google Scholar]
- 63.Caliceti P, Veronese FM. Pharmacokinetic and biodistribution properties of poly(ethylene glycol)-protein conjugates. Adv. Drug Deliv. Rev. 2003;55:1261–1277. doi: 10.1016/s0169-409x(03)00108-x. [DOI] [PubMed] [Google Scholar]
- 64.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 65.Lutolf MP, Lauer-Fields JL, Schmoekel HG, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc. Natl Acad. Sci. USA. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lei Y, Segura T. DNA delivery from matrix metalloproteinase degradable poly(ethylene glycol) hydrogels to mouse cloned mesenchymal stem cells. Biomaterials. 2009;30:254–265. doi: 10.1016/j.biomaterials.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 68.Hnia K, Gayraud J, Hugon G, et al. l-arginine decreases inflammation and modulates the nuclear factor-κB/matrix metalloproteinase cascade in mdx muscle fibers. Am. J. Pathol. 2008;172:1509–1519. doi: 10.2353/ajpath.2008.071009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bani C, Lagrota-Candido J, Pinheiro DF, et al. Pattern of metalloprotease activity and myofiber regeneration in skeletal muscles of mdx mice. Muscle Nerve. 2008;37:583–592. doi: 10.1002/mus.20970. [DOI] [PubMed] [Google Scholar]
- 70. Zimowska M, Brzoska E, Swierczynska M, Streminska W, Moraczewski J. Distinct patterns of MMP-9 and MMP-2 activity in slow and fast twitch skeletal muscle regeneration in vivo. Int. J. Dev. Biol. 2008;52:307–314. doi: 10.1387/ijdb.072331mz. • Different expression levels of MMP2 and MMP9 were demonstrated between slow twitch and fast twitch muscle cells emphasizing different regenerative capacities.
- 71.Fukushima K, Nakamura A, Ueda H, et al. Activation and localization of matrix metalloproteinase-2 and -9 in the skeletal muscle of the muscular dystrophy dog (CXMDJ) BMC Musculoskelet. Disord. 2007;8:54. doi: 10.1186/1471-2474-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ohtake Y, Tojo H, Seiki M. Multifunctional roles of MT1-MMP in myofiber formation and morphostatic maintenance of skeletal muscle. J. Cell Sci. 2006;119:3822–3832. doi: 10.1242/jcs.03158. •• MMP14 was demonstrated to serve as a checkpoint for the three stages of morphological differentiation (proliferation, elongation and fusion) as seen in this journal article.
- 73.Furochi H, Tamura S, Takeshima K, et al. Overexpression of osteoactivin protects skeletal muscle from severe degeneration caused by long-term denervation in mice. J. Med. Invest. 2007;54:248–254. doi: 10.2152/jmi.54.248. [DOI] [PubMed] [Google Scholar]
- 74.Pouw MH, Hosman AJ, van Middendorp JJ, Verbeek MM, Vos PE, van de Meent H. Biomarkers in spinal cord injury. Spinal Cord. 2009;47(7):519–525. doi: 10.1038/sc.2008.176. [DOI] [PubMed] [Google Scholar]
- 75.Ruff RL, McKerracher L, Selzer ME. Repair and neurorehabilitation strategies for spinal cord injury. Ann. NY Acad. Sci. 2008;1142:1–20. doi: 10.1196/annals.1444.004. [DOI] [PubMed] [Google Scholar]
- 76. Liu LY, Zheng H, Xiao HL, et al. Comparison of blood–nerve barrier disruption and matrix metalloprotease-9 expression in injured central and peripheral nerves in mice. Neurosci. Lett. 2008;434:155–159. doi: 10.1016/j.neulet.2007.12.052. • Examines how MMP9 expression varied between injuries to the central and peripheral nervous systems.
- 77.Tabesh H, Amoabediny G, Nik NS, et al. The role of biodegradable engineered scaffolds seeded with Schwann cells for spinal cord regeneration. Neurochem. Int. 2009;54:73–83. doi: 10.1016/j.neuint.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 78.Fleming JC, Norenberg MD, Ramsay DA, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- 79.Hsu JY, McKeon R, Goussev S, et al. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J. Neurosci. 2006;26:9841–9850. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lehmann HC, Kohne A, Bernal F, et al. Matrix metalloproteinase-2 is involved in myelination of dorsal root ganglia neurons. Glia. 2009;57:479–489. doi: 10.1002/glia.20774. [DOI] [PubMed] [Google Scholar]
- 81. Werner SR, Dotzlaf JE, Smith RC. MMP-28 as a regulator of myelination. BMC Neurosci. 2008:9–83. doi: 10.1186/1471-2202-9-83. •• Provides data examining one of the more poorly understood MMPs, MMP28 and its role in myelination.
- 82.Chattopadhya S, Shubayev VI. MMP-9 controls Schwann cell proliferation and phenotypic remodeling via IGF-1 and ErbB receptor-mediated activation of MEK/ERK pathway. Glia. 2009;57(12):1316–1325. doi: 10.1002/glia.20851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang LJ, Schnaar RL. Axon regeneration inhibitors. Neurol. Res. 2008;30:1047–1052. doi: 10.1179/174313208X362523. [DOI] [PubMed] [Google Scholar]
- 84.Werner SR, Mescher AL, Neff AW, et al. Neural MMP-28 expression precedes myelination during development and peripheral nerve repair. Dev. Dyn. 2007;236:2852–2864. doi: 10.1002/dvdy.21301. [DOI] [PubMed] [Google Scholar]
- 85.Toft-Hansen H, Nuttall RK, Edwards DR, Owens T. Key metalloproteinases are expressed by specific cell types in experimental autoimmune encephalomyelitis. J. Immunol. 2004;173:5209–5218. doi: 10.4049/jimmunol.173.8.5209. [DOI] [PubMed] [Google Scholar]
- 86.Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- 87.Toft-Hansen H, Babcock AA, Millward JM, Owens T. Downregulation of membrane type-matrix metalloproteinases in the inflamed or injured central nervous system. J. Neuroinflammation. 2007;4:24. doi: 10.1186/1742-2094-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin P. Wound healing – aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 89.Saarialho-Kere UK. Patterns of matrix metalloproteinase and TIMP expression in chronic ulcers. Arch. Dermatol. Res. 1998;290 Suppl:S47–S54. doi: 10.1007/pl00007453. [DOI] [PubMed] [Google Scholar]
- 90.Salo T, Makela M, Kylmaniemi M, Autio-Harmainen H, Larjava H. Expression of matrix metalloproteinase-2 and -9 during early human wound healing. Lab. Invest. 1994;70:176–182. [PubMed] [Google Scholar]
- 91.Saarialho-Kere UK, Chang ES, Welgus HG, Parks WC. Distinct localization of collagenase and tissue inhibitor of metalloproteinases expression in wound healing associated with ulcerative pyogenic granuloma. J. Clin. Invest. 1992;90:1952–1957. doi: 10.1172/JCI116073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Petersen MJ, Woodley DT, Stricklin GP, O’Keefe EJ. Enhanced synthesis of collagenase by human keratinocytes cultured on type I or type IV collagen. J. Invest. Dermatol. 1990;94:341–346. doi: 10.1111/1523-1747.ep12874471. [DOI] [PubMed] [Google Scholar]
- 93.Saarialho-Kere UK, Pentland AP, Birkedal-Hansen H, Parks WC, Welgus HG. Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J. Clin. Invest. 1994;94:79–88. doi: 10.1172/JCI117351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vaalamo M, Leivo T, Saarialho-Kere U. Differential expression of tissue inhibitors of metalloproteinases (TIMP-1, -2, -3, and -4) in normal and aberrant wound healing. Hum. Pathol. 1999;30:795–802. doi: 10.1016/s0046-8177(99)90140-5. [DOI] [PubMed] [Google Scholar]
- 95.Toriseva M, Kahari VM. Proteinases in cutaneous wound healing. Cell Mol. Life Sci. 2009;66:203–224. doi: 10.1007/s00018-008-8388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996;4:321–325. doi: 10.1046/j.1524-475X.1996.40307.x. [DOI] [PubMed] [Google Scholar]
- 97.Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J. Invest. Dermatol. 1993;101:64–68. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- 98.Vaalamo M, Kariniemi AL, Shapiro SD, Saarialho-Kere U. Enhanced expression of human metalloelastase (MMP-12) in cutaneous granulomas and macrophage migration. J. Invest. Dermatol. 1999;112:499–505. doi: 10.1046/j.1523-1747.1999.00547.x. [DOI] [PubMed] [Google Scholar]
- 99.Wilgus TA. Regenerative healing in fetal skin: a review of the literature. Ostomy. Wound Manage. 2007;53:16–33. [PubMed] [Google Scholar]
- 100.Dang CM, Beanes SR, Lee H, Zhang X, Soo C, Ting K. Scarless fetal wounds are associated with an increased matrix metalloproteinase-to-tissue-derived inhibitor of metalloproteinase ratio. Plast. Reconstr. Surg. 2003;111:2273–2285. doi: 10.1097/01.PRS.0000060102.57809.DA. [DOI] [PubMed] [Google Scholar]
- 101.Satoh A, Graham GM, Bryant SV, Gardiner DM. Neurotrophic regulation of epidermal dedifferentiation during wound healing and limb regeneration in the axolotl (Ambystoma mexicanum) Dev. Biol. 2008;319:321–335. doi: 10.1016/j.ydbio.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 102.Manuel JA, Gawronska-Kozak B. Matrix metalloproteinase 9 (MMP-9) is upregulated during scarless wound healing in athymic nude mice. Matrix Biol. 2006;25:505–514. doi: 10.1016/j.matbio.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 103.Chen W, Fu X, Ge S, Sun T, Sheng Z. Differential expression of matrix metalloproteinases and tissue-derived inhibitors of metalloproteinase in fetal and adult skins. Int. J. Biochem. Cell Biol. 2007;39:997–1005. doi: 10.1016/j.biocel.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 104.Xie J, Bian H, Qi S, et al. Effects of basic fibroblast growth factor on the expression of extracellular matrix and matrix metalloproteinase-1 in wound healing. Clin. Exp. Dermatol. 2008;33:176–182. doi: 10.1111/j.1365-2230.2007.02573.x. [DOI] [PubMed] [Google Scholar]
- 105.Kuramochi D, Unoki H, Bujo H, et al. Matrix metalloproteinase 2 improves the transplanted adipocyte survival in mice. Eur. J. Clin. Invest. 2008;38:752–759. doi: 10.1111/j.1365-2362.2008.02023.x. [DOI] [PubMed] [Google Scholar]
- 106.Dasu MR, Hawkins HK, Barrow RE, Xue H, Herndon DN. Gene expression profiles from hypertrophic scar fibroblasts before and after IL-6 stimulation. J. Pathol. 2004;202:476–485. doi: 10.1002/path.1539. [DOI] [PubMed] [Google Scholar]
- 107.Philips N, Keller T, Gonzalez S. TGF β-like regulation of matrix metalloproteinases by anti-transforming growth factor-β, and anti-transforming growth factor-β1 antibodies in dermal fibroblasts: implications for wound healing. Wound Repair Regen. 2004;12:53–59. doi: 10.1111/j.1067-1927.2004.012111.x. [DOI] [PubMed] [Google Scholar]
- 108.Yao XM, Zhao J, Li Y. Effects of bicyclol on liver regeneration after partial hepatectomy in rats. Dig. Dis. Sci. 2009;54:774–781. doi: 10.1007/s10620-009-0715-6. [DOI] [PubMed] [Google Scholar]
- 109.Chen MF, Hwang TL, Hung CF. Human liver regeneration after major hepatectomy. A study of liver volume by computed tomography. Ann. Surg. 1991;213:227–229. doi: 10.1097/00000658-199103000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Iimuro Y, Nishio T, Morimoto T, et al. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology. 2003;124:445–458. doi: 10.1053/gast.2003.50063. [DOI] [PubMed] [Google Scholar]
- 111.Svegliati-Baroni G, D’Ambrosio L, Ferretti G, et al. Fibrogenic effect of oxidative stress on rat hepatic stellate cells. Hepatology. 1998;27:720–726. doi: 10.1002/hep.510270313. [DOI] [PubMed] [Google Scholar]
- 112.Wallace K, Burt AD, Wright MC. Liver fibrosis. Biochem. J. 2008;411:1–18. doi: 10.1042/BJ20071570. [DOI] [PubMed] [Google Scholar]
- 113. Han YP, Yan C, Zhou L, Qin L, Tsukamoto H. A matrix metalloproteinase-9 activation cascade by hepatic stellate cells in trans-differentiation in the three-dimensional extracellular matrix. J. Biol. Chem. 2007;282:12928–12939. doi: 10.1074/jbc.M700554200. •• Using hepatic stellate cells, the authors of this paper reveal an MMP activation cascade where upon activation MMP14 activates MMP13 which then activates MMP9, thus promoting a positive feedback look to stimulate MMP13.
- 114.Kirimlioglu H, Kirimlioglu V, Yilmaz S. Expression of matrix metalloproteinases 2 and 9 in donor liver, cirrhotic liver, and acute rejection after human liver transplantation. Transplant Proc. 2008;40:3574–3577. doi: 10.1016/j.transproceed.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 115.Pham Van T, Couchie D, Martin-Garcia N, Laperche Y, Zafrani ES, Mavier P. Expression of matrix metalloproteinase-2 and -9 and of tissue inhibitor of matrix metalloproteinase-1 in liver regeneration from oval cells in rat. Matrix Biol. 2008;27:674–681. doi: 10.1016/j.matbio.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 116.Zhou X, Hovell CJ, Pawley S, et al. Expression of matrix metalloproteinase-2 and -14 persists during early resolution of experimental liver fibrosis and might contribute to fibrolysis. Liver Int. 2004;24:492–501. doi: 10.1111/j.1478-3231.2004.0946.x. [DOI] [PubMed] [Google Scholar]
- 117.Olle EW, Ren X, McClintock SD, et al. Matrix metalloproteinase-9 is an important factor in hepatic regeneration after partial hepatectomy in mice. Hepatology. 2006;44:540–549. doi: 10.1002/hep.21314. [DOI] [PubMed] [Google Scholar]
- 118.Alwayn IP, Verbesey JE, Kim S, et al. A critical role for matrix metalloproteinases in liver regeneration. J. Surg. Res. 2008;145:192–198. doi: 10.1016/j.jss.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Preaux AM, Mallat A, Nhieu JT, D’Ortho MP, Hembry RM, Mavier P. Matrix metalloproteinase-2 activation in human hepatic fibrosis regulation by cell-matrix interactions. Hepatology. 1999;30:944–950. doi: 10.1002/hep.510300432. [DOI] [PubMed] [Google Scholar]
- 120.Roderfeld M, Weiskirchen R, Wagner S, et al. Inhibition of hepatic fibrogenesis by matrix metalloproteinase-9 mutants in mice. FASEB J. 2006;20:444–454. doi: 10.1096/fj.05-4828com. [DOI] [PubMed] [Google Scholar]
- 121.Hu YB, Li DG, Lu HM. Modified synthetic siRNA targeting tissue inhibitor of metalloproteinase-2 inhibits hepatic fibrogenesis in rats. J. Gene Med. 2007;9:217–229. doi: 10.1002/jgm.1009. [DOI] [PubMed] [Google Scholar]
- 122.Mohammed FF, Pennington CJ, Kassiri Z, et al. Metalloproteinase inhibitor TIMP-1 affects hepatocyte cell cycle via HGF activation in murine liver regeneration. Hepatology. 2005;41:857–867. doi: 10.1002/hep.20618. [DOI] [PubMed] [Google Scholar]
- 123.Mohammed FF, Smookler DS, Taylor SE, et al. Abnormal TNF activity in Timp3−/− mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat. Genet. 2004;36:969–977. doi: 10.1038/ng1413. [DOI] [PubMed] [Google Scholar]
- 124.Krishnamurthy P, Peterson JT, Subramanian V, Singh M, Singh K. Inhibition of matrix metalloproteinases improves left ventricular function in mice lacking osteopontin after myocardial infarction. Mol. Cell Biochem. 2009;322:53–62. doi: 10.1007/s11010-008-9939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tessone A, Feinberg MS, Barbash IM, et al. Effect of matrix metalloproteinase inhibition by doxycycline on myocardial healing and remodeling after myocardial infarction. Cardiovasc. Drugs Ther. 2005;19:383–390. doi: 10.1007/s10557-005-5201-6. [DOI] [PubMed] [Google Scholar]
- 126.Cleutjens JP, Creemers EE. Integration of concepts: cardiac extracellular matrix remodeling after myocardial infarction. J. Card. Fail. 2002;8:S344–S338. doi: 10.1054/jcaf.2002.129261. [DOI] [PubMed] [Google Scholar]
- 127.Gaertner R, Jacob MP, Prunier F, Angles–Cano E, Mercadier JJ, Michel JB. The plasminogen–MMP system is more activated in the scar than in viable myocardium 3 months post-MI in the rat. J. Mol. Cell Cardiol. 2005;38:193–204. doi: 10.1016/j.yjmcc.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 128.Parfyonova YV, Plekhanova OS, Tkachuk VA. Plasminogen activators in vascular remodeling and angiogenesis. Biochemistry (Mosc.) 2002;67:119–134. doi: 10.1023/a:1013964517211. [DOI] [PubMed] [Google Scholar]
- 129.Lu L, Zhang JQ, Ramires FJ, Sun Y. Molecular and cellular events at the site of myocardial infarction: from the perspective of rebuilding myocardial tissue. Biochem. Biophys. Res. Commun. 2004;320:907–913. doi: 10.1016/j.bbrc.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 130.Mukherjee R, Bruce JA, McClister DM, Jr, Allen CM, Sweterlitsch SE, Saul JP. Time-dependent changes in myocardial structure following discrete injury in mice deficient of matrix metalloproteinase-3. J. Mol. Cell Cardiol. 2005;39:259–268. doi: 10.1016/j.yjmcc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 131.van Amerongen MJ, Harmsen MC, Petersen AH, Kors G, van Luyn MJ. The enzymatic degradation of scaffolds and their replacement by vascularized extracellular matrix in the murine myocardium. Biomaterials. 2006;27:2247–2257. doi: 10.1016/j.biomaterials.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 132.Moe GW, Laurent G, Doumanovskaia L, Konig A, Hu X, Dorian P. Matrix metalloproteinase inhibition attenuates atrial remodeling and vulnerability to atrial fibrillation in a canine model of heart failure. J. Card. Fail. 2008;14:768–776. doi: 10.1016/j.cardfail.2008.07.229. [DOI] [PubMed] [Google Scholar]
- 133.Okada M, Kikuzuki R, Harada T, Hori Y, Yamawaki H, Hara Y. Captopril attenuates matrix metalloproteinase-2 and -9 in monocrotaline-induced right ventricular hypertrophy in rats. J. Pharmacol. Sci. 2008;108:487–494. doi: 10.1254/jphs.08174fp. [DOI] [PubMed] [Google Scholar]
- 134.Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, Kishore R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ. Res. 2009;104:E9–E18. doi: 10.1161/CIRCRESAHA.108.188243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ogawa M, Suzuki J, Takayama K, Isobe M. Matrix metalloproteinase suppression induced by clarithromycin in murine cardiac allografts. Transplant Proc. 2009;41:395–397. doi: 10.1016/j.transproceed.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 136.Roten L, Nemoto S, Simsic J, et al. Effects of gene deletion of the tissue inhibitor of the matrix metalloproteinase-type 1 (TIMP-1) on left ventricular geometry and function in mice. J. Mol. Cell Cardiol. 2000;32:109–120. doi: 10.1006/jmcc.1999.1052. [DOI] [PubMed] [Google Scholar]
- 137.Nony PA, Schnellmann RG. Mechanisms of renal cell repair and regeneration after acute renal failure. J. Pharmacol. Exp. Ther. 2003;304:905–912. doi: 10.1124/jpet.102.035022. [DOI] [PubMed] [Google Scholar]
- 138.Thrailkill KM, Clay Bunn R, Fowlkes JL. Matrix metalloproteinases: their potential role in the pathogenesis of diabetic nephropathy. Endocrine. 2009;35:1–10. doi: 10.1007/s12020-008-9114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Munkert A, Helmchen U, Kemper MJ, Bubenheim M, Stahl RA, Harendza S. Characterization of the transcriptional regulation of the human MT1-MMP gene and association of risk reduction for focal-segmental glomerulosclerosis with two functional promoter SNPs. Nephrol. Dial. Transplant. 2009;24:735–742. doi: 10.1093/ndt/gfn576. [DOI] [PubMed] [Google Scholar]
- 140.Liu WH, Tang NN, Zhang QD. Could mycophenolate mofetil combined with benazapril delay tubulointerstitial fibrosis in 5/6 nephrectomized rats? Chin. Med. J. (Engl.) 2009;122:199–204. [PubMed] [Google Scholar]
- 141.Chromek M, Tullus K, Hertting O, et al. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinases-1 in acute pyelonephritis and renal scarring. Pediatr. Res. 2003;53:698–705. doi: 10.1203/01.PDR.0000057575.86337.CB. [DOI] [PubMed] [Google Scholar]
- 142.Yao XM, Ye SD, Chen Y, et al. Rosiglitazone protects diabetic rats against kidney injury through the suppression of renal matrix metalloproteinase-9 expression. Diabetes Obes. Metab. 2009;11:519–522. doi: 10.1111/j.1463-1326.2008.00990.x. [DOI] [PubMed] [Google Scholar]
- 143.Castro MM, Rizzi E, Rodrigues GJ, et al. Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic. Biol. Med. 2009;46:1298–1307. doi: 10.1016/j.freeradbiomed.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 144.Pulukuri SM, Rao JS. Matrix metalloproteinase-1 promotes prostate tumor growth and metastasis. Int. J. Oncol. 2008;32:757–765. [PMC free article] [PubMed] [Google Scholar]
- 145.Overall CM, Kleifeld O. Tumour microenvironment – opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 146.Pardo A, Selman M. Matrix metalloproteases in aberrant fibrotic tissue remodeling. Proc. Am. Thorac. Soc. 2006;3:383–388. doi: 10.1513/pats.200601-012TK. [DOI] [PubMed] [Google Scholar]