Abstract
An integrated and coordinated set of programs has been established to meet ICBG goals in Papua New Guinea (PNG). Here we give an overview of the PNG ICBG and focus on the key elements and major steps taken to establish a program necessary for the pharmacological assessment of botanicals and traditional medicines in PNG and, by extrapolation, in other developing countries.
Keywords: International Cooperative Biodiversity Group, botanical survey, traditional medicine, drug discovery, Papua New Guinea
Introduction
The Fogarty International Center of the NIH awards an unusual set of multi-institutional international program project grants called International Cooperative Biodiversity Groups (John E. Fogarty International Center for Advanced Study in the Health Sciences, 2007). The ICBGs are unusual because they require a mixture of scientific disciplines to synergize in the complicated task of natural products drug discovery. They are the only NIH grants with stated objectives that integrate scientific progress with social progress. The ICBG goals are:
To improve human health through the discovery of new pharmaceutical, agricultural and veterinary agents to treat diseases of importance in both developed and developing countries.
To promote scientific and economic activities in less developed countries by sharing the benefits of the drug discovery process and products.
To conserve biological diversity through understanding and valuation of diverse biological organisms and the development of local capacity to manage these natural resources.
The goals of the PNG ICBG reflect those of the overall program: To improve human health and well being through an integrated set of programs dedicated to the assessment, conservation, and rational utilization of the flora and fauna of Papua New Guinea (PNG). The central hypothesis of this work is that effective new therapeutics can be derived from the natural products of PNG flora and fauna. Such therapeutics, either as novel Western medicines or as traditional phytomedicines, will provide needed revenue to local stake-holders and responsible PNG institutions. This income would provide alternative, non-timber, economic value to PNG forests and reefs and an incentive for the preservation of biological diversity.
PNG is particularly well suited for biodiversity study (Sekhran & Miller, 1996). PNG consists of a central region, which is part of the largest tropical island in the world, and several smaller islands. It lies at the intersection of 5 tectonic plates, which drives a dynamic and varied topology. The main island is divided by the high central Owen Stanley mountain range (Mt. Wilhelm is almost 15,000 feet high) and contains two large river drainages (the Sepik to the north and the Fly to the south). PNG borders Indonesia on the west and faces Australia across the Torres Straight to the south. Its eastern territories stretch to the Solomon Islands and its northernmost islands almost reach the equator. Its coasts meet the Pacific Ocean and the Bismark, Solomon and Coral seas. The smaller islands of PNG are unusual. For instance, Goodenough Island reaches 8,000 ft, while spanning only 20 miles. Therefore, ecosystems in PNG vary dramatically from arid coastal plains to dense mangrove swamps and coastal, mountain and alpine forests. PNG boasts the third largest closed canopy rain forest in the world. It harbors approximately 6% of the world’s species in 1% of the world’s land area. Botanical studies suggest that PNG’s forests match those of Borneo, considered the most diverse in Asia. It is estimated that over 15,000 different vascular plants inhabit PNG, with more varieties of orchids, figs, and sugarcane than anywhere else on earth.
PNG is ethnically diverse as well. Originally settled over 40,000 years ago, it has been subjected to repeated rounds of colonization. It was the home of the Lapita people who went east to settle the Southern Pacific as the Melanesians, and returned later as new settlers. An estimated 800 languages are spoken in PNG reflecting its immense cultural diversity. Tribal socio-economic organization varies from hunter-gatherer to slash-and-burn agriculture, from subsistence farming to modern agriculture and mariculture. Approximately 95% of the land is privately owned and controlled by individual villages.
Several well organized biological survey expeditions have furthered appreciation for PNG’s diversity (summarized in Frodin & Gressitt, 1982; Allison, 1991). Such expeditions, especially those of the early and mid 1900s, resulted in several large voucher specimen collections being exported to London, Leiden and elsewhere. Nevertheless, large areas of PNG remain unexplored in terms of biological diversity. The lack of a national highway system and the still impassable regions in the highlands and some islands contribute to this remarkable state. In 1992 a comprehensive country-study workshop, called the Conservation Needs Assessment (CNA), was conducted in response to the perceived loss of forest due to clearing, mining and logging. Published in 1993 (Beehler, 1993), the study concluded that approximately 20% of PNG’s land area was still undocumented. Importantly, the CNA reported to the government and citizens that significant degradation of ecosystems in PNG had occurred and much of the country was threatened by development and exploitation pressures. A more recent GIS study (Shearman et al., 2008) reveals that this trend is continuing.
PNG’s economy struggled after gaining independence in 1975. Many of its institutions (including the university, herbaria and national health systems) that were being built up went under-funded. This economic situation is now improving, reflecting a renewed push by industrialized nations to partner in the exploitation of PNG’s extensive gold, gas, timber and fishery resources. Of course, this has negative impacts on PNG ecosystems. Still, approximately 40% of the population lives on less than $1 US per day, and the citizens of PNG suffer from the vicious cycle of poor health and poverty (Australian Government, 2007). Approximately one in 12 children dies before five years of age and overall the life expectancy is less than 60 years.
Tuberculosis is considered a leading cause of death in PNG. The World Health Organization (WHO) lists PNG as a Group 1 country (WHO Western Pacific Regional Offices Website, 2002), characterized by the highest level of tuberculosis burden. PNG faces other severe health threats. Malaria is the third leading cause of hospital admissions and deaths in PNG. Parasite rates have been reported to be 23% overall and up to 74% among children in the Eastern Sepik region of PNG (Lin et al., 2007; Hwaihwanje et al., 2007). Only the higher mountainous areas and Port Moresby are relatively malaria-free. In addition, according to AUSAID (2002), PNG is on the verge of an AIDS epidemic that could infect over 25% of the population by 2020. The 2006–2010 Papua New Guinea National Strategic Plan on HIV/AIDS estimates that approximately 1.5% of the women in the larger cities are infected (National Aids Council, 2006). AIDS-related diseases are the major cause of death at the general hospital in the capital Port Moresby. While other tropical diseases abound, Western maladies are becoming more common; especially cancer, cardio-pulmonary disease and diabetes. Access to Western health care for most of the population is limited to remote Aid Posts or regional Government Health Centers manned by a Health Officer, which are often undersupplied. Thus, well over 50% of the population relies on traditional medicines for health care. The long habitation of PNG’s diverse landscape by so many ethnic customs has led to a rich tradition of herbal medicine use.
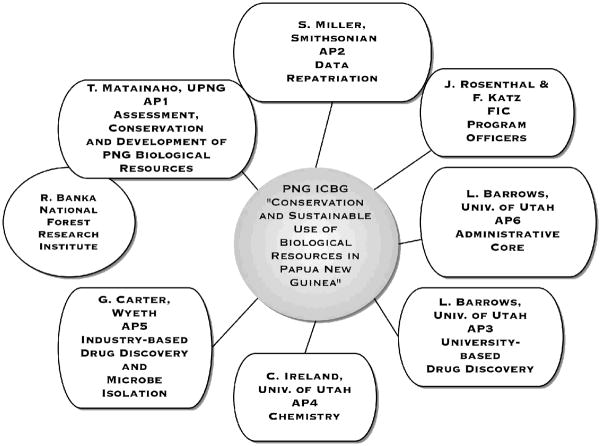
The PNG ICBG partnered faculty from two of PNG’s leading institutions, the University of Papua New Guinea (UPNG) and the Forest Research Institute (FRI, home of the Lae Herbarium) with faculty from the University of Utah. The ICBG has consisted of 6 Associated Programs (AP), AP1 “Assessment, Conservation and Development of PNG Biological Resources UPNG” is headed by Professor Teautolohi K. Matainaho of UPNG; AP2 “Data Repatriation” is headed by Dr. Scott Miller of the Smithsonian Institution; AP3 “Drug Discovery I” is headed by Dr. Louis R. Barrows of the University of Utah; AP4 “Chemistry” is headed by Dr. Chris M. Ireland of the University of Utah; AP5 “Drug Discovery II” is headed by Dr. Guy T. Carter of Wyeth Research; and AP6 which is the administrative core is also headed by Dr. Barrows (Fig 1). This presentation will focus on the efforts this ICBG has made toward linking botanical and ethnomedicinal survey with University based drug discovery activities. Some of the accomplishments of AP 2 and AP 5 are briefly described here, for completeness.
Fig. 1.
PNG ICBG “Conservation and Sustainable Use of Biological Resources in Papua New Guinea” participating institutions and organization.
Biodiversity conservation and data repatriation
AP 2 has been primarily dedicated to repatriation of data residing in a geometrid moth collection in Europe. Two critical issues guided this project. The first is that the collections gathered during the great PNG expeditions of the last century, documenting much of PNG’s biodiversity, do not reside in PNG and are not even accessible to scientists in PNG. Second, the identification and illustration of PNG moths is incomplete. One collection in the Museum of London contains approximately 1100 specimens of lowland PNG moths (Macrolepidoptera & Pyraloidea), only 80% of which were identified to species, and only 30% of which were illustrated in literature via types available only in Europe. The eventual goal of AP2 was to characterize the species not recognizable in the field today, dissect them and make the specimens accessible as digitized microscopic images available by the internet. Moths are important sentinel species and have been used successfully in Malaysia and elsewhere to monitor the environmental impact of changes in land use (Holloway & Stork, 1991; Intachat et al., 1999). Unfortunately, moths were not yet a useful tool in PNG due to incomplete species systematics and unavailable type vouchers. Completion of AP2 will empower identification of species in PNG and will make this important environmental monitoring tool available there.
Under Dr. Miller’s direction, and in collaboration with Dr. Holloway of the Museum of London and Dr. Weiblen, University of Minnesota, Lepidoptera species concepts developed in the field have been verified through consultation of type specimens, literature, and the preparation of 1,800 permanent, museum-quality genitalia dissections. Species concepts were further verified using cytochrome oxidase I (COI) DNA sequences to confirm sexual dimorphism and establish species limits in polyphagous complexes. The library now contains over 4,000 COI sequences from more than 500 New Guinea Lepidoptera species in the Barcodes of Life Database or BoLD (Ratnasingham & Herbert, 2007). Data were released to GenBank in 2008 and sequencing has recently been expanded to include more Lepidoptera, parasitoids, leaf miners, and ants. Miller and Holloway are preparing an atlas of New Guinea Geometridae to illustrate wings and genitalia of types for ~1,200 species linked to the taxonomic literature through the digital Biodiversity Heritage Library (e.g., Fig 2). The atlas extends the taxonomic coverage of Holloway’s “Moths of Borneo” across Wallace’s Line to the Solomon Islands, enabling the use of geometrids for ecological research and environmental monitoring.
Fig. 2.
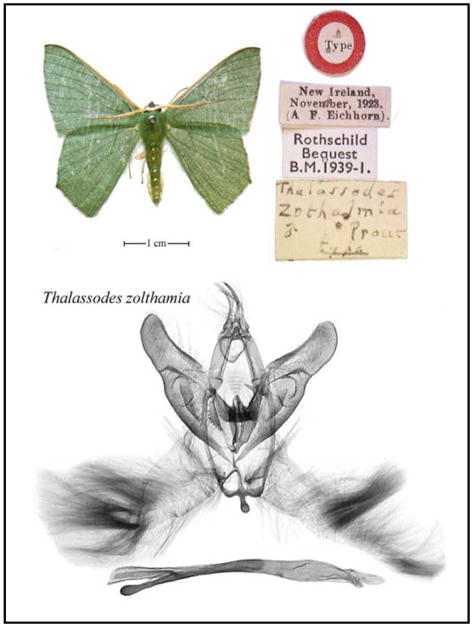
Dissection and digital imaging of a New Guinea Thalassodes moth.
A long term goal of AP 2 was to promote the establishment of a Smithsonian Center for Tropical Forest Sciences/Smithsonian Institute for Global Earth Observatories long term forest dynamics study plot in PNG. Collaborations were developed by Dr. Miller and Dr. George Weiblen, who represent the ICBG, the Binatang Research Centre (BRC), the Smithsonian Institution and the University of Minnesota, with the PNG Forest Research Institute (FRI), the PNG National Agriculture Research Institute (NARI), and the PNG Department of Environment and Conservation (DEC), provided the network required to begin serious discussions concerning study plot establishment. Customary landowners, the Madang Provincial Forestry Department, FRI and BRC proposed the Wanang Conservation Area in Madang Province (Fig 3), which was ultimately selected as the plot location. The choice was based on a thorough investigation by FRI and BRC of eight possible sites in four provinces. A survey of one hectare of lowland forest at Wanang, conducted by BRC scientists, proved pivotal in the selection of the Wanang plot site. This preliminary survey identified 43 families and 151 species of woody plants >10 cm in diameter. According to predictions from large plots in Malaysia, we expect 50 hectares to harbor >70 families and ~500 species of woody plants. Wanang is representative of lowland rainforest north of the central ranges of New Guinea, as shown by extensive CSIRO Land Surveys (Austraila’s Commonwealth Scientific and Industrial Research Organization) and BRC work (Novotny et al., 2007).
Fig. 3.
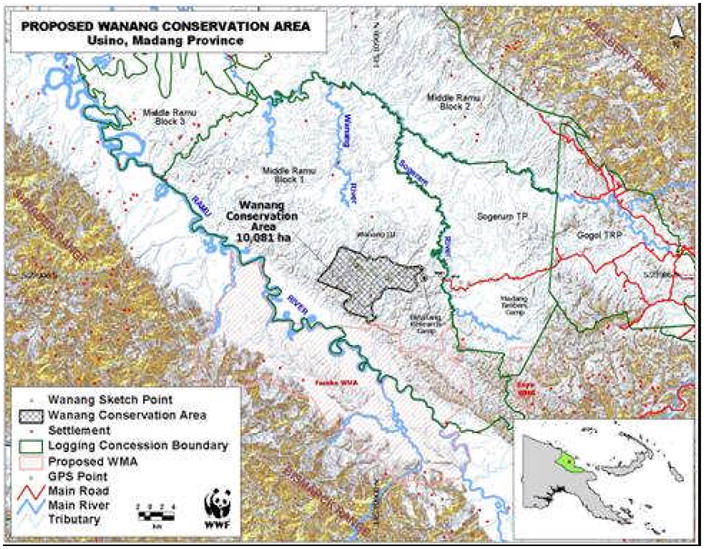
Wanang conservation area.
The decision to establish the 50-ha plot at the Wanang site was based on aspects of forest quality, land tenure, protection status, access, and facilities. The penultimate meeting amongst landowners, government representatives, stakeholders and concerned NOGs was held in conjunction with the ICBG’s 2007 annual meeting in Port Moresby. Recent awards to the University of Minnesota (National Science Foundation) and the CTFS (Swire Group) are currently supporting the first description of the large, permanent forest dynamics plot for PNG. All woody stems within the plot >1 cm diameter are in the process of being tagged, mapped, and identified by scientists from CTFS, FRI and collaborating institutions. Establishment of this plot is a lasting conservation investment, which will pay scientific dividends far into the future.
Industry-based drug discovery
AP5 has drawn on the experience and capacity of Wyeth Research, a leading pharmaceutical firm, and has focused on the isolation of unique actinomycetes and fungi from PNG marine fauna and terrestrial plants. Wyeth Research, known for products like Advil and Premarin, introduced its first antibiotic, chlorotetracycline (Aureomycin), in 1949. Wyeth Research brings a world-leading team of microbiologists to the project, with the capacity for rapid microbe isolation and culture optimization, coupled with genetic and metabolomic analysis. This analysis has yielded a unique microbial collection, which has been cultured and extracted to provide highly purified fractions that have gone directly into high-throughput drug discovery programs. The drug discovery efforts at Wyeth Research have focused on endocrine, cardiovascular, women’s health, cancer and other areas of global health concern. This complements the University of Utah drug discovery program, which focuses on infections diseases. The Wyeth Research program also shares its purified fractions with the University of Utah program for assessment against infectious diseases (described below).
Over 30,000 compounds have been described from microorganisms, with the majority being produced by actinomycetes and fungi. Historically, the majority of microbe-derived bioactive compounds have come from the Actinomycetales, with most of the effort focused on the genus Streptomyces Waksman & Henrici. Although Streptomyces spp. continue to provide new bioactive products, our program has discovered many new genera. Recent progress in molecular microbial ecology has shown that microbial diversity in nature is far greater than that reflected in microbe collections yet attained. Many microbial taxa are yet to be discovered and isolated in pure culture (Amann et al., 1995). Therefore, the expectation of isolating unexploited groups of actinomycetes from unexplored environmental niches is quite promising. In addition, only about 100,000 fungi have been described in the literature; however, Hawksworth (1991) estimates that there are at least 1.5 million fungal species worldwide. Wyeth has developed particular expertise in the isolation of endophytic fungi, which have been less intensely studied than most other groups of fungi (Wilson, 2000). So far, Wyeth’s efforts have resulted in the isolation of over 500 novel microbial isolates, including 10 new genera of endophytic actinomycetes alone (Table 1). As predicted, this extraordinary biological diversity is also producing extraordinary chemical diversity for assessment. As of spring 2009, Wyeth Research has screened ICBG plant and microbial samples in over 75,000 separate industry-based high throughput screens targeting anti-infectives, cancer, inflammation, women’s health, neurosciences, and metabolic diseases. This screening has resulted in 409 active compounds pursued in secondary assays. In addition, data generated in the University of Utah drug discovery program has shown that many of the Wyeth extracts possess potent anti-microbial, anti-viral and anti-protozoan activity (see below).
Table 1.
Summary of Microbial Isolations
| Purified Strains | Unique Strains | |
|---|---|---|
| Marine actinomycetes | 280 | 154 |
| Endophytic actinomycetes | 152 | 123 |
| Soil-derived actinomycetes | 95 | 57 |
| Endophytic fungi | 367 | 166 |
| Marine fungi | 54 | 36 |
| Total | 948 | 536 |
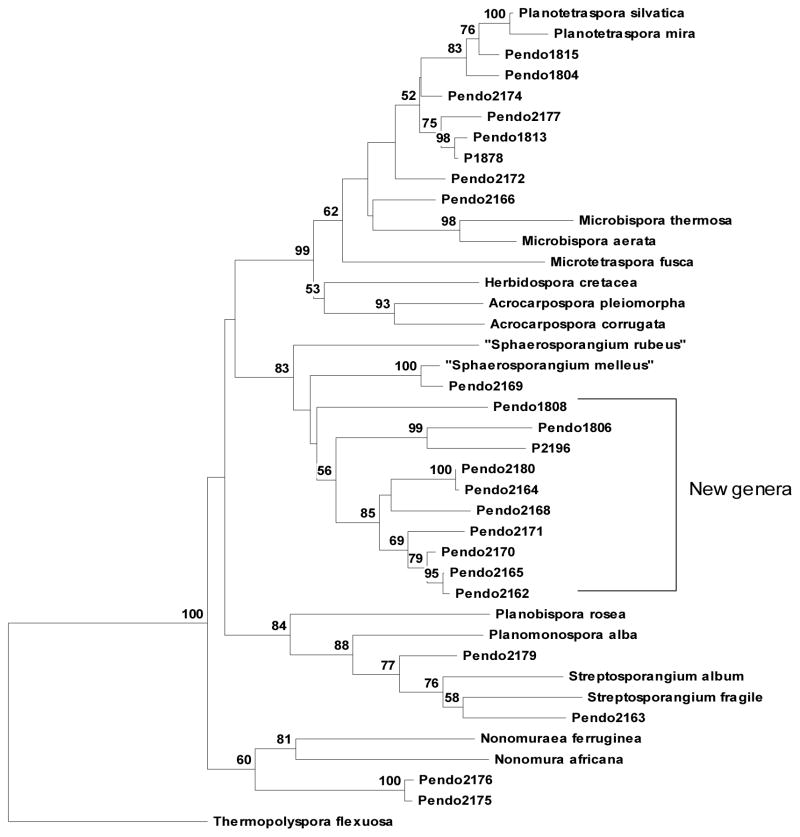
A total of 948 purified microbial strains from PNG plant and marine substrates were subjected to dereplication by ribotyping, ribosomal gene sequence comparisons, and morphology. As a result, 536 strains were determined to be genetically and/or biochemically unique. A highly effective process was developed for the isolation of endophytic actinomycetes from a variety of plant tissues. Remarkably, these procedures resulted in the isolation and characterization of at least five potentially new genera of plant-associated actinomycetes A total of 152 purified endophytic actinomycetes were dereplicated by ribotype analysis on the RiboPrinter® Microbial Characterization System (DuPont Qualicon, DE, USA), which is an automated instrument that provides a strain level “genetic fingerprint” of bacteria from only a few agar-grown colonies. Actinomycetes with unique riboprints were characterized to the genus level by nearly complete (~1400 bp) 16S rDNA sequence analysis. We found that rare genera such as Planotetraspora Hu Runmao, Wei Guizhen & Li Junying were isolated almost as frequently as Streptomyces, the most ubiquitous actinomycete. The most revealing discovery was the prevalence of isolates that formed their own clades by 16S, distinct from any known genera within Streptosporangiaceae and Thermomonosporaceae (see Fig 4). These strains form several potentially new genera that seem to have an association with plants. Using a molecular approach to estimate the biosynthetic capability of 34 unique isolates from Streptosporangiaceae and Thermomonosporaceae, genomic DNA was scanned for the presence of type I PKS, type II PKS, and NRPS genes. Of these, 82% were positive for putative type I PKS, 97% for putative type II PKS and 100% for putative NRPS genes. One hundred twenty-three unique endophytic actinomycetes were isolated, cultivated and processed for inclusion in the fractionated natural products library.
Fig. 4.
16S phylogenetic tree of the Streptosporangiaceae.
Most of the unique isolates could be classified to the genus level (54%) but may represent new species. At least 30% of the endophytic fungi could not be classified below the class level by ITS analysis and represent unique strains. We also found that by collecting less woody substrates and concentrating on isolating fungi from herbaceous plants like grasses and ferns, the diversity of fungal isolates increased due in part to the decrease in the number of xylariaceous fungi isolated. One hundred sixty-six unique fungi were isolated, cultivated and processed for the fractionated natural products library. For inclusion in high-throughput screening at Wyeth, 381 unique microbes were cultivated in several different media to generate over 4,000 pre-fractionated samples.
Botanical-based drug discovery
The botanically-linked drug discovery was designed to further the survey of PNG indigenous plants, and to use that activity as a source for drug discovery. As part of AP 1, plant survey and collection has been conducted by botanists at UPNG and FRI. This collection is formally permitted through the DEC. The DEC also supplies the necessary export permits for PNG genetic materials. For decades the herbaria at UPNG and FRI have been engaged in the survey of indigenous, endemic, invasive and economically important PNG plants for identification and voucher distribution. Unfortunately, their efforts had lagged due to funding constraints. Support through the ICBG reinvigorated their collection activity and has resulted in the recent identification of several new species from the D’Entrecasteaux Islands, with other potential new varieties from Cape Rodney, Western Province and Bougainville Island.
The DEC has formally adopted specific protocols developed by the DEC’s research evaluation arm, PNG BioNET, the ICBG and the Fogarty International Center, as prototypic for governing biological research activities. Explicit in these protocols are mechanisms for prior informed consent and benefit sharing in PNG. The expertise of the PNG botanists, their experience and familiarity with the traditions of the local landowners, has been critical to the execution of these protocols. ICBG collections occur only after reconnaissance visits have openly discussed the nature and expected extent of work, and the possible benefits and risks of such research activity, with local land owners, and collaboration is formally agreed upon.
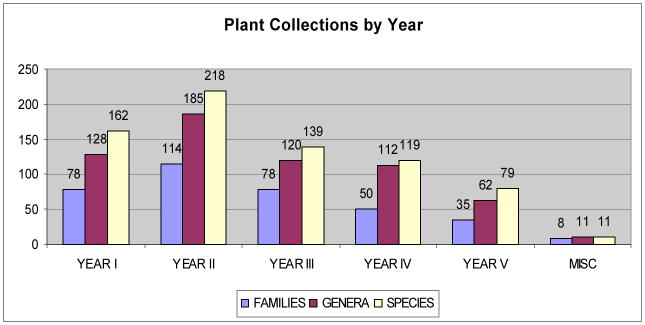
Herbaria activities include the collection of field voucher samples and their subsequent analysis, mounting and dissemination. For ICBG supported expeditions, botanists also direct the ecologically sensitive collection of approximately 1 kg of plant parts along with the field-pressed vouchers. The parts may include leaves, roots, twigs or stems, bark, flowers or fruit, depending on the plant and time of year. All plant samples are labeled with herbarium collection/accession numbers and subcodes, and then dried and stored at UPNG or FRI before milling. Collections have targeted 200 species per year. The current total is over 730 species, which represent approximately 500 genera and 300 families (Fig 5). To date, the herbaria in PNG have distributed over 1,700 specimen vouchers from ICBG collections internationally to herbaria including the Royal Botanic Gardens, Kew Palace, London; Nederland National Herbarium, Leiden University; Harvard University Herbaria, Boston; Australian National Herbarium, CSIRO, Canberra; Natural History Museum, Smithsonian Institution, Washington D.C.; Herbarium Bogoriense, Bogor, Indonesia; Philippine National Herbarium, Manila; and others.
Fig. 5.
Number of genera, family and species represented in our collection.
AP 4 (Chemistry) has developed an extraction and prefractionation protocol that facilitates rapid chemical dereplication and pharmacological analysis of plant extracts. Our laboratories have made several innovations in the development of a prefractionation procedure that yields botanical samples suitable for LCMS dereplication and that are compatible with high throughput screening technology (Bugni et al., 2008). Briefly, 20 g of ground material is extracted with 150 mL methanol for 24 h at room temperature. A 30 mL portion of the original methanol extract is filtered, dried onto 30 mg of pre-cleaned Diaion® HP20SS resin, and loaded into a polypropylene column. The HP20SS column is eluted with 15 mL each: 25% isopropanol:75% water, 50% isopropanol:50% water, 75% isopropanol:25% water, and 100% methanol, generating four fractions per sample. The more hydrophilic molecules, including nuisance polyphenolic compounds, elute in the first fraction while the more non-polar molecules usually elute in the later fractions. When strongly active fractions are detected in the most hydrophilic fraction, the extract is passed through a polyamide column to remove tannins and the fraction is retested. In either case, HP20SS-eluted fractions are dried under vacuum and aliquoted in DMSO at 10 mg/ml for biological assay. This pre-fractionation procedure effectively concentrates active compounds and thereby increases the likelihood of their detection in the bioactivity screens. Until recently, the extraction and pre-fractionation were performed exclusively at the University of Utah, but the technology has been transferred to UPNG and more of this work is performed there.
Over 4,000 fractions for biological activity testing have been generated from our botanically guided ICBG collections. Fractions active in the bioassays are subfractionated further by high performance liquid chromatography with simultaneous high resolution mass spectrometry for dereplication. This two dimensional chromatography successfully separates extract components throughout the LCMS chromatogram, most often yielding subfractions containing only one or two compounds that have determined accurate mass values. The mass values can efficiently identify known compounds, while the subfractions are perfectly suited for confirmatory bioassays. The plant extract fractions are also sent to Wyeth Research for high throughput screening. While most of the hits being followed at Wyeth Research have arisen from their fermentation projects, hits originating from PNG plant sources have been pursued in secondary assays of oncology and inflammation.
As mentioned above, PNG suffers from many of the diseases that plague third world countries. At the University of Utah, AP 3 has established three relatively high throughput drug discovery screens that focus on some of the worst: TB, malaria and HIV. We have also established a cytotoxicity assay that is useful for the identification of plant fractions containing cytotoxic products with potential as anti-cancer agents. The cytotoxicity assay is equally useful as a counterpoint to the infectious disease screens and is used routinely to identify the active anti-infective agents with the least cytotoxic potential.
To meet the demand for increased throughput, we adapted drug discovery assays to a uniform format for extract screening and data analysis. All of our screens are BSL-2 level containment and performed in 96-well culture plates. Three of the four screens use a simple colorimetric endpoint, while the malaria assay follows incorporation of tritium labeled phenylalanine into cultured parasites. Raw data is exported directly from plate readers into EXCEL© worksheets that have been custom designed to calculate results for the individual assays. This process minimizes transcription errors and greatly facilitates the speed of data calculation and the accuracy of lab notes. The worksheets also save the data in a format that can be directly uploaded into a database supported by the Fogarty ICBG program (NAPIS©). The NAPIS© database efficiently links field collection data with chemical and bioassay results, and can be searched efficiently from virtually any starting point (bioassay hit, collection site, plant name, chemical ID, lab book page, technician, dates, etc.).
The HIV assay we employ is a good example of the approach we have taken in developing our screens. This assay uses a biologically-contained HIV-1 strain that is deficient in making Tat and Rev proteins; both of which are essential for HIV infectivity (Chen et al., 1992). This virus can grow only in human T-cells that have been stably transformed to produce Tat and Rev. Thus, it is a whole virus assay system that is non-infectious. Kiser and coworkers at the NIH (Kiser et al., 1996), isolated a sub-clone of this engineered T-cell line (1A2) that is particularly sensitive to killing by infection with HIV. They then demonstrated the utility of the system to assess the ability of azidothymidine (AZT) and other anti-AIDS drugs to protect the T-cells from HIV killing. This cytoprotection assay is robust and can quantified with a colorimetric methylthiazolytetrazolium bromide (MTT) endpoint using a simple visible wavelength plate reader.
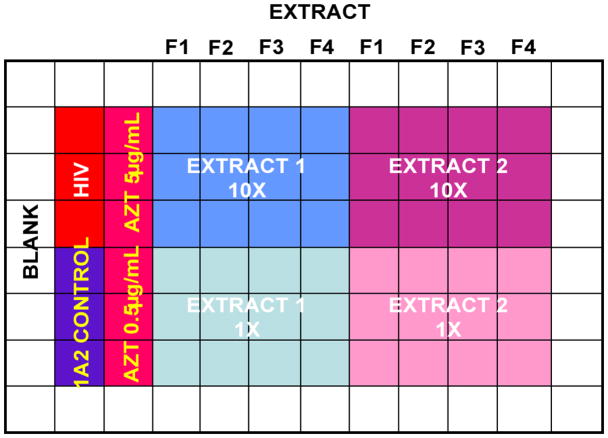
The plate layout (Fig 6) contributes to reproducible screening, these were determined through several years of experience with the assays. For HIV, each of the plates has a perimeter of medium-filled wells to minimize evaporative loss in the internior wells. The plate controls for 1A2 cell growth, as well as HIV infected 1A2 growth. The positive control AZT is tested at two concentrations (5 and 0.5 μg/mL). Each extract is tested at 10 and 1 μg/mL in triplicate. The AZT protection is calculated in the spreadsheet as a percentage of the 1A2 control growth and the AZT protection values are used as the positive control to which the test extracts are compared. Test extracts are considered active if they are statistically (p<0.05) more viable than HIV-infected controls and provide at least half the protection afforded by the better of the two positive control AZT concentrations. The spreadsheet identifies hits as questionable if they yield more than half the AZT protection but are not statistically different from HIV-infected controls, or if they are statistically better than controls but not half as active as the best AZT value. Everything else is denoted as inactive. The system still requires human interpretation (was the control 1A2 growth appropriate?, was the HIV titer potent enough to give a good signal?, did the AZT protect enough to make the comparisons relevant?, etc.), but the reproducibility of the system and the confirmation of hits it identifies argue that this is a robust and accurate way to evaluate screening data from the anti-HIV assay.
Fig. 6.
HIV cytoprotection assay plate layout. Four fractions of each extract are tested at 10 and 1 μg/ml alongside appropriate controls.
The HIV screen is the most complicated of the four assays we perform. Although each is unique in terms of design and layout, the other university-based screens (TB, adapted from Collins & Franzblau, 1997; malaria, adapted from Trager & Jensen, 1976, Geary et al., 1982; and cytotoxicity as described by Marshall et al., 2003) take advantage of similar data acquisition and calculator worksheets. As of summer 2007, we had completed well over 18,000 assays with a total of 703 non-toxic hits. Non-toxic hits are defined as active fractions yielding less than 30% growth inhibition of human T-cells in culture. For the HIV screen that amounted to 340 non-toxic hits out of 6,933 assays. Malaria and TB hits are defined as those fractions showing >70% malaria or TB growth inhibition. For malaria the total was 132 hits out of 3,397 assays and for TB our total was 164 out of 3,555 assays.
The assay development achieved in AP 3 improved throughput and allowed screening of Wyeth’s ICBG fractionated microbial library in the AP 3 assays. Initially, we tested 2,500 fractions from Wyeth’s microbial isolates in TB, our highest throughput assay. These TB actives were further tested for activity against two Gram− and three Gram+ bacterial strains using CLSI protocols (Jorgensen and colleagues, 1994). Of the 65 TB hits, 13 were TB selective and were not toxic to any of the other five bacterial strains. On the other hand, eight were active against all of the five additional strains, three of which exhibited no cytotoxicity to cultures human cells. On the basis of taxonomy and chemistry of the producing strains, prioritized hits were fractionated at Wyeth and guided by activity from AP 3 assays. Several rounds of this interaction resulted in Wyeth initiating patent filings in 2008 on the first two new antibiotics from ICBG isolates. An analysis of the microbial library in HIV and malaria was also productive. For HIV a final set of five highly active fractions were identified, two being as active as our AZT control. For malaria, 33 extracts, mostly from actinomycetes were confirmed active, but several were cytotoxic and not pursued. This exchange of material and data between an academic and industrial partner is extremely innovative and exemplifies the true collaborative and cooperative nature of this ICBG.
Prioritization of hits for confirmation, chemical isolation and structural analysis requires consideration of several issues. To obtain updated lists of active extracts to consider, we routinely query the NAPIS database for the 10 or 20 most potent and least cytotoxic fractions in a given screen. Once polyphenols are eliminated as the possible active constituent, LCMS generated sub-fractionations are re-assayed for activity. We use several search engines to retrieve literature pertaining to known chemistry and activity for the identified source plant genus and molecular mass values. This is done while performing dose response repeat assays for activity and cytotoxicity, in addition to secondary confirmatory assays (such as p24 production for HIV or CLSI MIC determination for TB). If the activity confirms and the mass values appear unique, or if little is known about the particular plant, effort is made to isolate the active constituents and to determine their three dimensional structure principally using NMR techniques. Particularly novel or interesting pharmacophores may then become the subject of pharmacologic investigations into molecular mechanism of action.
The above process has rewarded our efforts with the identification of exciting new activities being identified for plants and molecules. For instance, Caelospermum salomoniense (Engl.) J.T. Johansson (Rubiaceae) has yielded a known anthraquinone (Adesogan, 1973) with potent anti-TB and antimalarial activity. Exocarpus latifolius Labill. (Santalaceae) has yielded exocarpic acid (Hopkins & Chisholm, 1966) and several new derivatives that have potent and selective anti-TB activity (Koch et al., In Press). Fractions from Planchonia papuana R. Knuth (Lecythidaceae), and Myristica sp. (Myristicaceae) harvested for timber in PNG, both demonstrate potent anti-HIV activity. In fact, several trees in our collection that are harvested for timber in PNG (PNG Trees Website) show interesting activity; for example Rhus taitensis Guill. (Anacardiaceae) has antimalarial and antituberculin activity (Noro et al., 2008) and Dysoxylum gaudichaudianum Miq. (Meliaceae) has potent anti-TB activity. Finding non-timber value even in a few species will be an important impetus for forest preservation and future botanical exploration.
Traditional Medicines
Although a large part of our ICBG has been organized around botanical survey and screening for activity against infectious diseases, we have also supported the documentation and preservation of traditional knowledge concerning plant use. Traditional medicines have provided some of the most important medicines used by man, from morphine and aspirin for pain, to cardiac glycosides for congestive heart failure, from glitizones for diabetes to statins for high cholesterol, there are many examples (Gurub-Fakim, 2006). Traditional medicines are effective against infectious diseases as well, with two of the most effective current antimalarials, chloroquine and artemisinin, being derived from medicinal plants (Coatny, 1963; Meshnick, 1998). Prostratin, with exciting anti-HIV activity, is a key metabolite of a Samoan traditional medicine (Gustafson et al., 1992: Cox, 1993).
There are several reasons to study PNG medicinal plants. First, PNG is extraordinarily rich in plant and cultural diversity and there is a long tradition of plant use for health needs. PNG is a geographically segregated mountainous nation of at least 800 languages (Reilly & Phillpot, 2003). This geology has also resulted in isolated ecosystems and extraordinary biological diversity. Each culture in PNG is rich in their knowledge about the use of plants and animal materials for treating illnesses (Rai, 2007).
Second, there is a critical threat to the orally-based cultures of PNG. Generational knowledge of medicinal plant use in PNG is often transmitted in languages that are threatened by change of lifestyle. In some tribes, the art of traditional healing is dying as the older generations pass away. The traditional use of medicinal plants constitutes an important information reservoir that has been empirically tested and adopted through millennia of trial and error. The validation of medicinal plant use by confirmation of bioactivity can provide a powerful rationale for preservation of cultural traditions, the conservation of biological resources, and provide alternative healthcare options to needy populations.
Third, prior to the Traditional Medicines Database Surveys (described below), PNG medicinal plant use and corresponding pharmacological assessment was not well studied. The documentation of medicinal plants in PNG has been haphazard and the accrued knowledge not widely disseminated internationally. Dr Rai estimates that historically some 800 PNG plants have been described for treatment of various ailments, but this probably represents only a fraction of the total.
In 1999, the Ministers of Health of the Pacific Islands agreed that traditional medicine has an important role in health care systems and therefore should be encouraged (WHO Regional Office for the Western Pacific, 1999). The WHO developed an action plan (WHO Western Pacific Region, 2001) to assist the implementation of this action statement. The National Health Plan of PNG, 2001–2010, adopted by the National Department of Health, promoted a collaboration between the WHO and UPNG to create a Traditional Medicines Working Group to assist in the development of traditional medicines in the country. The first two goals of this Working Group were to initiate a country-wide survey on traditional medicine practice, and to develop a national database on native medicinal plants and traditional medicines. These efforts were undertaken by two of our colleagues at UPNG, Dr. Prem Rai and Dr. Teautulohi Matainaho, who enlisted UPNG researchers in the disciplines of Pharmacy, Pharmacology and Biology, including the Herbarium staff. A traditional medicines survey questionnaire was developed using WHO guidelines. In 2001 the surveys initiated with all the necessary approvals and endorsements from the Medical Research and Advisory Committee of the Department of Health. Similar endorsement was also obtained from the School of Medicine and Health Sciences Research & Ethics Committee, because the research involves human subject interviews and is carried out by UPNG faculty and students. The project now trains senior pharmacy or medical students who administer the questionnaire to their home community traditional medicine practitioners. The students are trained in the basics of questionnaire administration, traditional medicines, taxonomic nomenclature, herbarium specimen preparation, and instructed in the preservation and documentation of traditional knowledge and culture. They are supported for a one term elective, along with their travel and living costs. Under the guidance of Dr. Rai and Dr. Matainaho, the students write sophisticated reports (averaging four a year), which include analyses of interviews with traditional healers, pictures and documentation of the plants, and plant preparation and use. From 2001 to 2003 approximately 200 plants used medicinally were documented. From 2004 to the present, the project has been supported mainly by the ICBG. As of 2008, ICBG funded survey of traditional medicine practice and medicinal plants has covered 15 villages spanning 9 provinces, and documented approximately 720 medicinal plants. The total number of medicinal plant entries in the National Database on Traditional Medicines is over 1500, and documents approximately 850 plant species and 196 plant families. Approximately 400 practitioners are now listed in the database. While the ICBG now supports this program, the intellectual property contained within the database has remained proprietary and is accessible only to faculty at UPNG as legislation detailing benefit-sharing schedules for database use is approved. While additional empowering legislation is currently pending, the Traditional Medicines Taskforce is operating under the current UPNG benefit sharing protocol, which is generic and applicable to many areas of natural products research. This protocol includes guidelines on intellectual property rights (IPR) and access and benefit sharing (ABS), and has been approved by the national government.
The tradition of medicinal plant use was officially endorsed in 2005 by the PNG Ministry of Health through the announcement of a Traditional Medicines Health Care initiative. Since 1990, 50% of rural Aid Posts and 25% of all Health Centres have closed, causing a collapse of the rural health care system. In recognition of this, the new National Policy on Traditional Medicine was promoted by the Department of Health in order to:
improve the quality and delivery of health services to the people of Papua New Guinea;
develop traditional medicine and its practices;
incorporate traditional medicine into the primary health care system.
The National Policy on Traditional Medicine was officially adopted by the Government of PNG in 2007 (National Department of Health Waigani and NCD, PNG, 2007). The adopted policy identifies specific pathways for the development, promotion and integration of traditional medicine into the National Health System. This includes the preclinical assessment of medicinal plants that comprise the candidate traditional medicines selected for development. Dr. Rai has been appointed Chairman of the Traditional Medicines Taskforce. The Taskforce is charged with promoting the policy nationwide, selecting “safe and effective” traditional medicines, developing a training manual for incorporation of traditional practitioners in primary care, and formalizing Traditional Healer Associations in the different provinces of PNG (a major outreach activity).
The Traditional Medicines Health Care Initiative brings new importance to the Traditional Medicines Database, which is now viewed as a national resource (National Department of Health Waigani and NCD, PNG, 2007). The Traditional Medicines Taskforce is also charged with using the database to identify the candidate herbal medicines for inclusion in the primary health care formulary.
Additional opportunities to support the documentation and validation of traditional medicines arise from the tendency of UPNG students in pharmacy, pharmacology or pharmacognosy to focus elective research projects on traditional medicines from their home communities. Our ICBG has been able to support these activities with chemical and bioactivity analysis. The first published example of this collaboration was the study of Melicope (Evodia) elleryana F. Muell. (Rutaceae), used in the Kurti region of Manus Island for cough and fever. The antituberculin activity of extracts from this plant was published by a UPNG Master’s Student, Emma Powan (Barrows et al., 2007). Ms. Powan is now a lecturer at UPNG. In addition to continuing the documentation of medicinal plant use in PNG, the ICBG is poised to participate more actively in the validation of traditional medicine activity and chemical standardization of the different preparations. One of the first selections of the traditional medicines task force for further study is a topical analgesic and anti-inflammatory preparation made from two different species of Parmeliaceae lichen. About 95% of a hexane extract of the lichen was composed of two orsellinic acid derivatives, atranorin and chloroatranorin (Marante et al., 2003). These compounds have characteristic UV absorption spectra and will serve perfectly as standards to validate batch-to-batch consistency. Based upon use and similar chemical structures to other cyclooxygenase (COX) inhibitors, we used human COX 1 and 2 assays to show that both atranorins possess COX inhibitory activity. Inhibition was determined by measuring the decrease of prostaglandin production by these enzymes in the presence of the drugs. Atranorin showed a dose dependent inhibition of COX-1 with an IC50 of approximately 17 μg/mL (~45 μM). Atranorin and chloroatranorin showed partial inhibition of COX-2 and COX-1, respectively, but chloroatranorin did not inhibit COX 2 (Bugni et al., 2008).
Training
One of the great accomplishments of this ICBG has been student training. Approximately 33 undergraduate and graduate students have been supported at UPNG, with over 20 gaining diplomas. The Traditional Medicines Surveys have supported two Bachelor of Medicine students and 13 Bachelor of Pharmacy students. The UPNG Herbarium has trained one undergraduate student and is training one Master’s student, Ms. Gagul, in plant systematics.
The Bioassay laboratory, established with Fograrty funds, has supported training of 10 Bachelor or Honor’s level students. Three of these students, Mr. Kalit, Ms. Kerepi and Ms. Linge, have gone on to Medical School. Ms. Fabian is employed by a conservation oriented nongovernmental organization. ICBG-supported student Master’s and Honor’s projects have focused mostly on plants used as traditional medicines. Three of these students were hosted at the University of Utah for the laboratory research portion of their theses. They were trained in rigorous scientific methods, conducted advanced studies on traditional medicines, and generated publishable results. The first publication from this training partnership was the study of Melicope(Evodia) elleryana, used in the Kurti region of Manus Island for cough and fever (Barrows et al., 2007). The second reported a new anti-TB natural product from a tree that is harvested for timber in PNG (Noro et al., 2008), and a third reports anti-TB molecules from Exocarpus latifolius (Koch et al., in press). Two trainees that studied at the University of Utah, Ms. Emma Powan and Ms. Manah Dindi, have obtained their Master’s degrees, and now hold lectureships at UPNG. Mr. Jeffrey Noro has completed requirements for a Master’s degree, which should be awarded shortly. An additional student, Ms. Mungkaje, completed her Master’s degree working on an ICBG project with Dr. Rai, and is now a lecturer at a UPNG satellite campus. Ms. Powan and Mr. Noro have been accepted into Ph.D. programs starting next year at the University of Auckland and the University of New South Wales, respectively. Mr. Owen Paiva, a current Master’s student, has been accepted into the Ph.D. program at the University of Queensland, pending receipt of his degree. There are four additional students supported by ICBG funds currently enrolled in Master’s programs at UPNG. In addition, the ICBG has provided research opportunities for 13 University of Utah students and postdoctoral fellows. Three of these students have finished Ph.D. degrees and are currently postdoctoral fellows. One more will defend his thesis this spring.
ICBG financial and technical support of the Traditional Medicines Surveys, the UPNG Herbarium and the Bioassay Laboratory have provided numerous training opportunities for UPNG students. Clearly, the ICBG has already made an enduring contribution to PNG’s intellectual capital through the educational advancement of the next generation of scientists dedicated to excellence in their fields, and to conservation and social progress through education.
PNG BioNET IPR legislation and awareness
The development of an intellectual property, informed consent and benefit protocol has been an important goal for the ICBG and PNG BioNET, a secretariat established within the DEC to oversee Bioresource research in PNG. Pivotal in this process have been the two ICBG funded studies, conducted by PNG BioNET, and carried out by Dr. Eric Kwa of the School of Law, UPNG and Mr. Douveri Henao, formerly of the Department of Attorney General and Justice and now of the School of Law, UPNG. The initial study, carried out in 2005, assessed the different laws and policies related to the regulation and management of biodiversity under different government jurisdictions. The second study, commissioned in 2006, looked at the impacting issues of culture, economics, education, community and institutional practices on IPR and ABS and biodiversity conservation. These two studies, and two related national workshops, also funded by the ICBG, provided the legal background for development and drafting of the ABS legislation for biodiversity in PNG. In addition to funding legal reviews and workshops, the ICBG has provided practical examples of ABS principles. For instance, a portion of ICBG “appreciation” payments are designated specifically for local school funds (Fig. 7). The ICBG-developed standard operating procedures for prior informed consent and benefit sharing serve as a template for PNG BioNET.
Fig. 7.

Local school located in the Adelbert Mountains.
The issue of intellectual property rights is generally considered a challenging subject by developing nations with rich biodiversity. Through the establishment of PNG BioNET, progress has been made in the development of an ABS law under the guidance of the Department of the Attorney General. The PINBio Act is currently in draft form. It is being circulated to Parliament and stake holders for input and could be approval in early 2009. The bill, entitled “Papua New Guinea Institute of Biodiversity Act”, was created by the PNG National Parliament to come into operation upon certification by the Speaker of the National Parliament. The objective being an Act to establish the Papua New Guinea Institute of Biodiversity to:
coordinate access to the biological resources of the country;
coordinate the implementation of benefits derived from the sustainable utilization of biological resources;
monitor access to biological resources and their benefits derived from the sustainable utilization and management of biological resources; and
provide a means for carrying into effect obligations under the Convention on Biological Diversity or any other treaty or convention relating to biodiversity to which Papua New Guinea is a party, and for related purposes.
The ability of the ICBG collaborators to enable conservation minded institutions and to foster development of clear policies forwarding environmental, scientific and stakeholder goals illustrates how much can be achieved toward Fogarty International Center objectives in a brief period of time.
In conclusion, we have established an integrated set of programs promoting biological resource survey and conservation, drug discovery, technology transfer and education in PNG. This has been accomplished by empowering PNG institutions and researchers and linking them with capabilities found at the University of Utah. The work has required the parallel development of policies and legislation governing research concerning PNG genetic resources and intellectual property, and for prior informed consent and equitable benefit sharing. The activities of the associated programs have synergized to yield successful botanical and medicinal survey activities. The collaboration has also provided technology transfer and training opportunities for our partners in PNG and is documenting non-timber value to rainforest biodiversity. Advancements in chemistry and screening have yielded the extraction and fractionation, screening and data management protocols capable of identifying new plant chemistry and activities relevant to human health. Some of the active new molecules are moving ahead into more advanced stages of preclinical development, clearly confirming our overarching hypothesis and moving towards a tighter link between scientific and social progress.
Acknowledgments
Funding was provided by NIH through the ICBG 5UO1TW006671. The authors also acknowledge the NIH grants RR14768 and RR06262 for funding NMR instrumentation in the University of Utah, Health Sciences NMR Facility. C.D. Andjelic was partially supported by a 2004 AFPE predoctoral Fellowship and a 2005–2007 PhRMA predoctoral fellowship. M. Koch was partially supported by a 2007–2008 AFPE predoctoral fellowship.
Footnotes
Symposium: “Plants in the Service of Human Health: Continuing Search for Plant-based Therapies” - Society for Economic Botany 48th Annual Meeting in Chicago at Lake Forest College, June 4, 2007
References
- Adesogan EK. Anthraquinones and anthraquinols from Morinda lucida. Tetrahedron. 1973;29:4099–4102. [Google Scholar]
- Allison A. The role of museums and aoos in conserving biological diversity in Papua New Guinea. In: Pearl M, Beehler B, Allison A, Taylor M, editors. Conservation and Environment in Papua New Guinea: Establishing Research Priorities. Washington, D.C. and New York: Embassy of Papua New Guinea and Wildlife Conservation International; 1991. [Google Scholar]
- Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microb Rev. 1995;59:641–665. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUSAID, Canberra. Centre for International Economics. Potential Economic Impacts of an HIV/AIDS Epidemic in Papua New Guinea. 2002:v–ix. [Google Scholar]
- Australian Government. Overseas Aid. 2007 available at: http://www.ausaid.gov.au/country/papua.cfm.
- Barrows LR, Powan E, Pond CD, Matainaho T. Anti-TB activity of Evodia elleryana bark extract. Fitoterapia. 2007;78:250–252. doi: 10.1016/j.fitote.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beehler BM. PNG Conservation Needs Assessment. Vol. 2. Boroko: Papua New Guinea Department of Environment and Conservation, CNA Consensus Maps of Biodiversity in Papua New Guinea; 1993. pp. 11–20. [Google Scholar]
- Bugni TS, Harper MK, McCulloch MW, Reppart J, Ireland CM. Fractionated marine invertebrate extract libraries for drug discovery. Molecules. 2008;13:1372–1383. doi: 10.3390/molecules13061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Boyle TJ, Malim MH, Cullen BR, Lyerly HK. Derivation of a biologically contained replication system for human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992;89:7678–7682. doi: 10.1073/pnas.89.16.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coatney GR. Pitfalls in a discovery: The chronicle of chloroquine. Am J Trop Med Hyg. 1963;12:121–128. doi: 10.4269/ajtmh.1963.12.121. [DOI] [PubMed] [Google Scholar]
- Collins LA, Franzblau SG. Microplate alamar blue assay vs. BACTEC 460 system for high-throughput screening of compounds vs. Mycobacterium tuberculosis and M. avium. Antimicr Agents Chemoth. 1997;41:1004–1009. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox P. Saving the ethnopharmacological heritage of Samoa. J Ethnopharmacol. 1993;38:181–188. doi: 10.1016/0378-8741(93)90014-v. [DOI] [PubMed] [Google Scholar]
- Frodin DG, Gressitt JL. The Biological Exploration of New Guinea. In: Gressitt JL, editor. Biogeography and Ecology of New Guinea. Dr. W. Junk Publishers; The Hague: 1982. [Google Scholar]
- Geary TG, Divo AA, Jensen JB. An in vitro assay system for the identification of potential antimalarial drugs. J Parasit. 1983;69:577–583. [PubMed] [Google Scholar]
- Gurib-Fakim A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Gustafson KR, Cardellina JH, McMahon JB, Gulakowski RJ, Ishitoya J, Szallasi Z, Lewin NE, Blumberg PM, Weislow OS. A nonpromoting phorbol from the Samoan medicinal plant Homalanthus nutans inhibits cell killing by HIV-1. J Med Chem. 1992;35:1978–1986. doi: 10.1021/jm00089a006. [DOI] [PubMed] [Google Scholar]
- Hawksworth DL. The fungal dimensions of biodiversity: Magnitude, significance and conservation. Mycol Res. 1991;95:641–665. [Google Scholar]
- Holloway JD, Stork NE. The dimensions of biodiversity: The use of invertebrates as indicators of human impact. In: Hawksworth D, editor. Biodiversity Indicators. CAB International; Wallingford: 1991. pp. 37–62. [Google Scholar]
- Hopkins CY, Chisholm MJ. Acetylenic fatty acids from Buckleya seed oil. Natl Res Council, Ottawa, Can Chem Industry (London, United Kingdom) 1966;36:1533–1534. [Google Scholar]
- Hwaihwanje I, Tsukahara T, Hombhanje F, Lum K, Masta A, Sapuri M, Kobayakawa T, Tanihata T, Kaneko A. Eco-Epidemiological Characterization of Malaria Prevalence in High Endemicity Area of East Sepik Province, Papua New Guinea. Papua New Guinea, Annual Medical Symposium; Port Moresby: NCD; 2007. [Google Scholar]
- Intachat J, Holloway JD, Speight MR. The impact of logging on geometroid moth populations and their diversity in lowland forests of peninsular Malaysia. J Trop For Sci. 1999;11:61–78. [Google Scholar]
- John E. Fogarty International Center for Advanced Study in the Health Sciences. International Cooperative Biodiversity Groups (ICBG) 2007 (available at http://www.fic.nih.gov/programs/research_grants/icbg/index.htm)
- Jorgensen JH, Swenson JM, Tenover FC, Ferraro MJ, Hindler JA, Murray PR. Development of interpretive criteria and quality control limits for broth microdilution and disk diffusion antimicrobial susceptibility testing of Streptococcus pneumoniae. J Clin Microbiol. 1994;32:2448–2459. doi: 10.1128/jcm.32.10.2448-2459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser R, Makovsky S, Terpening SJ, Laing N, Clanton DJ. Assessment of a cytoprotection assay for the discovery and evaluation of anti-human immunodeficiency virus compounds utilizing a genetically-impaired virus. J Virol Meth. 1996;58:99–109. doi: 10.1016/0166-0934(95)01998-7. [DOI] [PubMed] [Google Scholar]
- Koch M, Bugni TS, Pond CD, Sondossi M, Dindi M, Piskaut P, Ireland CM, Barrows LR. Antimycobacterial activity of Exocarpus latifolius is due to exocarpic acid. Pl Med. 2009 doi: 10.1055/s-0029-1185687. in press. [DOI] [PubMed] [Google Scholar]
- Lin E, Kiniboro B, Schoepflin S, Iga J, Merkby J, Zimmerman P, Muller I. Epidemiology of Plasmodium infections in the Hahita Area of Papua New Guinea, Annual Medical Symposium. NCD; Port Moresby: 2007. [Google Scholar]
- Marante FJT, Castellano AG, Rosas FE, Aguiar JQ, Barerra JB. Identification and quantitation of allelochemicals from the lichen Lethaleriella canariensis. J Chem Ecol. 2003;29:2049–2071. doi: 10.1023/a:1025682318001. [DOI] [PubMed] [Google Scholar]
- Marshall KM, Matsumoto SS, Holden JA, Concepcion GP, Tasdemir D, Ireland CM, Barrows LR. The anti-neoplastic and novel topoisomerase II mediated cytotoxicity of neoamphimedine, a marine pyridoacridine. Biochem Pharmacol. 2003;16:447–458. doi: 10.1016/s0006-2952(03)00209-0. [DOI] [PubMed] [Google Scholar]
- Meshnick SR. In: From Quinine to Qinghaosu: Historical Perspectives, in Malaria, Parasite Biology, Pathogenesis, and Protection. Sherman IW, editor. American Society for Microbiology; Washington DC: 1998. pp. 341–353. [Google Scholar]
- National AIDS Council. Papua New Guinea National Strategic Plan on HIV/AIDS 2006–2010. 2006. p. 8. [Google Scholar]
- National Department of Health Waigani and NCD, P.N.G. National Policy on Traditional Medicine. 2007. p. 7. [Google Scholar]
- Noro JC, Barrows LR, Gideon OG, Ireland CM, Koch M, Matainaho T, Piskaut P, Pond CD, Bugni TS. Tetrahdroxysqualene from Rhus taitensis shows antimycobacterial activity against Mycobacterium tuberculosis. J Nat Prod. 2008;71:1623–1624. doi: 10.1021/np800082e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny V, Miller SE, Hulcr J, Drew RA, Basset Y, Janda M, Setliff GP, Darrow K, Stewart AJ, Auga J, Isua B, Molem K, Manumbor M, Tamtiai E, Mogia M, Weiblen GD. Low beta diversity of herbivorous insects in tropical forests. Nature. 2007;448:692–695. doi: 10.1038/nature06021. [DOI] [PubMed] [Google Scholar]
- PNG Trees. available at http://www.pngplants.org/PNGtrees.
- Rai PP. Traditional Medicine in Papua New Guinea. University of Papua New Guinea Press; 2007. pp. 8–13. [Google Scholar]
- Ratnasingham S, Hebert PDN. BOLD: The Barcode of Life Data System ( http://www.barcodinglife.org) Mol Ecol Notes. 2007;7:355–364. doi: 10.1111/j.1471-8286.2007.01678.x. [DOI] [PMC free article] [PubMed]
- Reilly B, Phillpot R. Making Democracy work in Papua New Guinea: Social Capital and Provincial Development in an Ethnically Fragmented Society. Asian Survey. 2002;42:902–927. [Google Scholar]
- Sekrhan N, Miller S. Papua New Guinea Department of Environment and Conservation. Colorcraft Ltd; Hong Kong: 1996. Papua New Guinea Country Study on Biological Diversity; pp. 67–96. [Google Scholar]
- Shearman P, Bryan J, Ash J, Hunnam P, Mackey B, Lokes B. The State of the Forests of Papua New Guinea; Mapping the extent and condition of forest cover and measuring the drivers of forest change in the period 1972–2002. 2008 http://www.scienceinpublic.com/State%20of%20Forests%20of%20PNG_Concise.pdf.
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- WHO Regional Office for the Western Pacific. The Palau Action Statement on Healthy Islands: Meeting of the Ministers of Health for Pacific Island Countries: Manila. 1999. p. 1. [Google Scholar]
- WHO Western Pacific Region. Apia Action Plan on Traditional Medicine in the Pacific Island Countries. WHO Regional Office for the Western Pacific; Manila: 2001. p. 1. [Google Scholar]
- WHO Western Pacific Regional Office Website. Stop TB, editor. Tuberculosis Control in WHO Western Pacific Region. 2002 available at http://www.wpro.who.int/pdf/StopTB/papua.pdf.
- Wilson D. Ecology of woody plant endophytes. In: Bacon CW, White JF, editors. Microbial Endophytes. CRC Press; Boca Raton, Florida: 2000. pp. 389–440. [Google Scholar]