Abstract
Therapeutics based on small interfering RNA (siRNA) have a great clinical potential; however, delivery problems have limited their clinical efficacy, and new siRNA delivery vehicles are greatly needed. In this report, we demonstrate that submicron particles (800–900 nm) composed of the polyketal PK3 and chloroquine, termed as the PKCNs, can deliver tumor necrosis factor-α (TNF-α) siRNA in vivo to Kupffer cells efficiently and inhibit gene expression in the liver at concentrations as low as 3.5 μg/kg. The high delivery efficiency of the PKCNs arises from the unique properties of PK3, which can protect siRNA from serum nucleases, stimulate cell uptake and trigger a colloid osmotic disruption of the phagosome and release encapsulated siRNA into the cell cytoplasm. We anticipate numerous applications of the PKCNs for siRNA delivery to macrophages, given their high delivery efficiency, and the central role of macrophages in causing diseases such as hepatitis, liver cirrhosis and chronic renal disease.
INTRODUCTION
The development of delivery vehicles that can efficiently deliver small interfering RNA (siRNA) in vivo remains a major challenge in the field of biotechnology (1–3). Delivering siRNA in vivo has been challenging because of its rapid hydrolysis by serum nucleases, membrane impermeability and sequestration in lysosomes after endocytosis (4,5). At present, siRNA delivery vehicles have been based around ‘soft materials’, which are composed of electrostatically held complexes, composed of cationic lipids or polycations (6–10). These delivery vehicles have had limited success in vivo, because they are easily destroyed by either charged serum proteins, the high shear forces in the blood or in cell lysosomes after endocytosis, and generally require high doses for efficacy in animals (>200 μg/kg siRNA) (6–8). In this report, we demonstrate that solid polymeric particles composed of the acid-sensitive polymer PK3 and chloroquine, termed as the PKCNs, can deliver siRNA to macrophages and inhibit gene expression in vivo at concentrations as low as 3.5 μg/kg. In contrast to cationic lipid or polycation siRNA complexes, the PKCNs are ‘hard’ materials, composed of the water-insoluble polymer PK3, and should maintain their integrity in vivo because of the high energetic cost of exposing PK3 to water. We anticipate numerous applications of the PKCNs given their high delivery efficacy and the central role of macrophages in human diseases.
MATERIALS AND METHODS
Materials
Double-stranded tumor necrosis factor-α (TNF-α) siRNA (mouse) (sense strand: 5′-GAC AAC CAA CUA GUG GUG CUU-3′) and scrambled siRNA (sense strand: 5′-GCG UCG UCA GUA CCA GGA AUU-3′) were synthesized by Dharmacon (Lafayette, CO, USA). Enzyme-linked immunosorbent assay (ELISA) kit (TNF-α ELISA Ready-Set-Go) for the detection of TNF-α was purchased from eBioscience (San Diego, CA, USA) and alanine aminotransferase (ALT) assay kit was purchased from Pointe Scientific Inc. (Canton, MI, USA). 1,2-Dioleoyl-3-trimethylammonium-propane (DOTAP; chloride salt) was purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). Lipofectamine2000 was purchased from Invitrogen (Carlsbad, CA, USA). Lipofectamine2000 and siRNA were formulated into Lipofectamine–siRNA complexes [5:1 (w/w)] following the manufacture’s instructions. All other chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA) and used as received unless otherwise specified.
Synthesis of PK3
PK3 was synthesized as described in Yang et al. (11). Briefly, the diols, cyclohexanedimethanol (1.04 g, 7.25 mmol) and 1,5-pentanediol (0.19 g, 1.81 mmol) were dissolved in 20 ml of distilled benzene at 100°C. Recrystallized p-toluenesulfonic acid (5.5 mg, 0.029 mmol) was dissolved in ethyl acetate (500 µl) and added to the benzene solution. The polymerization reaction was initiated by the addition of 2,2-dimethoxypropane (0.94 g, 9.06 mmol). Additional 2,2-dimethoxypropane (500 µl) and benzene (2 ml) were subsequently added to the reaction to compensate for 2,2-dimethoxypropane and benzene that had distilled off. After 24 h, the reaction was stopped with triethylamine (100 µl) and isolated by precipitation in hexanes. The number average molecular weight of PK3 was 2530 Da (polydispersity index: 1.43) as determined by gel permeation chromatography, using a Shimadzu system (Kyoto, Japan).
Preparation and characterization of siRNA-loaded PKCNs
PK3 (40 mg) with or without chloroquine (1 mg) was added to 1 ml of methylene chloride containing either an ion-paired DOTAP–siRNA complex (1.0 mg siRNA and 2.2 mg DOTAP) or fluorescein-labeled siRNA (Fl-siRNA) ion-paired with DOTAP (12). The mixture of PK3 and DOTAP–siRNA complex was emulsified by homogenization (24 200 r.p.m., 30 s) into 8 ml of a 5% (w/v) aqueous polyvinyl alcohol (PVA) solution. The resulting emulsion was then poured into 20 ml of 0.5% PVA solution, and the methylene chloride was removed with a rotary evaporator. The resulting particles were isolated by centrifugation (10 000 g for 10 min), washed twice and freeze-dried. Particle size and shape were determined by dynamic light scattering (DLS) using a 90plus particle size analyzer (Brookhaven, Holtsville, NY, USA) and scanning electron microscopy (SEM) using a Hitachi S-800 SEM (Hitachi, Pleasanton, CA, USA). In order to determine loading efficiency of siRNA in the PKCNs, the PKCNs (2.0 mg) containing Fl-siRNA were dispersed in HCl solution (0.12 M) and incubated until the PKCNs hydrolyzed completely. After neutralized with NaOH solution (0.12 M), the fluorescence of the total Fl-siRNA released from the PKCNs was measured using a fluorometer (λex/λem = 494/510 nm) and compared with the initial fluorescence of the Fl-siRNA used in making the particles. Poly(lactic-co-glycolic acid) (PLGA) particles containing chloroquine and siRNA were formulated exactly the same way as the PKCNs except the polymer PLGA was used instead of PK3 [RG 503H, 35.4 kDa (polydispersity index: 2.5), Boehringer Ingelheim].
The release of siRNA from the PKCNs in vitro
The release of siRNA from the PKCNs was evaluated using Fl-siRNA. Fl-siRNA-loaded PKCNs (1.5 mg) were suspended in either pH 5.0 or 7.4 buffer solutions (1.0 ml). For statistical analysis, three independent samples per group were prepared. The suspensions were kept at 37°C under gentle shaking. At specific time points, the suspensions were centrifuged at 12 000 g for 2 min, and the fluorescence of the supernatants was then analyzed with a Shimadzu spectrofluorophotometer (Kyoto, Japan) (λex/λem = 494/510 nm). The pellets were re-suspended with fresh buffer solutions (1.0 ml) and the procedure was repeated for each time point.
In vitro uptake of the PKCNs by macrophages
RAW264.7 macrophages (ATCC number: TIB-71) from the American Type Culture Collection (ATCC) (Manassas, VA, USA) were maintained at 37°C under a humidified atmosphere of 5% CO2 in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% (v/v) fetal bovine serum (FBS), supplemented with penicillin (100 U/ml) and streptomycin (100 µg/ml). Human umbilical vein endothelial cells (HUVECs; Genlantis; a gift from Dr Gang Bao) were cultured in endothelial cell growth medium (Genlantis) supplemented with 20% FBS, 13.3 U/ml heparin, 40 µg/ml endothelial mitogen (Biomedical Technologies), 1% l-glutamine and penicillin and streptomycin. For flow cytometry, the macrophages (1 × 106 cells/well, 12-well plate) and HUVECs (1 × 106 cells/well, 12-well plate) were incubated with free Fl-siRNA, DOTAP–Fl-siRNA, Lipofectamine–Fl-siRNA or PKCN–Fl-siRNA (183.4 pmol siRNA in each sample) for 4 h. Cells were washed three times with ice cold phosphate-buffered saline (PBS) and scraped into tubes for flow cytometry. The fluorescent cell population was measured by flow cytometer (BD LSR flow cytometer; BD Bioscience, San Jose, CA, USA) using a laser for fluorescein (λex/λem = 494/510 nm).
Confocal microscopy of siRNA delivered by the PKCNs
We investigated the intracellular distribution of siRNA delivered by the PKCNs to macrophages, using confocal microscopy. Macrophages (RAW264.7) were grown on chambered coverglasses (No. 1.5) and treated with either free Fl-siRNA, DOTAP–Fl-siRNA, Lipofectamine–Fl-siRNA or PKCN–Fl-siRNA (183.4 pmol siRNA in each sample) for 10 min, 30 min, 1 h and 2h. After each time point, the macrophages were washed three times with PBS and imaged with a confocal microscope (Zeiss LSM 510; Carl Zeiss Inc., Thornwood, NJ, USA).
Serum stability of siRNA in the PKCNs
The ability of the PKCNs to protect encapsulated siRNA from serum nucleases was investigated with electrophoresis, using fluorescein-labeled siRNA. siRNA samples, either free Fl-siRNA, DOTAP–Fl-siRNA, Lipofectamine–Fl-siRNA or PKCN–Fl-siRNA (468.5 pmol siRNA in each sample), were prepared in nuclease-free water (100 µl) and added to a 100 µl of 90% FBS. The siRNA samples were incubated at 37°C for 24 h, and then 10 µl of a 10% sodium dodecyl sulfate solution was added to them and the mixtures were heat-denatured for 5 min at 100°C. The siRNA from the samples were isolated by hot phenol extraction followed by ethanol precipitation. An aliquot of each sample (93.8 pmol per lane) was loaded on a 10–20% polyacrylamide gel (Lonza, Rockland, ME, USA) and the electrophoresis was performed at 100 V for 40 min. Gels were stained with ethidium bromide (0.1 μg/ml) and visualized by ChemiDoc XRS system (Bio-Rad Laboratories, Hercules, CA, USA).
In vivo biodistribution of the PKCNs
The biodistribution of the PKCNs was determined using Fl-siRNA. PKCNs encapsulating Fl-siRNA were formulated as described above. Either PKCN–Fl-siRNA (35 µg/kg siRNA) or DOTAP–Fl-siRNA complexes (35 µg/kg siRNA) were injected into three mice, respectively, via a jugular vein injection. The mice were sacrificed after 2 h and perfused using PBS (150 mM, pH 7.4). All organs were collected and placed in 5 ml of 20% (v/v) Triton X-100 and 80% (v/v) PBS (150 mM, pH 7.4). The organs were homogenized at 12 000 r.p.m. for 2 min and centrifuged at 5000 r.p.m. for 5 min. The fluorescence of fluorescein in the supernatant was then measured at 494 nm excitation and 510 nm emission, and the fluorescence from the organs of saline-injected mice was used as background. The fluorescent intensity of each organ was calculated based on the tissue weight.
Delivery of TNF-α siRNA with the PKCNs in vitro
RAW264.7 macrophages (1 × 105 cells/well, 96-well plate) in DMEM with 10% FBS were incubated with either PKCN–TNF-α siRNA, PKCN–scrambled siRNA, DOTAP–TNF-α siRNA complexes, Lipofectamine–TNF-α siRNA conjugates or free TNF-α siRNA for 4 h. All samples had 0.75 µg/ml of siRNA. The cells were washed three times and then incubated with fresh medium for 24 h. The cells were stimulated with 100 ng/ml lipopolysaccharide (LPS) for 4 h to induce TNF-α. The amount of extracellular TNF-α production was determined using an ELISA assay kit following the manufacture’s instructions in the kit.
Cytotoxicity of the PKCNs (MTT reduction assay)
An MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] reduction assay was performed to measure the cytotoxicity of the PKCNs. The macrophages (1 × 105 cells/well, 96-well plate) and HUVECs (1 × 106 cells/well, 12-well plate) were incubated with the PKCNs for 4 h. Cells were treated with the PKCNs at various particle concentrations (0.1–1 mg/ml). Next, 20 µl of MTT solution (5 mg/ml in PBS) was added to each well, and the cells were incubated for 2 h. Then, 200 µl of dimethyl sulfoxide was added to dissolve the resulting formazan crystals. After 10 min of incubation, the absorbance at 585 nm was measured using an Emax Microplate reader (Molecular Devices, Sunnyvale, CA, USA). Percentage cell viability was calculated by comparing the absorbance of the control cells to that of PKCN-treated cells.
Hemolysis with the PKCNs in vitro
A hemolysis assay was performed using the erythrocytes of mice. The erythrocytes were collected by centrifugation of mouse blood (6 ml) at 1500 g for 15 min and then washed five times with isotonic PBS (Dulbecco’s PBS, Gibco) at pH 7.4. The erythrocytes were resuspended in either PBS (three parts centrifuged erythrocytes plus 11 parts PBS) or distilled water (three parts centrifuged erythrocytes plus 11 parts water). The polyketal particle dispersions were prepared in PBS buffer with various concentrations (0.01–1 mg/ml). The erythrocyte stock dispersion (100 µl) was added to 1 ml of the polyketal particle dispersions. One milliliter of PBS without polyketal particles was used as the negative control (0% hemolysis), and 1 ml of distilled water was used as the positive control (100% hemolysis). The solutions were mixed and incubated for 4 h at 37°C in an incubator shaker and centrifuged at 13 000 r.p.m. for 15 min. The percentage of hemolysis was measured by UV–Vis analysis of the supernatant at 394 nm absorbance.
In vivo delivery of TNF-α siRNA with the PKCNs
A mouse model of acute liver failure was used to evaluate the ability of the PKCNs to deliver siRNA in vivo; 6- to 8-week-old female BALB/c mice were obtained from Jackson Laboratory (Bar Harbor, Maine). All procedures used in the animal studies were approved by the Institutional Animal Care and Use Committee at the Georgia Institute of Technology. Mice were anesthetized by isofluorane inhalation and were injected with either PKCN-TNF-α siRNA, PKCN–scrambled siRNA, DOTAP–TNF-α siRNA complexes, Lipofectamine–TNF-α siRNA conjugates or free TNF-α siRNA (either 35 µg/kg or 3.5 µg/kg siRNA) via a jugular vein injection. Immediately after the siRNA injection, LPS (2.5 µg/kg) from Escherichia coli 0111:B4 and 700 mg/kg d-galactosamine (GalN, 100 µl) were injected to induce acute liver failure via an intraperitoneal (i.p.) injection. After 24 h, blood was collected from the mice by a cardiac puncture and centrifuged at 4000 r.p.m. for 15 min. Plasma ALT levels were measured for liver function analysis using an ALT assay kit (Pointe Scientific Inc., Canton, MI, USA) and plasma TNF-α levels were also measured using an ELISA assay kit, using the protocols provided in the kit. For liver histology, livers were collected and frozen in optimal cutting temperature (OCT) solution. OCT solution-embedded livers were sliced to 10-μm sections using a Microm Cryo-Star HM 560MV Cryostat, and liver sections were stained with hematoxylin and eosin. For statistical analysis, all experiments were performed using six mice per group unless otherwise specified. Significance of results was determined via the paired t-test with P < 0.05.
Immunostaining of Kupffer cells in the liver
The ability of the PKCNs to target macrophages was determined qualitatively using immunohistochemistry. DiI (1,1′-dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate) [12.5% (w/w)]-loaded PKCNs (0.1 mg/mouse) were injected via a jugular vein injection and after 2 h, the livers were collected and preserved in OCT. The cryoprotective livers were then sliced into 10- m sections as described above. The 10- μm sections were fixed in ice-cold acetone for 2 min and rehydrated in tris-buffered saline (TBS, pH 7.0). To reduce nonspecific staining, the sections were incubated with 5% FBS in TBS for 30 min at room temperature. In order to visualize Kupffer cells, the sections were incubated with FITC-labeled anti-mouse F4/80 antigen (1:50; eBioscience) for 30 min. The sections were washed for another 10 min in TBS and imaged with a fluorescence microscope (Nikon E600, Nikon, Melville, NY, USA).
RESULTS AND DISCUSSION
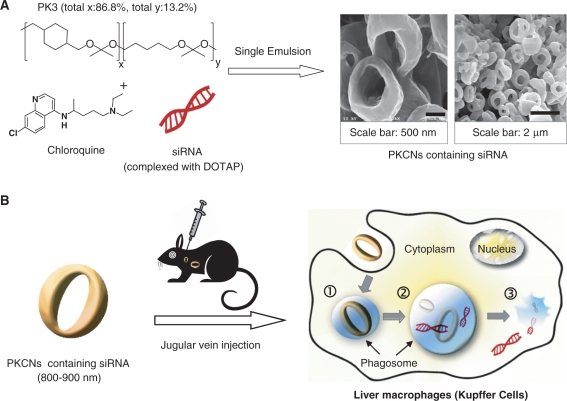
The PKCNs are a new delivery vehicle, designed to deliver siRNA to macrophages, they are composed of the acid-sensitive polymer PK3 and chloroquine. The mechanism by which the PKCNs are formulated and function are described in Figure 1. The PKCNs are formulated via a single emulsion solvent evaporation procedure, generating submicron particles, which have a donut shape. The PKCNs are designed to protect siRNA from serum nucleases, but after phagocytosis disrupt the phagosome and release siRNA into the cytoplasm. The PKCNs have this multifunctional capability because of the pH sensitivity of the ketal linkages in PK3 and chloroquine. PK3 has a hydrolysis half life of 1.8 days at pH 4.5 and 39 days at pH 7.4; therefore, the PKCNs should maintain their integrity at pH 7.4, but after phagocytosis, hydrolyze and cause an osmotic imbalance in the phagosome, leading to release of siRNA into the cytoplasm. The PKCNs can be freeze-dried and stored as a solid powder and also have excellent tissue biocompatibility because of their neutral degradation products. In summary, the PKCNs are a multifunctional siRNA delivery vehicle, which has the physical/chemical properties needed for translation into clinical trials.
Figure 1.
The PKCNs: a new siRNA delivery vehicle based on solid particles with high efficiency in vivo. (A) siRNA is encapsulated into the PKCNs via a single emulsion/solvent evaporation procedure, generating submicron particles (800–900 nm). (B) The PKCNs are injected into mice via a jugular vein injection and deliver siRNA to macrophages in vivo with high efficiency, due to their ability to protect siRNA from serum proteins, stimulate phagocytosis and disrupt phagosomes. (1) The PKCNs are phagocytosed by macrophages and trafficked into phagosomes; (2) the PKCNs degrade in the acidic environment of the phagosome because of the ketal linkages in PK3, and disrupt the phagosome via a colloid osmotic mechanism; and (3) siRNA is released into the cytoplasm.
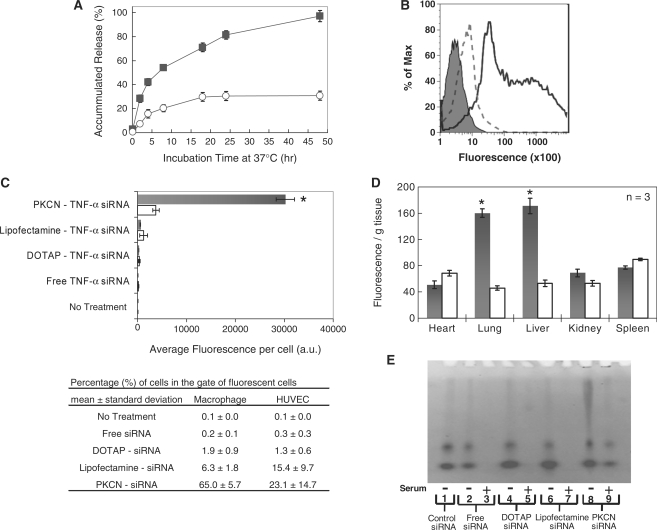
We investigated the pH-sensitive release kinetics of siRNA from the PKCNs to understand their behavior in the pH 7.4 environment of the blood and the acidic phagosome. Figure 2A demonstrates that the PKCNs release siRNA in a stimuli-responsive manner, at pH 7.4 they encapsulate siRNA with high stability, releasing only 20% after 48 h. In contrast, at pH 5.0 siRNA is rapidly released from the PKCNs, releasing 50% of the encapsulated siRNA within 7 h. Thus, the PKCNs should be able to protect siRNA from serum components and then release them after entering the acidic environment of the phagosome.
Figure 2.
The PKCNs are a pH-sensitive delivery vehicle that can enhance the uptake of siRNA in vitro and in vivo. (A) Accumulated release of Fl-siRNA from the PKCNs at phagosomal pH (pH 5.0) (black squares) and physiological pH (pH 7.4) (white circles) (each data represented as mean ± SD). (B) Enhanced cellular uptake of the PKCNs by macrophages in vitro (flow cytometry): macrophages (RAW264.7) were incubated with PKCNs encapsulating Fl-siRNA (solid line), DOTAP–Fl-siRNA complex (dashed line) and no treatment (shaded line) in DMEM with 10% FBS. All siRNA samples have 0.75 µg/ml siRNA. (C) Mean fluorescence of macrophages treated with PKCNs encapsulating Fl-siRNA and other delivery vehicles. Mean fluorescence was obtained by flow cytometry (mean ± SD). Statistical difference was performed between HUVEC and macrophages in microparticle uptake with P < 0.05 (asterisk). (D) In vivo biodistribution of PKCNs containing Fl-siRNA (35 µg/kg siRNA) (black bars) and DOTAP–Fl-siRNA complex (35 µg/kg siRNA; white bars) (mean ± SE); samples were injected into the jugular vein of mice (three mice per group), and the organs were collected and assayed after 2 h. Statistical difference of biodistribution was performed between PKCN–Fl-siRNA and DOTAP–Fl-siRNA in each organs with P < 0.05 (asterisks). (E) PKCNs protected siRNA from serum nucleases, whereas siRNA complexed with DOTAP and Lipofectamine degraded in serum. Samples were incubated in 37°C for 24 h. Lane 1: intact siRNA as a control; lane 2: free siRNA in nuclease-free water; lane 3: free siRNA in serum; lane 4: DOTAP–siRNA complex in nuclease-free water; lane 5: DOTAP–siRNA complex in serum; lane 6: Lipofectamine–siRNA conjugates in nuclease-free water; lane 7: Lipofectamine–siRNA conjugates in serum; lane 8: PKCN–siRNA in nuclease-free water; and lane 9: PKCN–siRNA in serum.
We performed experiments to determine if the PKCNs could enhance the delivery of siRNA into macrophages by phagocytosis in the presence of serum in vitro and in vivo. For in vitro experiments, the PKCNs were formulated with Fl-siRNA and incubated with macrophages (RAW264.7) or endothelial cells (HUVEC) in 10% serum. The uptake of siRNA was then measured by flow cytometry and compared against macrophages incubated with Fl-siRNA complexed with either DOTAP (a cationic lipid) (DOTAP–Fl-siRNA) or Lipofectamine2000 (Lipofectamine–Fl-siRNA). Figure 2B and C demonstrates that DOTAP–Fl-siRNA or Lipofectamine–Fl-siRNA by itself is inefficient at delivering Fl-siRNA into macrophages in the presence of serum, presumably due to their rapid degradation by serum proteins. In contrast, the PKCNs were two to three orders of magnitude more effective at delivering Fl-siRNA into macrophages than DOTAP–Fl-siRNA complexes. The PKCNs, therefore, have the physical stability needed to promote uptake through the phagocytic pathway in the presence of serum proteins.
We also investigated if the PKCNs could similarly enhance the uptake of siRNA in vivo. Mice were injected, via the jugular vein, with either PKCNs containing Fl-siRNA (PKCN–Fl-siRNA) or DOTAP–Fl-siRNA complexes. The biodistribution of the injected siRNA was then determined after 2 h. Figure 2D demonstrates that the PKCNs are capable of concentrating siRNA into tissues (liver and lung) with high macrophage content, suggesting that the PKCNs are efficiently taken up by phagocytosis in vivo. In contrast, DOTAP–Fl-siRNA complexes were relatively inefficient in delivering siRNA in vivo presumably because of their rapid degradation in serum. For example, mice treated with PKCN–Fl-siRNA had a 4-fold higher fluorescence in liver than DOTAP–Fl-siRNA.
A key requirement for the successful delivery of siRNA in vivo is protection of the siRNA from serum nucleases. We therefore investigated if the PKCNs could protect encapsulated siRNA from serum nucleases. siRNA samples, free siRNA, DOTAP-siRNA, Lipofectamine2000-siRNA, and PKCN–siRNA particles were incubated in either FBS or nuclease-free water at 37°C for 24 h and then analyzed by polyacrylamide gel electrophoresis. Figure 2E demonstrates that the PKCNs can protect siRNA from serum nucleases, presumably due to their solid nature, which prevents nucleases from accessing the siRNA. In contrast, self-assembled complexes of cationic lipids, such as DOTAP and Lipofectamine2000, were not able to protect siRNA from serum nucleases.
We investigated if the PKCNs could deliver functional siRNA into macrophages and inhibit gene expression in vitro and in vivo. siRNA targeting TNF-α siRNA was encapsulated into the PKCNs (Table 1), and their ability to inhibit TNF-α production from macrophages stimulated with LPS was investigated. The TNF-α siRNA sequence encapsulated in the PKCNs was originally developed by Sørensen et al. (13), who used this sequence to inhibit TNF-α gene expression in mice after an LPS injection. Figure 3A demonstrates that the PKCNs can significantly improve the functional delivery of siRNA therapeutics to macrophages in 10% serum. For example, at a concentration of 0.75 µg/ml siRNA, PKCN–TNF-α siRNA inhibited TNF-α production from macrophages by 80%, whereas DOTAP–TNF-α siRNA complexes and free TNF-α siRNA, at the same siRNA concentration, had no inhibitory effect, presumably because of their rapid degradation in serum and low uptake (4–6). PKCNs, which encapsulated scrambled siRNA, also had no inhibitory effect on TNF-α production from macrophages.
Table 1.
Loading efficiency of siRNA in PK3 or PLGA particles and particle sizes
| Submicron particles | PK3–siRNA particle without chloroquine | PK3–siRNA particle with chloroquine (PKCN–siRNA) | PLGA–siRNA particle with chloroquine |
|---|---|---|---|
| Average loading efficiency (%)a | 42.43 | 59.05 | 47.95 |
| Particle size (nm)b | 934.2 ± 59.3 | 887.7 ± 56.7 | 881.0 ± 514.9 |
aThe loading efficiency of Fl-siRNA in the PKCNs was determined by fluorescence of fluorescein (λex/λem = 494/518 nm). Loading efficiency (%) = (amount of Fl-siRNA loaded in submicron particle)/(initial amount of Fl-siRNA used to make submicron particle) × 100.
bParticle size was determined by DLS (mean ± SD).
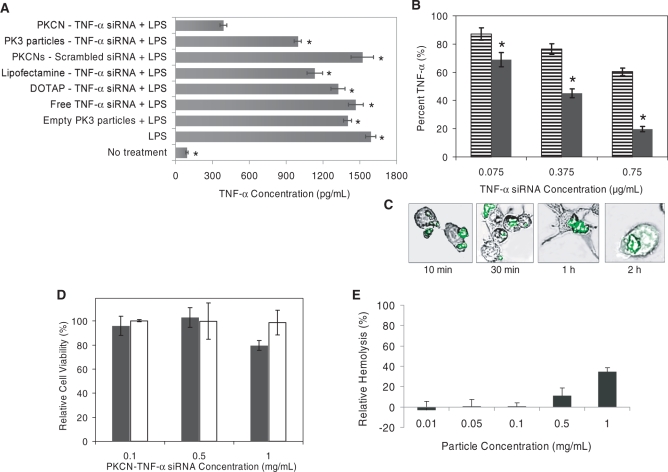
Figure 3.
The PKCNs can deliver siRNA to RAW264.7 macrophages efficiently in serum with low cytotoxicity, and chloroquine enhances their efficacy (all data given as mean ± SD). (A) The PKCNs reduced extracellular TNF-α from LPS-stimulated macrophage by 80%; macrophages were treated with either PKCN–TNF-α siRNA (0.1 mg/ml), PK3–TNF-α siRNA particles (without chloroquine) (0.1 mg/ml) or DOTAP–TNF-α siRNA complex (3.25 µg/ml) for 4 h. Cells were washed three times and TNF-α production was measured using an ELISA kit. All siRNA samples have 0.75 µg/ml siRNA. Significance of results was determined via the paired t-test between the PKCN–TNF-α siRNA and other delivery vehicles with P < 0.05 (asterisks). (B) Chloroquine enhances the efficacy of siRNA delivery: PKCN–TNF-α siRNA (black bars) and PK3–TNF-α siRNA particles (without chloroquine) (striped bars). Macrophages were treated with siRNA samples for 4 h. Cells were washed three times and TNF-α production was measured using an ELISA kit. Statistical difference was performed between PKCN–TNF-α siRNA and PK3–TNF-α siRNA particles (without chloroquine) at each concentration with P < 0.05 (asterisks). (C) Uptake of PKCNs by RAW264.7 macrophages with time in vitro (confocal microscopy). (D) PKCN–TNF-α siRNA caused no cellular toxicity at a concentration (0.1 mg/ml PKCN–TNF-α siRNA) required for 80% inhibition of TNF-α production (MTT assay). Macrophages (black bars) and HUVEC (white bars) were treated with PKCN–TNF-α siRNA for 4 h. (E) Hemolytic activity of PKCNs.
The cytoplasmic delivery of siRNA is also a major challenge in the delivery of siRNA. This is particularly important with macrophages, which rapidly degrade phagocytosed materials in their phagosomes. The PKCNs contain the endosomal disruptive molecule chloroquine, which should augment the delivery of siRNA. We investigated if chloroquine within the PKCNs enhanced the delivery of siRNA to macrophages in cell culture. Macrophages were incubated with either PKCN–TNF-α siRNA or PK3–TNF-α siRNA particles (without chloroquine) in 10% serum, stimulated with LPS, and then analyzed for TNF-α production. Figure 3B demonstrates that chloroquine enhances the delivery efficiency of the PKCNs. For example, at a concentration of 1 µg/ml siRNA, PKCN–TNF-α siRNA inhibited TNF-α production from macrophages by 80%, in contrast PK3–TNF-α siRNA particles without chloroquine caused only a 50% inhibition of TNF-α production. We performed further confocal microscopy experiments to determine if the PKCNs could deliver siRNA to the cytoplasm of macrophages. Confocal microscopy has been widely used to measure the intracellular distribution and efficacy of siRNA delivery vehicles, these studies demonstrate that optimal gene inhibition occurs when siRNA is delivered into the cytoplasm (14,15). Figure 3C demonstrates that RAW264.7 macrophages have internalized particles within 10 min of incubation and that these particles appear to be in phagosomes, based on their punctate appearance. However, after 2 h the cellular fluorescence distribution is dramatically altered, and now appears to diffuse through the cytoplasm, suggesting that the siRNAs have entered the cytoplasm.
We performed experiments with macrophages, endothelial cells and red blood cells, to determine the toxicity profile of the PKCNs. Macrophages and endothelial cells were incubated with various concentrations of the PKCNs, and cellular toxicity was determined with the MTT assay. Figure 3D demonstrates that the PKCNs caused no cellular toxicity to macrophages at a 0.1 mg/ml concentration, which is the concentration required for 80% inhibition of TNF-α production (in cell culture). The PKCNs also did not cause any cellular toxicity to HUVECs at concentrations up to 1.0 mg/ml. We also investigated the effects of the PKCNs on red blood cell membranes. The hemolysis of red blood cells is a common problem with drug delivery vehicles, particularly microparticles and cationic lipid-based delivery vehicles. Figure 3E demonstrates that the PKCNs do not induce hemolysis of RBCs at a concentration of 0.1 mg/ml, and that even at a high concentration of 1 mg/ml they only induced 34% hemolysis.
The ability of the PKCNs to enhance the delivery of TNF-α targeted siRNA in vivo was investigated in a mouse model of LPS-induced acute liver failure. Acute liver failure causes thousands of deaths each year and strategies that can enhance the treatment of acute liver failure are greatly needed. TNF-α secreted by Kupffer cells (liver macrophages) plays a major role in the development of acute liver failure, and we therefore investigated if the PKCNs could enhance the delivery of siRNA, targeted against TNF-α and alleviate acute liver failure. Acute liver failure was induced by an i.p. injection of LPS (2.5 µg/kg) and GalN (700 mg/kg), and siRNA samples were injected via a jugular vein injection.
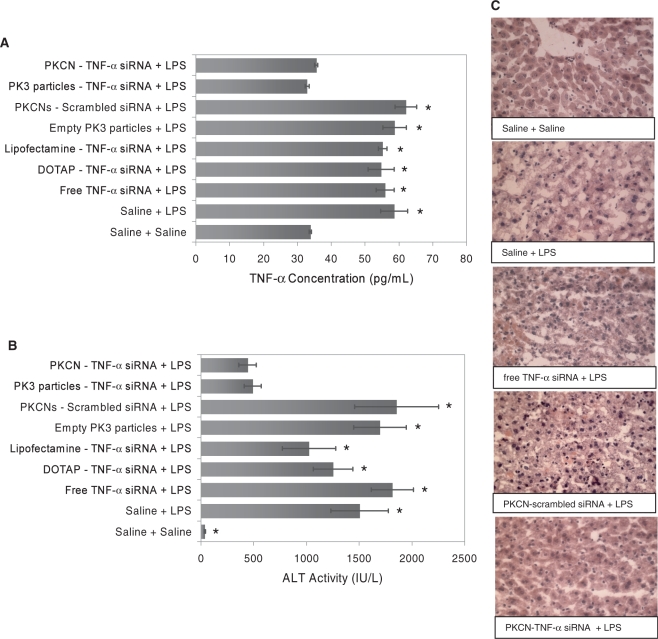
Figure 4A demonstrates that freeze-dried PKCNs can enhance the efficacy of TNF-α siRNA in vivo, inhibit TNF-α production and improve the therapeutic outcome of acute liver failure. For example, at a concentration of 35 µg/kg, PKCN–TNF-α siRNA reduced TNF-α levels in the serum to baseline levels, during LPS-induced acute liver failure. In contrast, PKCNs with scrambled siRNA, DOTAP–TNF-α siRNA, Lipofectamine–TNF-α siRNA or free TNF-α siRNA had no effect on serum levels of TNF-α. PKCN–TNF-α siRNA was also able to reduce the pathologic effects of LPS-induced liver failure. For example, at a concentration of 35 µg/kg, PKCN–TNF-α siRNA reduced the serum ALT values by 69.1%, whereas free TNF-α siRNA and PKCNs with scrambled siRNA (35 µg/kg siRNA) had no effect on reducing ALT levels (Figure 4B). Finally, histology sections of LPS-treated mice demonstrated that PKCNs with TNF-α siRNA protected the liver from LPS-induced liver damage, whereas PKCNs with scrambled siRNA, DOTAP–TNF-α siRNA complex or free TNF-α siRNA could not save hepatocytes from LPS-induced liver damage (Figure 4C).
Figure 4.
The PKCNs enhance the delivery of siRNA in vivo. Mice were injected with LPS (2.5 µg/kg) and GalN (700 mg/kg) i.p. to induce acute liver failure and injected with siRNA samples (35 µg/kg siRNA) via the jugular vein. The livers and blood of the mice were collected and assayed at 24 h after siRNA treatment. (A) PKCNs containing TNF-α siRNA reduce TNF-α levels in the serum to baseline levels (ELISA assay; TNF-α concentration in serum) (mean ± SE). Significance of results was determined via the paired t-test between the PKCN–TNF-α siRNA and other delivery vehicles with P < 0.05 (asterisks). (B) PKCNs containing TNF-α siRNA reduce the serum ALT values (mean ± SE). Significance of results was determined via the paired t-test between the PKCN–TNF-α siRNA and other delivery vehicles with P < 0.05 (asterisks). (C) Liver histology study using hematoxylin and eosin staining (tissue thickness sliced: 10 µm; magnification objectives: ×40) indicates that only PKCNs with TNF-α siRNA can protect the liver from LPS-induced liver damage.
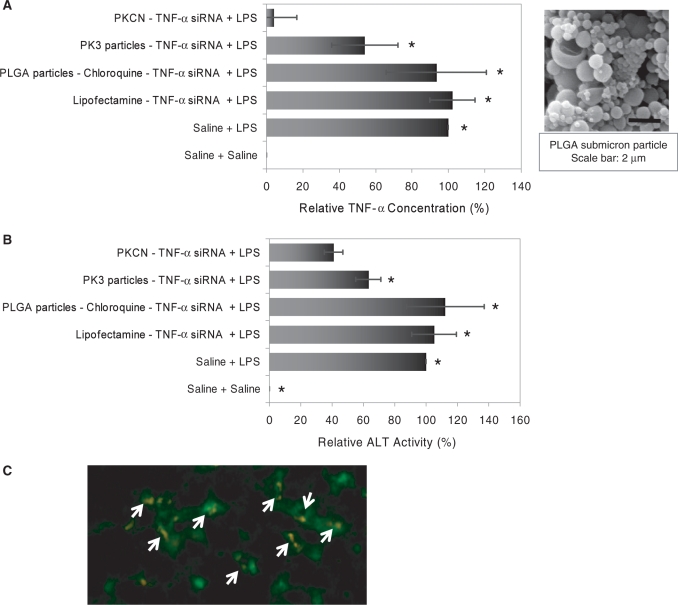
We performed further experiments to determine if the high efficacy of the PKCNs was due to their ‘hard’ solid nature that enhances their serum stability or if their acid-catalyzed degradation after phagocytosis was also important. We therefore investigated if PLGA submicron particles, containing chloroquine and TNF-α siRNA (PLGA–chloroquine–TNF-α siRNA), could also deliver siRNA to Kupffer cells in vivo, and compared their efficacy to the PKCNs. Figure 5A and B demonstrates that the acid sensitivity of the PKCNs is critical for in vivo efficacy, for example at a TNF-α siRNA dose of 3.5 µg/kg, PKCN–TNF-α siRNA-reduced serum ALT levels by 59.0% and serum TNF-α values by 96.0%, whereas PLGA–chloroquine–TNF-α siRNA had no effect presumably, because of their slower hydrolysis kinetics (16,17). At the same siRNA concentration, Lipofectamine–TNF-α siRNA had no effect on ALT and TNF-α levels.
Figure 5.
The PKCNs can deliver siRNA with high efficiency in vivo and the acid sensitivity of the PKCNs is critical for in vivo efficacy. Mice were injected with LPS (2.5 µg/kg) and GalN (700 mg/kg) i.p. to induce acute liver failure and injected with siRNA samples (3.5 µg/kg siRNA) via the jugular vein. Serum TNF-α concentration and ALT activity were measured at 24 h after treatment with siRNA samples. Significance of results was determined via the paired t-test between the PKCN–TNF-α siRNA and other delivery vehicles with P < 0.05 (asterisks). (A) The PKCNs reduced serum TNF-α values, whereas PLGA submicron particles had no effect. TNF-α concentration in the plasma was measured by ELISA (mean ± SE). (B) The PKCNs reduced serum ALT levels (mean ± SE), whereas PLGA submicron particles had no effect. (C) The PKCNs were localized in Kupffer cells. FITC-labeled F4/80 antibody (Pan macrophage marker) was used for Kupffer cell staining. DiI-loaded PKCNs (orange color) were taken up by Kupffer cells (green color) in vivo—white arrows. No PKCNs were found in hepatocytes—dark area (no green color). The picture was imaged by fluorescence microscopy (×20).
Kupffer cells (liver macrophages) play a key role in the development of acute liver failure. The ability of the PKCNs to target Kupffer cells in vivo was therefore determined using PKCNs that encapsulated the fluorescent dye DiI. DiI–PKCNs were injected into mice via the jugular vein, and after 2 h the mice were harvested and analyzed by histology for Kupffer cell staining. Figure 5C demonstrates that the DiI–PKCNs are localized in Kupffer cells after a systemic injection of DiI–PKCNs, whereas DiI–PKCNs (orange color) were not found in hepatocytes (dark area—no green color).
In summary, in this report we present a new siRNA delivery vehicle termed as the PKCNs, which can efficiently deliver siRNA to macrophages in vivo. In contrast to delivery vehicles composed of cationic lipids or polycations, the PKCNs are ‘hard’ materials composed of water-insoluble polymers and have a strong thermodynamic driving force to maintain their integrity in vivo. The PKCNs are acid sensitive, and hydrolyze after phagocytosis in the acidic environment of the phagosome, allowing them to rapidly release siRNA after cell internalization and potentially disrupt the phagosome through a colloid osmotic mechanism. The PKCNs were able to deliver TNF-α siRNA to Kupffer cells (liver macrophages) in vivo and rescue hepatocytes from LPS-induced toxicity at an siRNA dose of 3.5 µg/kg. Based on these observations, we anticipate numerous uses of the PKCNs for siRNA delivery to macrophages in vivo.
FUNDING
Georgia Tech/Emory Center for the Engineering of Living Tissues (NSF-EEC-9731643); National Science Foundation Career Award (NSF-BES-0546962); National Institutes of Health (NIH UO1 HL80711-01); National Institutes of Health (NIH R21 EB006418); Johnson & Johnson/Georgia Tech Health Care Innovation Seed Grant Proposal; Coulter Translational Research Award. Funding for open access charge: NSF-BES-0546962 Career Award (N.M.),National Institutes of Health UO1 HL80711-01 (N.M.), National Institutes of Health R21 EB006418 (N.M.).
Conflict of interest statement. None declared.
REFERENCES
- 1.Dykxhoorn DM, Palliser D, Lieberman J. The silent treatment: siRNAs as small molecule drugs. Gene Ther. 2006;13:541–552. doi: 10.1038/sj.gt.3302703. [DOI] [PubMed] [Google Scholar]
- 2.Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature. 2004;431:371–378. doi: 10.1038/nature02870. [DOI] [PubMed] [Google Scholar]
- 3.Li CX, Parker A, Menocal E, Xiang S, Borodyansky L, Fruehauf JH. Delivery of RNA interference. Cell Cycle. 2006;5:2103–2109. doi: 10.4161/cc.5.18.3192. [DOI] [PubMed] [Google Scholar]
- 4.Haupenthal J, Baehr C, Kiermayer S, Zeuzem S, Piiper A. Inhibition of RNAse A family enzymes prevents degradation and loss of silencing activity of siRNAs in serum. Biochem. Pharmacol. 2006;71:702–710. doi: 10.1016/j.bcp.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Turner JJ, Jones SW, Moschos SA, Lindsay MA, Gait MJ. MALDI-TOF mass spectral analysis of siRNA degradation in serum confirms an RNAse A-like activity. Mol. Biosyst. 2007;3:43–50. doi: 10.1039/b611612d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 2007;25:1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 7.Spagnou S, Miller AD, Keller M. Lipidic carriers of siRNA: differences in the formulation, cellular uptake, and delivery with plasmid DNA. Biochemistry. 2004;43:13348–13356. doi: 10.1021/bi048950a. [DOI] [PubMed] [Google Scholar]
- 8.Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat. Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desigaux L, Sainlos M, Lambert O, Chevre R, Letrou-Bonneval E, Vigneron JP, Lehn P, Lehn JM, Pitard B. Self-assembled lamellar complexes of siRNA with lipidic aminoglycoside derivatives promote efficient siRNA delivery and interference. Proc. Natl Acad. Sci. USA. 2007;104:16534–16539. doi: 10.1073/pnas.0707431104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozema DB, Lewis DL, Wakefield DH, Wong SC, Klein JJ, Roesch PL, Bertin SL, Reppen TW, Chu Q, Blokhin AV, et al. Dynamic polyconjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc. Natl Acad. Sci. USA. 2007;104:12982–12987. doi: 10.1073/pnas.0703778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang SC, Bhide M, Crispe IN, Pierce RH, Murthy N. Polyketal copolymers: a new acid-sensitive delivery vehicle for treating acute inflammatory diseases. Bioconjug. Chem. 2008;19:1164–1169. doi: 10.1021/bc700442g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel MM, Zeles MG, Manning MC, Randolph TW, Anchordoquy TJ. Degradation kinetics of high molecular weight poly(L-lactide) microspheres and release mechanism of lipid:DNA complexes. J. Pharm. Sci. 2004;93:2573–2584. doi: 10.1002/jps.20176. [DOI] [PubMed] [Google Scholar]
- 13.Sorensen DR, Leirdal M, Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J. Mol. Biol. 2003;327:761–766. doi: 10.1016/s0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- 14.Howard KA, Rahbek UL, Liu X, Damgaard CK, Glud SZ, Andersen MO, Hovgaard MB, Schmitz A, Nyengaard JR, Besenbacher F, et al. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol. Ther. 2006;14:476–484. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Shen X, Xu KF, Fan Q, Pacheco-Rodriguez G, Moss J, Vaughan M. Association of brefeldin A-inhibited guanine nucleotide-exchange protein 2 (BIG2) with recycling endosomes during transferrin uptake. Proc. Natl Acad. Sci. USA. 2006;103:2635–2640. doi: 10.1073/pnas.0510599103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollauf EJ, Berkland C, Kim KK, Pack DW. In vitro degradation of polyanhydride/polyester core-shell double-wall microspheres. Int. J. Pharm. 2005;301:294–303. doi: 10.1016/j.ijpharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Witschi C, Doelker E. Influence of the microencapsulation method and peptide loading on poly(lactic acid) and poly(lactic-co-glycolic acid) degradation during in vitro testing. J. Control. Release. 1998;51:327–341. doi: 10.1016/s0168-3659(97)00188-0. [DOI] [PubMed] [Google Scholar]