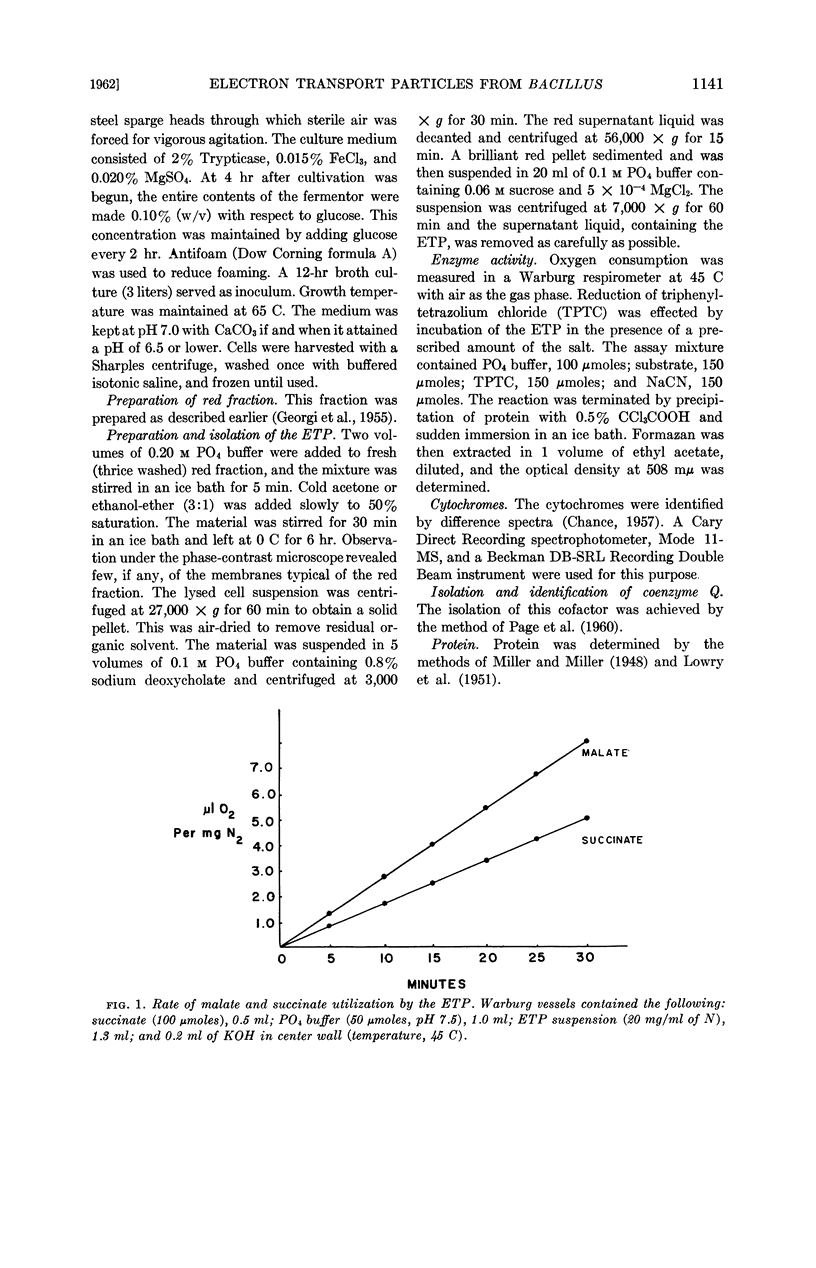
Abstract
Downey, Ronald J. (University of Nebraska, Lincoln), Carl E. Georgi, and Walter E. Militzer. Electron transport particles from Bacillus stearothermophilus. J. Bacteriol. 83:1140–1146. 1962—Electron-transport particles (ETP) have been isolated from Bacillus stearothermophilus. They are capable of oxidizing such substrates as succinate, malate, diphosphopyridine nucleotide, p-phenylenediamine, and hydroquinone. Difference spectra indicated bands of cytochromes a3, b, and c. A chromatographic procedure for purification of cytochrome c has been described.
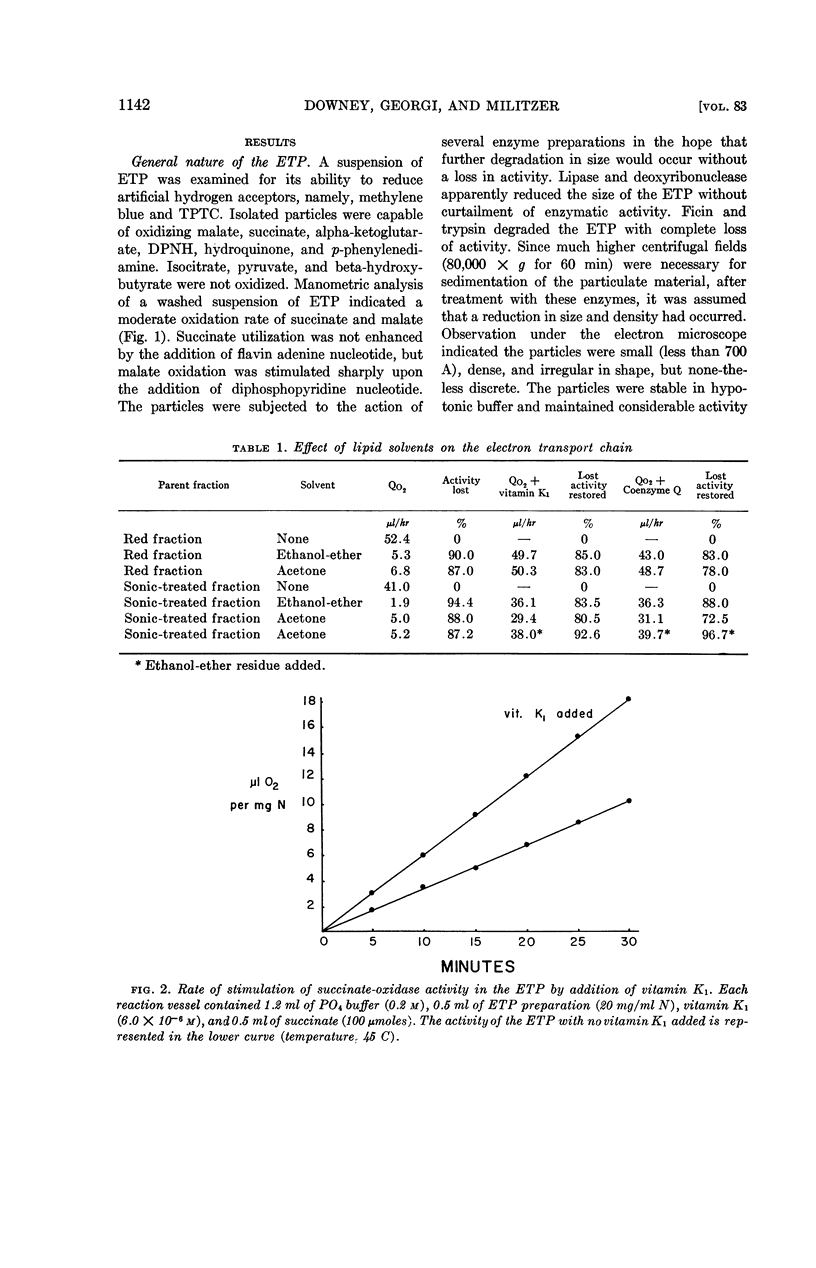
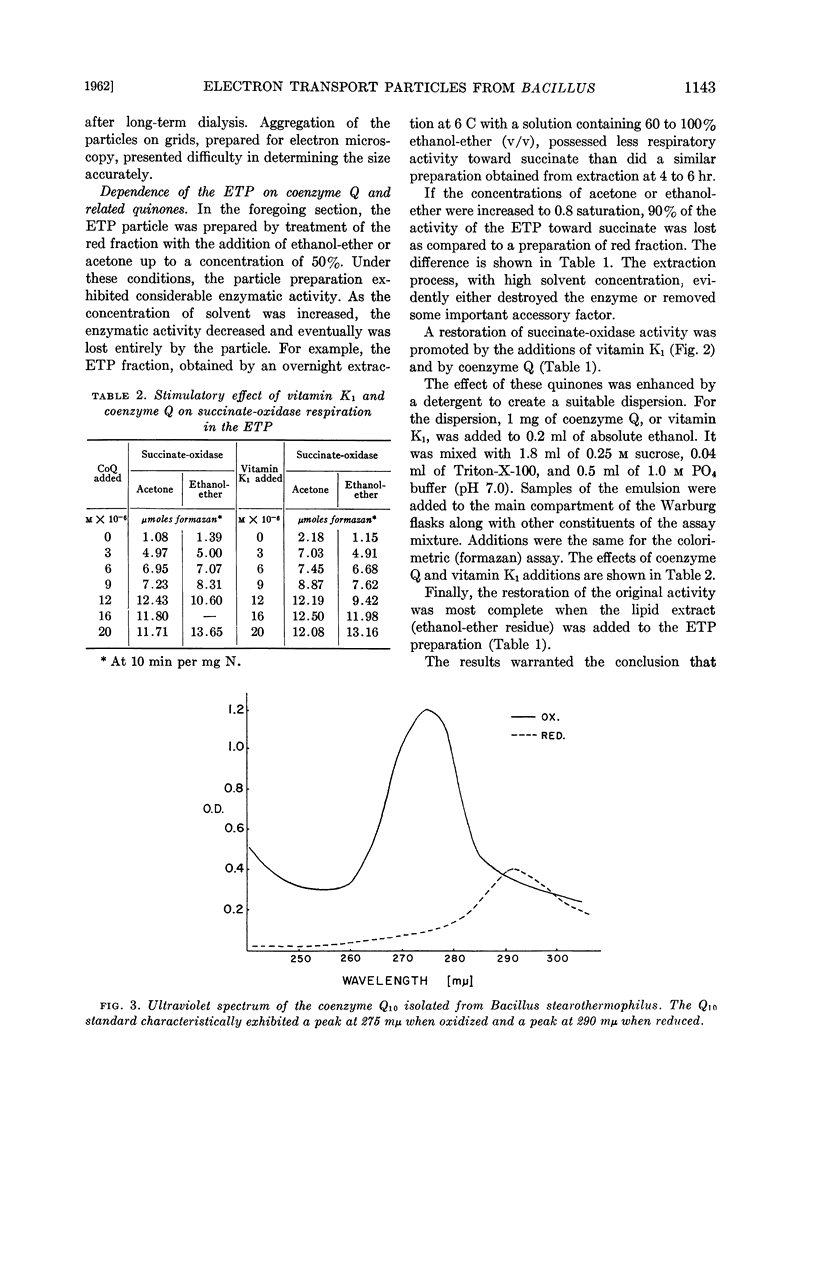
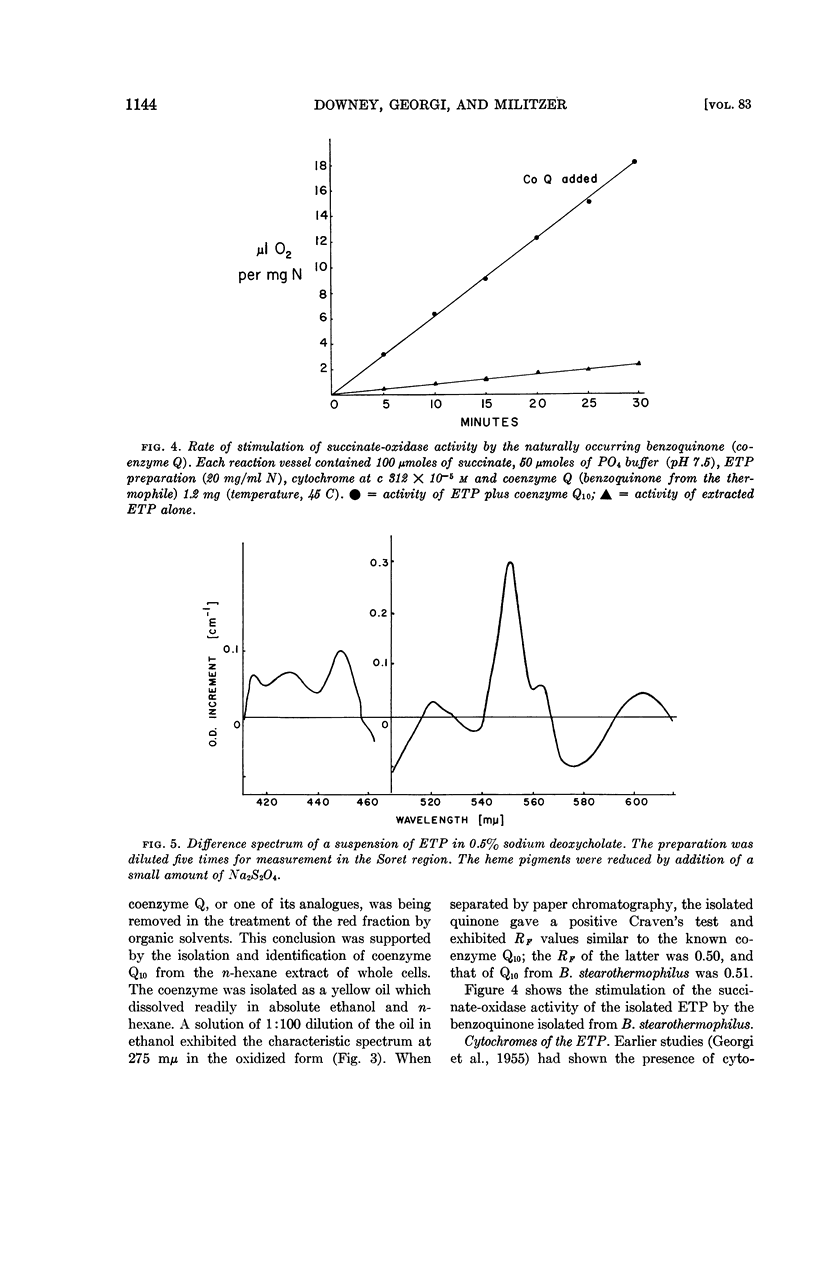
The role of quinonelike intermediates in the ETP was implied by the restorative effect of coenzyme Q and vitamin K1 on material treated with lipid solvent. Isolation and identification of coenzyme Q from the thermophile indicated it was similar to coenzyme Q10 of mammalian sources.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COTA-ROBLES E. H., MARR A. G., NILSON E. H. Submicroscopic particles in extracts of Azotobacter agilis. J Bacteriol. 1958 Mar;75(3):243–252. doi: 10.1128/jb.75.3.243-252.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGI C. E., MILITZER W. E., DECKER T. S. The organelle nature of a particle isolated from Bacillus stearothermophilus. J Bacteriol. 1955 Dec;70(6):716–725. doi: 10.1128/jb.70.6.716-725.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGI C. E., MILITZER W., BURNS L., HEOTIS J. On the existence of a cell granule in a thermophilic bacterium. Proc Soc Exp Biol Med. 1951 Mar;76(3):598–601. doi: 10.3181/00379727-76-18572. [DOI] [PubMed] [Google Scholar]
- KASHKET E. R., BRODIE A. F. Subcellular distribution of a biologically active naphthoquinone Mycobacterium phlei. Biochim Biophys Acta. 1960 Jun 3;40:550–552. doi: 10.1016/0006-3002(60)91404-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MUDD S. Cytology of bacteria. I. The bacterial cell. Annu Rev Microbiol. 1954;8:1–22. doi: 10.1146/annurev.mi.08.100154.000245. [DOI] [PubMed] [Google Scholar]
- PAGE A. C., Jr, GALE P., WALLICK H., WALTON R. B., McDANIEL L. E., WOODRUFF H. B., FOLKERS K. Coenzyme Q. 17. Isolation of coenzyme Q10 from bacterial fermentation. Arch Biochem Biophys. 1960 Aug;89:318–321. doi: 10.1016/0003-9861(60)90062-x. [DOI] [PubMed] [Google Scholar]
- WEIBULL C., BECKMAN H., BERGSTROM L. Localization of enzymes in Bacillus megaterium, strain M. J Gen Microbiol. 1959 Jun;20(3):519–531. doi: 10.1099/00221287-20-3-519. [DOI] [PubMed] [Google Scholar]


