Abstract
Crc is a key global translational regulator in Pseudomonads that orchestrates the hierarchy of induction of several catabolic pathways for amino acids, sugars, hydrocarbons or aromatic compounds. In the presence of amino acids, which are preferred carbon sources, Crc inhibits translation of the Pseudomonas putida alkS and benR mRNAs, which code for transcriptional regulators of genes required to assimilate alkanes (hydrocarbons) and benzoate (an aromatic compound), respectively. Crc binds to the 5′-end of these mRNAs, but the sequence and/or structure recognized, and the way in which it inhibits translation, were unknown. We have determined the secondary structure of the alkS mRNA 5′-end through its sensitivity to several ribonucleases and chemical reagents. Footprinting and band-shift assays using variant alkS mRNAs have shown that Crc specifically binds to a short unpaired A-rich sequence located adjacent to the alkS AUG start codon. This interaction is stable enough to prevent formation of the translational initiation complex. A similar Crc-binding site was localized at benR mRNA, upstream of the Shine–Dalgarno sequence. This allowed predicting binding sites at other Crc-regulated genes, deriving a consensus sequence that will help to validate new Crc targets and to discriminate between direct and indirect effects of this regulator.
INTRODUCTION
Free-living bacteria, particularly those species containing large genomes, usually have a versatile metabolism that allows the use of many different compounds as a source of carbon and energy. This facilitates survival in different habitats and adaptation to changing environmental conditions. Metabolic versatility is linked to a tight though flexible regulation of the expression of the different metabolic pathways involved, which occurs through both specific and global regulation systems. Several distinct global regulation networks can participate in coordinating gene expression programmes under different situations (1). Some of them allow cells to respond to stress conditions such as starvation or oxygen limitation. Many bacteria possess as well global regulation systems that coordinate metabolism under conditions of feast, i.e. when several possible carbon sources are available at high concentrations. The regulatory outcome, named catabolite repression control, allows cells to induce the genes required for the assimilation of a preferred compound, inhibiting at the same time the uptake and/or the expression of genes required for the catabolism of other non-preferred compounds, adapting metabolism accordingly. Catabolite repression can have an important impact on cell behaviour, modifying for example the expression of virulence factors and the infecting ability in pathogenic bacteria (2).
The molecular mechanisms responsible for catabolite repression control can differ markedly in distinct bacterial species (2,3). In Pseudomonads, which are characterized by a great metabolic flexibility, catabolite repression is still poorly understood, in spite of the importance of these bacteria in biotechnology, in medicine and in the environment. Available evidence indicates that, in these bacterial species, catabolite repression occurs through mechanisms that are very different from those present in other model organisms such as Escherichia coli or Bacillus subtilis (4). Several global regulatory mechanisms leading to catabolite repression control have been identified in Pseudomonas aeruginosa and in P. putida. One of these systems relies on the Crc protein, a master regulator of carbon metabolism. Crc represses the expression of genes involved in the assimilation of some sugars, nitrogenated compounds, aromatic compounds and hydrocarbons when other preferred substrates are present (5–12). Contrary to what has been observed in other bacterial species, succinate or some amino acids are preferred over glucose in Pseudomonads. A recent genomic and proteomic analysis has shown that Crc coordinates the hierarchical use of amino acids in P. putida when cells grow in a complete medium, affecting the expression of more than 130 genes (13). Most of these effects are probably indirect. The molecular mechanism through which Crc inhibits gene expression has been determined for the alkane and for the benzoate degradation pathways (14,15). In both cases, Crc directly controls the expression of the transcriptional activator of the pathway genes (AlkS for the alkane pathway, and BenR for the benzoate pathway). Crc is an RNA-binding protein that binds to the 5′-end of the alkS and benR mRNAs, inhibiting their translation in vivo (14,15). Thus, by decreasing the synthesis of AlkS, or of BenR, Crc indirectly reduces the mRNA levels of all the genes that are under the influence of these two specific transcriptional activators. Crc does not contain a known specific RNA-binding motif. Moreover, no information exists on the RNA determinants that are specifically recognized by Crc.
In this work, we report the characterization of the Crc-binding site at the alkS mRNA by using a combination of footprinting techniques and mutagenesis analysis. Our data show that Crc binds to an unpaired AU-rich sequence motif in the coding sequence of alkS mRNA and prevents the formation of active ribosomal initiation complex. Crc was found to bind to a similar AU-rich motif at the benR mRNA. Based on these two targets, other potential Crc-binding sites were predicted for several Crc-repressed genes. These data will help to discriminate between direct and indirect effects of this global regulator, in order to unravel the Crc-dependent regulatory network.
MATERIALS AND METHODS
Protein purification
The Crc protein containing a 6xHis tag at the C-terminus, which is biologically active, was overproduced in E. coli BL21(DE3) (pLysS) containing plasmid pCRCH, and purified using an Ni–NTA column, as described previously (14). E. coli BL21(DE3) (pLysS) (16) and plasmid pCRCH, which contains the crc(6xHis) gene (14), have been described earlier.
RNA fragments for in vitro analyses
The 26 or 33 nt RNA oligonucleotides used were chemically synthesized and obtained from Sigma. Larger RNA fragments were obtained by in vitro transcription with T7 RNA polymerase, using as substrate PstI-linearized plasmids palkS-78 or palkS-200. These plasmids, which derive from pGEM-T-Easy (Promega), contain different segments of the 5′ leader of alkS mRNA (positions 1–78 or −37 to 200, respectively, relative to promoter PalkS2 transcription start site) cloned between the ApaI and PstI sites of the vector. These alkS segments can be transcribed from the T7 promoter of the vector. The run-off transcripts obtained contain, in addition to the mentioned alkS mRNA segments, 14 extra nts at the 5′-end and one extra nt at the 3′-end, derived from vector sequences. Transcription was performed for 1 h at 37°C; reactions (1 ml) contained 40 mM Tris–HCl (pH 8), 15 mM MgCl2, 50 mM NaCl, 5 mM spermidine, 5 mM DTT, 4 mM ATP, CTP GTP and UTP, bovine serum albumin at 50 µg/ml, RNasin (300 U), 0.1 µg/ml of DNA template (linearized with PstI), and 800 U of T7 RNA polymerase. The RNA transcript was phenol-extracted and purified on a denaturing 8 M urea–8% polyacrylamide gel. The full-length RNA transcript corresponding to the alkS leader region was eluted in a buffer containing 0.5 M ammonium acetate, 1 mM EDTA, 16% phenol and 0.1% SDS overnight at 4°C, phenol-extracted and precipitated with ethanol. Gel-purified RNA was dephosphorylated with calf intestine alkaline phosphatase (Roche).
The 5′-end labelling of RNA fragments, RNA oligonucleotides, or DNA oligonucleotides was performed with T4 polynucleotide kinase and [γ-32P]ATP, as described (17). Labelled RNAs were purified on a denaturing 8% polyacrylamide–urea gel, eluted and precipitated with ethanol. Small RNA oligonucleotides were purified though MicroSpin G-25 columns (GE Healthcare).
Probing of mRNA secondary structure
RNA secondary structure probing was performed essentially as described (18). Prior to its use, 5′-end-labelled RNA was denatured for 1 min at 90°C, chilled on ice for 3 min, and allowed to fold at 20°C in 50 mM Tris–HCl (pH 7.5), 5 mM MgCl2, 50 mM KCl. One microgram of tRNA was then added and RNA was digested with RNase T1 (0.5 and 1 U), RNase T2 (0.05 and 0.1 U), RNase V1 (0.005 and 0.0025 U) or RNase A (0.0025–0.01 µg/ml) for 6 min at 20°C. RNases were added at concentrations optimized to yield no more than one cut per RNA molecule. Reactions were stopped by adding 20 µl of stop solution (1 M guanidine thyocyanate, 0.167% N-lauryl-sarcosine, 10 mM DTT, 83% isopropanol) and precipitated with ethanol. Digestion products were resolved on denaturing 8% urea–polyacrylamide gels.
Footprinting assays
Footprinting was performed with either unlabelled RNA, or with 5′-end-labelled RNA, prepared as described above. The RNA, previously renatured, was incubated for 15 min at 20°C in 10 µl reactions containing 50 mM Tris–HCl (pH 7.5), 5 mM MgCl2, 50 mM KCl, 1 µg of total tRNA, in the absence or in the presence of purified Crc protein (3 nM to 2 µM). The free mRNA and the protein-mRNA complexes were then probed for 6 min at 20°C with RNase T1, RNase T2, or RNase V1 as described above. For dimethylsulfate (DMS) modification, 1 µl DMS (freshly diluted 1:16 in ethanol) was added to 25 µl of 50 mM Tris–HCl (pH 7.5), 5 mM MgCl2, 50 mM KCl, 1 µg of total tRNA, alkS mRNA (2 pmol) and, when added, Crc (3 nM to 2 µM). Reactions were allowed for 5 min at 20°C. Analysis of the sensitivity of riboses towards modification by the 1M7 reagent (SHAPE assays) was also performed using non-labelled alkS mRNA, alone or bound to Crc as described above, in the presence of 2 and 4 mM of 1M7 reagent dissolved extemporaneously in DMSO. The reaction was performed for 70 s at 20°C following the conditions described earlier (19). In all cases, reactions were stopped by phenol–chloroform extraction, followed by ethanol precipitation.
When an end-labelled RNA was used, the digestion products were analyzed by electrophoresis on denaturing 8% polyacrylamide–urea gels. For enzymatic and chemical reactions performed with non-labelled RNAs, the sites of digestion, and of DMS and 1M7 modifications, were revealed by primer extension using AMV reverse transcriptase, as previously described (18), and an end-labelled oligonucleotide (5′-gcaactatgaactttggc-3′) which is complementary to nts +120 to +103 of alkS mRNA. Incubation controls (in the absence of the RNases, DMS, or 1M7) were also performed in parallel to detect unspecific nicks in the RNA or pauses of reverse transcriptase. To assign the cleavages or modifications, sequencing ladders were run in parallel.
Quantification of the reactivity of the 2′-OH group of riboses towards 1M7 was performed with the SAFA software after scanning the autoradiographies (20). The global intensity of each lane was first normalized according to the intensities of the full-length extension product bands. The intensity of each band was determined by using a scale in which the value 0 indicates an unreactive site and the value 1.0 corresponds to the average intensity at highly reactive sites (21). The normalization factor for each dataset was determined after exclusion of the most-reactive peak intensities (2%) and the average was thus calculated for the next 8% of peak intensities. All reactivities were then divided by this average. The reactivity towards 1M7 of riboses in free RNA was quantified by susbtracting the unspecific pauses observed in the incubation control lane of unmodified RNA, while the reactivity of riboses of the RNA complexed with Crc was subtracted from the densities found in the control of unmodified RNA bound to Crc. Reactivity of each ribose was classified as high, medium or low. Comparison of ribose reactivity on the RNA in the absence or presence of Crc reveals the changes induced by Crc binding.
RNA band shift assays
Radioactively labelled RNA (0.1 nM) was incubated for 30 min at 20°C, in the absence or presence of purified Crc (53–1700 nM), in 20 µl reactions containing 10 mM HEPES–KOH (pH 7.9), 35 mM KCl, 2 mM MgCl2 and 1 µg of yeast tRNA. When indicated, competing unlabelled RNA oligonucleotides were also added. After adding 4 µl of loading buffer (60% glycerol, 0.025% xylene cyanol), complexes were resolved by electrophoresis under non-denaturing conditions using 4 or 6% polyacrylamide gels, as described (14).
Determination of the benR transcription start site
Cells of P. putida KT2440 (22) were collected by centrifugation, and total RNA was obtained using the RNeasy Mini kit (Qiagen). Thirty micrograms of RNA were denatured by heating to 85°C for 3 min, and annealed at 20°C with 2.5 pmol of the oligonucleotide 5′-gaaacacgctactgcgctcg-3′, which was end labelled at the 5′-end with [γ32P]ATP (6000 Ci/mmol) and T4 polynucleotide kinase. The oligonucleotide is complementary to nts 40 to 21 relative to the benR translational start site. The RNA was precipitated with ethanol and resuspended in 10 µl of water. Primer extension reactions, performed essentially as described (23), were conducted for 1 h at 42°C using 10 U of AMV reverse transcriptase. The extended cDNA products were analyzed by electrophoresis on 6% polyacrylamide–urea gels. A DNA sequence ladder was performed by chemical sequencing of a DNA fragment of the benR promoter region, obtained by PCR with oligonucleotides 5′-gaaacacgctactgcgctcg-3′ (labelled at the 5′-end) and 5′-gaccgtgatgaccgcaacca-3′. The same labelled oligonucleotide was used for primer extension and the sequence reactions allowing the precise identification of the 5′ start of benR mRNA.
Extension inhibition (Toeprint) assays
Formation of simplified translational initiation complexes was performed using E. coli 30S ribosome subunits, essentially as described (24,25). The alkS mRNA was obtained by in vitro transcription with plasmid palkS-200 (see ‘RNA fragments for in vitro analyses’ section). Reactions contained, in 10 µl, 25 nM of renatured mRNA, the 5′-end-labelled primer (complementary to nts +103 to +120 relative to PalkS2 transcription start site), which was pre-annealed to the mRNA, 200 nM 30S subunits, 20 mM Tris–HCl (pH 7.5), 10 mM MgCl2, 60 mM KCl, 1 mM DTT and, when indicated, Crc protein at various concentrations (16 nM to 2 µM). After 15 min at 37°C, all four dNTPs (each up to 50 µM) and tRNAfMet (2 µM) were added and the assays were incubated for a further 5 min at 37°C. Primer extension was conducted with 1 U of AMV reverse transcriptase for 15 min at 37°C. Reactions were stopped by phenol–chloroform extraction, followed by ethanol precipitation. The cDNA formed was fractionated on denaturing 8% polyacrylamide–urea gels.
RESULTS
The secondary structure of the alkS mRNA leader sequence
The alkS gene can be expressed from two promoters (Figure 1). In the absence of alkanes, alkS is transcribed from promoter PalkS1 (26). The AlkS protein binds to this promoter, acting as a repressor and allowing for a low basal expression. When alkanes become available, AlkS turns into a transcriptional activator and, from its site at PalkS1, it activates the expression of promoter PalkS2, located 38 bp downstream from PalkS1 (27). We have previously shown that Crc can efficiently bind in vitro to an RNA fragment that includes the first 43 nt of the alkS mRNA that starts at promoter PalkS2 (14). Binding is also efficient when the 3′-end of the mRNA extends up to position +99. The Crc-RNA interaction is specific, since it can be competed by an excess of specific RNA, but not by a similar amount of a non-specific RNA (14).
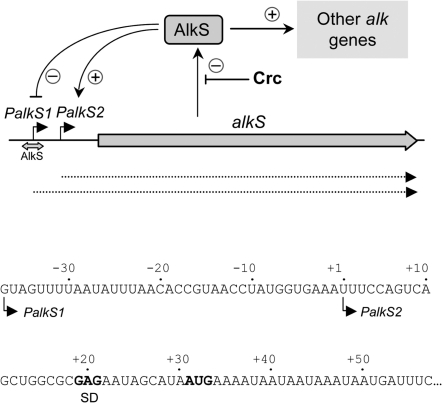
Figure 1.
Transcription of the alkS gene. The alkS gene can be transcribed from two promoters, PalkS1 and PalkS2. In the absence of alkanes, alkS is expressed from promoter PalkS1, which is recognized by σS-RNA polymerase. AlkS binds to a site overlapping the PalkS1 −10 region, repressing this promoter but allowing for a low basal expression. When alkanes become available, AlkS activates the expression of promoter PalkS2, located 38 bp downstream from PalkS1. This leads to a self-amplification process that significantly increases alkS mRNA levels. AlkS also activates the expression of other genes of the alkane degradation pathway from promoter PalkB (data not shown). Under conditions generating catabolite repression control (i.e. in the presence of amino acids that are preferred to alkanes), translation of the alkS mRNA is inhibited by the Crc global regulatory protein. The sequence of the alkS mRNA is shown (the start sites corresponding to promoters PalkS1 or PalkS2 are indicated; coordinates are referred to the PalkS2 start site). The Shine–Dalgarno (SD) sequence and the initiation codon AUG are indicated in bold characters.
Prior to characterizing the Crc-binding site, the secondary structure of the alkS mRNA 5′-end was analyzed using the double-strand-specific RNase V1 and several single-strand-specific RNases (RNases T1, T2, A). Results were interpreted with the help of computer simulations of RNA folding at http://mfold.bioinfo.rpi.edu (28). The RNA molecule used, which includes nts +1 to +78 of alkS mRNA (abbreviated as 1–78 alkS RNA; coordinates are referred always relative to promoter PalkS2 transcription start site), was labelled at the 5′-end and renatured in the presence of magnesium ions. The secondary structure model derived from the enzymatic cleavage patterns show two stem-loop structures (Figure 2). The largest one includes the AUG start site and a 14 nt A-rich apical loop spanning positions +33 to +46. Most of the residues located in unpaired regions were strongly cleaved by RNases A and T2. Conversely, the helical regions interrupted by an internal loop were supported by the existence of several RNase V1 cleavages (at +18, +20, +29, +51 and +59). However, the helix that closes the apical loop is of weak stability, as deduced from the simultaneous presence of RNase V1 and RNase T2 cleavages (Figure 2B).
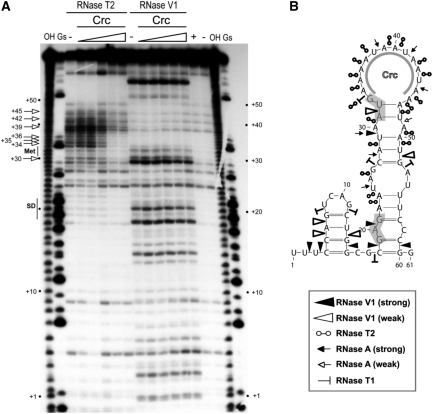
Figure 2.
Secondary structure of the alkS mRNA leader region and effect of Crc binding monitored by enzymatic probing. (A) Fractionation of a 5′-end-labelled alkS mRNA fragment (nts +1 to +78) digested with RNase T2 and RNase V1 on a 12% polyacrylamide–urea gel. The enzymatic digestions were performed in the absence (−) or in the presence of increasing concentrations of Crc (3, 16, 80, 400 nM or 2 µM). Incubation controls (in the absence of RNase) were done in the absence (−) and in the presence (+) of Crc (80 nM). Lanes ‘OH’ and ‘Gs’ correspond, respectively, to an alkaline ladder and an RNase T1 ladder performed under denaturing conditions. Arrows indicated the nucleotides protected by Crc from the attack of RNase T2. Positions relative to PalkS2 transcription start site, as well as the Shine–Dalgarno sequence (SD) and the AUG codon (Met), are indicated on the sides of the gel. (B) Summary of the cleavage patterns generated by RNase V1, RNase T2, RNase A and RNase T1 depicted on the secondary structure model of the alkS mRNA. The symbols used for each RNase are indicated in the insert. The region protected by Crc in the apical loop, as deduced from (A), is indicated. The SD sequence and the AUG start codon are shaded.
The experiments were also performed with a longer fragment of alkS mRNA that includes the PalkS1 start site, and thus carries 37 additional nucleotides at the 5′-end. Assays were performed on the non-labelled RNA and the modifications revealed by primer extension with AMV reverse transcriptase. The accessibility of unpaired regions of the RNA were analyzed using RNases A and T2 (Figures 2A and 3A). In addition, we probed the reactivity of adenines at N1 and cytosines at N3 using DMS (Figure 3A, summarized in Figure 3D), as well as the reactivity of the 2′-OH group of riboses using 1M7 reagent (Figure 3B and C). This chemical gives insight on the local nucleotide flexibility, since a nucleotide involved in Watson–Crick base pairing or in tertiary interactions does not react with 1M7, while flexible regions (unpaired nucleotides, or a helix of weak stability) are sensitive to 1M7. This compound has several advantages: it reacts in the same manner with the four nucleotides, the reaction is very fast (below 1 min), and undergoes a self-inactivating hydrolysis (19,29).
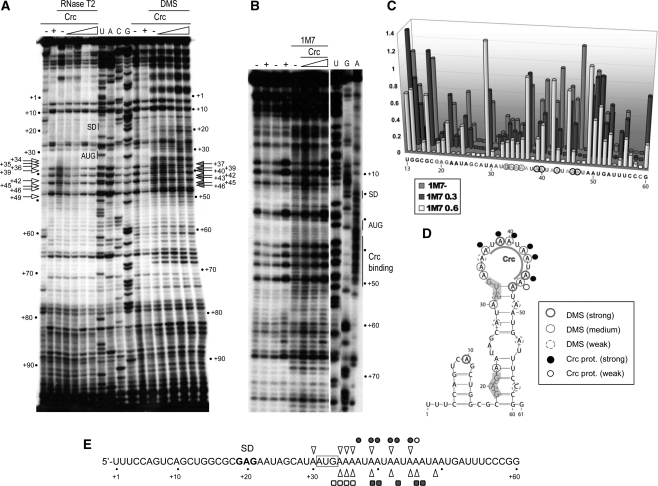
Figure 3.
Secondary structure of the alkS mRNA leader region and effect of Crc binding monitored by chemical probing. (A) RNase T2 hydrolysis and dimethylsulfate (DMS) modification were done on an unlabelled alkS mRNA fragment (nts −37 to +200), in the absence (−) or in the presence of Crc (16, 80, 400 nM or 2 µM). The sites of modification or cleavage were revealed by primer extension. The extension products were resolved on an 8% polyacrylamide–urea gel with sequence ladders of alkS mRNA (lanes U, A, C, G). Incubation controls (no probe), were performed with alkS mRNA in the absence (−) or in the presence (+) of 2 µM Crc. Nucleotides protected by Crc from methylation by DMS (filled arrows), or from RNase T2 digestion (open arrows), are indicated. (B) Modifications of the riboses by 1M7. The RNA was incubated in the absence (−) or in the presence of Crc (300 nM, 600 nM or 1.2 µM). Incubation controls (no 1M7) were performed in the absence (−) or in the presence (+) of Crc. DMSO (the solvent used for 1M7) was added in the incubation controls presented in the third and fourth lanes. Lanes U, G, A are sequencing ladders. (C) Quantification of the reactivity of riboses towards 1M7 on the RNA (1M7−), and on the RNA bound to Crc (300 or 600 nM, indicated as ‘1M7 0.3’ or ‘1M7 0.6’, respectively). Modifications at riboses 26–28, 38, 41, 44, 48 and 59 were not considered due to strong pauses in the control lanes. The sequence of alkS mRNA is indicated below the diagram. (D) Summary of the DMS footprinting data represented on the secondary structure of alkS mRNA. Nucleotides reactive towards DMS in the absence of Crc are encircled. Nucleotides protected from DMS by Crc binding are indicated by black dots (strong) and empty dots (moderate). (E) Summary of the positions protected by Crc from digestion by RNase T2 (open triangles), and from modification by DMS (filled circles, strong protection; open circles, weak protection) or by the 1M7 reagent (filled squares, strong protection; open squares, weak protections). The AUG initiation codon is boxed. “SD”, Shine-Dalgarno sequence.
The nucleotides showing strong or medium reactivity towards DMS (Figure 3A and D) were all located at regions that were also sensitive to RNases T2 and A (Figure 2B), thus confirming that these positions are mostly in an unpaired configuration. Modification of riboses by 1M7 gave correlated changes showing a high degree of flexibility in the regions 33–47, flanked by regions of restricted flexibility (see Figure 3B and C). The weak reactivity of riboses in the stems of this hairpin towards 1M7, and of several N1 positions of adenines towards DMS (Figure 3), strongly suggests that the hairpin including the AUG start codon is of weak stability. These results also show that the 37 additional residues in the 5′-end of the mRNA do not affect the formation of this hairpin.
Crc protects the AU-rich apical loop at hairpin II of alkS mRNA
To identify the Crc-binding site at alkS mRNA, the 5′-end-labelled 1–78 alkS RNA fragment was incubated in the presence of increasing concentrations of purified Crc protein prior to addition of the probe. A clear footprint was detected with RNase T2 (Figure 2), which cuts preferentially at unpaired A residues. The main protections were observed from positions 30–45. Conversely, no Crc footprint was detected with RNase V1 (Figure 2A), or with RNase T1, which cleaves unpaired guanines (not shown).
The Crc–RNA complex was further characterized by monitoring the modifications of bases and riboses with DMS and 1M7, respectively. Due to their small sizes, these two reagents are less sensitive to steric hindrance than the bulky RNases. These experiments were performed on the non-labelled (−37/+200) alkS mRNA, which includes PalkS1 start site, and the modifications were revealed by primer extension with AMV reverse transcriptase. Binding of Crc to alkS mRNA induced a strong reduction of DMS reactivity at position N1 of adenines +37, +39, +40, +42, +43 and +45 and, to a lesser extent, at +46 (Figure 3A and D). These data correlate well with those obtained with reagent 1M7, since Crc binding generated strong protections towards the attack of 1M7 at riboses +39, +40, +43, +46 and +47, and weaker protections at riboses from +33 to +36 (Figure 3B and C). In addition, all these nucleotides lie within the region protected by Crc from RNase T2 (Figure 3E).
Altogether, these results indicate that Crc interacts with the unpaired RNA region located between positions +37 and +47 (Figures 2 and 3), and that its binding does not significantly alter the overall secondary structure of the RNA.
A minimal RNA fragment containing the AU-rich sequence binds to Crc
To assess the relevance of the RNA regions located upstream and downstream of the identified Crc-binding sequence, the ability of Crc to recognize different RNA segments of alkS mRNA was analyzed by band-shift assays. We have previously shown that the 3′-end of alkS mRNA can be deleted up to nt +43 without impairing Crc binding to the RNA, whereas further deletion up to position +33 eliminates Crc binding (14). On the basis of the footprinting data, a 26 bp RNA spanning positions +27–+52 (oligonucleotide 27–52_aS) was synthesized and labelled at the 5′-end. As shown in Figure 4, Crc could bind to this 26 nt RNA. Binding was competed by a 50-fold excess of the same non-labelled RNA, but not by a similar amount of a non-labelled RNA that lacks the sequences protected by Crc. Hence, the regions upstream of nt 27 or downstream of nt 52 are not strictly required for Crc binding.
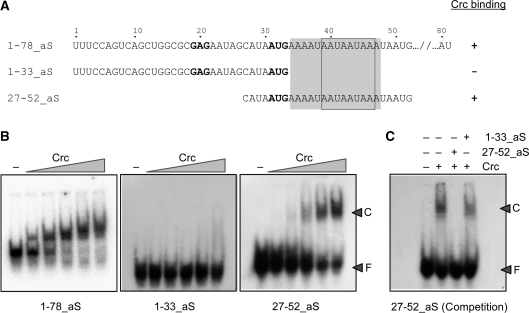
Figure 4.
Determination of alkS mRNA minimal fragment required for Crc binding. (A) Sequence of the alkS mRNA fragments and ability to bind to Crc, as deduced from (B). The Crc footprint is shaded. The Crc-consensus recognition site, as defined in Figure 8, is boxed. The SD sequence and the AUG initiation codon are indicated in bold characters. (B) Band shift assays showing the ability of Crc to bind to the different alkS mRNA fragments. The mRNAs were incubated in the absence (−) or in the presence of increasing concentrations of Crc protein (53, 106, 212, 425 or 850 nM) in the presence of 1 µg of tRNA. ‘F’ is for free RNA and ‘C’ is for the Crc–RNA complex. (C) Ability of the unlabelled RNA oligonucleotides 1–33_aS and 27–52_aS to compete the binding of Crc (212 nM) to the 5′-end-labelled 27–52_aS RNA. Competition was performed by adding a 50-fold excess of the non-labelled 27–52_aS RNA (specific competitor), or of the 1–33_aS RNA (non-specific competitor).
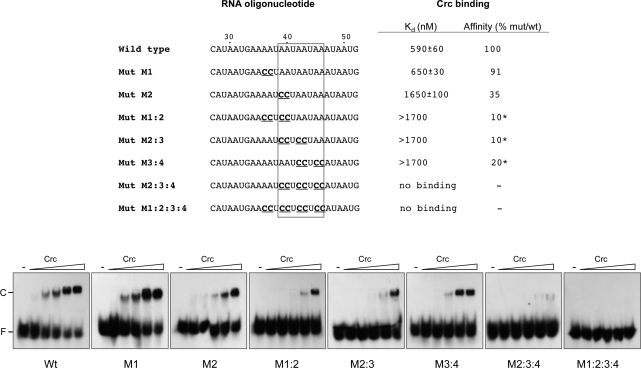
To further characterize the Crc-binding region delimited by the footprinting assays, seven RNA oligonucleotides were synthesized with a sequence derived from that of oligo 27–52_aS (wild-type sequence), but containing mutations at several positions within those protected by Crc from the attack of DMS or of 1M7. The ability of Crc to bind to these RNAs was determined by band-shift assays. As shown in Figure 5, mutation of the adenines located at positions 36 and 37 into cytosines (mutant M1) did not alter Crc binding. However, mutation of residues A39 and A40 (mutant M2) reduced Crc-binding affinity by threefold. The simultaneous mutation of four A residues in several combinations within positions 36–46 (mutant RNAs M1:2, M2:3 and M3:4), had a more pronounced effect, reducing Crc affinity by 5- to 10-fold. Mutations affecting simultaneously six A residues (mutant M2:3:4), or eight A residues (mutant M1:2:3:4), abolished Crc binding. We conclude that the sequence integrity of this RNA region is important for Crc binding, and that residues A39 and A40 are particularly critical for an optimal recognition.
Figure 5.
Mutational analysis of the Crc-binding site at the alkS gene. The ability of Crc (106, 212, 425, 850 or 1700 nM) to bind to the indicated radioactively labelled RNA oligonucleotides was determined by band-shift assays in the presence of 1 µg of tRNA. The free RNA and retarded band corresponding to the Crc–RNA complex are indicated as ‘F’ and ‘C’, respectively. The wild-type RNA includes the alkS positions 27–52 (oligo 27–52_aS), while the mutant oligonucleotides indicated below have different substitutions within this sequence (indicated in bold and underlined). The boxed sequence corresponds to the Crc-consensus recognition site (as defined in Figure 8). The apparent binding constant (Kd) for the wild-type RNA, and for the mutant RNAs M1 and M2, was evaluated after quantification of the radioactive bands of the free and Crc-bound RNAs with a Molecular Imager and the QuantityOne software (BIORAD), and corresponds to the concentration of the protein allowing 50% binding, since the concentration of the labelled RNA was negligible. The Kd value could not be evaluated for mutant RNAs M1:2, M2:3 and M3:4 in the Crc concentration range tested. The effect of the mutations on Crc binding is indicated as percent relative to that observed for the wild-type RNA. For mutants M1:2, M2:3 and M3:4, the value was estimated by comparing the amount of Crc needed to retard 15% of the free RNA relative to that needed for the wild-type RNA (indicated with an asterisk).
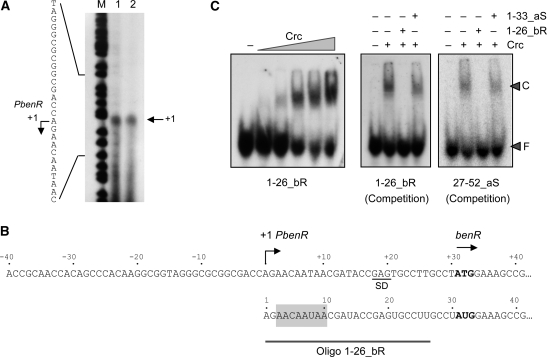
Identification of the Crc-binding site at the benR mRNA
Crc is known to inhibit translation of the P. putida benR mRNA and can bind to an RNA fragment containing nts −35 to +16 relative to the benR translation initiation site (15). This RNA region should therefore include a Crc-binding site. Since the benR transcription start site had not been determined accurately, we first determined the 5′-end of this mRNA by primer extension. Transcription started at an A residue located 30 nt upstream from the AUG translation start site (Figure 6A). The mRNA leader region showed a sequence spanning nts +3 to +9 relative to the 5′-end that was highly similar to the central region of the Crc-binding site determined at alkS (AACAAUAA in benR as compared to AAUAAUAA in alkS; shaded in Figure 6B). To determine whether this sequence represents a true Crc-binding site, a 26 nt RNA spanning positions +1–+26 of benR mRNA (named 1–26_bR) was synthesized and labelled at the 5′-end. Band-shift assays showed that Crc could bind to this RNA minimal site (Figure 6C, left panel). Binding was specific since it was competed when using an excess of the same non-labelled RNA, while an unlabelled RNA lacking a Crc-binding site was not able to compete (Figure 6C, central panel). Furthermore, the unlabelled 1–26_bR RNA could compete the binding of Crc to the end-labelled RNA containing positions 27–52 of the alkS mRNA (Figure 6C, right panel), which provides an additional evidence that the 5′-end of the benR mRNA contains a Crc-binding site.
Figure 6.
Identification of the Crc binding site at benR mRNA. (A) Determination of the benR transcription start site by primer extension. Two identical primer extension reactions were analyzed on a urea–polyacrylamide gel, in parallel to a DNA sequence ladder obtained by chemical sequencing (the bands indicate C and T residues) (43). (B) Sequence of the promoter for the benR gene, indicating the transcription start site and the AUG initiation codon (in bold); the 5′-end of the benR mRNA is shown below; the sequence showing similarity to Crc binding site at alkS mRNA is shaded, and the RNA used in panel C is indicated. (C) Binding of purified Crc protein to 26 nt end-labelled RNAs spanning positions from +1 to +26 of benR mRNA (oligo 1–26_bR), or from +27 to +52 of alkS mRNA (27–52_aS). Left panel: the end-labelled 1–26_bR (0.1 nM) was incubated with 1 µg of tRNA as non-specific competitor, and in the absence (−) or in the presence of increasing concentrations of Crc (53, 106, 212, 425 or 850 nM). Competition experiments contained end-labelled oligonucleotide 1–26_bR (central panel), or end-labelled oligonucleotide 27–52_aS (right panel), Crc (212 nM), 1 µg of tRNA and, when indicated, a 50-fold excess of the unlabelled RNA oligonucleotides 1–26_bR (specific competitor) or 1–33_aS (non-specific competitor, corresponding to nts +1 to +33 of alkS mRNA).
The interaction of Crc with alkS mRNA prevents the formation of the translation initiation complex
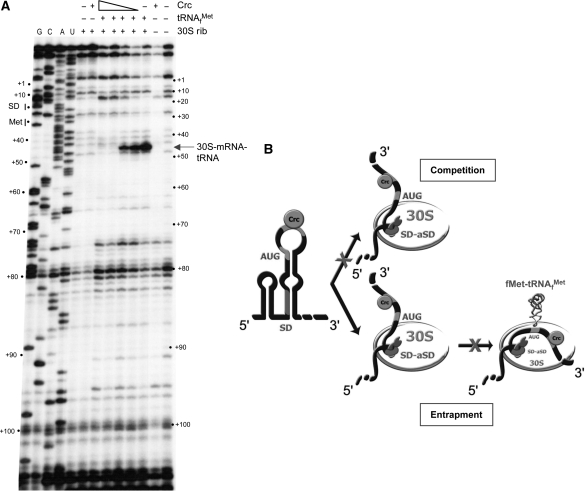
Although it was previously shown that Crc regulates the synthesis of AlkS at a post-transcriptional level in vivo, it was not known which steps of translation were affected by the binding of Crc. This is of interest since Crc binds to the coding sequence of alkS mRNA. To investigate this issue, we analyzed the effect of Crc on the formation of the translation initiation complex at alkS mRNA using toeprinting assays. This technique detects ribosomal complexes by their ability to block the extension of a cDNA from a complementary oligonucleotide primer, when the reverse transcriptase collides with the translational initiation complex (30). An alkS RNA fragment containing nts −37 to +200 was incubated with the E. coli ribosomal 30S subunit and tRNAfMet, in the presence or absence of Crc. The complexes formed were monitored by extension of a primer hybridizing between positions +120 and +103. As shown in Figure 7A, in the absence of Crc a clear stop of reverse transcriptase (toeprint) was detected at position +16 relative to the AUG start codon, which indicates that the translation initiation complex has formed, in spite of the weak SD sequence. No such stop was detected when either 30S ribosome or tRNAfMet were omitted in the reaction. The presence of increasing concentrations of Crc progressively inhibited the toeprint at position +16, which indicates that Crc binding is sufficiently stable to impair formation of the translation initiation complex. A toeprint corresponding to Crc binding was not clearly observed, suggesting that reverse transcriptase is effective at displacing Crc from its binding site.
Figure 7.
Crc binding to alkS mRNA prevents the formation of the ternary ribosomal initiation complex. (A) Toeprinting analysis showing the effect of Crc on the formation of the ternary complex formed between alkS mRNA, E. coli ribosomal 30S subunit and the initiator tRNAfMet. The unlabelled alkS mRNA (−37 to +200) was incubated in the absence (−) or in the presence of increasing concentrations of Crc (16 nM, 80 nM, 400 nM or 2 µM), tRNAfMet, or 30S ribosomes, as indicated. The primer extension products were resolved on a 12% urea–polyacrylamide gel, side by side with sequence ladders (lanes G, C, A, U). The toeprint corresponds to tRNAfMet-mRNA-30S complex that stops the elongation of reverse transcriptase (16 nts downstream the A of the AUG initiation codon). Positions relative to PalkS2 transcription start sites are indicated on the sides of the gel. (B) Possible models of the effect of Crc on the formation of the ribosomal initiation complex. The formation of the 30S initiation complex involves as the first step the docking of mRNA structure (formation of the SD helix), followed by the accommodation step that correctly positions the rest of the mRNA to promote stable codon–anticodon interactions in the P-site. Crc binds to the AU rich sequence downstream the AUG codon of alkS mRNA and prevents formation of the ternary complex by blocking the accommodation process. Two mechanisms are possible: the 30S subunit cannot access the mRNA bound to Crc (competition mechanism), or Crc blocks the 30S subunit into an inactive complex (entrapment complex) and prevents the accommodation step to promote the codon-anticodon interaction at the P site. The model of the mRNA binding to the 30S subunit is taken from ref. (37).
DISCUSSION
The results presented show that the Crc global regulatory protein represses translation of the alkS mRNA by sequestering part of the coding sequence just downstream of the AUG start codon. Footprinting assays indicate that Crc binds specifically to the sequence motif (+37)AUAAUAAUAAA(+47) of alkS mRNA. Analysis of the 2D structure of this mRNA region suggested that the Crc-binding site has an unpaired configuration, and lies within the apical loop of an unstable stem-loop structure. Mutational analysis of this RNA region supports that the sequence of this AU-rich motif is important for an efficient binding of Crc, being residues A39 and A40 particularly significant. The data also show that the changes induced by Crc on the RNA are restricted to the AU-rich unpaired region, since Crc binding did not alter significantly the overall secondary structure of the mRNA upstream or downstream of the binding site. This is further supported by the fact that a small RNA substrate containing the AU-rich motif (nts +27 to +52) specifically binds to Crc, albeit with a weaker affinity than for the longer RNA fragments.
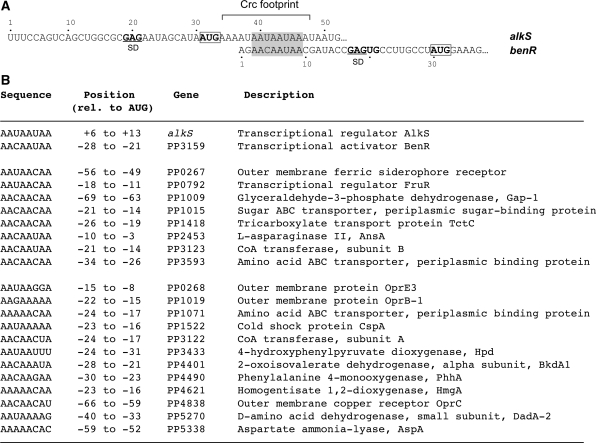
Crc can also bind to the 5′-end of the P. putida benR mRNA, inhibiting translation (15). The results presented herein have allowed identifying a Crc-binding site at benR mRNA located between nucleotides +3 and +9. Computer analyses predict that this Crc-binding site lies within an unpaired RNA region. Unlike in the case of alkS, the Crc-binding site at benR is located a few nt upstream of the predicted Shine–Dalgarno (SD) sequence. The similarity between the alkS and benR Crc-binding sites is restricted to the sequence AA(C/U)AAUAA (Figure 8A), which includes most of the nucleotides protected by Crc from the attack of DMS at alkS mRNA. This consensus motif offers the possibility to search for additional Crc targets. Using a proteomic approach, we recently identified 58 P. putida proteins, the levels of which increase in cells growing exponentially in LB medium upon inactivation of the crc gene (13). Thus, increased protein levels in the Δcrc mutant strain suggest that Crc could directly inhibit translation of some of the corresponding mRNAs. Several translational regulatory proteins recognize conserved sequence or structure signatures on their target mRNAs, in this way coordinating the expression of functionally related genes (31,32). Therefore, based on our study on alkS and benR mRNAs, we searched for a conserved Crc sequence motif in the translation initiation regions of the mRNAs coding for the 58 Crc-repressed proteins. This search was done within a window comprising nts −70 to +16 of the mRNAs since the translational initiation sites can be quite extended (31,33). Interestingly, we found 20 mRNAs that contained a sequence resembling the alkS and benR Crc-binding sites close to the ribosome binding site (Figure 8B). Eight of the genes contain the consensus sequence AA(C/U)AA(C/U)AA, while the other ones show a degenerated sequence rich in A residues. It is worth noting that, in many cases, the presumed Crc-binding site was present at the first gene of an operon (oprB-1, bkdA1, phhA, hmgA, dadA-2 or aspA). One of the mRNA targets encodes the transcriptional regulator FruR, which regulates genes for the assimilation of fructose. The functional significance of this consensus and the mechanism by which Crc might repress these genes remain to be experimentally determined. Furthermore, the remaining 38 proteins detected in the proteomic assays as being repressed by Crc, but which do not show a putative Crc-binding site in their mRNA, are probably regulated by Crc in an indirect way. Taken together, our prediction will likely be helpful to validate new direct Crc targets and to discriminate between direct and indirect effects of this global regulatory protein.
Figure 8.
Identification of sequences similar to the alkS and benR Crc-binding sites in the proximity of the AUG start site of genes that are repressed by Crc. (A) Alignment of the 5′ regions of alkS and benR mRNAs. The AUG initiation codon is boxed. The Crc binding site at the alkS mRNA is indicated. The nucleotides of the alkS and benR Crc binding sites that show sequence similarity are shaded. (B) Sequences similar to the Crc binding region common to alkS and benR mRNAs (shaded in panel A) found in the proximity of the AUG start site of known Crc-regulated genes; the position relative to the AUG start site, as well as the name and description of the genes, is indicated. The potential Crc-targets are divided into two classes, eight of them carry the consensus sequence AAYAAYAA and all others contain a divergent consensus rich in A residues. The transcription start sites are only available for phhA (44), hpd (45) and bkdA1 (9) mRNAs, and the presumed Crc-target lies within the transcribed region.
The results presented also indicate that Crc impedes the formation of the 30S- tRNAfMet-RNA ternary complex at the alkS mRNA. Inhibition of translation initiation by a trans-acting repressor can occur through different mechanisms (31–33). One is a direct competition process in which the repressor protein inhibits binding of the 30S to the SD sequence at the RNA, either by steric hindrance, or by modifying RNA conformation in such a way that the SD sequence becomes inaccessible to the ribosome. In an alternative mechanism, called entrapment (34–36), the repressor protein allows the 30S subunit to bind to RNA, but traps it into an inactive complex that cannot proceed to further steps of the translation initiation process (Figure 7B). Whether Crc prevents or traps the initial binding of the 30S subunit on the mRNA remains unsolved. The footprinting assays indicate that Crc neither modifies the 2D structure of the leader part of alkS mRNA nor stabilizes the hairpin motif that may sequester the ribosome binding site. Hence, it seems likely that Crc poses a direct steric hindrance on ternary complex formation. The crystal structure of several ribosomal complexes shows that ∼30 nt of the mRNA wrap around the neck of the 30S subunit (37–39). It was further proposed that, in the first step of the initiation, the mRNA binds to the 30S by establishing contacts between the SD sequence and the 3′-end of 16S rRNA on the 30S subunit platform. The following step involves a simultaneous positioning of the initiation codon into the P site to form the codon–anticodon interaction, and of the 3′-end of the mRNA (36–39). In this scenario, it is tempting to propose that Crc would prevent the accommodation step required to correctly position the AUG codon and the 3′-end of the mRNA into the normal ribosomal track. As a consequence, the codon–anticodon could not form (Figure 7B). A recent systematic antisense interference with 30S subunit binding to mRNA revealed that masking of the coding sequence down to the fifth codon, leaving the SD and the AUG codon unpaired, strongly inhibits the formation of the ternary initiation complex (40). In addition, the Salmonella typhimurium non-coding RNA (ncRNA) RybB represses the translation of ompN mRNA by forming a short duplex with the coding sequence just downstream the AUG codon (40,41). Therefore, as for ncRNAs, regulatory proteins also prevent formation of the active initiation ribosomal complex by direct masking of the coding sequence close by to the AUG codon. We note, however, that Crc may not work in the same way with all target mRNAs, the mechanism differing depending on the location of the Crc-binding site relative to the AUG start site, and on mRNA structure. In benR mRNA, the Crc-binding site is located just upstream the SD sequence. Of interest, the S1 ribosomal protein in Gram-negative bacteria is known to bind to an unpaired AU rich motif located in the leader regions of mRNAs, i.e. upstream the SD. This r-protein facilitates the initial ribosome binding of structured mRNAs, or of mRNAs having weak SD sequences as for alkS and benR mRNAs (42). Hence, in that particular case, Crc may prevent the initial ribosome binding to the benR mRNA by competing with ribosomal protein S1.
Several bacterial proteins can inhibit translation initiation by binding to the mRNA leader sequences (31–33). Translational regulation is believed to provide a fast response to changes in environmental or physiological conditions, and is therefore complementary to other regulatory mechanisms. The mechanisms of catabolite repression control described to date typically inhibit either the uptake of the non-preferred substrates by blocking the relevant permeases through processes based on protein–protein contacts (inducer exclusion), or the transcription initiation of the genes involved in the catabolism of the non-preferred compounds (2,3). The results presented in this work, together with previous evidence (14,15 and references therein), show that a key catabolite repression control network from Pseudomonads ultimately relies on regulating translation initiation. This is in contrast to what has been found for other model bacteria like E. coli or B. subtilis, and may perhaps be related to the extreme metabolic versatility of Pseudomonads.
FUNDING
Spanish Ministry of Science and Innovation (BFU2006-00767/BMC and BFU2009-07009/BMC) and Instituto de Salud Carlos III (Spanish Network for the Research in Infectious Diseases; REIPI RD06/0008) to F.R. Centre National de la Recherche Scientifique (CNRS) and the French National Agency for Research (ANR-07-blan-0351-02) to P.R. Funding for open access charge: Grants from the Spanish Ministry of Science and Innovation (BFU2006–00767/BMC and BFU2009–07009/BMC to F.R.).
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
The authors are grateful to E. Martínez-Salas and M. Yusupov for helpful discussions and to L. Yuste and AC. Helfer for excellent technical assistance. We are indebted to K. Weeks for providing 1M7 reagent and advices for performing the chemical reactions and quantification of the data. R.M. was holder of a short-term fellowship from the European Molecular Biology Organization (ASTF 224.00-2008).
REFERENCES
- 1.Cases I, de Lorenzo V. Promoters in the environment: transcriptional regulation in its natural context. Nat. Rev. Microbiol. 2005;3:105–118. doi: 10.1038/nrmicro1084. [DOI] [PubMed] [Google Scholar]
- 2.Görke B, Stülke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev. Microbiol. 2008;6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 3.Deutscher J. The mechanisms of carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 2008;11:87–93. doi: 10.1016/j.mib.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Rojo F, Dinamarca MA. Catabolite repression and physiological control. In: J. L. Ramos JL, editor. Pseudomonas. Vol. 2. New York: Kluwer Academic/Plenum Publishers; 2004. pp. 365–387. [Google Scholar]
- 5.Wolff JA, MacGregor CH, Eisenberg RC, Phibbs PV., Jr Isolation and characterization of catabolite repression control mutants of Pseudomonas aeruginosa PAO. J. Bacteriol. 1991;173:4700–4706. doi: 10.1128/jb.173.15.4700-4706.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collier DN, Hager PW, Phibbs PV., Jr Catabolite repression control in Pseudomonads. Res. Microbiol. 1996;147:551–561. doi: 10.1016/0923-2508(96)84011-3. [DOI] [PubMed] [Google Scholar]
- 7.MacGregor CH, Arora SK, Hager PW, Dail MB, Phibbs PV., Jr The nucleotide sequence of the Pseudomonas aeruginosa pyrE-crc-rph region and the purification of the crc gene product. J. Bacteriol. 1996;178:5627–5635. doi: 10.1128/jb.178.19.5627-5635.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hester KL, Lehman J, Najar F, Song L, Roe BA, MacGregor CH, Hager PW, Phibbs PV, Jr, Sokatch JR. Crc is involved in catabolite repression control of the bkd operons of Pseudomonas putida and Pseudomonas aeruginosa. J. Bacteriol. 2000;182:1144–1149. doi: 10.1128/jb.182.4.1144-1149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hester KL, Madhusudhan KT, Sokatch JR. Catabolite repression control by crc in 2xYT medium is mediated by posttranscriptional regulation of bkdR expression in Pseudomonas putida. J. Bacteriol. 2000;182:1150–1153. doi: 10.1128/jb.182.4.1150-1153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuste L, Rojo F. Role of the crc gene in catabolic repression of the Pseudomonas putida GPo1 alkane degradation pathway. J. Bacteriol. 2001;183:6197–6206. doi: 10.1128/JB.183.21.6197-6206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morales G, Linares JF, Beloso A, Albar JP, Martínez JL, Rojo F. The Pseudomonas putida Crc global regulator controls the expression of genes from several chromosomal catabolic pathways for aromatic compounds. J. Bacteriol. 2004;186:1337–1344. doi: 10.1128/JB.186.5.1337-1344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aranda-Olmedo I, Ramos JL, Marqués S. Integration of signals through Crc and PtsN in catabolite repression of Pseudomonas putida TOL plasmid pWW0. Appl. Environ. Microbiol. 2005;71:4191–4198. doi: 10.1128/AEM.71.8.4191-4198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno R, Martínez-Gomariz M, Yuste L, Gil C, Rojo F. The Pseudomonas putida Crc global regulator controls the hierarchical assimilation of amino acids in a complete medium: evidence from proteomic and genomic analyses. Proteomics. 2009;9:2910–2928. doi: 10.1002/pmic.200800918. [DOI] [PubMed] [Google Scholar]
- 14.Moreno R, Ruiz-Manzano A, Yuste L, Rojo F. The Pseudomonas putida Crc global regulator is an RNA binding protein that inhibits translation of the AlkS transcriptional regulator. Mol. Microbiol. 2007;64:665–675. doi: 10.1111/j.1365-2958.2007.05685.x. [DOI] [PubMed] [Google Scholar]
- 15.Moreno R, Rojo F. The target for the Pseudomonas putida Crc global regulator in the benzoate degradation pathway is the BenR transcriptional regulator. J. Bacteriol. 2008;190:1539–1545. doi: 10.1128/JB.01604-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg AH, Lade BN, Chui DS, Lin SW, Dunn JJ, Studier FW. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56:125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. 3rd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 2001. [Google Scholar]
- 18.Brunel C, Romby P. Probing RNA structure and RNA-ligand complexes with chemical probes. Methods Enzymol. 2000;318:3–21. doi: 10.1016/s0076-6879(00)18040-1. [DOI] [PubMed] [Google Scholar]
- 19.Mortimer SA, Weeks KM. A fast-acting reagent for accurate analysis of RNA secondary and tertiary structure by SHAPE chemistry. J. Am. Chem. Soc. 2007;129:4144–4145. doi: 10.1021/ja0704028. [DOI] [PubMed] [Google Scholar]
- 20.Laederach A, Das R, Vicens Q, Pearlman SM, Brenowitz M, Herschlag D, Altman RB. Semiautomated and rapid quantification of nucleic acid footprinting and structure mapping experiments. Nat. Protocols. 2008;3:1395–1401. doi: 10.1038/nprot.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson KA, Gorelick RJ, Vasa SM, Guex N, Rein A, Mathews DH, Giddings MC, Weeks KM. High-throughput SHAPE analysis reveals structures in HIV-1 genomic RNA strongly conserved across distinct biological states. PLoS Biol. 2008;6:e96. doi: 10.1371/journal.pbio.0060096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagdasarian M, Lurz R, Ruckert B, Franklin FC, Bagdasarian MM, Frey J, Timmis KN. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981;16:237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- 23.Monsalve M, Mencía M, Rojo F, Salas M. Transcription regulation in Bacillus subtilis phage ϕ29: expression of the viral promoters throughout the infection cycle. Virology. 1995;207:23–31. doi: 10.1006/viro.1995.1048. [DOI] [PubMed] [Google Scholar]
- 24.Moine H, Romby P, Springer M, Grunberg-Manago M, Ebel JP, Ehresmann B, Ehresmann C. Escherichia coli threonyl-tRNA synthetase and tRNA(Thr) modulate the binding of the ribosome to the translational initiation site of the thrS mRNA. J. Mol. Biol. 1990;216:299–310. doi: 10.1016/S0022-2836(05)80321-3. [DOI] [PubMed] [Google Scholar]
- 25.Fechter P, Chevalier C, Yusupova G, Yusupov M, Romby P, Marzi S. Ribosomal initiation complexes probed by toeprinting and effect of trans-acting translational regulators in bacteria. Methods Mol. Biol. 2009;540:247–263. doi: 10.1007/978-1-59745-558-9_18. [DOI] [PubMed] [Google Scholar]
- 26.Canosa I, Yuste L, Rojo F. Role of the alternative sigma factor sigma S in expression of the AlkS regulator of the Pseudomonas oleovorans alkane degradation pathway. J. Bacteriol. 1999;181:1748–1754. doi: 10.1128/jb.181.6.1748-1754.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canosa I, Sánchez-Romero JM, Yuste L, Rojo F. A positive feedback mechanism controls expression of AlkS, the transcriptional regulator of the Pseudomonas oleovorans alkane degradation pathway. Mol. Microbiol. 2000;35:791–799. doi: 10.1046/j.1365-2958.2000.01751.x. [DOI] [PubMed] [Google Scholar]
- 28.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkinson KA, Merino EJ, Weeks KM. Selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE): quantitative RNA structure analysis at single nucleotide resolution. Nat. Protocols. 2006;1:1610–1616. doi: 10.1038/nprot.2006.249. [DOI] [PubMed] [Google Scholar]
- 30.Ringquist S, Gold L. Toeprinting assays. Mapping by blocks to reverse transcriptase primer extension. Methods Mol. Biol. 1998;77:283–295. doi: 10.1385/0-89603-397-X:283. [DOI] [PubMed] [Google Scholar]
- 31.Romby P, Springer M. Bacterial translational control at atomic resolution. Trends Genet. 2003;19:155–161. doi: 10.1016/S0168-9525(03)00020-9. [DOI] [PubMed] [Google Scholar]
- 32.Babitzke P, Baker CS, Romeo T. Regulation of translation initiation by RNA binding proteins. Annu. Rev. Microbiol. 2009;63:27–44. doi: 10.1146/annurev.micro.091208.073514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlax PJ, Worhunsky DJ. Translational repression mechanisms in prokaryotes. Mol. Microbiol. 2003;48:1157–1169. doi: 10.1046/j.1365-2958.2003.03517.x. [DOI] [PubMed] [Google Scholar]
- 34.Philippe C, Eyermann F, Benard L, Portier C, Ehresmann B, Ehresmann C. Ribosomal protein S15 from Escherichia coli modulates its own translation by trapping the ribosome on the mRNA initiation loading site. Proc. Natl Acad. Sci. USA. 1993;90:4394–4398. doi: 10.1073/pnas.90.10.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlax PJ, Xavier KA, Gluick TC, Draper DE. Translational repression of the Escherichia coli alpha operon mRNA: importance of an mRNA conformational switch and a ternary entrapment complex. J. Biol. Chem. 2001;276:38494–38501. doi: 10.1074/jbc.M106934200. [DOI] [PubMed] [Google Scholar]
- 36.Marzi S, Myasnikov AG, Serganov A, Ehresmann C, Romby P, Yusupov M, Klaholz BP. Structured mRNAs regulate translation initiation by binding to the platform of the ribosome. Cell. 2007;130:1019–1031. doi: 10.1016/j.cell.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Yusupova GZ, Yusupov MM, Cate JH, Noller HF. The path of messenger RNA through the ribosome. Cell. 2001;106:233–241. doi: 10.1016/s0092-8674(01)00435-4. [DOI] [PubMed] [Google Scholar]
- 38.Yusupova G, Jenner L, Rees B, Moras D, Yusupov M. Structural basis for messenger RNA movement on the ribosome. Nature. 2006;444:391–394. doi: 10.1038/nature05281. [DOI] [PubMed] [Google Scholar]
- 39.Korostelev A, Trakhanov S, Laurberg M, Noller HF. Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements. Cell. 2006;126:1065–1077. doi: 10.1016/j.cell.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 40.Bouvier M, Sharma CM, Mika F, Nierhaus KH, Vogel J. Small RNA binding to 5′ mRNA coding region inhibits translational initiation. Mol. Cell. 2008;32:827–837. doi: 10.1016/j.molcel.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 41.Papenfort K, Pfeiffer V, Mika F, Lucchini S, Hinton JC, Vogel J. SigmaE-dependent small RNAs of Salmonella respond to membrane stress by accelerating global omp mRNA decay. Mol. Microbiol. 2006;62:1674–1688. doi: 10.1111/j.1365-2958.2006.05524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komarova AV, Tchufistova LS, Dreyfus M, Boni IV. AU-rich sequences within 5′ untranslated leaders enhance translation and stabilize mRNA in Escherichia coli. J. Bacteriol. 2005;187:1344–1349. doi: 10.1128/JB.187.4.1344-1349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maxam AM, Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65:499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- 44.Herrera MC, Ramos JL. Catabolism of phenylalanine by Pseudomonas putida: the NtrC-family PhhR regulator binds to two sites upstream from the phhA gene and stimulates transcription with sigma70. J. Mol. Biol. 2007;366:1374–1386. doi: 10.1016/j.jmb.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Arias-Barrau E, Olivera ER, Luengo JM, Fernández C, Galán B, García JL, Díaz E, Miñambres B. The homogentisate pathway: a central catabolic pathway involved in the degradation of L-phenylalanine, L-tyrosine, and 3-hydroxyphenylacetate in Pseudomonas putida. J. Bacteriol. 2004;186:5062–5077. doi: 10.1128/JB.186.15.5062-5077.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]