Introduction
Breast cancer is the most common non-skin cancer and the second leading cause of cancer death in women [1]. There are 40,000 women per year dying of breast cancer in the US, and most breast cancer victims die of progressive metastatic disease [1]. The optimal treatment of patients with recurrent breast cancer depends on knowing the true extent of disease. Conventional imaging (CI) for restaging breast cancer which includes mammography, ultrasound and MR for locoregional recurrence and contrast-enhanced CT and bone scintigraphy for distant metastasis covers likely sites of breast cancer recurrence and spread, but may miss sites of recurrence and particularly sites of disease spread. The addition of FDG PET to CI for detection of recurrent neoplasm after primary treatment of breast cancer has proven to be a complementary imaging technique, overcoming many of the limitations CI for re-staging. The additional metabolic information provided by FDG PET increases the accuracy of detecting recurrent or metastatic lesions [2-16]. This is particularly true in the evaluation of extra-axillary regional lymph nodes [2,8,9,11,13,14] and for some bone metastases that are osteolytic [17-22]. Integrated PET/CT systems, with their ability to accurately map foci of elevated FDG uptake to anatomic structures, have provided additional diagnostic confidence and a modest level accuracy in the evaluation of breast cancer recurrences compared to FDG PET alone [23-27].
The recognition that breast cancer is a systemic disease, even in its early stages, led to the current approach to treatment which combines local measures such as surgery and radiotherapy with systemic treatment [28]. For most clinical trial studies, local failure is defined as any recurrence of tumor in the ipsilateral chest wall or mastectomy scar; regional failure is defined as any recurrence of tumor in the ipsilateral supraclavicular, infraclavicular, axillary, or internal mammary (IM) nodes; and recurrence of tumor in any other site is considered as distant failure [29]. In general, systemic therapy is used at almost all disease stages; however, isolated locoregional disease or single sites of metastatic recurrence are also treated with surgery and radiation therapy [30,31].
The improvement in restaging accuracy for FDG PET and PET/CT compared to CI in patients suspected of having local or distant recurrence has led to changes in treatment strategies in clinical practice [32,33]. This is mostly a result of improved detection of local and distant nodal involvement occult at other imaging studies. The detection of more widespread disease by FDG PET can, particularly in patients thought to have limited locoregional disease based on CI, select patients more appropriately for systemic treatment alone rather than more aggressive, curative treatments like surgery and radiation [33]. The potential of FDG PET to provide more accurate and earlier detection of breast cancer recurrences will hopefully translate into more effective treatment strategies and better health outcomes for these patients in the future.
Locoregional Recurrences
Recurrence in the breast, skin of the breast, axillary nodes, chest wall and supraclavicular nodes are the most common sites of first locoregional recurrence (LRR) after primary surgical resection [34]. The majority of LRR occurs within 5 years following primary surgical resection. Although LRR may be an ominous event after mastectomy, data on incidence and outcomes are limited by heterogenous patient populations and the time period studied. Besides extent of primary tumor (size ≥ 4cm) and axillary node involvement (four or more positive nodes or extranodal extension), several other risk factors for LRR in post-mastectomy patients have been identified [34]. In a large, single-institution retrospective study of patients who underwent mastectomy and multimodality adjuvant treatment [35], the incidence of locoregional recurrence was 9% with median 6 year follow-up; multivariate analysis showed age less than 35 years, lymphovascular invasion, and multicentricity as major predictors for isolated LRR. The shift towards breast-conserving surgery and local radiation therapy for early breast cancer in recent years has heightened concern over locoregional recurrence [36]. The incidence of locoregional recurrence after breast conservation treatment ranges from 5 to 22% [37,38]. Independent risk factors associated with locoregional recurrence in this group of patients include close or positive margins at surgical resection, tumors with extensive intraductal component, high grade DCIS, patient age under 40 years and absence of radiation after breast conservation therapy [37].
Locoregional recurrence involving the chest wall and supraclavicular nodes is associated with poor prognosis in terms of survival after recurrence [39]. However, for patients presenting with LRR and no evidence of distant disease, aggressive multimodality treatment, including surgery, radiation, chemotherapy and hormonal therapy, may be warranted because many of these patients can be rendered disease-free [35]. Supraclavicular node recurrence is technically considered stage IV disease and generally considered a harbinger to more widely disseminated disease. However, patients with supraclavicular node involvement as the sole site of disseminated disease may benefit from aggressive local radiotherapy [40,41].
The evaluation of local recurrence in the breast, skin or chest wall with FDG PET can be problematic (Figures 1 and 2). A number of studies evaluating FDG PET and PET/CT have reported both false positive and false negative cases of recurrent neoplasm in the skin, residual breast and chest wall [7,10,11,24,25,42]. Inflammation in these previously treated areas can be a source of FDG avidity, leading to a false positive result. The tissue volume of some local recurrences may be too small for detection or avidity of the recurrence too low (particularly in cases of lobular carcinoma), leading to a false negative result [42]. Physical exam and conventional imaging, including a combination of mammography, ultrasound, and MR, remains the mainstay for the evaluation of locoregional recurrence. Since the majority of suspected local recurrences are easily accessible to percutaneous needle biopsy, the definitive status of the suspected lesion can be made by histologic analysis.
Figure 1.
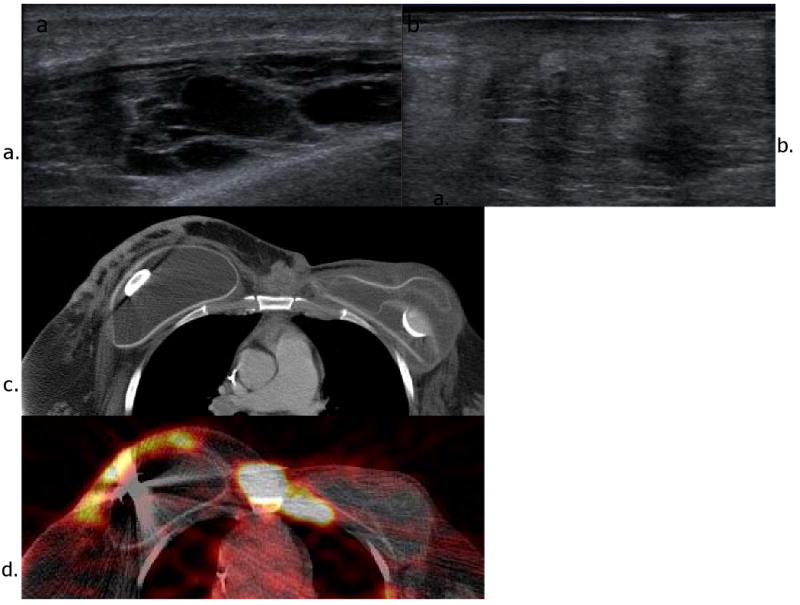
Recurrence in the contralateral breast in a patient with history of left breast cancer and left partial mastectomy 10 years ago. She also underwent left total mastectomy and prophylactic right mastectomy when she had recurrence 6 years ago. Ultrasound images (a and b) show multiple hypoechoic and heterogeneous mixed echogenic lesions with shadowing in the right breast. Axial CT (c) and FDG PET-CT images (d) reveal hypermetabolic soft tissue mass and nodules with irregular skin thickening in the right breast.
Figure 2.
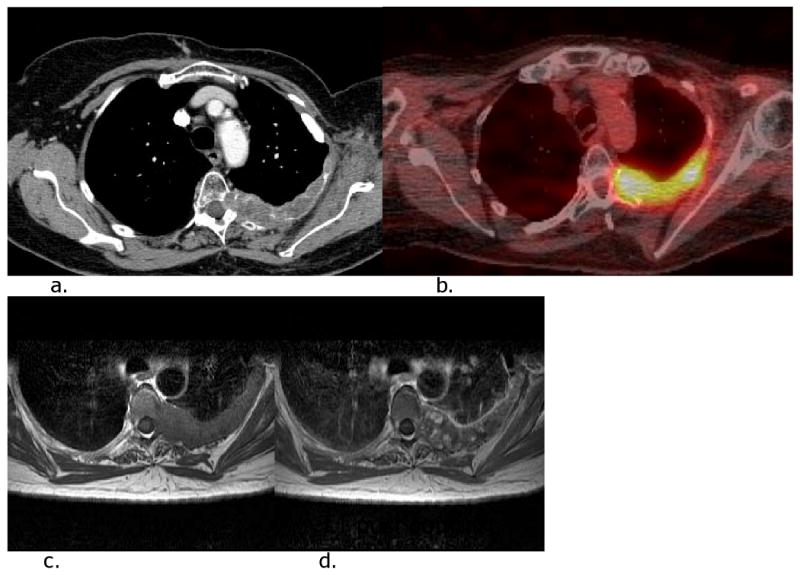
Locoregional recurrence in the left posterior chest wall in a patient who had undergone left radical mastectomy 13 years ago. She had left anterolateral chest wall recurrence 5 years ago, which had been resected. CT (a), FDG PET-CT (b) and MR (c and d) images show hypermetabolic irregular mass in the left posterior chest wall extending to the epidural space of T5.
A particularly vexing clinical problem occurs in the patient with symptoms of brachial plexopathy suspected of axillary or supraclavicular tumor recurrence, since either tumor recurrence or treatment-induced scarring can be responsible for the symptoms. Hathaway et al. [43] showed the value of combining the functional information of FDG PET and the anatomic information from dedicated MR imaging to decide whether patients would benefit from further surgery. These results have been confirmed by a subsequent study [44].
Internal mammary and other extra-axial regional lymph node evaluation
Lymphatic drainage to the IM nodal chain is an important pathway of spread of disease both at the time of initial diagnosis and after primary treatment of breast cancer. Data from two large sentinel node lymphoscintigraphy series in patients with early breast cancer reveals that overall prevalence of drainage to the IM nodes is 17% [45,46]. This is a similar prevalence to that shown in early extended radical mastectomy series where histologically-proven metastasis to IM nodes occurred in close to one in five women with operable (stage II-III) breast cancer [47,48]. Metastasis to IM nodes can occur from tumor located anywhere in the breast; however, in our series, IM drainage was significantly less frequent in tumors located in the upper outer quadrant (10%) compared with the other three quadrants and subareolar portion of the breast (17-29%) [45]. Metastasis to the IM and axillary nodes usually occurs synchronously but infrequently (4-6% incidence) may be isolated to the IM chain [47].
Neoplastic spread to the IM nodes in axillary node-positive patients is associated with worse prognosis compared to patients with axillary disease only. Early extended radical mastectomy series showed a significantly worse 10-year overall survival among patients with IM and axillary nodal metastasis (30%) compared with patients having only axillary node disease (55%) [48]. Similar prognostic information for drainage to IM nodes at sentinel node lymphoscintigraphy has been shown. Yao et al. [46] showed that among axillary node-positive patients with early breast cancer, lymphatic drainage to IM nodes is associated with worse overall survival compared to patients without IM drainage. The IM nodal basins are not routinely sampled due to their relative inaccessibility and their uncertain clinical significance and treatment [49]. Neoplastic involvement of IM nodes may go undetected at initial diagnosis and, if not adequately treated, may give rise to intrathoracic recurrence in the follow-up period. Although the utility of FDG PET and PET/CT in detecting IM nodes in early stage disease remains to be proven, a number of studies have pointed towards the value in new or recurrent locally advanced breast cancer (LABC) [11,50-52]. Our experience with imaging patients with LABC shows that the prevalence of IM FDG uptake can be as high as 25% and that the presence of IM FDG uptake predicts treatment failure patterns of disease consistent with IM nodal involvement and progression [53]. A study by Tran et al. [54] showed that the likelihood of extraaxillary lymph node findings on FDG PET was affected by the position of the primary tumor (medial versus lateral). The presence of extraaxillary nodal uptake on FDG PET combined with medial tumor location indicated a high risk of subsequent disease progression. These studies suggest that the IM nodal chain is a conduit for more widespread dissemination of disease.
Distant metastases
FDG PET can be helpful in the evaluation for distant metastases in patients who have been previously treated for their primary disease, particularly in those with advanced stages of disease, equivocal CI findings and in asymptomatic patients with elevated tumor markers. In terms of diagnostic performance, several studies have shown FDG PET to have a relative advantage over CI in the evaluation of distant metastases in previously treated patients [2-11,13,15,16]. The largest of these studies are summarized in Table 1. In a meta-analysis of FDG PET for the evaluation of breast recurrence and metastases, Isasi et al. [55] reported a median sensitivity and specificity of 93% and 82%, respectively, in a patient-based analysis.
Table 1.
Largest series comparing the ability of whole-body FDG PET with conventional imaging to detect locoregional and distant recurrences in patients who have previously undergone primary treatment for breast cancer.
| Series | Number of patients | Confirmed positive/negative cases | FDG PET Sensitivity (TP/TP+FN)* |
FDG PET Specificity (TN/TN+FP)* |
|---|---|---|---|---|
| Bender 19972 | 75 | 60/15 | 95% (41/43) | 96% (213/221) |
| Moon 19983 | 57 | 29/28 | 93% (27/29) | 79% (22/28) |
| Lonneux 20004† | 39 | 33/6 | 94% (31/33) | 50% (3/6) |
| Kim 20015 | 27 | 17/10 | 94% (16/17) | 80% (8/10) |
| Lin 20026 | 36 | 11/25 | 85% (23/27) | 96% (85/89) |
| Liu 20027† | 30 | 28/2 | 96% (25/28) | 50% (1/2) |
| Suarez 20028† | 38 | 26/12 | 92% (24/26) | 75% (9/12) |
| Vranjesevic13 2002 | 61 | 42/19 | 93% (39/42) | 84% (16/19) |
| Gallowitsch 20039 | 62 | 34/28 | 97% (33/34) | 82% (23/28) |
| Siggelkow 200310 | 57 | 31/26 | 81% (25/31) | 98% (25/26) |
| Kamel 200311 | 60 | 43/17 | 89% (24/27) LRR** 100% (26/26) DM†† |
84% (16/19) LRR 97% (30/31) DM |
| Wolfort 200615 | 23 | 16/7 | 81% (13/16) | 100% (7/7) |
| Mahner 200814*** | 119 | 71/48 | 87% (62/71) | 83% (40/48) |
Values calculated on patient analysis except for Bender and Lin series which are calculated on lesion analysis; TP=true positive, TN=true negative, FP=false positive, FN=false negative
Patients were mostly or all asymptomatic with elevated tumor markers.
LRR=Locoregional recurrence
DM=Distant metastases
50/119 patients had undergone primary treatment
A common finding in these studies comparing FDG PET to CI for the detection of recurrent disease is that FDG PET detects a significantly greater number (about two-fold) of extra-axial lymph node metastases (Figure 3) [2,8,9,11,13,14]. Neoplastic spread to mediastinal nodes is common in patients with advanced disease and as a site of recurrence in patients who have undergone axillary node dissection and radiation. As with IM nodes, mediastinal nodes are rarely sampled in breast cancer patients. CT, the conventional method of staging these nodes, relies on size criteria to determine the presence or absence of disease; this method has been proven significantly less accurate than FDG PET in patients with non-small cell lung cancer where histologic analysis is used as the gold standard [56,57]. In our retrospective series of 73 patients with recurrent or metastatic breast cancer who underwent both FDG PET and chest CT [58], FDG uptake in mediastinal or IM nodes was two times more prevalent than suspiciously enlarged nodes by CT, suggesting that PET is a much more sensitive technique at detecting nodal disease. In the subset of patients with confirmation, the sensitivity of FDG PET was significantly higher (85%) than CT (50%) with nearly the same level of specificity (90% for PET and 83% for CT).
Figure 3.
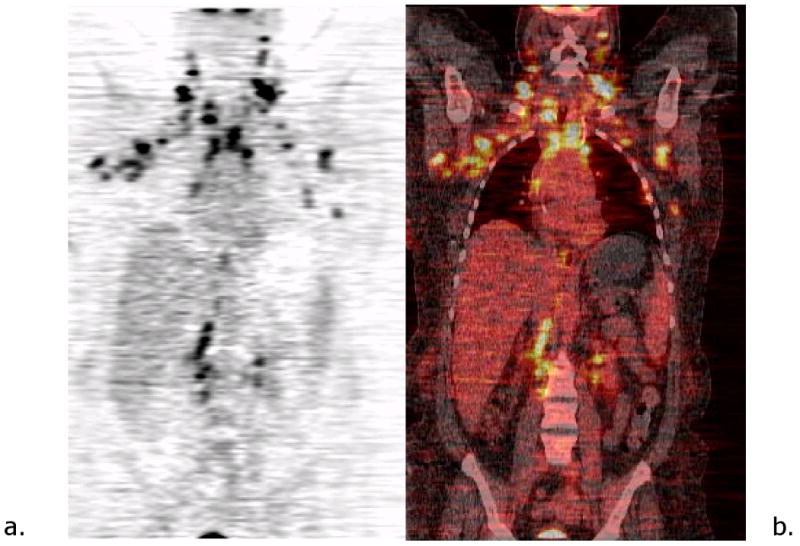
Patient with history of breast cancer. Coronal PET (a) and PET-CT images (b) show extensive bilateral axillary, mediastinal, supraclavicular and retroperitoneal nodal involvement.
Bone is the most frequent site of recurrence after treatment for primary breast cancer; nearly 70% of patients with advanced disease have skeletal metastases [59]. Metastases from breast cancer can produce a varied physiologic response in bone; lesions can be osteolytic, osteoblastic or a mixture of the two. Bone scintigraphy has been the standard initial imaging method for the detection of skeletal metastases in oncologic patients because of its ability to survey the entire skeleton with high sensitivity for detection of metastatic lesions. Retrospective studies comparing the sensitivity of bone scintigraphy to FDG PET for the detection of skeletal metastases in patients with advanced breast cancer have shown conflicting results [3,4,9,14,17-19,60]. Some studies have shown FDG PET to be equal or superior to planar bone scintigraphy in the detection of skeletal metastases [14,18,19] while others have shown FDG PET to be less sensitive [3,4,9,60] on a lesion-based analysis. Cook et al. [17] were the first investigators to correlate the diagnostic performance of FDG PET and bone scintigraphy with the morphologic appearance of individual skeletal metastases at plain film radiography or CT. They showed that FDG PET was superior to bone scintigraphy in the detection of osteolytic metastases and bone scintigraphy detected significantly more osteoblastic metastases. Their findings suggest that the physiologic basis of tracer uptake in skeletal metastases with FDG PET and bone scintigraphy is different; FDG uptake is a more direct reflection of metabolically active tumor cells in bone while bone scintigraphy reflects a reparative process occurring in bone tissues adjacent to tumor cells. Cook et al. also found that patients with lytic disease had significantly worse survival from time of diagnosis of skeletal metastases than patients with either sclerotic or mixed disease. Others have corroborated these findings [18-22], leading to the general conclusion that FDG PET and bone scintigraphy are complementary methods for the detection of skeletal metastases in breast cancer patients. These results also suggest that FDG PET and bone scan should not be considered substitutes for each other for skeletal metastasis staging in breast cancer. In our center, bone scintigraphy remains one of the routine studies in breast cancer metastatic staging, with FDG PET to help clarify staging in the case of difficult or equivocal conventional staging.
Evolving data suggest that [F-18]-fluoride PET may improve skeletal metastasis detection compared to bone scintigraphy [61] and may play a role in breast cancer skeletal metastasis staging in the future. Two advantages that [F-18]-fluoride PET has over routine bone scintigraphy are improved image quality due to more favorable pharmacokinetic characteristics and better spatial resolution of PET. [F-18]-fluoride PET has been shown to be more accurate than bone scintigraphy for the detection of both lytic and sclerotic skeletal metastases in oncologic patients [61,62]. In a prospective study comparing [F-18]-fluoride PET with bone scintigraphy in 34 breast cancer patients being evaluated for skeletal metastases, Schirrmeister et al. [61] found that [F-18]-fluoride PET was nearly two times more sensitive in detecting metastases and accurately depicted the extent of disease in all 17 patients with proven metastases whereas 11 out of the 17 patients were undetected with bone scintigraphy. There was also, however a two-fold increase in the detection of benign bone lesions with [F-18]-fluoride PET in this study, raising concern over the potential poor specificity of this modality. Even-Sapir et al. [63] showed that this limitation can be overcome with the use of [F-18]-fluoride PET/CT. In their study of 44 oncologic patients, [F-18]-fluoride PET/CT had a significantly better specificity than [F-18]-fluoride PET alone (97% vs 72%). The exact role of [F-18]-fluoride PET and [F-18]-fluoride PET/CT has not yet been determined and is not widely available. However, it does show promise in making earlier detection of skeletal metastases in breast cancer patients and selecting them for trials of newer therapeutic agents for skeletal metastases that are on the horizon.
The combined PET and CT system (PET/CT) has emerged as a routine method of restaging many oncologic patients [64]. In an early retrospective review of 75 breast cancer patients who underwent PET/CT, Tatsumi et al. [23] showed PET/CT added incremental diagnostic confidence in 60% of patients who had positive FDG uptake compared to FDG PET alone. The boost in confidence was due to better anatomic localization of FDG foci in over half of the cases and fewer equivocal readings. The increase in confidence was particularly evident for the evaluation of lymph nodes (57%) and skeletal lesions (77%) in this study. Several of the large retrospective studies of patients being restaged after primary treatment for breast cancer comparing diagnostic performance of PET/CT to either contrast-enhanced CT alone, PET alone or side by side evaluation of CT and PET are summarized in Table 2 [23-27,65-67]. Fused PET/CT data consistently detected more malignant foci in these studies, however, on patient-based analysis, the studies comparing PET/CT to PET alone or side by side evaluation of PET and CT showed marginal (no statistically difference) improvement in sensitivity and specificity for the detection of recurrences. Improvement in accuracy of PET/CT compared to PET alone was due mainly to: (1) better localization of FDG uptake foci, particularly in the mediastinum and cervical regions [24,25], and in the skeleton [25,26] with a decrease in the number of false positive findings and (2) an increase in sensitivity to osteoblastic skeletal metastases [24,26]. For the evaluation of pulmonary metastases (Figure 4), there was a tendency for the readers of PET/CT or CT alone to overestimate disease [24,66,67] probably since any pulmonary nodule seen on CT in this patient group is considered suspicious and to underestimate disease with PET alone [24,27] due to the small size of these lesions. Slightly fewer hepatic metastases (Figure 5) were detected at CT alone compared with PET/CT or PET alone [24,26,67], indicating that FDG PET enhances slightly the sensitivity for detecting metastatic disease in the liver. Some investigators have recommended the use of PET/CT for the detection of local and distant recurrences in patients previously treated for primary breast cancer since the combination of CT and PET overcomes the limitations of each modality when used separately [24,67]. This needs to be confirmed in larger prospective trials using FDG-PET/CT-based algorithms.
Table 2.
Largest series comparing the ability of PET/CT with FDG-PET alone or conventional imaging to detect locoregional and distant recurrences in patients who have previously undergone primary treatment for breast cancer.
| Series | Number of patients | Confirmed positive/negative cases | PET/CT Sensitivity (TP/TP+FN)* |
PET/CT Specificity (TN/TN+FP)* |
Comparison imaging |
|---|---|---|---|---|---|
| Grahek 200465† | 75 | 57/18 | 84% | 78% | Physical exam, CI** |
| Fueger 200526 | 58 | 33/25 | 94% | 84% | PET alone |
| Tatsumi 200623 | 69 | 58/11 | 84% | 88% | NCCT*** |
| Radan 200666 | 46 | 30/16 | 90% | 71% | CECT |
| Haug 200724†† | 34 | 26/8 | 96% | 89% | CECT, PET |
| Veit-Haibach 200725 | 44 | 19/25 | Correct staging: 91% (n=40) | Overstaged: 9% (n=4) | CECT, PET, and PET+CECT |
| Piperkova 200767 | 34 (restaging) lesions, n=257 | 15/19 | 98% | 94% | CECT |
| Dirisamer 200927 | 52 lesions, n=150 | 42/10 | 93% | 100% | CECT, PET |
Values calculated on patient analysis except for Piperkova and Dirisamer series which are calculated on lesion analysis; TP=true positive, TN=true negative, FP=false positive, FN=false negative
CI=conventional imaging
NCCT=noncontrast CT
CECT=contrast enhanced CT
PET gamma camera used
fusion software used; all patients were asymptomatic with elevated tumor markers
Figure 4.
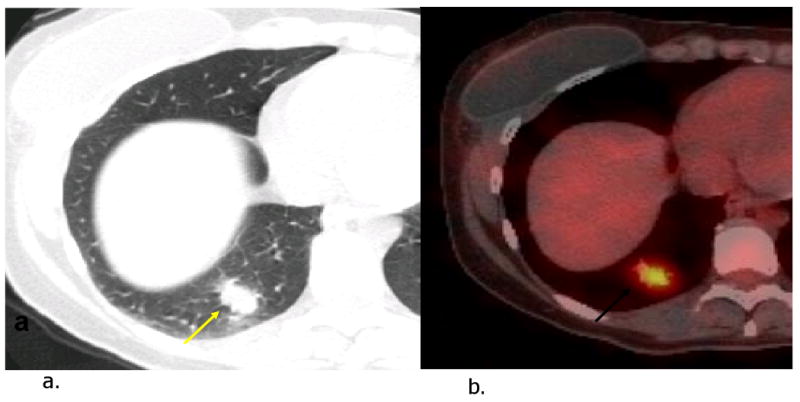
Pulmonary metastasis in a patient with history of recurrent right breast cancer and widespread metastases to brain and bones. CT image (a) shows spiculated nodule in the right lower lobe (arrow). FDG PET-CT image (b) reveals hypermetabolic activity in the nodule.
Figure 5.
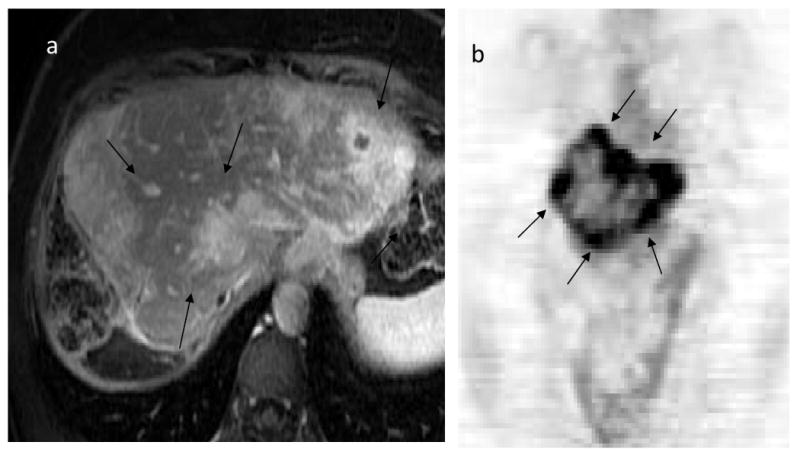
Recurrence after resection of prior liver metastasis in a patient with history of breast cancer. Axial image of liver MRI following gadolinium enhancement (a) demonstrate multiple enhancing masses (arrows) that correspond to the hypermetabolic activity involving entire liver on coronal FDG PET image (b).
Treatment response of metastatic disease
Metastatic breast cancer is often responsive to systemic therapy, and although cure is rarely achieved, with appropriate therapy, patients often have prolonged survival and improved quality of life. The use of FDG PET to assess response to treatment has been studied more extensively in the setting of neoadjuvant systemic therapy in patients with LABC [68-70] compared to the metastatic setting [71-74]. As for the assessment of treatment response of LABC, conventional methods used to assess treatment response in metastases, namely whole-body CT or MR and bone scintigraphy, can be problematic. Morphologic changes detected at CT or MR and skeletal scintigraphic abnormalities may persist or be slow to decrease despite good response to systemic therapy. Metabolic imaging with FDG PET has shown promise in making predictions of treatment response or nonresponse earlier and more accurately than CI. Two small prospective studies including patients with multi-organ metastatic disease [71,72] showed that FDG PET was able to accurately predict treatment response as early as after the first cycle of chemotherapy, with average decline in lesion SUV of 28-42% in responders compared to no change in SUV among nonresponders. Nonresponders were detected earlier than CI during their course of chemotherapy and patients who had no change on follow-up CI were more accurately classified with FDG PET after the first cycle of chemotherapy. In a more recent study of 20 patients with hormone receptor-negative or hormone therapy-refractive metastatic disease, Couturier et al. [73] showed that serial FDG PET could accurately predict response to chemotherapy after the third cycle of therapy; SUV decrease was 52-56% in responders compared to decrease of 16-26% in nonresponders. They found that semi-quantitative changes after the first cycle were not predictive nor was visual assessment at any time point after the start of therapy. FDG PET results following completion of chemotherapy also carries strong prognostic information. In a study of 47 patients with metastatic breast cancer having completed high-dose chemotherapy with autologous stem cell transplantation, Cachin et al. [75] showed that patients without residual FDG uptake on whole-body scans (complete metabolic response) had significantly longer survival times than those with a positive FDG PET (median survival time 24 months vs 10 months, respectively). The results of these early studies, along with determining the most accurate method of predicting response in the metastatic setting, need to be confirmed in larger prospective trials. The main potential benefit of using FDG PET as a method of assessing treatment response in patients with metastatic breast cancer is the earlier recognition of those who do not respond to systemic chemotherapy, allowing the earlier withdrawal of this often toxic from of therapy.
Since there are many therapeutic options available to patients with skeletal metastases, including both local treatment (surgery and/or radiation) and systemic treatment (chemotherapy, endocrine therapy, diphosphonates or a combination), accurate assessment of treatment response is crucial to ensure prolonged quality of life and survival. Following patients with bone disease on systemic therapy with serial bone scintigraphy, the standard method, and MR is unsuitable since the disease is not measurable [76]. Tracer uptake on scintigraphy or morphologic change on CT or MR do not accurately represent disease burden [77] or can be misleading as exemplified by the phenomenon of bone scan “flare” [78]. In a retrospective study, Stafford et al. [74] showed that in patients with FDG-positive bone-dominant metastatic disease, SUV changes of an index lesion on serial FDG PET correlated with clinical assessment of response and change in tumor marker value. Comparing serial changes in SUV of index lesions to patient outcome measures, Specht et al. [79] showed that percentage change in SUV is predictive of time to progression. In this retrospective study, a median decline of 41% or greater was associated with a longer time to progression.
PET/CT is ideally suited for evaluating treatment response of skeletal metastases in breast cancer patients since this technique provides accurate registration of metabolic and morphologic information. Du et al. [80] made some interesting observations on the natural history of 146 bone lesions in 25 breast cancer patients with metastatic skeletal disease being treated with systemic therapy and followed serially with PET/CT. An osteoblastic response was seen in the majority of FDG–avid lesions that were either osteolytic (n=77) or invisible (n=17) by CT to start with and converted to FDG-negative with additional systemic therapy. The change in CT appearance with additional treatment appears to be helpful, in some cases, in confirming treatment response based on FDG SUV change. Furthermore, Tateishi et al. [81] showed, in a retrospective study of 102 patients with breast cancer skeletal metastasis undergoing systemic treatment, that a concomitant increase in CT attenuation and decrease in FDG SUV of the index lesion is predictive of a more durable response to therapy. Larger prospective trials are again warranted to confirm these initial observations.
Impact on management
More important than the diagnostic performance of FDG PET and PET/CT compared to CI is the impact they have on the management of patients with suspected recurrent breast cancer. Unlike patients with some other advanced-stage malignancy, patients with advanced breast cancer can benefit from a variety of therapies including surgery, radiation, chemotherapy and hormonal therapy. Choosing the most appropriate therapy depends primarily on accurately defining the extent of disease. As of October, 2002, PET has been approved for payment by the Center for Medicare and Medicaid Services in the US for staging or re-staging of patients with recurrent or metastatic disease, especially when conventional staging studies are equivocal. Despite this approval, there is relatively little insight into which patients with recurrent or metastatic breast cancer benefit the most from FDG PET. In a prospective study of 50 women undergoing staging studies for suspected recurrent breast cancer [32], FDG PET had a significant impact on defining the extent of disease by changing the clinical stage in 36% of patients and on management by inducing changes in therapy in 58% of the patients. In our retrospective study of 125 patients with advanced breast cancer undergoing conventional imaging and FDG PET for staging [33], the extent of disease was changed in 67% (increased in 43% and decreased in 24%) of patients and the therapeutic plan was altered in 32% of patients based on FDG PET findings. Among different referral categories, FDG PET altered therapy most frequently in patients suspected of locoregional recurrence, under consideration for aggressive local therapy (44%), by demonstrating more widespread disease than expected and avoiding local surgical therapy. Patients with known metastases being evaluated for response to therapy (33%) was another subgroup of patients in whom FDG PET helped the oncologic team make decisions to change therapy. In our study, these two subgroups of patients with advanced disease were most likely to benefit from staging with FDG PET. The more recent retrospective studies comparing PET/CT to contrast-enhanced CT have also shown that PET/CT impacts treatment choices in 20-51% of patients [27,66]. The need for a more sensitive staging tool in patients with first-episode locoregional recurrence was also corroborated by van Oost et al. [82]; their study of 175 patients showed that 16% had distant metastases at the time of locoregional recurrence and 24% developed distant metastases within 18 months of confirmation of recurrence. They estimated that FDG PET would upstage, and likely change the therapeutic plan, in up to 29% of patients with negative conventional staging studies. These results indicate that FDG PET should not be used as the sole restaging tool in patients with recurrent or metastatic disease but to answer specific questions that will likely impact their management.
PET/CT also shows promise in radiation treatment planning by modifying target tissue volumes selected for irradiation based on the combination of morphologic and metabolic data [83]. For example, PET/CT may help to further define the supraclavicular or internal mammary nodal region in patients at risk for recurrence in these regions. In a study that included 32 patients with advanced breast cancer and positive FDG uptake in the supraclavicular region [41], five patients had FDG-positive nodes posterior to the vertebral body transverse process, a location not covered by a typical radiation field to the supraclavicular region and several other patients had nodes close to the medial border of the field. The impact of PET/CT on patient outcomes and which patients will benefit from more aggressive radiation coverage will need to be addressed in future studies.
Summary
FDG PET and PET/CT are useful tools for restaging breast cancer patients who have undergone primary therapy, particularly in those with advanced stages, equivocal findings at conventional staging studies or asymptomatic with elevated tumor markers. In the clinical setting, they should be used to answer specific clinical questions and to complement conventional staging studies, not as a replacement. Evaluation of locoregional recurrences involving the skin, breast and chest wall with FDG PET and PET/CT can be problematic due to poor accuracy and diagnosis is usually made by histologic confirmation. For evaluation of distant metastases, FDG PET and PET/CT perform significantly better than conventional staging studies, particularly for the detection of nodal disease and osteolytic skeletal metastases, and therefore a more accurate method of determining the true extent of disease. One exception is the detection of sclerotic bone metastases; these lesions are not metabolically active enough for FDG PET detection but readily are detected by bone scan. PET/CT enhances diagnostic confidence (with marginal improvement in accuracy) compared to FDG PET alone for the evaluation of metastatic disease. FDG PET and PET/CT can also help in the assessing the treatment response of metastases earlier than conventional imaging. Serial FDG PET or PET/CT more accurately reflects disease status in patients with bone-dominant disease undergoing systemic treatment compared to conventional methods. Preliminary investigations show that FDG PET has the greatest impact on the choice of treatment in patients with suspected or proven locoregional recurrence who are being considered for aggressive curative treatment and in the evaluation of treatment response in patients with metastatic disease.
Synopsis
One of the major strengths of FDG PET and PET/CT in breast cancer imaging is in the evaluation of patients with suspected locoregional recurrence or distant metastasis. In general, FDG PET is more sensitive than conventional imaging for the detection of recurrent disease. Due to its ability to more accurately stage patients with advanced breast cancer, FDG PET has a significant impact on choice of treatment and management in this patient group.
Acknowledgments
This work was supported in part by NIH grants RO1CA42045, RO1CA72064, RO1CA90771 and S10RR177229
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Bender H, Kirst J, Palmedo H, et al. Value of [F-18]-fluoro-deoxyglucose positron emission tomography in the staging of recurrent breast carcinoma. Anticancer Res. 1997;17(3B):1687–92. [PubMed] [Google Scholar]
- 3.Moon DH, Maddahi J, Silverman DH, et al. Accuracy of whole-body [fluorine-18]-FDG PET for the detection of recurrent or metastatic breast carcinoma. J Nucl Med. 1998;39(3):431–5. [PubMed] [Google Scholar]
- 4.Lonneux M, Borbath II, Berliere M, et al. The Place of Whole-Body PET FDG for the Diagnosis of Distant Recurrence of Breast Cancer. Clin Positron Imaging. 2000;3(2):45–9. doi: 10.1016/s1095-0397(00)00042-x. [DOI] [PubMed] [Google Scholar]
- 5.Kim TS, Moon WK, Lee DS, et al. Fluorodeoxyglucose positron emission tomography for detection of recurrent or metastatic breast cancer. World J Surg. 2001;25(7):829–34. doi: 10.1007/s002680020095. [DOI] [PubMed] [Google Scholar]
- 6.Lin WY, Tsai SC, Cheng KY, et al. [Fluorine-18]-FDG-PET in detecting local recurrence and distant metastases in breast cancer--Taiwanese experiences. Cancer Invest. 2002;20(56):725–9. doi: 10.1081/cnv-120003541. [DOI] [PubMed] [Google Scholar]
- 7.Liu CS, Shen YY, Lin CC, et al. Clinical impact of [F-18]-FDG-PET in patients with suspected recurrent breast cancer based on asymptomatically elevated tumor marker serum levels: a preliminary report. Jpn J Clin Oncol. 2002;32(7):244–7. doi: 10.1093/jjco/hyf052. [DOI] [PubMed] [Google Scholar]
- 8.Suarez M, Perez-Castejon MJ, Jimenez A, et al. Early diagnosis of recurrent breast cancer with FDG-PET in patients with progressive elevation of serum tumor markers. Q J Nucl Med. 2002;46(2):113–21. [PubMed] [Google Scholar]
- 9.Gallowitsch HJ, Kresnik E, Gasser J, et al. [F-18]-fluorodeoxyglucose positron-emission tomography in the diagnosis of tumor recurrence and metastases in the follow-up of patients with breast carcinoma: a comparison to conventional imaging. Invest Radiol. 2003;38(5):250–6. doi: 10.1097/01.RLI.0000063983.86229.f2. [DOI] [PubMed] [Google Scholar]
- 10.Siggelkow W, Zimny M, Faridi A, et al. The value of positron emission tomography in the follow-up for breast cancer. Anticancer Res. 2003;23(2C):1859–67. [PubMed] [Google Scholar]
- 11.Kamel EM, Wyss MT, Fehr MK, et al. [F-18]-Fluorodeoxyglucose positron emission tomography in patients with suspected recurrence of breast cancer. J Cancer Res Clin Oncol. 2003;129(3):147–53. doi: 10.1007/s00432-003-0424-z. [DOI] [PubMed] [Google Scholar]
- 12.Eubank WB, Mankoff DA, Vesselle HJ, et al. Detection of locoregional and distant recurrences in breast cancer patients by using FDG PET. Radiographics. 2002;22(1):5–17. doi: 10.1148/radiographics.22.1.g02ja055. [DOI] [PubMed] [Google Scholar]
- 13.Vranjesevic D, Filmont JE, Meta J, et al. Whole-body [F-18]-FDG PET and conventional imaging for predicting outcome in previously treated breast cancer patients. J Nucl Med. 2002;43(3):325–9. [PubMed] [Google Scholar]
- 14.Mahner S, Schirrmacher S, Brenner W, et al. Comparison between positron emission tomography using 2-[fluorine-18]-fluoro-2-deoxy-D-glucose, conventional imaging and computed tomography for staging of breast cancer. Ann Oncol. 2008;19(7):1249–54. doi: 10.1093/annonc/mdn057. [DOI] [PubMed] [Google Scholar]
- 15.Wolfort RM, Li BD, Johnson LW, et al. The role of whole-body [fluorine-18]-FDG positron emission tomography in the detection of recurrence in symptomatic patients with stages II and III breast cancer. World J Surg. 2006;30(8):1422–7. doi: 10.1007/s00268-005-0207-6. [DOI] [PubMed] [Google Scholar]
- 16.Weir L, Worsley D, Bernstein V. The value of FDG positron emission tomography in the management of patients with breast cancer. Breast J. 2005;11(3):204–9. doi: 10.1111/j.1075-122X.2005.21625.x. [DOI] [PubMed] [Google Scholar]
- 17.Cook GJ, Houston S, Rubens R, et al. Detection of bone metastases in breast cancer by [F-18]-FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998;16(10):3375–9. doi: 10.1200/JCO.1998.16.10.3375. [DOI] [PubMed] [Google Scholar]
- 18.Yang SN, Liang JA, Lin FJ, et al. Comparing whole body [F-18]-2-deoxyglucose positron emission tomography and [technetium-99m]-methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128(6):325–8. doi: 10.1007/s00432-002-0342-5. [DOI] [PubMed] [Google Scholar]
- 19.Ohta M, Tokuda Y, Suzuki Y, et al. Whole body PET for the evaluation of bony metastases in patients with breast cancer: comparison with [99mTc]-MDP bone scintigraphy. Nucl Med Commun. 2001;22(8):875–9. doi: 10.1097/00006231-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Abe K, Sasaki M, Kuwabara Y, et al. Comparison of [F-18]-FDG-PET with [99mTc]-HMDP scintigraphy for the detection of bone metastases in patients with breast cancer. Ann Nucl Med. 2005;19(7):573–9. doi: 10.1007/BF02985050. [DOI] [PubMed] [Google Scholar]
- 21.Nakai T, Okuyama C, Kubota T, et al. Pitfalls of FDG-PET for the diagnosis of osteoblastic bone metastases in patients with breast cancer. Eur J Nucl Med Mol Imaging. 2005;32(11):1253–8. doi: 10.1007/s00259-005-1842-8. [DOI] [PubMed] [Google Scholar]
- 22.Uematsu T, Yuen S, Yukisawa S, et al. Comparison of FDG PET and SPECT for detection of bone metastases in breast cancer. AJR Am J Roentgenol. 2005;184(4):1266–73. doi: 10.2214/ajr.184.4.01841266. [DOI] [PubMed] [Google Scholar]
- 23.Tatsumi M, C C, Mortzikos KA, Fishman EK, Wahl RL. Initial Experience With FDG-PET/CT in the Evaluation of Breast Cancer. European Journal of Nuclear Medicine and Molecular Imaging. 2006;33(3):254–62. doi: 10.1007/s00259-005-1835-7. [DOI] [PubMed] [Google Scholar]
- 24.Haug AR, Schmidt GP, Klingenstein A, et al. [F-18]-fluoro-2-deoxyglucose positron emission tomography/computed tomography in the follow-up of breast cancer with elevated levels of tumor markers. J Comput Assist Tomogr. 2007;31(4):629–34. doi: 10.1097/01.rct.0000284394.83696.42. [DOI] [PubMed] [Google Scholar]
- 25.Veit-Haibach P, Antoch G, Beyer T, et al. FDG-PET/CT in restaging of patients with recurrent breast cancer: possible impact on staging and therapy. Br J Radiol. 2007;80(955):508–15. doi: 10.1259/bjr/17395663. [DOI] [PubMed] [Google Scholar]
- 26.Fueger B, Weber WA, Quon A, et al. Performance of 2-Deoxy-2-[F-18]-fuoro-D-glucose Positron Emission Tomography and Integrated PET/CT in Restaged Breast Cancer Patients. Mol Imaging Biol. 2005;7:369–76. doi: 10.1007/s11307-005-0013-4. [DOI] [PubMed] [Google Scholar]
- 27.Dirisamer A, Halpern BS, Flory D, et al. Integrated contrast-enhanced diagnostic whole-body PET/CT as a first-line restaging modality in patients with suspected metastatic recurrence of breast cancer. Eur J Radiol. 2009 doi: 10.1016/j.ejrad.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 28.Hortobagyi GN. Developments in chemotherapy of breast cancer. Cancer. 2000;88(12 Suppl):3073–9. doi: 10.1002/1097-0142(20000615)88:12+<3073::aid-cncr26>3.3.co;2-i. [DOI] [PubMed] [Google Scholar]
- 29.Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004;22(21):4247–54. doi: 10.1200/JCO.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 30.Schwaibold F, Fowble BL, Solin LJ, et al. The results of radiation therapy for isolated local regional recurrence after mastectomy. Int J Radiat Oncol Biol Phys. 1991;21(2):299–310. doi: 10.1016/0360-3016(91)90775-y. [DOI] [PubMed] [Google Scholar]
- 31.Niibe Y, Kuranami M, Matsunaga K, et al. Value of high-dose radiation therapy for isolated osseous metastasis in breast cancer in terms of oligo-recurrence. Anticancer Res. 2008;28(6B):3929–31. [PubMed] [Google Scholar]
- 32.Yap CS, Seltzer MA, Schiepers C, et al. Impact of whole-body [F-18]-FDG PET on staging and managing patients with breast cancer: the referring physician's perspective. J Nucl Med. 2001;42(9):1334–7. [PubMed] [Google Scholar]
- 33.Eubank W, Mankoff D, Bhattacharya M, et al. Impact of [F-18]-Fluorodeoxyglucose PET on defining the extent of disease and management of patients with recurrent or metastatic breast cancer. AJR Am J Roentgen. 2004;183:479–86. doi: 10.2214/ajr.183.2.1830479. [DOI] [PubMed] [Google Scholar]
- 34.Katz A, Strom EA, Buchholz TA, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000;18(15):2817–27. doi: 10.1200/JCO.2000.18.15.2817. [DOI] [PubMed] [Google Scholar]
- 35.Buchanan CL, Dorn PL, Fey J, et al. Locoregional recurrence after mastectomy: incidence and outcomes. J Am Coll Surg. 2006;203(4):469–74. doi: 10.1016/j.jamcollsurg.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Fodor J, Major T, Polgar C, et al. Prognosis of patients with local recurrence after mastectomy or conservative surgery for early-stage invasive breast cancer. Breast. 2008;17(3):302–8. doi: 10.1016/j.breast.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Huston TL, Simmons RM. Locally recurrent breast cancer after conservation therapy. Am J Surg. 2005;189(2):229–35. doi: 10.1016/j.amjsurg.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 38.Touboul E, Buffat L, Belkacemi Y, et al. Local recurrences and distant metastases after breast-conserving surgery and radiation therapy for early breast cancer. Int J Radiat Oncol Biol Phys. 1999;43(1):25–38. doi: 10.1016/s0360-3016(98)00365-4. [DOI] [PubMed] [Google Scholar]
- 39.Aristei C, Marsella AR, Chionne F, et al. Regional node failure in patients with four or more positive lymph nodes submitted to conservative surgery followed by radiotherapy to the breast. Am J Clin Oncol. 2000;23(3):217–21. doi: 10.1097/00000421-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Chen SC, Chang HK, Lin YC, et al. Prognosis of breast cancer after supraclavicular lymph node metastasis: not a distant metastasis. Ann Surg Oncol. 2006;13(11):1457–65. doi: 10.1245/s10434-006-9012-1. [DOI] [PubMed] [Google Scholar]
- 41.Reed VK, Cavalcanti JL, Strom EA, et al. Risk of subclinical micrometastatic disease in the supraclavicular nodal bed according to the anatomic distribution in patients with advanced breast cancer. Int J Radiat Oncol Biol Phys. 2008;71(2):435–40. doi: 10.1016/j.ijrobp.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heusner TA, Freudenberg LS, Kuehl H, et al. Whole-body PET/CT-mammography for staging breast cancer: initial results. Br J Radiol. 2008;81(969):743–8. doi: 10.1259/bjr/69647413. [DOI] [PubMed] [Google Scholar]
- 43.Hathaway PB, Mankoff DA, Maravilla KR, et al. The value of combined FDG-PET and magnetic resonance imaging in the evaluation of suspected recurrent local-regional breast cancer: preliminary experience. Radiology. 1999;210:807–14. doi: 10.1148/radiology.210.3.r99mr43807. [DOI] [PubMed] [Google Scholar]
- 44.Ahmad A, Barrington S, Maisey M, et al. Use of positron emission tomography in evaluation of brachial plexopathy in breast cancer patients. Br J Cancer. 1999;79(34):478–82. doi: 10.1038/sj.bjc.6690074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byrd DR, Dunnwald LK, Mankoff DA, et al. Internal mammary lymph node drainage patterns in patients with breast cancer documented by breast lymphoscintigraphy. Ann Surg Oncol. 2001;8(3):234–40. doi: 10.1007/s10434-001-0234-y. [DOI] [PubMed] [Google Scholar]
- 46.Yao MS, Kurland BF, Smith AH, et al. Internal mammary nodal chain drainage is a prognostic indicator in axillary node-positive breast cancer. Ann Surg Oncol. 2007;14(10):2985–93. doi: 10.1245/s10434-007-9473-x. [DOI] [PubMed] [Google Scholar]
- 47.Donegan WL. The influence of untreated internal mammary metastases upon the course of mammary cancer. Cancer. 1977;39(2):533–8. doi: 10.1002/1097-0142(197702)39:2<533::aid-cncr2820390222>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 48.Veronesi U, Cascinelli N, Greco M, et al. Prognosis of breast cancer patients after mastectomy and dissection of internal mammary nodes. Ann Surg. 1985;202(6):702–7. doi: 10.1097/00000658-198512000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sugg SL, Ferguson DJ, Posner MC, et al. Should internal mammary nodes be sampled in the sentinel lymph node era? Ann Surg Oncol. 2000;7:188–92. doi: 10.1007/BF02523652. [DOI] [PubMed] [Google Scholar]
- 50.Danforth DN, Jr, Aloj L, Carrasquillo JA, et al. The role of [F-18]-FDG-PET in the local/regional evaluation of women with breast cancer. Breast Cancer Res Treat. 2002;75(2):135–46. doi: 10.1023/a:1019664126220. [DOI] [PubMed] [Google Scholar]
- 51.Carkaci S, Macapinlac HA, Cristofanilli M, et al. Retrospective study of [F-18]-FDG PET/CT in the diagnosis of inflammatory breast cancer: preliminary data. J Nucl Med. 2009;50(2):231–8. doi: 10.2967/jnumed.108.056010. [DOI] [PubMed] [Google Scholar]
- 52.Groheux D, Moretti JL, Baillet G, et al. Effect of [F-18]-FDG PET/CT imaging in patients with clinical Stage II and III breast cancer. Int J Radiat Oncol Biol Phys. 2008;71(3):695–704. doi: 10.1016/j.ijrobp.2008.02.056. [DOI] [PubMed] [Google Scholar]
- 53.Bellon JR, Livingston RB, Eubank WB, et al. Evaluation of the internal mammary lymph nodes by FDG-PET in locally advanced breast cancer (LABC) Am J Clin Oncol. 2004;27(4):407–10. doi: 10.1097/01.coc.0000128869.19357.9b. [DOI] [PubMed] [Google Scholar]
- 54.Tran A, Pio BS, Khatibi B, et al. [F-18]-FDG PET for staging breast cancer in patients with inner-quadrant versus outer-quadrant tumors: comparison with long-term clinical outcome. J Nucl Med. 2005;46(9):1455–9. [PubMed] [Google Scholar]
- 55.Isasi CR, Moadel RM, Blaufox MD. A meta-analysis of FDG-PET for the evaluation of breast cancer recurrence and metastases. Breast Cancer Res Treat. 2005;90(2):105–12. doi: 10.1007/s10549-004-3291-7. [DOI] [PubMed] [Google Scholar]
- 56.Vansteenkiste JF, Stroobants SG, De Leyn PR, et al. Lymph node staging in non-small-cell lung cancer with FDG-PET scan: a prospective study on 690 lymph node stations from 68 patients. J Clin Oncol. 1998;16(6):2142–9. doi: 10.1200/JCO.1998.16.6.2142. [DOI] [PubMed] [Google Scholar]
- 57.Stroobants S, Verschakelen J, Vansteenkiste J. Value of FDG-PET in the management of non-small cell lung cancer. Eur J Radiol. 2003;45(1):49–59. doi: 10.1016/s0720-048x(02)00282-6. [DOI] [PubMed] [Google Scholar]
- 58.Eubank WB, Mankoff DA, Takasugi J, et al. [F-18]-fluorodeoxyglucose positron emission tomography to detect mediastinal or internal mammary metastases in breast cancer. J Clin Oncol. 2001;19(15):3516–23. doi: 10.1200/JCO.2001.19.15.3516. [DOI] [PubMed] [Google Scholar]
- 59.Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55(1):61–6. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kao CH, Hsieh JF, Tsai SC, et al. Comparison and discrepancy of [F-18]-2-deoxyglucose positron emission tomography and [99m Tc]- MDP bone scan to detect bone metastases. Anticancer Res. 2000;20(3B):2189–92. [PubMed] [Google Scholar]
- 61.Schirrmeister H, Guhlmann A, Kotzerke J, et al. Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. J Clin Oncol. 1999;17(8):2381–9. doi: 10.1200/JCO.1999.17.8.2381. [DOI] [PubMed] [Google Scholar]
- 62.Petren-Mallmin M, Andreasson I, Ljunggren O, et al. Skeletal metastases from breast cancer: uptake of [F-18]-fluoride measured with positron emission tomography in correlation with CT. Skeletal Radiol. 1998;27(2):72–6. doi: 10.1007/s002560050340. [DOI] [PubMed] [Google Scholar]
- 63.Even-Sapir E, Metser U, Flusser G, et al. Assessment of malignant skeletal disease: initial experience with [F-18]-fluoride PET/CT and comparison between [F-18]-fluoride PET and [F-18]-fluoride PET/CT. J Nucl Med. 2004;45(2):272–8. [PubMed] [Google Scholar]
- 64.Antoch G, Saoudi N, Kuehl H, et al. Accuracy of whole-body dual-modality [fluorine-18]-2-fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography (FDG-PET/CT) for tumor staging in solid tumors: comparison with CT and PET. J Clin Oncol. 2004;22(21):4357–68. doi: 10.1200/JCO.2004.08.120. [DOI] [PubMed] [Google Scholar]
- 65.Grahek D, Montravers F, Kerrou K, et al. [F-18]-FDG in recurrent breast cancer: diagnostic performances, clinical impact and relevance of induced changes in management. Eur J Nucl Med Mol Imaging. 2004;31(2):179–88. doi: 10.1007/s00259-003-1348-1. [DOI] [PubMed] [Google Scholar]
- 66.Radan L, B-H S, Bar-Shalom R, et al. The Role of FDG-PET/CT in Suspected Recurrence of Breast Cancer. Cancer. 2006;107:2545–51. doi: 10.1002/cncr.22292. [DOI] [PubMed] [Google Scholar]
- 67.Piperkova E, Raphael B, Altinyay ME, et al. Impact of PET/CT in comparison with same day contrast enhanced CT in breast cancer management. Clin Nucl Med. 2007;32(6):429–34. doi: 10.1097/RLU.0b013e31805375e0. [DOI] [PubMed] [Google Scholar]
- 68.Schelling M, Avril N, Nahrig J, et al. Positron emission tomography using [18F]-fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol. 2000;18:1689–95. doi: 10.1200/JCO.2000.18.8.1689. [DOI] [PubMed] [Google Scholar]
- 69.Mankoff DA, Dunnwald LK, Gralow JR, et al. Blood flow and metabolism in locally advanced breast cancer: relationship to response to therapy. J Nucl Med. 2002;43(4):500–9. [PubMed] [Google Scholar]
- 70.Schwarz-Dose J, Untch M, Tiling R, et al. Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [F-18]-fluorodeoxyglucose. J Clin Oncol. 2009;27(4):535–41. doi: 10.1200/JCO.2008.17.2650. [DOI] [PubMed] [Google Scholar]
- 71.Gennari A, Donati S, Salvadori B, et al. Role of 2-[F-18]-fluorodeoxyglucose (FDG) positron emission tomography (PET) in the early assessment of response to chemotherapy in metastatic breast cancer patients. Clin Breast Cancer. 2000;1(2):156–61. doi: 10.3816/cbc.2000.n.014. discussion 62-3. [DOI] [PubMed] [Google Scholar]
- 72.Dose Schwarz J, Bader M, Jenicke L, et al. Early prediction of response to chemotherapy in metastatic breast cancer using sequential [F-18]-FDG PET. J Nucl Med. 2005;46(7):1144–50. [PubMed] [Google Scholar]
- 73.Couturier O, Jerusalem G, N'Guyen JM, et al. Sequential positron emission tomography using [F-18]-fluorodeoxyglucose for monitoring response to chemotherapy in metastatic breast cancer. Clin Cancer Res. 2006;12(21):6437–43. doi: 10.1158/1078-0432.CCR-06-0383. [DOI] [PubMed] [Google Scholar]
- 74.Stafford SE, Gralow JR, Schubert EK, et al. Use of serial FDG PET to measure the response of bone-dominant breast cancer to therapy. Acad Radiol. 2002;9(8):913–21. doi: 10.1016/s1076-6332(03)80461-0. [DOI] [PubMed] [Google Scholar]
- 75.Cachin F, Prince HM, Hogg A, et al. Powerful prognostic stratification by [F-18]-fluorodeoxyglucose positron emission tomography in patients with metastatic breast cancer treated with high-dose chemotherapy. J Clin Oncol. 2006;24(19):3026–31. doi: 10.1200/JCO.2005.04.6326. [DOI] [PubMed] [Google Scholar]
- 76.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 77.Hamaoka T, Madewell JE, Podoloff DA, et al. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22(14):2942–53. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 78.Schneider JA, Divgi CR, Scott AM, et al. Flare on bone scintigraphy following Taxol chemotherapy for metastatic breast cancer. J Nucl Med. 1994;35(11):1748–52. [PubMed] [Google Scholar]
- 79.Specht JM, Tam SL, Kurland BF, et al. Serial 2-[F-18]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) to monitor treatment of bone-dominant metastatic breast cancer predicts time to progression (TTP) Breast Cancer Res Treat. 2007;105(1):87–94. doi: 10.1007/s10549-006-9435-1. [DOI] [PubMed] [Google Scholar]
- 80.Du Y, Cullum I, Illidge TM, et al. Fusion of metabolic function and morphology: sequential [F-18]-fluorodeoxyglucose positron-emission tomography/computed tomography studies yield new insights into the natural history of bone metastases in breast cancer. J Clin Oncol. 2007;25(23):3440–7. doi: 10.1200/JCO.2007.11.2854. [DOI] [PubMed] [Google Scholar]
- 81.Tateishi U, Gamez C, Dawood S, et al. Bone metastases in patients with metastatic breast cancer: morphologic and metabolic monitoring of response to systemic therapy with integrated PET/CT. Radiology. 2008;247(1):189–96. doi: 10.1148/radiol.2471070567. [DOI] [PubMed] [Google Scholar]
- 82.van Oost FJ, van der Hoeven JJ, Hoekstra OS, et al. Staging in patients with locoregionally recurrent breast cancer: current practice and prospects for positron emission tomography. Eur J Cancer. 2004;40(10):1545–53. doi: 10.1016/j.ejca.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 83.Kruser TJ, Bradley KA, Bentzen SM, et al. The impact of hybrid PET-CT scan on overall oncologic management, with a focus on radiotherapy planning: a prospective, blinded study. Technol Cancer Res Treat. 2009;8(2):149–58. doi: 10.1177/153303460900800208. [DOI] [PubMed] [Google Scholar]


