Abstract
The values of the second dissociation constant pK2 and related thermodynamic quantities of [N-(2-acetamido)-2-aminoethanesulfonic acid] (ACES) have already been reported over the temperature range 5 to 55°C including 37°C. This paper reports the paH values of four chloride ion free buffer solutions and eight buffer solutions with I = 0.16 mol·kg −1, matching closely to that of the physiological sample. Conventional paH values for all twelve buffer solutions from 5 to 55°C, are reported. The residual liquid junction potential correction for two widely used temperatures, 25 and 37°C, has been made. The flowing-junction calomel cell method has been utilized to measure Ej, the liquid junction potential. The operational pH values for four buffer solutions at 25 and 37°C are calculated using the physiological phosphate buffer standard based on NBS/NIST convention. These solutions are recommended as pH standards in the pH range of 6.8 to 7.2 for physiological fluids.
Keywords: Buffers, ACES, Liquid junction, Ionic strength, Emf, Zwitterions, pH, Acidity function
1 Introduction
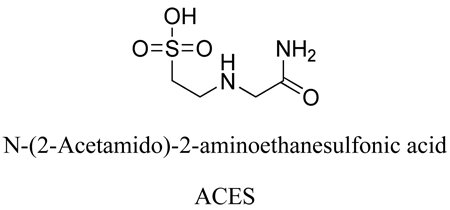
We have reported the pK2 values of N-(2-acetamido)-2-aminoethanesulfonic acid (ACES) [1] and TABS [2], at temperatures from 5 to 55°C including 37°C. The zwitterionic compounds have been recommended by Good and coworkers [3–4] for use as biological buffers. The structure of ACES is given below:
In 1961[5], the National Bureau of Standards (NBS) certified the widely used phosphate buffer as a physiological pH standard in the pH range between 7.3 and 7.5. The pH of this physiological phosphate buffer standard is 7.415 at 25°C and 7.395 at 37°C.
Some of the problems with its use are explained as follows: (i) phosphates interact unfavorably with biological media, (ii) precipitation occurs with some polyvalent cations in the blood constituents such as Mg+2 and Ca+2, (iii) phosphates, may also act as an inhibitor to enzymatic processes, and (iv) the temperature coefficient of the phosphate buffer is −0.0028 pH unit/°C which is not close to that of whole blood (−0.015 pH unit/°C) [6].
Wu and coworkers [7] have published the values of pK2 and pH of the zwitterionic buffer N-(2-hydroxyethyl)piperazine-N-2-ethanesulfonic acid (HEPES). The National Institute of Standards and Technology (NIST) has certified the HEPES buffer as a primary reference standard in the range of physiological application. For two-point calibrations of pH measurements for physiological fluids, a second zwitterionic buffer, 3-(N-morpholino)-2-hydroxypropanesulfonic acid (MOPSO), has been studied by Wu et al.[8]. In 1973, Bates et al. [9–10] suggested the use of tris(hydroxymethyl)methylglycine (TRICINE) as a buffer standard for the physiological range of pH 7.2 to 8.5. Roy et al. [11] reported results for pK2 and pH for 3-(N-morpholino)propanesulfonic acid (MOPS) in the temperature range 5 to 55°C. Another zwitterionic buffer material, N-(2-hydroxyethyl)piperazine-N′-2-hydroxypropanesulfonic acid (HEPPSO) was investigated by Roy et al. [12] for the control of acidity in the biochemically important pH range. Goldberg et al. [13], in their comprehensive review of the thermodynamic quantities of the biological buffers, indicates that no reliable pH data are available for ACES buffer.
In continuation of research in the area of pH values for physiological pH standards, we have studied ACES, with the following compositions in units of molality m, where m = mol·kg−1:
ACES (0.01 mol·kg −1) + NaACES (0.03 mol·kg −1), I = 0.03 mol·kg −1
ACES (0.02 mol·kg −1) + NaACES (0.04 mol·kg −1), I = 0.04 mol·kg −1
ACES (0.03 mol·kg −1) + NaACES (0.06 mol·kg −1), I = 0.06 mol·kg −1
ACES (0.04 mol·kg −1) + NaACES (0.08 mol·kg −1), I = 0.08 mol·kg −1
ACES (0.01 mol·kg −1) + NaACES (0.03 mol·kg −1) + NaCl (0.13 mol·kg −1), I = 0.16 mol·kg −1
ACES (0.02 mol·kg −1) + NaACES (0.04 mol·kg −1) + NaCl (0.12 mol·kg −1), I = 0.16 mol·kg −1
ACES (0.03 mol·kg −1) + NaACES (0.06 mol·kg −1) + NaCl (0.10 mol·kg −1), I = 0.16 mol·kg −1
ACES (0.04 mol·kg −1) + NaACES (0.08 mol·kg −1) + NaCl (0.08 mol·kg −1), I = 0.16 mol·kg −1
ACES (0.04 mol·kg −1) + NaACES (0.04 mol·kg −1) + NaCl (0.12 mol·kg −1), I = 0.16 mol·kg −1
ACES (0.05 mol·kg −1) + NaACES (0.05 mol·kg −1) + NaCl (0.11 mol·kg −1), I = 0.16 mol·kg −1
ACES (0.06 mol·kg −1) + NaACES (0.06 mol·kg −1) + NaCl (0.10 mol·kg −1), I = 0.16 mol·kg −1
ACES (0.08 mol·kg −1) + NaACES (0.08 mol·kg −1) + NaCl (0.08 mol·kg −1), I = 0.16 mol·kg −1
The detailed procedure for the preparation of these buffer solutions for ACES is described below.
2. Experimental
ACES was obtained from Sigma Chemical Co. (St. Louis, Missouri). The details of purification by further crystallization as well as the determinations of the assay have been reported in earlier papers [1]. The assays showed that the ACES was (99.94 ± 0.03) % pure. The buffer solutions were prepared by weighing required amounts of ACES, some standard solutions of NaOH subsequently used to prepare the buffer solutions, and calculated amounts of CO2-free doubly distilled water. ACS reagent grade NaCl was recrystalized from water. Buoyancy corrections were applied to all for all masses used.
The preparation of the hydrogen electrodes, the silver-silver chloride, the design of the all-glass cells, and the purification of the hydrogen gas have been described previously [11, 14]. The description of the equipment, such as a digital platinum resistance thermometer (Guildline Model 9540), digital voltmeter (Hewlett-Packard 2000 multimeter), constant temperature bath (controlled to ±0.005°C), etc. have been published [ 11–12].
Four types of electrochemical cells were employed in the present investigation. The schematic diagram of these is shown below:
| (A) |
where the buffer ACES, in indicated molalities of the respective species, and 1 atm = 101.325 kPa in SI units. The estimation of the liquid junction potential was made by using the flowing junction cell (B):
| (B) |
where the double vertical line represents a liquid junction, and the abbreviations (s), (l), and (g) denote solid, liquid, and gaseous state, respectively.
This primary reference with the composition [KH2PO4 (0.008695 mol·kg −1) + Na2HPO4 (0.03043 mol·kg −1)] was used. The schematic design of cell (C) is:
| (C) |
The values of the standard electrode potential, E°SCE, of the saturated calomel electrode were given [15–17] to be: −0.2415 V, and −0.2335 V at 25 and 37°C [15–17], respectively. The values of Ej for the physiological phosphate as well as for the other buffer solutions for cell (B) were calculated using the following equation [11]:
| (1) |
where k = 0.059156 and pH = 7.415 (physiological phosphate buffer solution) at 25°; k = 0.061538 and pH = 7.395 at 37°C. The operational definition of pH, namely pH (x), is:
| (2) |
where x refers to the unknown buffers (ACES + NaACESate;), s is the reference solution of known pH, and δEj = Ej(s) − Ej(x). For δEj = 0, Eq. (2) becomes:
| (3) |
As a trial run, the procedure used to determine pK2 values for ACES differed in employing the following cell with silver-silver bromide electrodes:
| (D) |
The results of pK2 obtained by using cell (A) containing a Ag-AgCl reference electrode and cell (D) with Ag-AgBr reference electrodes lie within experimental uncertainties (within ±0.001). Thus, there was no indication of the elevated solubility of silver chloride in presence of solutions involving nitrogen bases. Hence, silver-silver chloride electrodes were used throughout the experiments in order to determine pH values of buffer solutions. Duplicate cells usually gave readings within 0.02 mV on the average in the temperature range 5 to 55°C.
3. Methods and Results
The emf values for cell (A) containing four buffer solutions, and eight dilute buffer solutions in which NaCl had been added to make I = 0.16 mol·kg−1, have been corrected to a hydrogen pressure of 1 atm. These values are entered in Table 1 and Table 2, respectively.
Table 1.
Emf of Cell A: Pt(s); H2 (g, 1 atm) | ACES (m1), NaACES (m2), NaCl (m3) | AgCl(s), Ag(s)
| m1a | m2a | m3a | 5°C | 10°C | 15°C | 20°C | 25°C | 30°C | 35°C | 37°C | 40°C | 45°C | 50°C | 55°C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.03 | 0.005 | 0.78699 | 0.78819 | 0.78956 | 0.79065 | 0.79160 | 0.79256 | 0.79333 | 0.79360 | 0.79397 | 0.79455 | 0.79508 | 0.79565 |
| 0.01 | 0.03 | 0.010 | 0.77132 | 0.77231 | 0.77351 | 0.77430 | 0.77498 | 0.77569 | 0.77598 | 0.77643 | 0.77661 | 0.77680 | 0.77704 | 0.77729 |
| 0.01 | 0.03 | 0.015 | 0.76265 | 0.76359 | 0.76439 | 0.76498 | 0.76564 | 0.76619 | 0.76653 | 0.76665 | 0.76676 | 0.76697 | 0.76714 | 0.76725 |
| 0.01 | 0.03 | 0.020 | 0.75690 | 0.75761 | 0.75850 | 0.75901 | 0.75928 | 0.75975 | 0.75995 | 0.76030 | 0.76023 | 0.76015 | 0.76021 | 0.76031 |
| 0.02 | 0.04 | 0.005 | 0.77832 | 0.77950 | 0.78061 | 0.78149 | 0.78237 | 0.78309 | 0.78373 | 0.78401 | 0.78425 | 0.78475 | 0.78490 | 0.78507 |
| 0.02 | 0.04 | 0.010 | 0.76281 | 0.76364 | 0.76447 | 0.76515 | 0.76571 | 0.76621 | 0.76661 | 0.76668 | 0.76681 | 0.76704 | 0.76683 | 0.76680 |
| 0.02 | 0.04 | 0.015 | 0.75406 | 0.75474 | 0.75530 | 0.75589 | 0.75636 | 0.75672 | 0.75695 | 0.75687 | 0.75693 | 0.75707 | 0.75663 | 0.75645 |
| 0.02 | 0.04 | 0.020 | 0.74831 | 0.74891 | 0.74933 | 0.74960 | 0.75020 | 0.75047 | 0.75058 | 0.75038 | 0.75051 | 0.75055 | 0.74995 | 0.74962 |
| 0.03 | 0.06 | 0.005 | 0.77864 | 0.77991 | 0.78090 | 0.78185 | 0.78260 | 0.78326 | 0.78382 | 0.78402 | 0.78421 | 0.78447 | 0.78453 | 0.78464 |
| 0.03 | 0.06 | 0.010 | 0.76264 | 0.76355 | 0.76433 | 0.76491 | 0.76530 | 0.76551 | 0.76584 | 0.76590 | 0.76595 | 0.76595 | 0.76574 | 0.76556 |
| 0.03 | 0.06 | 0.015 | 0.75343 | 0.75405 | 0.75470 | 0.75500 | 0.75513 | 0.75516 | 0.75534 | 0.75531 | 0.75524 | 0.75514 | 0.75474 | 0.75439 |
| 0.03 | 0.06 | 0.020 | 0.74716 | 0.74761 | 0.74819 | 0.74821 | 0.74821 | 0.74813 | 0.74825 | 0.74814 | 0.74803 | 0.74786 | 0.74735 | 0.74690 |
| 0.04 | 0.08 | 0.005 | 0.77830 | 0.77956 | 0.78060 | 0.78151 | 0.78223 | 0.78295 | 0.78370 | 0.78393 | 0.78429 | 0.78448 | 0.78507 | 0.78534 |
| 0.04 | 0.08 | 0.010 | 0.76237 | 0.76325 | 0.76396 | 0.76441 | 0.76506 | 0.76545 | 0.76577 | 0.76570 | 0.76601 | 0.76581 | 0.76617 | 0.76612 |
| 0.04 | 0.08 | 0.015 | 0.75312 | 0.75377 | 0.75418 | 0.75450 | 0.75507 | 0.75523 | 0.75523 | 0.75527 | 0.75523 | 0.75482 | 0.75501 | 0.75476 |
| 0.04 | 0.08 | 0.020 | 0.74680 | 0.74736 | 0.74767 | 0.74785 | 0.74833 | 0.74834 | 0.74808 | 0.74806 | 0.74790 | 0.74715 | 0.74741 | 0.74702 |
Units of m, mol·kg−1
Table 2.
Electromotive force of the Cell A (in volts): Pt(s); H2 (g, 1 atm) | ACES (m1), NaACES (m2), NaCl (m3) | AgCl(s), Ag(s)
| m1a | m2a | m3a | 5°C | 10°C | 15°C | 20°C | 25°C | 30°C | 35°C | 37°C | 40°C | 45°C | 50°C | 55°C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.03 | 0.13 | 0.71363 | 0.71367 | 0.71356 | 0.71342 | 0.71285 | 0.71236 | 0.71172 | 0.71152 | 0.71106 | 0.71018 | 0.70922 | 0.70837 |
| 0.02 | 0.04 | 0.12 | 0.70601 | 0.76214 | 0.70586 | 0.70529 | 0.70466 | 0.70392 | 0.70318 | 0.70283 | 0.70223 | 0.70130 | 0.70017 | 0.69913 |
| 0.03 | 0.06 | 0.10 | 0.70216 | 0.70344 | 0.70436 | 0.70483 | 0.70545 | 0.70554 | 0.70544 | 0.70532 | 0.70517 | 0.70419 | 0.70338 | 0.70240 |
| 0.04 | 0.08 | 0.08 | 0.71561 | 0.71577 | 0.71547 | 0.71522 | 0.71478 | 0.71422 | 0.71355 | 0.71326 | 0.71281 | 0.71198 | 0.71099 | 0.71010 |
| 0.04 | 0.04 | 0.12 | 0.68876 | 0.68851 | 0.68775 | 0.68706 | 0.68617 | 0.68523 | 0.68420 | 0.68374 | 0.68303 | 0.68177 | 0.68043 | 0.67917 |
| 0.05 | 0.05 | 0.11 | 0.69261 | 0.69229 | 0.69166 | 0.69076 | 0.68977 | 0.68867 | 0.68756 | 0.68699 | 0.68619 | 0.68479 | 0.68328 | 0.68186 |
| 0.06 | 0.06 | 0.10 | 0.69343 | 0.69315 | 0.69251 | 0.69180 | 0.69111 | 0.69023 | 0.68923 | 0.68881 | 0.68817 | 0.68688 | 0.68555 | 0.68425 |
| 0.08 | 0.08 | 0.08 | 0.69920 | 0.69894 | 0.69842 | 0.69790 | 0.69713 | 0.69631 | 0.69541 | 0.69504 | 0.69441 | 0.69317 | 0.69202 | 0.69087 |
Units of m, mol·kg−1
3.1 The Conventional paH of ACES Buffer
Conventional paH values have been calculated by the method of Bates et al. [9, 17] for twelve buffer solutions with the following compositions, where the numbers in the parentheses, and the ionic strengths of each solution, are in units of molality:
ACES (0.01 mol·kg −1) + NaACES (0.03 mol·kg −1), I = 0.03 mol·kg −1
ACES (0.02 mol·kg −1) + NaACES (0.04 mol·kg −1), I = 0.04 mol·kg −1
ACES (0.03 mol·kg −1) + NaACES (0.06 mol·kg −1), I = 0.06 mol·kg −1
ACES (0.04 mol·kg −1) + NaACES (0.08 mol·kg −1), I = 0.08 mol·kg −1
ACES (0.01 mol·kg −1) + NaACES (0.03 mol·kg −1) + NaCl (0.13 mol·kg −1), I = 0.16 mol·kg −1
ACES (0.02 mol·kg −1) + NaACES (0.04 mol·kg −1) + NaCl (0.12 mol·kg −1), I = 0.16 mol·kg −1
ACES (0.03 mol·kg −1) + NaACES (0.06 mol·kg −1) + NaCl (0.10 mol·kg −1), I = 0.16 mol·kg −1
ACES (0.04 mol·kg −1) + NaACES (0.08 mol·kg −1) + NaCl (0.08 mol·kg −1), I = 0.16 mol·kg −1
ACES (0.04 mol·kg −1) + NaACES (0.04 mol·kg −1) + NaCl (0.12 mol·kg −1), I = 0.16 mol·kg −1
ACES (0.05 mol·kg −1) + NaACES (0.05 mol·kg −1) + NaCl (0.11 mol·kg −1), I = 0.16 mol·kg −1
ACES (0.06 mol·kg −1) + NaACES (0.06 mol·kg −1) + NaCl (0.10 mol·kg −1), I = 0.16 mol·kg −1
ACES (0.08 mol·kg −1) + NaACES (0.08 mol·kg −1) + NaCl (0.08 mol·kg −1), I = 0.16 mol·kg −1
The conventional paH values for all twelve buffer solutions were calculated using the Eq. (4–Eq. 7). Values of the acidity function p(aHγCl) were derived at each temperature from the emf (E) listed in Table 1 and Table 2, the molality of the chloride, and E°, the standard potential of the silver-silver chloride electrode [1], by the following equation:
| (5) |
where k is the Nernst slope.
| (6) |
| (7) |
Where C is an adjustable parameter, Ba° was assumed to be 1.38 [11] at the experimental temperature, corresponding to an (ion-size parameter) a° of 4.1 – 4.2 Ǻ [11].
| (8) |
where C25 = 0.032 [11]. For the calculation of paH, the procedures used for other are the same as those reported in preceding papers for HEPES and DIPSO.
The values of (paHγCl) for four buffer solutions are listed in Table 3. The values of p(aHγCl) for the eight isotonic saline solutions are entered in Table 4 and Table 5. The values of paH, listed in Table 6, Table 7, and Table 8 for twelve buffer solutions of ACES with and without NaCl were computed from Eq. (5 – Eq. 8) and are represented by the following equations:
| (8) |
| (9) |
| (10) |
| (11) |
Where 5 ≤ t ≤ 55°C. The standard deviations of regression for the paH of the chloride-free buffer solutions, obtained from the fits with Eq. (8 – Eq. 11), are 0.0012, 0.0015, 0.0012, and 0.0018 respectively. For eight buffer solutions containing NaCl at an isotonic saline media of the ionic strength I = 0.16 mol·kg−1, the values of paH listed in Table 7 and Table 8 are expressed by the equations:
| (12) |
| (13) |
| (14) |
| (15) |
| (16) |
| (17) |
| (18) |
| (19) |
where t is the temperature in °C. The standard deviations of regression for these eight equations are: 0.0011, 0.0011, 0.0015, 0.0008, 0.0010, 0.0009, 0.0011, and 0.0008, respectively.
Table 3.
| t(°C) | 0.01 m ACES + 0.03 m NaACES I = 0.03 m |
0.02 m ACES + 0.04 m NaACES I = 0.04 m |
0.03 m ACES + 0.06 m NaACES I = 0.06 m |
0.04 m ACES + 0.08 m NaACES I = 0.08 m |
|---|---|---|---|---|
| 5 | 7.696 | 7.540 | 7.554 | 7.549 |
| 10 | 7.589 | 7.434 | 7.452 | 7.445 |
| 15 | 7.494 | 7.335 | 7.349 | 7.345 |
| 20 | 7.394 | 7.239 | 7.255 | 7.248 |
| 25 | 7.302 | 7.144 | 7.162 | 7.152 |
| 30 | 7.215 | 7.055 | 7.072 | 7.064 |
| 35 | 7.127 | 6.970 | 6.984 | 6.982 |
| 37 | 7.093 | 6.939 | 6.951 | 6.948 |
| 40 | 7.045 | 6.887 | 6.900 | 6.902 |
| 45 | 6.966 | 6.809 | 6.817 | 6.822 |
| 50 | 6.890 | 6.731 | 6.737 | 6.748 |
| 55 | 6.817 | 6.656 | 6.661 | 6.675 |
Units of m, mol·kg−1
Table 4.
p(aHγCl) of (ACES + NaACES) buffer solutions from 5 to 55°C computed using eq. (4)a
| t(°C) | 0.01 m ACES + 0.03 m NaACES + 0.13 m NaCl I = 0.16 m |
0.02 m ACES + 0.04 m NaACES + 0.12 m NaCl I = 0.16 m |
0.03 m ACES + 0.06 m NaACES + 0.10 m NaCl I = 0.16 m |
0.04 m ACES + 0.08 m NaACES + 0.08 m NaCl I = 0.16 m |
|---|---|---|---|---|
| 5 | 7.802 | 7.629 | 7.480 | 7.627 |
| 10 | 7.697 | 8.525 | 7.400 | 7.523 |
| 15 | 7.596 | 7.426 | 7.321 | 7.418 |
| 20 | 7.500 | 7.326 | 7.239 | 7.321 |
| 25 | 7.404 | 7.231 | 7.165 | 7.226 |
| 30 | 7.314 | 7.139 | 7.087 | 7.134 |
| 35 | 7.226 | 7.052 | 7.010 | 7.045 |
| 37 | 7.194 | 7.018 | 6.979 | 7.011 |
| 40 | 7.144 | 6.967 | 6.935 | 6.961 |
| 45 | 7.063 | 6.887 | 6.854 | 6.881 |
| 50 | 6.985 | 6.809 | 6.780 | 6.802 |
| 55 | 6.912 | 6.735 | 6.706 | 6.727 |
Units of m, mol·kg−1
Table 5.
p(aHγCl) of (ACES + NaACES) buffer solutions from 5 to 55°C computed using eq. (4)a
| t(°C) | 0.04 m ACES + 0.04 m NaACES + 0.12 m NaCl I = 0.16 m |
0.05 m ACES + 0.05 m NaACES + 0.11 m NaCl I = 0.16 m |
0.06 m ACES + 0.06 m NaACES + 0.10 m NaCl I = 0.16 m |
0.04 m ACES + 0.08 m NaACES + 0.08 m NaCl I = 0.16 m |
|---|---|---|---|---|
| 5 | 7.316 | 7.348 | 7.322 | 7.330 |
| 10 | 7.214 | 7.244 | 7.218 | 7.218 |
| 15 | 7.109 | 7.140 | 7.114 | 7.120 |
| 20 | 7.012 | 7.038 | 7.015 | 7.023 |
| 25 | 6.918 | 6.944 | 6.922 | 6.927 |
| 30 | 6.828 | 6.848 | 6.832 | 6.836 |
| 35 | 6.741 | 6.759 | 6.745 | 6.749 |
| 37 | 6.708 | 6.723 | 6.711 | 6.715 |
| 40 | 6.658 | 6.671 | 6.661 | 6.665 |
| 45 | 6.578 | 6.588 | 6.580 | 6.583 |
| 50 | 6.501 | 6.507 | 6.502 | 6.506 |
| 55 | 6.429 | 6.432 | 6.428 | 6.432 |
Units of m, mol·kg−1
Table 6.
paH of (ACES + NaACES) buffer solutions from 5 to 55°C computed using eq. (4)a
| t(°C) |
0.01 m ACES + 0.03 m NaACES I = 0.03 m |
0.02 m ACES + 0.04 m NaACES I = 0.04 m |
0.03 m ACES + 0.06 m NaACES I = 0.06 m |
0.04 m ACES + 0.08 m NaACES I = 0.08 m |
|---|---|---|---|---|
| 5 | 7.628 | 7.463 | 7.464 | 7.449 |
| 10 | 7.520 | 7.357 | 7.362 | 7.346 |
| 15 | 7.422 | 7.258 | 7.259 | 7.245 |
| 20 | 7.325 | 7.161 | 7.165 | 7.148 |
| 25 | 7.232 | 7.066 | 7.070 | 7.051 |
| 30 | 7.144 | 7.976 | 6.980 | 6.962 |
| 35 | 7.055 | 6.890 | 6.891 | 6.880 |
| 37 | 7.022 | 6.859 | 6.858 | 6.846 |
| 40 | 6.973 | 6.807 | 6.806 | 6.799 |
| 45 | 6.893 | 6.728 | 6.723 | 6.718 |
| 50 | 6.817 | 6.650 | 6.642 | 6.644 |
| 55 | 6.744 | 6.574 | 6.565 | 6.569 |
Units of m, mol·kg−1
Table 7.
paH of (ACES + NaACES) buffer solutions from 5 to 55°C computed using eq. (4)a
| t(°C) | 0.01 m ACES + 0.03 m NaACES + 0.13 m NaCl I = 0.16 m |
0.02 m ACES + 0.04 m NaACES + 0.12 m NaCl I = 0.16 m |
0.03 m ACES + 0.06 m NaACES + 0.10 m NaCl I = 0.16 m |
0.04 m ACES + 0.08 m NaACES + 0.08 m NaCl I = 0.16 m |
|---|---|---|---|---|
| 5 | 7.676 | 7.504 | 7.355 | 7.501 |
| 10 | 7.571 | 7.399 | 7.275 | 7.398 |
| 15 | 7.470 | 7.301 | 7.195 | 7.293 |
| 20 | 7.375 | 7.201 | 7.114 | 7.196 |
| 25 | 7.277 | 7.104 | 7.038 | 7.099 |
| 30 | 7.187 | 7.012 | 6.960 | 7.007 |
| 35 | 7.099 | 6.924 | 6.882 | 6.918 |
| 37 | 7.066 | 6.890 | 6.851 | 6.883 |
| 40 | 7.015 | 6.839 | 6.807 | 6.833 |
| 45 | 6.934 | 6.758 | 6.725 | 6.751 |
| 50 | 6.855 | 6.679 | 6.650 | 6.671 |
| 55 | 6.781 | 6.604 | 6.575 | 6.596 |
Units of m, mol·kg−1
Table 8.
paH of (ACES + NaACES) buffer solutions from 5 to 55°C computed using Eq. (4)a
| t(°C) | 0.04 m ACES + 0.04 m NaACES + 0.12 m NaCl I = 0.16 m |
0.05 m ACES + 0.05 m NaACES + 0.11 m NaCl I = 0.16 m |
0.06 m ACES + 0.06 m NaACES + 0.10 m NaCl I = 0.16 m |
0.08 m ACES + 0.08 m NaACES + 0.08 m NaCl I = 0.16 m |
|---|---|---|---|---|
| 5 | 7.191 | 7.223 | 7.196 | 7.204 |
| 10 | 7.089 | 7.118 | 7.092 | 7.098 |
| 15 | 6.984 | 7.014 | 6.988 | 6.994 |
| 20 | 6.887 | 6.913 | 6.890 | 6.898 |
| 25 | 6.792 | 6.815 | 6.796 | 6.801 |
| 30 | 6.701 | 6.721 | 6.705 | 6.709 |
| 35 | 6.614 | 6.631 | 6.617 | 6.621 |
| 37 | 6.580 | 6.595 | 6.583 | 6.587 |
| 40 | 6.530 | 6.543 | 6.533 | 6.637 |
| 45 | 6.449 | 6.459 | 6.451 | 6.453 |
| 50 | 6.371 | 6.377 | 6.371 | 6.376 |
| 55 | 6.297 | 6.301 | 6.296 | 6.301 |
Units of m, mol·kg−1
The values of pH were also determined from cells with liquid-junction for cells (B and C) at 25 and 37°C. The trend in pH values for Table 2 and Table 3 is irrelevant because the compositions of the buffer solutions have the molar ratios of two solutes fixed at 1 : 3 and 1 : 2 of varying ionic strengths. The emf values of the cells (B and C) at 25 and 37° are given in Table 9. Also, the values of Ej shown in Table 10 were obtained by using Eq. (1). Finally, the values of pH listed in Table 11 were obtained by using Eq. (2 and Eq. 3). Various sources of errors for accurate values of pH have been described by Bates et al. [17–18]. The excellent agreement between the calculated pH values and the values obtained from Ej corrections are within ±0.002 pH units. The overall uncertainty for the pH values was estimated by combining the systematic uncertainties due to (i) assumption for the calculation of the single-ion-activity coefficient (±0.003 pH unit), (ii) extrapolation to p(aHγCl)° by plotting to mCl = 0, (iii) liquid junction potential measurement using the flow junction cell, and (iv) error in the experimental emf measurement (±0.005 mV). Because the pH range lies in the physiological region, the buffer compound ACES is recommended as a pH buffer standard in the range of biochemical application.
Table 9.
Emf of Cell B for ACES buffer
| m1 | m2 | m3 | E, V | ||
|---|---|---|---|---|---|
| ACES | 25°C | 37°C | |||
| 0.04 | 0.08 | 0.00 | 0.66065 | 0.65704 | |
| 0.08 | 0.08 | 0.08 | 0.64427 | 0.63950 | |
| 0.03 | 0.06 | 0.10 | 0.65823 | 0.65563 | |
| 0.02 | 0.04 | 0.12 | 0.68695 | 0.65490 | |
| Emf of Cell Ca | |||||
| Cell C | E, V | ||||
| 0.008695 m KH2PO4 | 25°C | 37°C | |||
| + | |||||
| 0.03043 m Na2HPO4 | 0.68275 | 0.69147 | |||
Corrected to a hydrogen pressure of 1 atm, for physiological phosphate buffer solutions (primary reference standard buffer) at 25 and 37°C
Table 10.
Values of the liquid junction potentials for ACES, and physiological phosphate buffers at 25 and 37°C, from eq. (1)
| Ej a, mV | ||
|---|---|---|
| System | 25°C | 37°C |
| Physiological phosphate (0.008695m KH2PO4 + 0.03043 m Na2HPO4) | 2.6 | 2.9 |
| 0.04 m ACES + 0.08 m NaACES + 0.00 m NaCl | 2.1 | 2.3 |
| 0.08 m ACES + 0.08 m NaACES + 0.08 m NaCl | 0.5 | 0.7 |
| 0.03 m ACES + 0.06 m NaACES + 0.10 m NaCl | 0.4 | 0.6 |
| 0.02 m ACES + 0.04 m NaACES + 0.12 m NaCl | 0.4 | 0.6 |
Ej= E + E°sce-kpH, where E°sce= −0.2415V, k (Nernst slope) = 0.059156, and pH = 7.415 (physiological phosphate as standard reference solution) at 25°C; E°sce = −0.2335V, k = 0.061538, and pH = 7.395 at 37°C for the physiological phosphate buffer. The pH for the phosphate buffer is that assigned by the NIST (National Institute of Standards and Technology) based on the Bates-Guggenheim convention [5, 17].
Table 11.
Values of pH at 25 and 37°C for ACES buffer solutions using data for Ej corrections
| Cell B | 25°C | 37°C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| m1 | m2 | m3 | Ionic strength, I |
Withouta Ej corr |
Withb Ej corr |
Calcc | Withouta Ej corr |
Withb Ej corr |
Calcc |
| 0.04 | 0.08 | 0.00 | 0.08 | 7.041 | 7.050 | 7.051 | 6.836 | 6.846 | 6.846 |
| 0.08 | 0.08 | 0.08 | 0.16 | 6.765 | 6.800 | 6.801 | 6.550 | 6.586 | 6.587 |
| 0.03 | 0.06 | 0.10 | 0.16 | 7.001 | 7.037 | 7.038 | 6.813 | 6.850 | 6.851 |
| 0.02 | 0.04 | 0.12 | 0.16 | 7.066 | 7.103 | 7.104 | 6.801 | 6.838 | 6.839 |
pH = 7.415 + [(E – 0.68275)/ 0.059156] where 7.415 is the physiological phosphate buffer standard at 25°C, and pH = 7.395 + [(E−0.69144) / 0.061538] where 7.395 is the pH of phosphate buffer at 37°C; for example 0.68275 and 0.69147 are the emf values (in volts) from Table 9 at 25 and 37°C, respectively. This is based on the NIST convention for γCl.
Calculation by Eq. (2) where reported liquid junction potentials apply for cells (B and C)
Acknowledgments
The authors are grateful to the late Dr. R.G. Bates for useful discussions and important suggestions incorporated in this revised manuscript; and the grant fund from the National Institute of Health (AREA Fund), under grant R15 GM 066866-02 and R 15 GM 066866-02S2 (NIH supplemental grant).
Footnotes
This original publication is available at springerlink.com by using the following link: http://www.springerlink.com/content/fq4tk14754406071/?p=6801c2bafc064e67b85a230ea76d619b&pi=7
References
- 1.Roy RN, Bice J, Greer J, Carlsten JA, Smithson J, Good WS, Moore CP, Roy LN, Kuhler KM. Buffers for the physiological pH range: Acidic dissociation constants of zwitterionic compounds (ACES and CHES) in water from 5 to 55 °C. J. Chem. Eng. Data. 1997;42:41–44. [Google Scholar]
- 2.Roy RN, Roy LN, Simon AN, Moore AC, Seing LA, Richards SJ, Craig HD, Childers BA, Tabor BJ, Himes CA, Viele KE. Thermodynamics of the second dissociation constant of N-bis(hydroxymethyl) piperazine-N-4-butanesulfonic acid from 5 to 55°C. J. Solution Chem. 2004;33:351–362. [Google Scholar]
- 3.Good NE, Winget GD, Connolly TN, Izawa S, Singh RMM. Hydrogen ion buffers for biological research. Biochemistry. 1966;5:467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson WJ, Braunschweiger KI, Braunschweiger WR, Smith JR, McCormick JJ, Wasmann CC, Jarvis NP, Bell DH, Good NE. Hydrogen ion buffers for biological research. Anal. Biochem. 1980;104:300–310. doi: 10.1016/0003-2697(80)90079-2. [DOI] [PubMed] [Google Scholar]
- 5.Bower VE, Paabo M, Bates RG. A standard for the measurement of the pH of blood and other physiological media. J. Res. Nat. Bur. Std. 1961;65A:267–270. doi: 10.6028/jres.065A.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durst RA, Staples BR. Tris/Tris HCl: Standard buffer for use in the physiological pH range. Clin. Chem. 1972;18:206–208. [PubMed] [Google Scholar]
- 7.Feng D, Koch WF, Wu YC. Second dissociation constant and pH of N-(2-hydroxyethyl)piperazine-N’-2-ethanesulfonic acid from 0 to 50°C. Anal. Chem. 1989;61:1400–1405. [Google Scholar]
- 8.Wu YC, Berezansky PA, Feng D, Koch WF. Second dissociation constant of 3-(N-morpholino)-2-hydroxypropanesulfonic acid and pH of its buffer solutions. Anal. Chem. 1993;65:1084–1087. [Google Scholar]
- 9.Bates RG, Roy RN, Robinson RA. Buffer standards of tris(hydroxymethyl)methylglycine (“Tricine”) for the physiological range pH 7.2 to 8.5. Anal. Chem. 1973;45:1663–1666. doi: 10.1021/ac60331a022. [DOI] [PubMed] [Google Scholar]
- 10.Roy RN, Robinson RA, Bates RG. Thermodynamics of the two dissociation steps of N-tris (hydroxymethyl)methylglycine (“Tricine”) in water from 5° to 50°C. J. Amer. Chem. Soc. 1973;95:8231–8235. doi: 10.1021/ja00806a004. [DOI] [PubMed] [Google Scholar]
- 11.Roy RN, Mrad DR, Lord PA, Carlsten JA, Good WS, Allsup P, Roy LN, Kuhler KM, Koch WF, Wu YC. Thermodynamics of the second dissociation constant and standards for pH of 3-(N-morpholino)propanesulfonic acid (MOPS) from 5 to 55°C. J. Solution Chem. 1998;27:73–87. [Google Scholar]
- 12.Roy RN, Cramer J, Rendon V, Willard D, Walter JL, Good WS, Kilker A, Roy LN. Buffers for the physiological pH range: Thermodynamics of the second dissociation of N-(2-hydroxyethyl)piperazine-N’-2-hydroxypropanesulfonic acid (HEPPSO) from 5 to 55°C. J. Solution Chem. 1998;27:425–434. [Google Scholar]
- 13.oldberg RN, Kishore RN, Lennen RM. Thermodynamic quantities for the ionization reactions of buffers. J. Phys. Chem. Ref. Data. 2002;31:231–370. [Google Scholar]
- 14.Bates RG. Determination of pH. 2nd ed. New York: Wiley; 1973. Chap. 4, 10. [Google Scholar]
- 15.Latimer WM. Oxidation Potentials. 2nd ed. New York: Prentice Hall; 1952. [Google Scholar]
- 16.Wu YC, Feng D, Koch WF. Evaluation of liquid junction potentials and determination of pH values of strong acids at moderate ionic strengths. J. Solution Chem. 1989;18:641–649. [Google Scholar]
- 17.Bates RG. Revised standard values for pH measurements from 0 to 95°C. J. Res Nat. Bur. Stand. 1962;66A:179–184. [Google Scholar]
- 18.Bates RG, Vega CA, White DR., Jr Standards for pH Measurements in Isotonic Saline Media of Ionic Strength I = 0.16. Anal. Chem. 1978;50:1295–1300. [Google Scholar]