Abstract
Many genes are responsible for the modulation of lifespan in model organisms. In addition to regulating adaptive biologic responses that control stress signaling and longevity, some of these genes participate in tumor formation. The mechanisms that determine longevity and link regulation of lifespan with tumorigenesis are poorly understood. Here, we show that the tumor suppressor von Hippel-Lindau (VHL), which has widely known roles in renal carcinogenesis and the formation of kidney cysts, controls longevity in Caenorhabditis elegans. Loss of vhl-1 significantly increased lifespan and resulted in accelerated basal signaling of the p38 mitogen-activated protein kinase PMK-3. Furthermore, the VHL-1 effect on the regulation of lifespan was independent of the insulin/IGF-1–like signaling pathway, suggesting a mechanism for stress resistance that controls both lifespan and tumorigenesis. These findings define VHL-1 as a player in longevity signaling and connect aging, regulation of lifespan, and stress responses with formation of renal cell carcinomas.
Mutations in the tumor suppressor von Hippel-Lindau (VHL) disease gene are responsible for almost all hereditary and most sporadic renal cell carcinomas.1 Restoration of vhl gene expression is sufficient to suppress kidney tumor formation in vivo, suggesting that tumorigenesis is a direct effect of the loss of both VHL alleles.2,3 pVHL, the protein encoded by the VHL gene, is evolutionarily conserved and serves as part of an E3 ubiquitin ligase complex that inhibits hypoxic signaling in the presence of oxygen through degradative ubiquitination of proteins of the hypoxia-inducible factor (HIF) family.4,5 In addition, loss of VHL induces the formation of renal cysts and the development of polycystic kidney disease. Studies demonstrated a critical role for pVHL in controlling microtubule polarization and the formation of monocilia on various cell types linking pVHL to known pathways of cystogenesis.6–9 These findings provided a novel link between the formation of kidney cysts and renal carcinogenesis.10
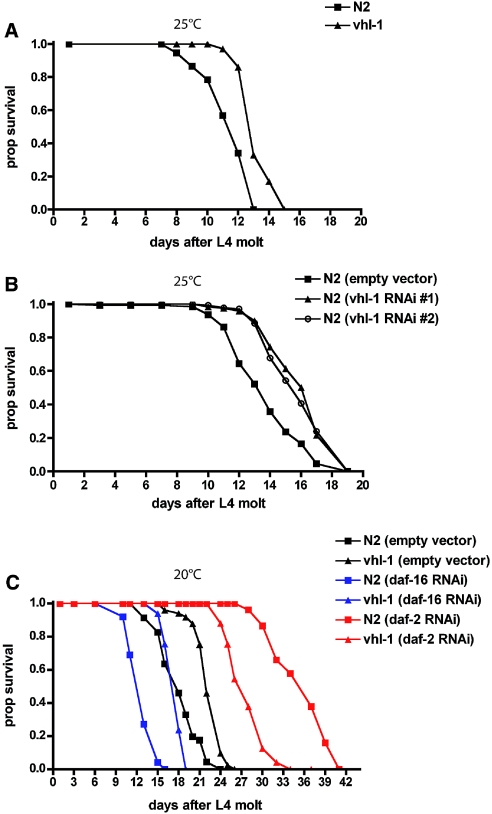
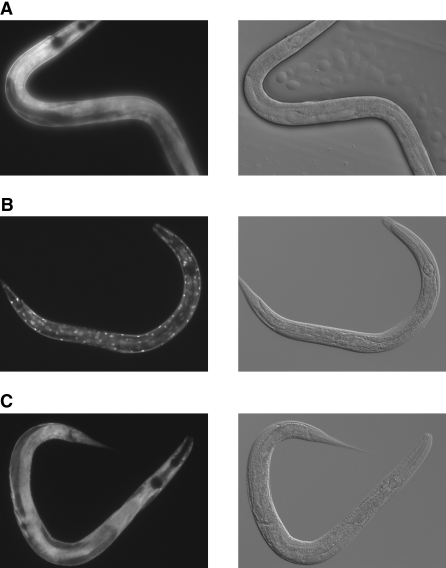
Despite recent advances in the understanding of pVHL function, the cell biologic and molecular basis of VHL-dependent cancer formation is far from being entirely understood. To address the in vivo function of pVHL, we analyzed the phenotype of vhl-1–defective nematode worms. Surprising, knockout of vhl-1 significantly increased lifespan in these animals as compared with wild-type controls (Figure 1A). This result could be confirmed by knocking down vhl-1 through RNA interference (RNAi; Figure 1B). Taking into account the well-conserved role of vhl-1 in the degradative ubiquitination of hif-1, it may be speculated that altered levels of hif-1 are involved in the longevity phenotype as well.11,12 Using hif-1;vhl-1 double knockout worms, we could show that the increase in lifespan is partly dependent on hif-1. In contrast to observations of Mehta et al.,12 loss of hif-1 partially but not entirely abrogated increased longevity (Supplemental Figure 1), suggesting a combination of hif-1–dependent and hif-1–independent effects. The lifespan of Caenorhabditis elegans (and of other species) is regulated by both genetic and environmental influences.13–15 One study identified specific signaling components that regulate lifespan and demonstrated that loss-of-function mutations in the insulin/IGF-1–like (DAF-2) signaling pathway can dramatically increase the lifespan of the nematode.16 Insulin signaling negatively regulates the forkhead (FOXO) transcription factor DAF-16, which ultimately functions to regulate both positively and negatively transcription of metabolic, chaperone, cellular defense, and other genes.17 To test whether the longevity phenotype of vhl-1–defective C. elegans could be explained by modulation of the insulin/IGF-1–like signaling pathway, we used RNAi technology to downregulate DAF-2 (insulin receptor) and DAF-16 (FOXO) expression in wild-type N2 and vhl-1–defective worms (Figure 1C). As expected, knockdown of daf-2 resulted in an increased lifespan in the wild-type N2 worms, whereas knockdown of daf-16 shortened their life; however, downregulation of daf-16 did not abrogate the life-extending effect of vhl-1 deletion, indicating that pVHL acts in a pathway distinct from insulin-FOXO signaling. Consistent with these findings, vhl-1 deletion did not affect DAF-16 nuclear localization (Figure 2). DAF-16 activity is strongly controlled by its subcellular localization.18 Activation of the insulin signaling pathway results in phosphorylation of DAF-16 and prevents its nuclear translocation and activation. To visualize DAF-16 localization, we used a transgenic strain expressing a DAF-16::GFP fusion protein. In wild-type N2, the vast majority of DAF-16 protein was dispersed throughout the cytoplasm of the cells (Figure 2A). Knockdown of daf-2 induced the activation and translocation of DAF-16 to the nucleus (Figure 2B). In contrast, RNAi against vhl-1 did not affect DAF-16 localization, which is consistent with the conclusion that VHL-1 does not affect insulin signaling (Figure 2C). Activation of DAF-16 induces entry of C. elegans into the Dauer larval stage.19 Dauer describes an alternative developmental stage of C. elegans whereby the larva goes into a type of stasis and can survive harsh conditions. As expected, vhl-1–negative worms did not show a constitutive Dauer larval phenotype as described for nematodes lacking daf-2, again supporting the conclusion that VHL-1 acts in a pathway distinct from repressed insulin/IGF-1–like signaling (Table 1).
Figure 1.
Loss of vhl-1 increases longevity. (A) Worms lacking vhl-1 (▲) show an extended lifespan as compared with the wild-type N2 strain (■). (B) RNAi-mediated knockdown of vhl-1 using two different RNAi constructs from either the Ahringer (#1) or the Vidal (#2) library confirms the VHL-1 effect on lifespan observed in vhl-1 knockout worms. (C) Lifespan extension mediated by VHL-1 does not depend on insulin/IRS-1–like signaling. Wild-type N2 (squares) or vhl-1 −/− worms (triangles) were grown on RNAi plates for downregulation of either daf-16/FOXO (blue line), simulating increased insulin signaling, or daf-2/insulin receptor (red line), which reduces insulin signaling.
Figure 2.
Loss of vhl-1 is without effect on nuclear translocation of DAF-16. Activity of DAF-16 is dependent on its subcellular localization, because DAF-16 exerts its effects only in the nucleus. (A through C) In contrast to animals grown on bacteria expressing empty vector (A), knockdown of daf-2 leads to strong nuclear translocation of DAF-16 (B), whereas knockdown of vhl-1 is without effect (C).
Table 1.
Loss of vhl-1 does not induce Dauer formation
| Strain | No. of Worms | No. of Dauers |
|---|---|---|
| vhl-1 −/− | 61 | 0 |
| vhl-1 −/− | 24 | 0 |
| daf-2 −/− | 72 | 60 |
| daf-2 −/− | 95 | 66 |
daf-2 −/− worms showed approximately 100% Dauer formation when grown at 25°C; however, vhl-1 −/− worms, similar to wild-type controls, did not enter the Dauer larval stage at this temperature.
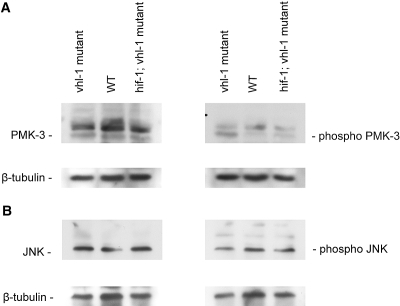
Recent work suggested a striking but not completely understood role for stress-dependent mitogen-activated protein kinase (MAPK) signaling pathways for the control of longevity.20–23 Some of these effects on lifespan regulation are dependent and other aspects are independent of a modulation of insulin/IGF-1–like signaling.21,22,24 We therefore tested p38 and c-Jun N-terminal kinase (JNK) MAPK phosphorylation with phosphospecific antibodies in lysates of N2 wild-type versus vhl-1–defective worms (Figure 3). Interestingly, vhl-1–deleted worms displayed an enhanced basal PMK-3/p38 MAPK phosphorylation, whereas the JNK pathway and the p42/44 MAPK pathways were unaffected. C. elegans contains three isoforms of p38 MAPKs, PMK1 through 3. The three genes are contiguous on chromosome IV and compose an operon.25 Activity of these genes has been demonstrated to mediate increased longevity,22 suggesting that p38 may be involved in mediating the VHL-1 lifespan effect. Worms deficient for both vhl-1 and hif-1 did not show this enhanced phosphorylation of PMK-3, suggesting that hif-1–dependent effects on longevity may be mediated through this pathway (Figure 3); however, future studies will be required to definitively this issue address and to shed light on the mechanism involved in hif-1–independent regulation of nematode lifespan.
Figure 3.
Loss of vhl-1 is associated with enhanced PMK-3/p38 MAPK signaling. Phosphorylation of p38 (A, right) and JNK (B, right) MAPKs was assessed using phosphospecific antibodies on Western blots of whole lysates of control versus both vhl-1–deficient and hif-1;vhl-1 double-knockout worms. Equal total amounts of the two MAPKs independent of their phosphorylation status was determined using p38 (A, left) and JNK (B, left) antibodies on blots with the identical lysates.
During the past 20 yr, fundamental insights into the biology of aging have emerged through the study of model genetic organisms, for which undoubtedly the nematode C. elegans has led the way.26 Importantly, the discovery that the single-gene mutants age-1 and daf-2 could extend the short 3-wk lifespan one- to two-fold revealed that longevity is under genetic control.27,28 The identification of components of the insulin/IRS-1–like signaling pathway led to the striking realization that a modest downregulation of insulin signaling promotes stress resistance and longevity and ameliorates aging-related pathologies.16,29,30 Aside from this signaling system, several other signaling pathways have been shown to contribute to longevity, including the MAPKs p38 and JNK.20–23 Here we add to these findings and demonstrate that deletion of the homolog of the mammalian VHL tumor suppressor gene in C. elegans increases longevity. Long life was accompanied by an elevated level of phospho-PMK-3/p38 in vhl-1–deficient animals; however, additional studies will have to address whether VHL-1–dependent longevity is indeed mediated through increased rates of basal MAPK activity and how this might affect DAF-2/DAF-16 insulin/IGF-1–like signaling. So far, there is no evidence of a VHL-1 effect on the insulin signaling pathway in C. elegans. The finding that the deletion of a tumor suppressor gene influences lifespan is remarkable for several reasons. Considering that loss of pVHL through the promotion of tumor formation has a limiting effect on human life expectancy, the finding that the loss of this protein leads to lifespan extension in C. elegans is peculiar; however, because of the short lifespan and a limited capacity for cell division, tumor formation is not one of the lifespan-limiting factors in the nematode. This fact allowed us to unravel an entirely novel function of the VHL tumor suppressor gene, providing an exciting link among tumorigenesis, longevity, and age-related pathology. This important yet peculiar link was suggested previously,31,32 predominantly focusing on p53 as a ubiquitously active model tumor suppressor.33,34
pVHL is the most important tumor suppressor in the kidney; therefore, the finding that pVHL is also involved in pathways regulating longevity is intriguing. Elucidating the molecular mechanisms through which pVHL and most likely a series of additional genes influence aging will be required for a better understanding of aging-related kidney disease. Both apoptosis and cellular senescence are crucial for tumor suppression and regression, yet both processes probably severely impair the regenerative capacity of tissues and whole organisms.31 Thus, it is intriguing to speculate that increased tumor suppressor activity, which is vital in the aging individual who accumulates DNA damage over time, induces an aging phenotype. This fascinating hypothesis suggests that a stable protection from tumorigenesis (in this case, kidney cancer) may come at the expense of losing tissue-regenerative capacity. Such a decrease in regenerative capacity may well contribute to the high susceptibility to acute kidney injury observed in older patients. Interestingly, HIF-1 has been shown to upregulate factors that protect from cellular damage and cell death after acute ischemic kidney injury.35 The VHL gene therefore may be not only the most prominent tumor suppressor gene of the kidney but also a detrimental factor in aging-related renal failure and fibrosis. This concept suggests that a number of additional tumor suppressor proteins may show a similar functional pleiotropy in other organs. The molecular characterization of VHL-mediated lifespan regulation will be key to understanding this novel link of cancer and lifespan and is needed to unveil conserved aspects in lifespan determination and human aging.
Concise Methods
Strains and Growth Conditions
All of the C. elegans strains were maintained according to standard methods.36 The following strains were used in this study: N2 Bristol as wild-type strain; vhl-1(ok161) (strain CB5602); hif-1(ia4);vhl-1(ok161) (strain CB6090); DAF-16::GFP (strain TJ356); and daf-2 (e1370)III (strain CB1370). All of the strains were provided by the C. elegans Genetics Center (http://www.cbs.umn.edu/CGC/).
RNAi Technology
RNAi by feeding was performed as described by Fire et al.37 Two different F08G12.4 RNAi clones derived from the Ahringer library and the Vidal library were used for vhl-1 knockdown. RNAi clones for daf-16 (R13H8.1) and daf-2 knockdown were provided by Frank Slack (Yale University).
Lifespan Assays
Lifespan assays were performed as described by Boehm and Slack.38 Briefly, synchronized L1 were fed with Escherichia coli OP50 or the RNAi strain HT115 (DE3). Young adults (approximately 30 worms) were then transferred to fresh NGM feeding plates containing 0.1 mg/ml 5-fluorodeoxyuridine (Sigma) to prevent progeny production. For RNAi experiments, worms were transferred every 5 to 7 d to fresh plates containing 1 mM IPTG, 50 μg/ml carbenicillin, 5-fluorodeoxyuridine, and fresh-grown RNAi expressing bacteria. Worms were checked every 2 to 3 d and were scored as dead when they did not respond to repeated gentle prodding with a platinum wire. Worms that crawled off the plate or burst were censored.
Dauer Formation Assay
Dauer formation assays were performed as described by Gottlieb and Ruvkun.19 Briefly, synchronized egg broods of the respective strains were grown at 25°C and scored for Dauer formation after 24 and 48 h. At these time points, worms were treated with 1% SDS. Dauer formation was measured as the percentage of worms surviving this treatment.
Fluorescence Imaging
Fluorescence microscopy was performed using an Axiovert 200M (Carl Zeiss), equipped with the Filter Set 38HE (BP filter, with excitation filter at 470/40 nm and emission filter at 525/50 nm) and an objective EC Plan-Neofluor 40×/1.30 Oil DIC. Briefly, worms were paralyzed in 30 mM sodium azide, placed on a 2.5% agarose pad, and imaged immediately.
Western Blot Analysis
Synchronized young adult worms were collected from nearly starved plates and pelleted, frozen in liquid nitrogen, and then lysed in SDS-PAGE sample buffer (50 mM Tris-Cl [pH 6.8], 2% wt/vol SDS, 10% vol/vol glycerol, 0.1% bromophenol blue, and 50 mM dithiothreitol) for immunoblotting. Anti-p38 monoclonal rabbit antibodies, anti–phospho-p38 (Thr180/Tyr182) monoclonal rabbit antibodies, anti-SAPK/JNK monoclonal rabbit antibodies, and anti–phospho-SAPK/JNK (Thr183/Tyr185) monoclonal rabbit antibodies were used at a 1:1000 dilution. Both antibodies were purchased from Cell Signaling Technology. Anti–tubulin-β monoclonal mouse antibody was purchased from Sigma (clone D66) and used at a 1:1000 dilution. In the Western blots shown, identical lysates were used for anti-JNK and anti-p38 stainings.
Disclosures
None.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (BE2212 to T.B., SCHE581 to B.S., and SFB 832) and the BMBF (FRISYS to T.B.).
We thank members of the Benzing laboratory for helpful discussions and critically reading the manuscript. Special thanks to Dr. Frank Slack (Yale University), Dr. Thorsten Hoppe (University of Cologne), and Dr. Bjoern Schumacher (University of Cologne) for providing reagents and critical discussions.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Kaelin WG, Jr: The von Hippel-Lindau tumor suppressor gene and kidney cancer. Clin Cancer Res 10: 6290S–6295S, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Iliopoulos O, Kibel A, Gray S, Kaelin WG, Jr: Tumour suppression by the human von Hippel-Lindau gene product. Nat Med 1: 822–826, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Schoenfeld A, Davidowitz EJ, Burk RD: A second major native von Hippel-Lindau gene product, initiated from an internal translation start site, functions as a tumor suppressor. Proc Natl Acad Sci U S A 95: 8817–8822, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaelin WG, Jr: The von Hippel-Lindau gene, kidney cancer, and oxygen sensing. J Am Soc Nephrol 14: 2703–2711, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Mandriota SJ, Turner KJ, Davies DR, Murray PG, Morgan NV, Sowter HM, Wykoff CC, Maher ER, Harris AL, Ratcliffe PJ, Maxwell PH: HIF activation identifies early lesions in VHL kidneys: Evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 1: 459–468, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Esteban MA, Harten SK, Tran MG, Maxwell PH: Formation of primary cilia in the renal epithelium is regulated by the von Hippel-Lindau tumor suppressor protein. J Am Soc Nephrol 17: 1801–1806, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Lutz MS, Burk RD: Primary cilium formation requires von Hippel-Lindau gene function in renal-derived cells. Cancer Res 66: 6903–6907, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Schermer B, Ghenoiu C, Bartram M, Muller RU, Kotsis F, Hohne M, Kuhn W, Rapka M, Nitschke R, Zentgraf H, Fliegauf M, Omran H, Walz G, Benzing T: The von Hippel-Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J Cell Biol 175: 547–554, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thoma CR, Frew IJ, Krek W: The VHL tumor suppressor: Riding tandem with GSK3beta in primary cilium maintenance. Cell Cycle 6: 1809–1813, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Schraml P, Frew IJ, Thoma CR, Boysen G, Struckmann K, Krek W, Moch H: Sporadic clear cell renal cell carcinoma but not the papillary type is characterized by severely reduced frequency of primary cilia. Mod Pathol 22: 31–36, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Chen D, Thomas EL, Kapahi P: HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet 5: e1000486, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta R, Steinkraus KA, Sutphin GL, Ramos FJ, Shamieh LS, Huh A, Davis C, Chandler-Brown D, Kaeberlein M: Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science 324: 1196–1198, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finch CE, Ruvkun G: The genetics of aging. Annu Rev Genomics Hum Genet 2: 435–462, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Partridge L: Some highlights of research on aging with invertebrates, 2008. Aging Cell 7: 605–608, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Tatar M, Bartke A, Antebi A: The endocrine regulation of aging by insulin-like signals. Science 299: 1346–1351, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G: The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389: 994–999, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Henderson ST, Johnson TE: daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol 11: 1975–1980, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Partridge L, Bruning JC: Forkhead transcription factors and ageing. Oncogene 27: 2351–2363, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Gottlieb S, Ruvkun G: daf-2, daf-16 and daf-23: Genetically interacting genes controlling Dauer formation in Caenorhabditis elegans. Genetics 137: 107–120, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizuno T, Hisamoto N, Terada T, Kondo T, Adachi M, Nishida E, Kim DH, Ausubel FM, Matsumoto K: The Caenorhabditis elegans MAPK phosphatase VHP-1 mediates a novel JNK-like signaling pathway in stress response. EMBO J 23: 2226–2234, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neumann-Haefelin E, Qi W, Finkbeiner E, Walz G, Baumeister R, Hertweck M: SHC-1/p52Shc targets the insulin/IGF-1 and JNK signaling pathways to modulate life span and stress response in C. elegans. Genes Dev 22: 2721–2735, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Troemel ER, Chu SW, Reinke V, Lee SS, Ausubel FM, Kim DH: p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet 2: e183, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf M, Nunes F, Henkel A, Heinick A, Paul RJ: The MAP kinase JNK-1 of Caenorhabditis elegans: Location, activation, and influences over temperature-dependent insulin-like signaling, stress responses, and fitness. J Cell Physiol 214: 721–729, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Wang MC, Bohmann D, Jasper H: JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121: 115–125, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Berman K, McKay J, Avery L, Cobb M: Isolation and characterization of pmk-(1–3): Three p38 homologs in Caenorhabditis elegans. Mol Cell Biol Res Commun 4: 337–344, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antebi A: Genetics of aging in Caenorhabditis elegans. PLoS Genet 3: 1565–1571, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman DB, Johnson TE: A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 118: 75–86, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang RA: C. elegans mutant that lives twice as long as wild type. Nature 366: 461–464, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G: daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277: 942–946, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Morris JZ, Tissenbaum HA, Ruvkun G: A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature 382: 536–539, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Campisi J, Sedivy J: How does proliferative homeostasis change with age? What causes it and how does it contribute to aging? J Gerontol A Biol Sci Med Sci 64: 164–166, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collado M, Blasco MA, Serrano M: Cellular senescence in cancer and aging. Cell 130: 223–233, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Serrano M: Cancer regression by senescence. N Engl J Med 356: 1996–1997, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Rodier F, Kim SH, Nijjar T, Yaswen P, Campisi J: Cancer and aging: The importance of telomeres in genome maintenance. Int J Biochem Cell Biol 37: 977–990, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Haase VH: The VHL/HIF oxygen-sensing pathway and its relevance to kidney disease. Kidney Int 69: 1302–1307, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Brenner S: The genetics of Caenorhabditis elegans. Genetics 77: 71–94, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC: Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391: 806–811, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Boehm M, Slack F: A developmental timing microRNA and its target regulate life span in C. elegans. Science 310: 1954–1957, 2005 [DOI] [PubMed] [Google Scholar]