Abstract
Aldosterone promotes glomerular and tubular sclerosis independent of angiotensin II in animal models of diabetic nephropathy. Most human studies testing the renoprotective benefit of adding an angiotensin receptor blocker or a mineralocorticoid receptor antagonist to a regimen based on inhibition of angiotensin-converting enzyme (ACE) used relatively low doses of ACE inhibitors. Furthermore, these studies did not determine whether antiproteinuric effects were independent of BP lowering. We conducted a double-blind, placebo-controlled trial in 81 patients with diabetes, hypertension, and albuminuria (urine albumin-to-creatinine ratio ≥300 mg/g) who all received lisinopril (80 mg once daily). We randomly assigned the patients to placebo, losartan (100 mg daily), or spironolactone (25 mg daily) for 48 wk. We obtained blood and urine albumin, urea, creatinine, electrolytes, A1c, and ambulatory BP at baseline, 24, and 48 wk. Compared with placebo, the urine albumin-to-creatinine ratio decreased by 34.0% (95% CI, −51.0%, −11.2%, P = 0.007) in the group assigned to spironolactone and by 16.8% (95% CI, −37.3%, +10.5%, P = 0.20) in the group assigned to losartan. Clinic and ambulatory BP, creatinine clearance, sodium and protein intake, and glycemic control did not differ between groups. Serum potassium level was significantly higher with the addition of either spironolactone or losartan. In conclusion, the addition of spironolactone, but not losartan, to a regimen including maximal ACE inhibition affords greater renoprotection in diabetic nephropathy despite a similar effect on BP. These results support the need to conduct a long-term, large-scale, renal failure outcomes trial.
Diabetic nephropathy is the leading cause of ESRD worldwide.1 The presence of nephropathy in diabetes is associated not only with excessive cardiovascular risk but also with increased risk for progression to ESRD.2,3 It is well established that suboptimal BP control, activation of the renin–angiotensin–aldosterone system (RAAS), and proteinuria are important factors in the progression of diabetic nephropathy. Intensive BP lowering and administration of drugs that block the RAAS, such as angiotensin-converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs), can slow progression of diabetic nephropathy.4–11 Renoprotection afforded by these agents is linked strongly and inextricably to reduction in proteinuria. Moreover, residual proteinuria is a strong predictor of adverse renal outcomes in long-term studies of patients treated with either an ACEi or an ARB.7,12,13 Unfortunately, renoprotection afforded by these agents is limited; thus most patients continue to progress toward ESRD.14–16
Studies in animal models have demonstrated that aldosterone can cause renal injury leading to glomerular and tubular sclerosis independent of angiotensin II.17,18 Adding either an angiotensin receptor antagonist or a mineralocorticoid receptor antagonist (MRA) to an ACEi-based regimen in patients with diabetic nephropathy may further reduce proteinuria and thereby afford additional renoprotection.19–24 However, most studies included small numbers of subjects who were treated for a short duration and used relatively low doses of ACEi.19–21 Importantly, these studies were not designed to determine whether the antiproteinuric effect of a MRA was independent of BP lowering. We hypothesized that, in patients with diabetic nephropathy, the addition of either an ARB or a MRA to a maximally dosed ACEi-based regimen will afford greater renoprotection than an ACEi-based regimen alone. We further hypothesized that the added value of a MRA is specific for aldosterone and is not explained solely on the basis of reduced time-integral BP burden.
Results
The baseline characteristics were similar among the randomized groups (Table 1). The mean age of the study subjects was approximately 50 yr. There was a slight female predominance, and the majority of subjects were either African-American or Hispanic. The median creatinine clearance was 64.5 ml/min, and the mean 24-h urine albumin-to-creatinine ratio (UACR) was approximately 1000 mg/g. The mean number of days of lisinopril (80 mg daily) administered during run-in before randomization was 31.6 for placebo, 32.7 for losartan, and 35.5 for spironolactone. Most subjects were overweight or obese, and the mean duration of diabetes was approximately 15 yr. There was a trend toward lower creatinine clearance and higher UACR in the spironolactone group, but the difference was not statistically significant. There were no differences in baseline BP or UACR when comparing patients with type 1 and type 2 diabetes (data not shown). Ten percent of subjects had a prior history of myocardial infarction or coronary revascularization.
Table 1.
Baseline characteristicsa
| Placebo n = 27 | Losartan n = 26 | Spironolactone n = 27 | |
|---|---|---|---|
| Female, no. | 15 | 13 | 14 |
| Race, no. | |||
| Black | 10 | 8 | 7 |
| Hispanic | 12 | 12 | 17 |
| Non-Hispanic white | 4 | 6 | 2 |
| Native American | 1 | 0 | 1 |
| Age, yr | 49.3 (8.8) | 52.3 (9.1) | 51.7 (9.3) |
| Body mass index, kg/m2 | 32.3 (7.1) | 30.3 (5.4) | 33.7 (7.1) |
| Duration of diabetes, yr | 14.4 (9.6) | 17.0 (7.7) | 17.0 (9.1) |
| Diabetes type (type1/type 2), no. | 4/23 | 4/22 | 4/23 |
| 24-h ambulatory BP, mmHg | |||
| Systolic | 138 (15) | 143 (15) | 135 (11) |
| Diastolic | 75 (9) | 75 (9) | 71 (9) |
| Clinical BP, mmHg | |||
| Systolic | 132 (18) | 136 (14) | 132 (16) |
| Diastolic | 74 (9) | 72 (11) | 73 (10) |
| Prior history of myocardial infarction/CABG/PTCA | 3 | 2 | 2 |
| UACR, mg/g | 917 [633–1329] | 897 [611–1316] | 1094 [758–1579] |
| Serum creatinine, mg/dl | 1.4 (0.7) | 1.7 (0.7) | 1.8 (0.9) |
| Normalized protein catabolic rate, g/kg/d | 0.97 (0.21) | 1.07 (0.26) | 0.91 (0.21) |
| Blood urea nitrogen, mg/dl | 30.5 (16.3) | 39.1 (18.6) | 43.4 (22.8) |
| Serum potassium, mEq/L | 4.5 (0.7) | 4.5 (0.4) | 4.5 (0.7) |
| Hemoglobin A1c, % | 8.1 (1.3) | 7.6 (1.3) | 7.4 (1.6) |
| Plasma C-reactive protein, mg/L | 3.6 [1.9–6.5] | 2.4 [1.4–4.2] | 3 [1.6–5.5] |
| Triglycerides, mg/dl | 183 [145–232] | 175 [136–225] | 191 [156–235] |
| Total cholesterol, mg/dl | 189 (49) | 198 (75) | 176 (44) |
| LDL cholesterol, mg/dl | 95 (38) | 100 (45) | 75 (29) |
| HDL cholesterol, mg/dl | 43 (10) | 46 (15) | 45 (11) |
CABG, coronary artery bypass graft; PCTA, percutaneous transluminal coronary angioplasty.
aResults are presented as mean (SD) or geometric mean [95% CI].
Urine Albumin-to-Creatinine Ratio
Overall there was a significant difference in the percent change in UACR between the three treatment groups (P = 0.02). The mean difference percentage change in the UACR in spironolactone as compared with that in placebo was −34.0% (95% CI, −51.0%, −11.2%, P = 0.007) (Figure 1A). This difference in UACR was persistent throughout the 48-wk double-blind phase. The mean difference in percentage change in losartan as compared with that in placebo was −16.8% (95% CI, −37.3%, +10.5%, P = 0.20). In covariance models controlling for clinical BP, ambulatory BP, creatinine clearance, and dietary sodium and protein intake, the mean difference in the percentage change in the UACR between the spironolactone and the placebo groups persisted. For example, after adjustment for the change from the baseline in 24-h systolic BP and creatinine clearance, the between-group difference in the UACR percentage change was −29.6% in spironolactone as compared with that in placebo (95% CI, −45.1%, −9.7%, P = 0.006). After adjustment for absolute change, percentage change, and lag change in ambulatory BP modeled as a static or a time-varying covariate, reduction in UACR in spironolactone as compared with that of placebo remained robust with virtually identical results.
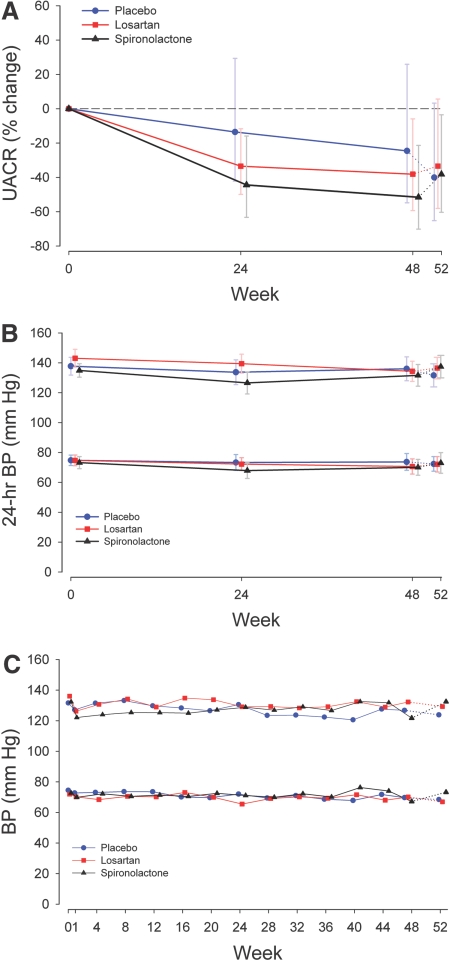
Figure 1.
The UACR and BP treatment responses for each study group. Solid lines indicate active treatment weeks, and the dotted lines indicate the washout phase. (A) The UACR percentage change for spironolactone versus placebo (−34.0%, 95% CI, −51.0%, −11.2%, P = 0.007) and losartan versus placebo (95% CI, −37.3%, +10.5%, P = 0.2). Data are presented as the percentage change from the baseline geometric mean and 95% CI. The P values are from mixed-model repeated-measures analysis. See the text for within-group differences. (B) Twenty-four-hour systolic BP percentage change was not statistically different between groups. Data are presented as the mean and 95% CI. (C) Clinical BP responses were not statistically different between groups. Data are presented as the mean and 95% CI.
During the 48 wk of treatment, the UACR decreased significantly from the baseline in the losartan (P = 0.001) and spironolactone (P < 0.0001) groups but not in the placebo group (P = 0.08) (Figure 1A). At 48 wk, the percentage change from the baseline was −24.6% (95% CI, −54.8%, +25.9%) in those assigned to placebo, −38.2% (95% CI, −59.3%, −5.9%) in those assigned to losartan, and −51.6% (95% CI, −70.2%, −21.4%) in those assigned to spironolactone. The response to intervention for each individual who completed 48 wk of follow-up demonstrated that all (with one exception) subjects randomized to spironolactone experienced a decrease in the UACR. To assess the potential impact of patient discontinuation on the primary outcome, the baseline characteristics of those who discontinued the blinded phase of the study before 48 wk of follow-up were compared with those who completed 48 wk of follow-up. In this analysis, those who discontinued the study early showed similar trends in reductions in the UACR when compared with those who completed the study (data not shown).
BP Control
Overall, 24-h ambulatory systolic BP decreased significantly from the baseline at 24 and 48 wk in all three groups, but these decreases were not statistically different between groups (P = 0.26) (Figure 1B). Similarly, clinical systolic and diastolic BP decreased in each group; however, the decrease was not significantly different between groups (Figure 1C). Table 2 illustrates the concomitant antihypertensive use at randomization and during the active phase of the study. On average, three add-on antihypertensive agents were used to achieve and maintain the BP goal in all three groups. The antihypertensive regimen included a diuretic agent in 90 to 95% of the study subjects during this phase.
Table 2.
Concomitant antihypertensive medicationsa
| Placebo n = 27 | Losartan n = 26 | Spironolactone n = 27 | |
|---|---|---|---|
| At randomization (week 0) | |||
| Diuretic | 23 (85.2) | 23 (88.5) | 27 (96.3) |
| β-Blocker | 19 (70.4) | 17 (63.4) | 21 (77.8) |
| α-Blocker | 8 (29.6) | 8 (30.8) | 7 (25.9) |
| Central adrenergic agonist | 2 (7.4) | 3 (11.5) | 3 (11.1) |
| Vasodilator | 1 (3.7) | 0 (0) | 0 (0) |
| During active treatment (weeks 1 to 48) | |||
| Diuretic | 25 (92.6) | 24 (92.3) | 25 (92.6) |
| β-Blocker | 21 (77.8) | 22 (84.6) | 22 (81.5) |
| α-Blocker | 15 (55.6) | 16 (61.5) | 13 (48.2) |
| Central adrenergic agonist | 9 (33.3) | 11 (42.3) | 5 (18.5) |
| Vasodilator | 2 (7.4) | 2 (7.7) | 0 (0) |
| No. of concomitant antihypertensive medications during active treatment (weeks 1 to 48) | |||
| 0 | 2 (7.4) | 1 (3.9) | 2 (7.4) |
| 1 | 4 (14.8) | 4 (15.4) | 3 (11.1) |
| 2 | 5 (18.5) | 4 (15.4) | 9 (33.3) |
| 3 | 7 (25.9) | 7 (26.9) | 8 (29.6) |
| ≥4 | 9 (33.3) | 10 (38.5) | 5 (18.5) |
aData are presented as n (%). Treatment group differences are nonsignificant.
Renal Function, Dietary Intake, and Serum Potassium and Bicarbonate Levels
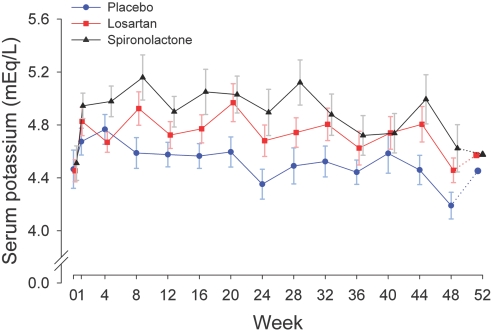
Overall, creatinine clearance decreased slightly from the baseline in all three groups, but there were no differences between groups (P = 0.8) (Table 3). The mean percentage change in creatinine clearance from the baseline over 48 wk was −16.0% (95% CI, −23.3%, −7.9%) for placebo, −16.8% (95% CI, −23.9%, −9.1%) for losartan, and −13.1% (95% CI, −21.3%, −3.9%) for spironolactone. Twenty-four-hour urinary sodium excretion and protein catabolic rate did not change significantly within or between groups (Table 3). Mean serum potassium concentration was significantly higher in both active treatment arms and was higher in spironolactone as compared with that in losartan (Figure 2). A serum potassium level ≥6.0 mEq/L occurred at least once in 2, 10, and 14 subjects in the placebo, losartan, and spironolactone groups, respectively (spironolactone versus placebo, P < 0.001; losartan versus placebo, P = 0.009). Two subjects in the spironolactone and none in placebo and losartan groups discontinued the study drug because of recurrent hyperkalemia (serum potassium level ≥6.0 mEq/L). During treatment, the mean serum bicarbonate level, adjusted for the baseline, was 25.5 mEq/L in the placebo group versus 23.7 mEq/L in the spironolactone group (difference for placebo minus spironolactone 1.8 mEq/L, 95% CI, 0.5, 3.1, P = 0.007).
Table 3.
Creatinine clearance and dietary sodium, potassium, and protein intakea
| Variable | Treatment | Week |
P valueb | ||
|---|---|---|---|---|---|
| 0 | 24 | 48 | |||
| Sample sizec | |||||
| Placebo | 27 | 22 | 21 | ||
| Losartan | 26 | 23 | 21 | ||
| Spironolactone | 27 | 20 | 17 | ||
| Creatinine clearance, ml/min | 0.80 | ||||
| Placebo | 73.0 [59.6–89.4] | 67.5 [54.4–83.8] | 64.3 [49.6–83.4] | ||
| Losartan | 64.8 [53–79.2] | 60.5 [49.6–73.7] | 54.1 [40.5–72.2] | ||
| Spironolactone | 51.4 [40.1–65.8] | 48.1 [36.2–64.0] | 51.6 [39.6–67.4] | ||
| 24-h urinary sodium, mEq/d | 0.28 | ||||
| Placebo | 193 [161–225] | 223 [169–278] | 196 [147–245] | ||
| Losartan | 220 [189–251] | 206 [169–243] | 213 [164–262] | ||
| Spironolactone | 219 [169–270] | 220 [171–268] | 221 [165–277] | ||
| 24-h urinary potassium, mEq/d | 0.11 | ||||
| Placebo | 56.8 [49.5–64] | 59 [48.5–69.4] | 53.4 [43.3–63.5] | ||
| Losartan | 62.7 [56.6–68.9] | 58.3 [51.4–65.1] | 51.8 [44.5–59.1] | ||
| Spironolactone | 57.6 [50.2–65] | 53.8 [45.3–62.4] | 54.5 [42.6–66.5] | ||
| Normalized protein catabolic rate, g/kg/d | 0.18 | ||||
| Placebo | 0.97 [0.89–1.06] | 1.03 [0.9–1.15] | 0.98 [0.88–1.09] | ||
| Losartan | 1.07 [0.96–1.18] | 1.02 [0.86–1.19] | 1.02 [0.83–1.21] | ||
| Spironolactone | 0.91 [0.83–1] | 0.93 [0.83–1.03] | 0.98 [0.87–1.09] | ||
aData are presented as mean [95% CI]. Geometric means are reported for creatinine clearance.
bP value represents the between-group treatment effect from a mixed-model repeated-measures analysis of weeks 0 to 48.
cSample size indicates the number of subjects remaining at each evaluation.
Figure 2.
Serum potassium treatment response by study group. Serum potassium for losartan versus placebo, P = 0.03; spironolactone versus placebo, P < 0.0001; losartan versus spironolactone, P = 0.05. Solid lines indicate active treatment weeks, and the dotted lines indicate the washout phase. Data are presented as the mean and standard error.
Glycemic Control
There were no differences in hemoglobin A1c within or between groups at the baseline or during treatment. Fasting glucose was significantly lower in the spironolactone group as compared with those in the losartan and placebo groups at the baseline. During treatment, fasting glucose fluctuated similarly across groups, tending to increase at week 24 then decrease and level off at week 48. The (geometric mean model estimate) values for fasting glucose during treatment were as follows: 166 mg/dl for placebo, 139 mg/dl for losartan, and 158 mg/dl for spironolactone.
Washout Period
There was a trend for ambulatory BP, albuminuria, and serum potassium to return to baseline at 52 wk in the spironolactone group (Figures 1 and 2). However, there was no significant change in the UACR, ambulatory or clinical systolic BP, or serum potassium at 52 as compared with 48 wk in any group.
Adverse Events
During the double-blind phase, the incidence of a transient increase in serum creatinine of ≥50% from the baseline was similar among study groups (10 in placebo and 13 each in losartan and spironolactone). Hospitalization for a cardiovascular event occurred in nine subjects comprising 3.7%, 7.7%, and 22.2% in the placebo, losartan, and spironolactone groups, respectively (P = 0.11). These events included one subject in the placebo group (stroke), two subjects in the losartan group (heart failure), and six subjects in the spironolactone group (two strokes, two heart failures, one myocardial infarction, and one coronary artery bypass graft). In addition to recurrent hyperkalemia (see above), two subjects in the spironolactone group required discontinuation of the study drug, one each for symptomatic hypotension and gynecomastia.
Discussion
The principal new finding in this study is that spironolactone affords greater renoprotection than a maximally dosed ACEi-based regimen alone. Use of a supramaximal dose of the once daily ACEi lisinopril was chosen to ensure that add-on therapy would reflect effects beyond what is achievable with an ACEi blockade alone. We also demonstrated that this renoprotection was not explained solely on the basis of reduced time-integral BP burden as assessed by 24-h ambulatory BP monitoring. In addition, this is the first study to demonstrate the added antiproteinuric effect of a MRA when carefully controlling for factors known to affect protein excretion in diabetes including BP, sodium and protein intake, and glycemia. We did not find a significant decrease in proteinuria in those assigned to losartan as compared with those assigned to placebo. Possible explanations include the fact that our subjects were on higher doses of an ACEi at the time losartan was added and the lack of effect of an ARB on the mineralocorticoid receptor.
Strict and similar control of systolic BP was achieved by design and is a distinct feature of our study. This allowed us to examine whether effects of add-on therapy were solely due to the observed reduction in BP. Previous investigators have reported significant reductions in proteinuria after adding a mineralocorticoid antagonist onto either an ACEi- or an ARB-based regimen.19,21,23–26 However, those studies were not designed to maintain BP at a similar level among treatment arms. The decrease in ambulatory systolic BP at 24 wk, but not at 48 wk, was numerically but not significantly greater in the spironolactone group as compared with those in the placebo and losartan groups (Figure 1). Recognizing that small differences in ambulatory BP could be biologically important, albeit not statistically significant, we conducted additional analyses controlling for the baseline and follow-up clinical as well as ambulatory BP. These analyses consistently found significant differences in the UACR of approximately 30% between placebo and spironolactone groups, confirming that the clinically relevant reduction in proteinuria observed during spironolactone administration is not explained solely on the basis of BP (Supplemental Table S1).
Aldosterone can cause cardiac, renal, and vascular injury and subsequent fibrosis by promoting tissue inflammation.27–29 Aldosterone receptors are present in glomerular endothelial and epithelial cells, and in experimental animal models aldosterone infusion can cause direct injury to glomerular epithelial cells, leading to proteinuria and glomerulosclerosis.30 These effects are believed to be mediated, in part, by upregulation of plasminogen activator inhibitor-1, TGF-β, serum- and glucocorticoid regulated kinase 1, and NADPH oxidase and downregulation of nitric oxide.31 The exact mechanism by which spironolactone reduces proteinuria in humans has not been elucidated. In animal studies, mineralocorticoid antagonists such as spironolactone prevent fibrosis and reduce glomerulosclerosis and proteinuria, presumably by antagonizing the mineralocorticoid receptor.28,32 In humans, MRAs have been reported to lower both BP and GFR in some patients with diabetic nephropathy.19,21,23,24,26 Our findings suggest that nonhemodynamic effects, possibly at the level of the glomerular filter, play an important role in reducing proteinuria. Further studies on the mechanism of spironolactone's antiproteinuric effect are needed to identify potential novel therapeutics that might lower proteinuria while minimizing hyperkalemia.
Dietary sodium and protein intake and glycemia can influence protein excretion in patients with chronic kidney disease. To the best of our knowledge, no prior study using a MRA or an ARB added onto an ACEi attempted to control for all of these important variables. In the present study, we controlled for BP, renal function, ACEi dose, dietary sodium and protein intake, and glycemia. After controlling for these variables, we observed a persistent significant reduction in proteinuria during spironolactone treatment.
The beneficial effect of spironolactone in this study was tempered by the greater incidence of hyperkalemia. This is an important finding for clinicians managing patients with diabetic kidney disease with combinations of drugs that block the RAAS at multiple sites. Most instances of hyperkalemia were asymptomatic, not accompanied by EKG changes, and were manageable with dietary counseling and short-term administration of a sodium–potassium exchange resin. Still, this could be a rate-limiting side effect in clinical practice. One possible explanation for the higher average serum potassium level and higher incidence of hyperkalemia in the spironolactone group is the slightly greater decline in serum bicarbonate concentration observed in the spironolactone group. However, we did not measure blood pH or other acid–base parameters in this study. It is possible that lower doses, for example, 12.5 mg once daily of spironolactone, could both reduce proteinuria and mitigate hyperkalemia in this patient population. Given the beneficial effect of MRA administration in patients with heart disease, identifying strategies to reduce the risk for hyperkalemia in patients with kidney disease (e.g., combining it with thiazide or loop diuretics) could allow the extension of the cardiovascular benefit, and perhaps a renal benefit, to those with diabetic nephropathy.33,34 The slightly higher incidence of nonhyperkalemia complications in the spironolactone group did not follow a pattern, or cluster; thus, we were unable to establish potential causality. Our study was not powered to compare effects of study drug on cardiovascular events, underscoring the need for a larger, longer-term study.
Albuminuria is a strong predictor for the progression of diabetic nephropathy, and greater reductions in albuminuria are associated with a greater reduction in risk for ESRD.35,36 Given the strong association between changes in albuminuria and reduction in risk for ESRD in patients with diabetic nephropathy, our study suggests that the addition of spironolactone also might reduce the risk for future ESRD. However, changes in the UACR are not necessarily associated with ESRD risk, and our study was not powered to detect differences in the slope of creatinine clearance, doubling of serum creatinine, or ESRD. Also, despite its inherent limitations with regard to renal outcomes, post hoc analysis of the ONTARGET study revealed dissociation between overall proteinuria reduction and rates of acute kidney injury requiring at least temporary dialysis.37 Therefore, establishing the long-term renoprotective effect of spironolactone will require a large-scale trial with hard clinical end points such as ESRD and death.
Our study has some limitations. First, the overall dropout rate was higher than expected. Still, the baseline characteristics of patients who dropped out during the blinded phases of the study were similar to those of the patients who finished the study. Second, we did not study lower doses of spironolactone (e.g., 12.5 mg/d) that may mitigate hyperkalemia but still be effective for reducing proteinuria. However, only two patients in this group reached a stop point for hyperkalemia. Third, we excluded calcium channel blockers from our antihypertensive regimens, because of their known variable effects on albuminuria. In addition, we note that the UACR did not return to the baseline during the washout period of the study. We believe that this was likely due to the fact that the washout duration may not have been sufficient to observe a reversible effect. Our study population was largely Hispanic and African-American, which may limit the generalizability of our findings. Still, these populations are among those at the highest risk for progression of diabetic nephropathy to ESRD and could benefit from spironolactone therapy if our findings are confirmed in additional studies. Finally, we did not perform renal biopsy as a part of the protocol; therefore, we cannot entirely exclude the possibility that some of our study subjects had hypertensive nephrosclerosis or other renal diseases.
In summary, we found that the addition of spironolactone (25 mg once daily) afforded greater renoprotection than a maximally dosed ACEi-based regimen alone in patients with diabetic nephropathy. Moreover, the benefit of spironolactone was not explained solely on the basis of a reduction in time-integral BP burden as assessed by 24-h BP monitoring. The addition of spironolactone was associated with a higher serum potassium concentration overall. Because hyperkalemia can cause serious cardiotoxicity, clinicians should be cautious whenever using RAAS drugs in the management of patients with diabetes and kidney disease, especially when using combinations of drugs that block the RAAS at multiple sites. A large-scale randomized trial is needed to determine whether spironolactone or other MRAs added onto an ACEi-based regimen is safe and effective for reducing the incidence of ESRD in patients with diabetic nephropathy.
Concise Methods
Study Population
The study population consisted of male and female subjects between the ages of 20 and 65 yr with type 1 or type 2 diabetes mellitus who satisfied the following criteria: (1) seated systolic BP >130 mmHg and (2) proteinuria defined as a 24-h UACR ≥300 mg/g despite treatment with an ACEi or an ARB for at least 3 mo. None of the participants was on both an ACEi and an ARB at screening. We enrolled subjects with either type 1 or 2 diabetes based on published data indicating similar BP and proteinuria lowering effects of adding either an ARB or an MRA to an ACEi. In addition, an effort was made to recruit younger patients with type 2 diabetes as recommended by the study sponsor (Table 1). Major exclusion criteria included body mass index >45 kg/m2, serum creatinine >3.0 mg/dl in females and >4.0 mg/dl in males, known nondiabetic kidney disease, serum potassium concentration >5.5 mEq/L, hemoglobin A1c >11%, stroke or myocardial infarction within the preceding 12 mo, heart failure, known adverse reaction to losartan or spironolactone, or anticipated need for dialysis within 12 mo.
Study Design
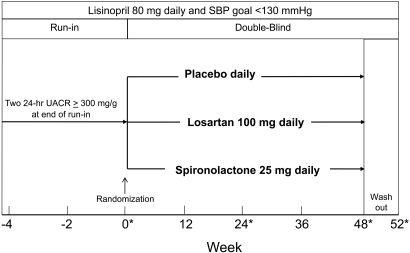
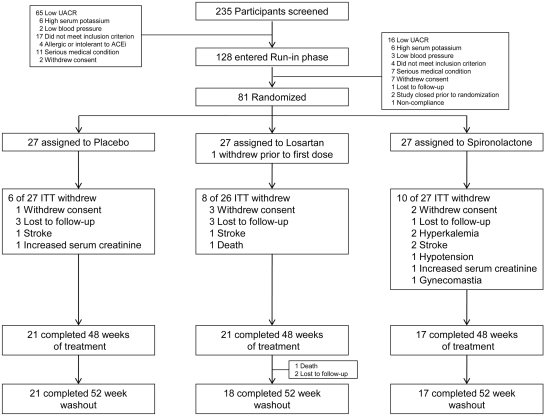
The study was a prospective, randomized, double-blind placebo-controlled trial consisting of three phases (Figure 3). Between August 2003 and March of 2007, we screened a total of 235 subjects of whom 128 qualified for entry into the run-in phase and 81 were randomized (Figure 4). After qualifying, study subjects entered a run-in period of 4 to 8 wk during which an initial dose of lisinopril (20 to 40 mg daily) was substituted for the subject's ACEi or ARB and gradually increased to 80 mg daily. Additional antihypertensives (excluding other ACEis, ARBs, MRAs, and calcium channel blockers) were added to reach a clinical systolic BP <130 mmHg before randomization. A research dietitian counseled each study subject on the recommended daily dietary restrictions, including 4 g of sodium, 0.8 g/kg protein, and 0.8 mEq/kg potassium. Two 24-h urine samples were obtained at the end of the run-in period to confirm a persistent UACR ≥300 mg/g while taking 80 mg daily of lisinopril and to estimate dietary sodium and potassium intake. At run-in visits, BP, serum creatinine, and potassium were measured, and medication adherence was assessed by pill count. Subjects were then admitted to the inpatient our Clinical Translational Research Center (CTRC) for baseline measurements including 24-h ambulatory BP and 24-h urine for albumin, urea, creatinine, sodium, and potassium level. After completion of baseline measurements, study subjects were assigned randomly, in equal proportion, to a blinded study medication. The study was approved by the UT Southwestern Medical Center Institutional Review Board (ClinicalTrials.gov Identifier: NCT00381134).
Figure 3.
Study Design. Asterisks denote inpatient CTRC admission.
Figure 4.
Number of study subjects who were screened, enrolled, randomized, and completed the study.
Dose and Administration of Blinded Study Medication
The initial dose of the blinded study medication was administered in a single capsule containing placebo, losartan (50 mg), or spironolactone (12.5 mg) once daily for the first week. Thereafter, the study drug dose was doubled by administering two capsules of the study drug—placebo once daily, losartan 100 mg once daily, or spironolactone 25 mg once daily—for the remainder of the study. The 100 mg once daily dose of losartan was chosen based on its efficacy in clinical trials and on clinical practice guidelines. The 25 mg once daily dose of spironolactone was chosen based on the results of clinical trials in patients with heart disease and chronic kidney disease. Blocked randomization, stratified by diabetes type, was programmed to determine treatment assignment. The Investigational Study Drug Unit of Parkland Memorial Hospital performed allocation of the study drug.
Follow-Up
Follow-up visits were conducted routinely at 4-wk intervals at which interval history, BP, physical examination, and serum creatinine and potassium levels were obtained. Inpatient visits for repeated measures of ambulatory BP and 24-h urine variables were performed at weeks 24 and 48 (Figure 3). In addition, 24-h urine collections and ambulatory BP were completed in the outpatient setting at weeks 12 and 36. To assess the effects of study drug discontinuation, subjects were invited to return for a final evaluation after a 4-wk washout period (52 wk).
Study Procedures
Blood Pressure Control.
A unique design feature of our study was to maintain equal BP control among study groups (Figure 3). To accomplish this, add-on antihypertensive medications, including diuretics, β and α blockers, central acting α agonists, and vasodilators, were used to achieve and maintain a goal systolic BP <130 mmHg.38 In addition, dietary sodium intake was monitored as estimated by 24-h urine collections, and subjects were reminded at each visit to adhere to the 4 g of sodium diet. Twenty-four-hour ambulatory BP was measured with a SpaceLab model 90207 device. Staff trained and certified according to JNC VII guidelines using a mercury sphygmomanometer performed clinical BP measurements.39,40
Laboratory Measurements.
Urine albumin was measured from an aliquot obtained from each 24-h urine collection and quantified by immunoprecipitation (DiaSorin, Stillwater, MN). The within-assay coefficient of variation (CV) range was 1.7 to 2.8%, and the between-assay CV range was 2.4 to 4.2%. Serum and urine chemistries were assayed in the CTRC laboratory on a Beckman model CX-9 autoanalyzer. Hemoglobin A1c was measured by affinity chromatography. Protein catabolic rate was estimated from 24-h urine urea and protein excretion and normalized for body weight as described previously.41
Statistical Analysis
Sample Size and Power.
The sample size was calculated to detect a 30% difference in the change in the UACR from the baseline between the placebo and the active treatment (losartan or spironolactone) groups. This study was not designed nor powered to detect differences between losartan and spironolactone groups. On the basis of the estimate of the variability of the change in the UACR of 33% (CV) and with an α of 0.05, the study had 80 and 90% power to detect this effect size with 20 and 26 subjects per group, respectively. We enrolled and randomized a total of 81 subjects (Figure 4).
Primary Analysis.
An intent-to-treat analysis was performed including 80 of the 81 randomized subjects, excluding one subject who did not complete the baseline evaluation and who never received the allocated losartan intervention. The primary outcome variable was percentage change in the 24-h UACR comparing losartan versus placebo and spironolactone versus placebo. The UACR and other skewed variables were log-transformed before analysis, but back-transformed values are presented to ease interpretation. Repeated-measures analyses utilizing all of the UACR and BP measurements obtained during the 48 wk of treatment were conducted using a mixed linear model analysis of covariance approach.42 This model consisted of the treatment effect, study week, and baseline value as a covariate, with the subject modeled as a random effect. An unstructured covariance pattern was selected for the UACR analyses.43 Creatinine clearance, systolic BP, and serum potassium levels were modeled similarly. Treatment-by-time interaction terms were evaluated for all models. Mixed -model repeated-measures analysis was performed to compare results at week 52 with those at week 48.
The UACR treatment response was further examined in adjusted mixed models including BP, creatinine clearance, serum potassium levels, urinary sodium levels, urinary potassium levels, and protein catabolic rate as baseline and time-varying covariates. The purpose of this latter analysis was to evaluate interactions between BP lowering, as well as blood and urine biochemistry, and change in the UACR. To rigorously test our hypothesis that changes in the UACR are not explained solely on the basis of reduced time-integral BP burden, ambulatory BP measurements were modeled as static and time-varying covariates using absolute change, percentage change, and lag change for 24-h measurements. Similar analyses were done using clinical BP. The Fisher exact test was applied for comparisons of adverse event frequencies. All tests were two-tailed with a P value <0.05 considered significant. Statistical analyses were performed with SAS version 9.1.3 (SAS Institute, Cary, NC).
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Diabetes Digestive and Kidney Diseases (2-R01 DK6301001) and the National Center for Research Resources General Clinical Research Center (M01-RR-00633 and CTSA UL1-RR-024982).
The authors wish to acknowledge the assistance of Ms. Martha Cruz and Mr. Robert Butsch in the preparation of the manuscript. In addition, we acknowledge the assistance of the following individuals who contributed to this project: Dr. Justin Teiwes, Dr. Koohsa Mortazavi, Dr. Hector Reyes, Dr. Thai Nguyen, Dr. Anupama Mohanram, Dr. Sandeep Singh, Ms. Tammy Lightfoot, Ms. Zhengnan Wang, Ms. Kim Jones, and Ms. Suzan Morrison.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Aldosterone Blockade in Diabetic Nephropathy: Relative Risks and Potential Promise,” on pages 2487–2489.
References
- 1.Adler S: Diabetic nephropathy: Linking histology, cell biology, and genetics. Kidney Int 66: 2095–2106, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR: Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63: 225–232, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O: Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 348: 383–393, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Andersen S, Tarnow L, Rossing P, Hansen BV, Parving HH: Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney Int 57: 601–606, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Lewis JB, Berl T, Bain RP, Rohde RD, Lewis EJ: Effect of intensive blood pressure control on the course of type 1 diabetic nephropathy. Am J Kidney Dis 34: 809–817, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Rossing P, Hommel E, Smidt UM, Parving HH: Impact of arterial blood pressure and albuminuria on the progression of diabetic nephropathy in IDDM patients. Diabetes 42: 715–719, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Rossing P, Hommel E, Smidt UM, Parving HH: Reduction in albuminuria predicts a beneficial effect on diminishing the progression of human diabetic nephropathy during antihypertensive treatment. Diabetologia 37: 511–516, 1994 [DOI] [PubMed] [Google Scholar]
- 12.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia): Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349: 1857–1863, 1997 [PubMed] [Google Scholar]
- 13.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Parving HH, Hommel E: Prognosis in diabetic nephropathy. BMJ 299: 230–233, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, Drury PL, Esmatjes E, Hricik D, Pohl M, Raz I, Vanhille P, Wiegmann TB, Wolfe BM, Locatelli F, Goldhaber SZ, Lewis EJ: Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol 16: 2170–2179, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Keane WF, Zhang Z, Lyle PA, Cooper ME, de Zeeuw D, Grunfeld J-P, Lash JP, McGill JB, Mitch WE, Remuzzi G, Shahinfar S, Snapinn SM, Toto R, Brenner BM: Risk scores for predicting outcomes in patients with type 2 diabetes and nephropathy: The RENAAL study. Clin J Am Soc Nephrol 1: 761–767, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Juknevicius I, Segal Y, Kren S, Lee R, Hostetter TH: Effect of aldosterone on renal transforming growth factor-β. Am J Physiol Renal Physiol 286: F1059–F1062, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Ponda MP, Hostetter TH: Aldosterone antagonism in chronic kidney disease. Clin J Am Soc Nephrol 1: 668–677, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Rossing K, Schjoedt KJ, Smidt UM, Boomsma F, Parving HH: Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: A randomized, double-masked, cross-over study. Diabetes Care 28: 2106–2112, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Sato A, Hayashi K, Naruse M, Saruta T: Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension 41: 64–68, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, Patni R, Beckerman B: Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol 1: 940–951, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Epstein M: Aldosterone receptor blockade and the role of eplerenone: Evolving perspectives. Nephrol Dial Transplant 18: 1984–1992, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Chrysostomou A, Pedagogos E, MacGregor L, Becker GJ: Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin II receptor blocker. Clin J Am Soc Nephrol 1: 256–262, 2006 [DOI] [PubMed] [Google Scholar]
- 24.van den Meiracker AH, Baggen RG, Pauli S, Lindemans A, Vulto AG, Poldermans D, Boomsma F: Spironolactone in type 2 diabetic nephropathy: Effects on proteinuria, blood pressure and renal function. J Hypertens 24: 2285–2292, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ: Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: A systematic review. Am J Kidney Dis 51: 199–211, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Schjoedt KJ, Rossing K, Juhl TR, Boomsma F, Tarnow L, Rossing P, Parving HH: Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy. Kidney Int 70: 536–542, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Hollenberg NK: Aldosterone in the development and progression of renal injury. Kidney Int 66: 1–9, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Rocha R, Chander PN, Zuckerman A, Stier CT, Jr: Role of aldosterone in renal vascular injury in stroke-prone hypertensive rats. Hypertension 33: 232–237, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Zhou X, Ono H, Ono Y, Frohlich ED: Aldosterone antagonism ameliorates proteinuria and nephrosclerosis independent of glomerular dynamics in L-NAME/SHR model. Am J Nephrol 24: 242–249, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Viengchareun S, Le Menuet D, Martinerie L, Munier M, Pascual-Le Tallec L, Lombès M: The mineralocorticoid receptor: Insights into its molecular and (patho)physiological biology. Nucl Recept Signal 5: e012, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagase M, Fujita T: Aldosterone and glomerular podocyte injury. Clin Exp Nephrol 12: 233–242, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Rocha R, Chander PN, Khanna K, Zuckerman A, Stier CT, Jr: Mineralocorticoid blockade reduces vascular injury in stroke-prone hypertensive rats. Hypertension 31: 451–458, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J: The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341: 709–717, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Pitt B, Williams G, Remme W, Martinez F, Lopez-Sendon J, Zannad F, Neaton J, Roniker B, Hurley S, Burns D, Bittman R, Kleiman J: The EPHESUS trial: Eplerenone in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction. Cardiovasc Drugs Ther 15: 79–87, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Pohl MA, Blumenthal S, Cordonnier DJ, De Alvaro F, Deferrari G, Eisner G, Esmatjes E, Gilbert RE, Hunsicker LG, Lopes de Faria JB, Mangili R, Moore J, Jr, Reisin E, Ritz E, Schernthaner G, Spitalewitz S, Tindall H, Rodby RA, Lewis EJ: Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: Clinical implications and limitations. J Am Soc Nephrol 16: 3027–3037, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Toto RD: Proteinuria reduction: Mandatory consideration or option when selecting an antihypertensive agent? Curr Hypertens Rep 7: 374–378, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S: Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 372: 547–553, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Kidney Disease Outcomes Quality Initiative (K/DOQI): K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 43: S1–S290, 2004 [PubMed] [Google Scholar]
- 39.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Fox JC, Leight K, Sutradhar SC, Demopoulos LA, Gleim GW, Lewin AJ, Bakris GL: The JNC 7 approach compared to conventional treatment in diabetic patients with hypertension: a double-blind trial of initial monotherapy vs. combination therapy. J Clin Hypertens 6: 437–442, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maroni BJ, Steinman TI, Mitch WE: A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 27: 58–65, 1985 [DOI] [PubMed] [Google Scholar]
- 42.Hedeker DG, RD: Longitudinal Data Analysis New York, Wiley-Interscience, 2006 [Google Scholar]
- 43.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O: SAS for Mixed Models, 2nd Ed., Cary, NC, SAS Institute, 2006 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.