Abstract
Kidney function predicts cardiovascular and all-cause mortality, but little is known about the association of changes in estimated GFR (eGFR) with clinical outcomes. We investigated whether 3- and 9-yr changes in eGFR associated with risk for coronary heart disease (CHD) and all-cause mortality among 13,029 participants of the Atherosclerosis Risk in Communities (ARIC) Study. After adjustment for baseline covariates including eGFR in Cox proportional hazards models, the quartile of participants with the greatest annual decline (annual decline ≥5.65%) in eGFR were at significantly greater risk for CHD and all-cause mortality (hazard ratio 1.30 [95% confidence interval 1.11 to 1.52] and 1.22 [95% confidence interval 1.06 to 1.41], respectively) compared with the third quartile (annual decline between 0.33 and 0.47%). We observed similar results when we analyzed 9-yr changes in eGFR. Adjustment for covariates at the second eGFR used to estimate change reduced the association with CHD but not with mortality. Among participants with stage 3 chronic kidney disease, an increase in eGFR during the first 3 yr also associated with a higher risk for mortality, perhaps as a result of clinical instability. In conclusion, a steeper than average decline in eGFR associates with a higher risk for CHD and all-cause mortality. Increases in eGFR among participants with chronic kidney disease associate with similar increased risks.
Chronic kidney disease (CKD) affects >26 million adults in the United States.1 Numerous articles have reported that decreased kidney function is associated with incident cardiovascular disease (CVD) and all-cause mortality independent of traditional risk factors.2,3 Consequently, current clinical guidelines categorize individuals with CKD as a “high-risk group” for CVD.2,4,5
Clinicians often monitor kidney function over a period of years, but in contrast to the large number of studies documenting an independent association between baseline kidney function and future cardiovascular events,3,6–8 little is known about how sequential changes in kidney function may be related to future risk. A few studies have reported that deterioration in kidney function is associated with CVD.9–13 Interestingly, two studies showed that change in kidney function was a better predictor of clinical outcomes than baseline kidney function12,13; however, previous studies were conducted among select populations of Eastern Asian individuals,9 elderly Americans,10 or patients with hypertension or diabetes.11–13
Furthermore, only a few studies have investigated the association between a rapid decline in estimated GFR (eGFR), the best overall measure of kidney function, and future outcomes.9,10 Although Go et al.14 reported that low eGFR is associated with adverse outcomes independent of time-varying conventional risk factors, no study, to our knowledge, has taken into account the deteriorations of other risk factors to evaluate the independent association of a change in eGFR with cardiovascular outcomes.
The objective of this study was to investigate the independent association of a change in eGFR with incident coronary heart disease (CHD) and all-cause mortality in a community-based, middle-aged population. We examined both intermediate (3-yr) and long-term (9-yr) changes. We also examined whether the adjustments for covariates at baseline and at follow-up provided different results.
Results
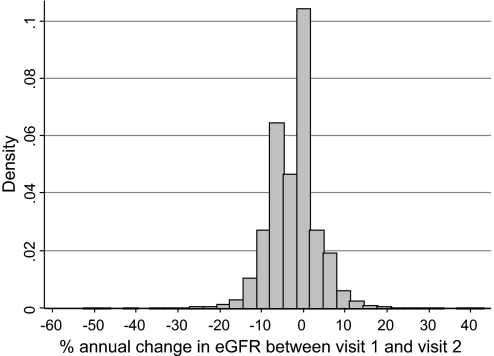
The distribution of percentage annual change in eGFR between visit 1 and visit 2 is shown in Figure 1. The mean (SD) of percentage annual change was −2.49% (5.66%) with median (interquartile range) of −0.47% (−5.65 to −0.33%). Demographic characteristics of participants according to the quartiles of percentage annual changes in eGFR are shown in Table 1. Participants with the largest declines in eGFR (Q1) were more likely to be women and to be black. Q1 had higher average systolic BP but lower LDL cholesterol and higher HDL cholesterol than Q2 to Q4. As might be expected, Q1 had the highest mean baseline eGFR among the quartiles. Participants in Q1 and Q4 were similar with respect to most characteristics: lower education level, more likely to be current smokers, higher prevalence of diabetes, and more likely to be receiving treatment for hypertension as compared with individuals in Q2 or Q3 (stable eGFR).
Figure 1.
Distribution of percentage annual change in eGFR on the basis of 3-yr change among 13,029 participants of the ARIC Study is shown. Cutoff points of quartiles were −5.65, −0.47, and −0.33%/yr.
Table 1.
Characteristics of participants according to quartiles of change in eGFR from visit 1 to visit 2
| Characteristic | Quartiles of % Annual Change in eGFR |
|||
|---|---|---|---|---|
| Q1 (−52.76 to −5.65; n = 3258) | Q2 (−5.65 to −0.47; n = 3257) | Q3 (−0.47 to −0.33; n = 3257) | Q4 (−0.33 to 42.94; n = 3257) | |
| Age (yr; mean ± SD) | 54.0 ± 5.8 | 53.8 ± 5.8 | 53.2 ± 5.1 | 54.9 ± 6.0 |
| Male gender (%) | 32.3 | 51.7 | 40.7 | 47.1 |
| Black race (%) | 27.8 | 25.8 | 20.4 | 24.4 |
| Educational level (%)a | ||||
| <12 yr completed | 22.4 | 20.7 | 18.4 | 22.2 |
| 12 to 16 yr completed | 41.8 | 40.7 | 42.9 | 41.5 |
| >16 yr completed | 35.8 | 38.6 | 38.7 | 36.3 |
| Current smokers (%)a | 24.7 | 23.7 | 24.1 | 24.9 |
| Current alcohol drinkers (%)a | 54.4 | 57.7 | 61.1 | 57.4 |
| BMI (kg/m2; mean ± SD)a | 27.5 ± 5.5 | 27.5 ± 4.9 | 27.3 ± 5.2 | 27.9 ± 5.4 |
| Diabetes mellitus (%) | 12.2 | 8.6 | 8.0 | 11.5 |
| Antihypertensive medication (%)a | 28.5 | 26.4 | 24.9 | 29.9 |
| ACEI (%)a | 3.0 | 2.7 | 2.7 | 3.0 |
| SBP (mmHg; mean ± SD)a | 121.9 ± 20.1 | 121.2 ± 17.8 | 118.5 ± 16.9 | 120.3 ± 17.5 |
| DBP (mmHg; mean ± SD)a | 73.5 ± 11.4 | 74.4 ± 10.9 | 72.8 ± 10.6 | 73.3 ± 10.7 |
| LDL cholesterol (mg/dl; mean ± SD)a | 134.0 ± 38.8 | 137.9 ± 39.1 | 136.9 ± 38.5 | 138.7 ± 39.1 |
| HDL cholesterol (mg/dl; mean ± SD)a | 53.7 ± 17.3 | 51.3 ± 16.3 | 52.8 ± 17.4 | 51.4 ± 16.9 |
| TG (mg/dl; median [IQR])a | 106 (75, 152.5) | 107 (78, 152) | 106 (78, 152) | 113 (80, 158) |
| Left ventricular hypertrophy (%)a | 2.4 | 2.0 | 1.2 | 2.0 |
| Carotid atherosclerosis (%)a | 8.5 | 7.4 | 5.5 | 7.0 |
| Serum creatinine (mg/dl; mean ± SD) | ||||
| visit 1 | 0.75 ± 0.20 | 0.87 ± 0.15 | 0.85 ± 0.17 | 0.93 ± 0.19 |
| visit 2 | 0.96 ± 0.47 | 0.97 ± 0.16 | 0.85 ± 0.17 | 0.84 ± 0.18 |
| eGFR (ml/min per 1.73 m2; mean ± SD) | ||||
| visit 1 | 105.9 ± 23.2 | 91.5 ± 16.0 | 91.1 ± 16.9 | 84.0 ± 17.0 |
| visit 2 | 80.3 ± 18.5 | 80.0 ± 14.0 | 90.1 ± 16.7 | 94.3 ± 20.5 |
All comparisons were significant at P < 0.001, except for current smoking (P = 0.016), angiotensin-converting enzyme inhibitors (ACEI; P = 0.829), and left ventricular hypertrophy (P = 0.002). BMI, body mass index; SBP, systolic BP; DBP, diastolic BP; IQR, interquartile range; TG, triglycerides.
aMissing values (number missing): Educational level, 20; current smokers, 8; current drinkers, 43; BMI, 7; diabetes, 19; antihypertensive medication, 6; ACEI, 1; SBP, 3; DBP, 4; LDL cholesterol, 258; HDL cholesterol, 84; TG, 83; left ventricular hypertrophy, 303; carotid atherosclerosis, 386.
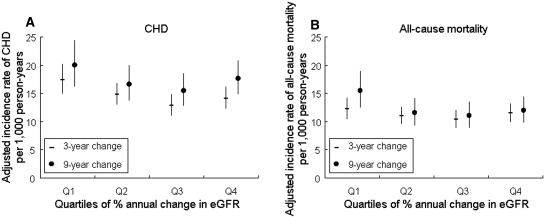
During 16 to 18 yr of follow-up, there were 1627 cases of CHD and 2042 deaths before January 1, 2006. The overall incidence rates of CHD and all-cause mortality were 9.8 per 1000 person-years and 11.7 per 1000 person-years. The age-, race-, and gender-adjusted incidence rates of CHD and all-cause mortality were calculated among the quartiles of percentage annual change of eGFR (Figure 2). Despite the greater mean eGFR in Q1, this group had the greatest incidence rate of CHD and all-cause mortality. As we hypothesized, Q4—consisting of individuals with minimal decrease or an increase in eGFR—showed a higher incidence rate of both outcomes compared with Q3.
Figure 2.
(A and B) Adjusted incidence rates and their 95% CIs of CHD (A) and all-cause mortality (B) are shown by quartiles of percentage annual changes in eGFR (Q1 through Q4) from visit 1 to visit 2 (3-yr change) or visit 4 (9-yr change). Cutoff points of quartiles were −5.65, −0.47, and −0.33%/yr for 3-yr change and −2.46, −0.74, and −0.56%/yr for 9-yr change. Adjusted to mean age (54.0 yr), race (white), and gender (male).
Because these incidence rates are presumably confounded by other risk factors and baseline eGFR, we computed the hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for CHD and all-cause mortality by quartiles of change using Cox proportional hazards models, adjusting for covariates including eGFR at visit 1 (Table 2). Overall, Q1 had higher HRs for both CHD and all-cause mortality (1.30 [95% CI 1.11 to 1.52] and 1.22 [95% CI 1.06 to 1.41], respectively). When participants were stratified by baseline eGFR category, higher HRs for incident CHD and all-cause mortality were observed in Q1 compared with Q3 among those with eGFR between 60 and 89 (1.39 [95% CI 1.09 to 1.76] and 1.40 [95% CI 1.12 to 1.76], respectively). Among the 716 participants in this group, 36.3% (n = 260) dropped below an eGFR of 60 by the second visit. Q1 also had a higher risk for mortality compared with Q3 among those with eGFR between 30 and 59 (HR 4.69; 95% CI 1.28 to 17.16) at baseline. Changes in eGFR were not significantly associated with higher risk for CHD or all-cause mortality among participants with eGFR ≥90 at baseline. The interaction terms of baseline eGFR and quartiles of change in eGFR were statistically significant only in the model for all-cause mortality (P = 0.03). We did not observe a significant gender interaction for either CHD (P = 0.795 for interaction) or all-cause mortality (P = 0.078 for interaction). Similar results for both outcomes were observed when we removed baseline eGFR from the models or adjusted for mean values of eGFR at visit 1 and visit 2 (data not shown).
Table 2.
Adjusted HRs (95% CIs) of CHD and all-cause death by quartiles of % annual change in eGFR from visit 1 to visit 2, overall, and eGFR category at visit 1
| Outcomes | Quartiles of % Annual Change in eGFR |
|||
|---|---|---|---|---|
| Q1 (−52.76 to −5.65) | Q2 (−5.65 to −0.47) | Q3 (−0.47 to −0.33) | Q4 (−0.33 to 42.94) | |
| All | ||||
| na | 3016 | 3012 | 3004 | 2985 |
| CHD | 1.30 (1.11 to 1.52) | 1.16 (1.00 to 1.35) | Reference | 1.04 (0.90 to 1.22) |
| all-cause mortality | 1.22 (1.06 to 1.41) | 1.05 (0.92 to 1.21) | Reference | 1.10 (0.96 to 1.27) |
| eGFR ≥90 ml/min per 1.73 m2 | ||||
| na | 2262 | 1363 | 1247 | 826 |
| CHD | 1.13 (0.91 to 1.41) | 1.19 (0.95 to 1.49) | Reference | 0.98 (0.75 to 1.28) |
| all-cause mortality | 0.96 (0.80 to 1.16) | 0.93 (0.76 to 1.13) | Reference | 1.00 (0.80 to 1.23) |
| eGFR 60 to <90 ml/min per 1.73 m2 | ||||
| na | 716 | 1604 | 1720 | 1996 |
| CHD | 1.39 (1.09 to 1.76) | 1.10 (0.90 to 1.36) | Reference | 1.06 (0.87 to 1.29) |
| all-cause mortality | 1.40 (1.12 to 1.76) | 1.10 (0.90 to 1.35) | Reference | 1.18 (0.97 to 1.42) |
| eGFR 30 to <60 ml/min per 1.73 m2 | ||||
| na | 37 | 44 | 37 | 163 |
| CHD | 1.47 (0.46 to 4.67) | 1.68 (0.56 to 4.99) | reference | 1.16 (0.43 to 3.10) |
| all-cause mortality | 4.69 (1.28 to 17.16) | 4.98 (1.36 to 18.25) | reference | 2.52 (0.73 to 8.74) |
Adjusted for following covariates at visit 1: Age, race, gender, level of education, carotid atherosclerosis, SBP, antihypertensive medication, diabetes, smoking, alcohol intake, BMI, LDL cholesterol, HDL cholesterol, left ventricular hypertrophy, and eGFR.
aParticipants included in the fully adjusted analysis.
We repeated our analyses adjusting for covariates at visit 2 and obtained a similar result for all-cause mortality (HR 1.28; 95% CI 1.11 to 1.47 in Q1), although the association of change in eGFR with incident CHD was attenuated (HR 1.13; 95% CI 0.96 to 1.31; P = 0.134). Similarly, Q1 was significantly associated with a higher risk for all-cause mortality but not with incident CHD in individuals with eGFR at visit 2 between 30 and 59 and between 60 and 89 (data not shown). In contrast, no associations were observed between change in eGFR and either outcome in the group with eGFR ≥90 at visit 2. In the participants with eGFR at visit 2 between 30 and 59, Q4—a group with minimal decrease or an increase in eGFR—was also associated with a higher risk for all-cause mortality (HR 7.16; 95% CI 1.63 to 31.36).
We conducted the same analysis after dividing the participants into the following three groups: ≤−5%, >−5 to ≤0, and >0. The group with percentage annual changes between −5 and 0% was set as the reference group. The results comparing the group with annual change in eGFR ≤−5% were almost identical to our results observed for Q1 in our previous analyses (data not shown). We also obtained very similar results in analyses of quartiles of annual difference in eGFR (using cut points of −5.40, −0.52, and −0.27 ml/min per 1.73 m2/yr). In addition, the group with larger eGFR declines defined using cut points from published guidelines (≤−4 or <−5 ml/min per 1.73 m2/yr) had a higher risk for all-cause mortality regardless of the covariate adjustment approach we used. In the case of incident CHD, a statistically significant association was observed only when adjusting for covariates at visit 1. Again, among those with eGFR at visit 2 between 30 and 59, individuals with increased eGFR in the past (difference in eGFR >0 ml/min per 1.73 m2/yr) demonstrated a higher risk for death, similar in magnitude to the association for Q4 observed in the main analysis (data not shown).
Finally, we analyzed 9-yr change in eGFR from visit 1 to visit 4 and risk for subsequent CHD and all-cause mortality (Figure 2, Table 3). In this analysis, quartiles (Q1 to Q4) of percentage annual change calculated using eGFR levels at visit 1 and visit 4 were ≤−2.46, −2.46 to −0.74, −0.74 to <−0.56 and ≥−0.56% per year. Q1 was associated with a statistically significantly higher risk for both outcomes compared with Q3 in the models with eGFR and covariates at visit 1 (1.32 [95% CI 1.08 to 1.63] for CHD and 1.41 [95% CI 1.16 to 1.72] for all-cause mortality). When adjusted for covariates at visit 4, the HRs of Q1 compared with Q3 were 1.17 (95% CI 0.87 to 1.58) for CHD and 1.27 (95% CI 0.97 to 1.67) for all-cause mortality. Similar results were obtained when we used quartiles of an annual change estimated from a least-squares regression model using eGFR measurements at all three time points (data not shown).
Table 3.
Adjusted HR (95% CIs) of CHD and all-cause mortality by quartiles of % annual change in eGFR from visit 1 to visit 4
| Outcomes | Quartiles of % Annual Change in eGFR |
|||
|---|---|---|---|---|
| Q1 (−25.53 to −2.46) | Q2 (−2.46 to −0.74) | Q3 (−0.74 to −0.56) | Q4 (−0.56 to 13.54) | |
| Adjustment for covariates at visit 1 | ||||
| na | 2326 | 2316 | 2339 | 2335 |
| CHD | 1.32 (1.08 to 1.63) | 1.06 (0.86 to 1.30) | Reference | 1.18 (0.97 to 1.45) |
| all-cause mortality | 1.41 (1.16 to 1.72) | 1.11 (0.90 to 1.36) | Reference | 1.11 (0.90 to 1.36) |
| Adjustment for covariates at visit 4 | ||||
| na | 1190 | 1130 | 1131 | 1048 |
| CHD | 1.17 (0.87 to 1.58) | 1.11 (0.84 to 1.48) | Reference | 1.32 (0.99 to 1.74) |
| all-cause mortality | 1.27 (0.97 to 1.67) | 0.98 (0.74 to 1.30) | Reference | 1.05 (0.80 to 1.39) |
Adjusted for following covariates at either visit: Age, race, gender, level of education, carotid atherosclerosis, SBP, antihypertensive medication, diabetes, smoking, alcohol intake, BMI, LDL cholesterol, HDL cholesterol, left ventricular hypertrophy, and eGFR.
aParticipants included in the fully adjusted analysis.
We obtained similar results for percentage change in eGFR between visit 1 and visit 2 or between visit 1 and visit 4 after adjusting for use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers at each visit or excluding participants who had hospitalizations with acute kidney injury during a period elapsed between visits (n = 3 between visit 1 and 2 and n = 14 between visit 1 and visit 4; data not shown). Further adjustment for albuminuria in the analyses with covariates measured at visit 4 also did not alter the results (data not shown). There was no evidence of interaction between change in eGFR and inhibitors of renin-angiotensin system or albuminuria (P = 0.663 for CHD and P = 0.122 for all-cause mortality).
Discussion
In this prospective analysis of data from the Atherosclerosis Risk in Communities (ARIC) Study, individuals in the quartile with a steeper than average decline in eGFR (Q1) had a higher risk for incident CHD and all-cause mortality even after adjustment for baseline covariates and eGFR. Similar results were obtained among participants with mildly or moderately low kidney function (eGFR 30 to 89 ml/min per 1.73 m2). On the whole, our results suggest there may be clinical value in sequential eGFR data, often measured in routine care, even among individuals with mildly reduced eGFR (60 to 89).
These findings are generally consistent with other recently published studies.9,10 Cheng et al.9 reported that a decrease in eGFR >20% during 18 mo of follow-up was associated with a higher risk for cardiovascular and all-cause mortality in a middle-aged Taiwanese population. Rifkin et al.10 demonstrated that an annual decrease in eGFR >3 ml/min per 1.73 m2 over 3 to 7 yr was associated with a higher risk for all-cause and cardiovascular mortality in elderly adults. We extended these associations to a middle-aged, biethnic, community-based population of the United States.
A rapid decline in eGFR and increased mortality risk were previously observed among elderly adults both above and below an eGFR of 60 ml/min per 1.73 m2.10 We did not find that a rapid decrease in eGFR was associated with risk for future adverse events for levels of eGFR ≥90 ml/min per 1.73 m2; however, this may partially or wholly reflect imprecision of the Modification of Diet in Renal Disease (MDRD) Study equation in this range of eGFR.15
Participants in Q1, the group with a rapid decline in eGFR, were more likely to be female as compared with the other groups. In general, progression of kidney disease is considered to be faster in men compared with women16; however, it has been suggested that kidney function may decrease faster in postmenopausal women as compared with men of similar age.17
Although we found that the association of a decrease in eGFR with all-cause mortality persisted even adjusting for eGFR and covariates at follow-up, past changes of eGFR are somewhat less useful in estimating risk for incident CHD, because the higher risk for incident CHD observed in those with the most rapid decline (Q1) was NS after adjustment for current (follow-up) eGFR and covariate levels. It is important to note, however, that a large decline in the past (Q1) necessarily means that many of these individuals had previously better kidney condition than individuals in the other quartiles. An important caveat to our results is that adjustment for eGFR at visit 2 or visit 4 may be a conservative approach to investigate clinically important changes in eGFR.18 Indeed, when eGFR at visit 2 was removed from the model, Q1 was borderline significantly associated with a higher risk for CHD compared with Q3 overall (HR 1.15; 95% CI 0.99 to 1.34). It is not evident why changes of eGFR in the past were more strongly associated with all-cause mortality than incident CHD; however, this result is consistent with previous research demonstrating that impaired kidney function is more strongly associated with all-cause mortality than cardiovascular risk.2
The causative mechanisms by which impaired kidney function contributes to CHD and other causes of mortality are not fully elucidated. CKD itself is considered a risk factor for ventricular and vascular remodeling and may serve to exacerbate the effects of other risk factors.3,8 Some researchers have suggested that CKD is a marker of subclinical atherosclerosis.8 We found that change in eGFR was independently associated with clinical outcomes even after adjustment for rigorously measured cardiovascular risk factors, left ventricular hypertrophy by electrocardiogram, and a measure of subclinical atherosclerosis. The association of a change in eGFR and CHD was weakened after adjustment for covariates at follow-up, suggesting that deterioration of cardiovascular risk factors or ventricular/vascular remodeling may mediate the association between change in kidney function and cardiovascular risk.3 Other possible mediators are oxidative stress,8 inflammation,2 increased leptin,19 and/or activation of the renin-angiotensin system7 induced by renal impairment.
Interestingly, among individuals with current eGFR between 30 and 59, a past increase in eGFR over 3 yr was also associated with higher risk for all-cause mortality. This positive association was not entirely unexpected, because individuals with an increase in eGFR to 30 to 59 are likely to have had more severe impairment in kidney function in the past (mean eGFR at visit 1 48.7 in Q4 versus 56.1 ml/min per 1.73 m2 in the reference group). Otherwise, this increase may be related to instability of kidney function. An increase in eGFR might result from decreased serum creatinine as a result of muscle loss related to malnutrition or ill health. Mean triglyceride and LDL cholesterol concentrations and body mass index only slightly decreased or were unchanged from visit 1 to visit 2 for Q4 among participants with eGFR 30 to 59 ml/min per 1.73 m2 at visit 2 (triglycerides from 183 at visit 1 to 176 mg/dl at visit 2; LDL cholesterol from 148 to 138 mg/dl; and body mass index from 27.7 to 28.0); however, a contribution of muscle loss could not be eliminated, because we did not have direct measures of body composition. Nevertheless, the positive association of a past increase in eGFR with all-cause mortality suggests that once individuals have reached an eGFR of <60, it may be useful to track their kidney function even when a subsequent eGFR measurement is higher. Overall, our results suggest that changes in eGFR over time provide additional prognostic information to current eGFR.
Some limitations of this study should be mentioned. First, the MDRD equation has been shown systematically to underestimate eGFR in the normal range.15 In addition, eGFR by this formula has been shown to be imprecise for evaluating sequential changes.20 Nonetheless, because eGFR by the MDRD equation is broadly used in clinical practice,2,4,5 our results have direct clinical applicability. Second, random fluctuations in creatinine levels over time would tend to increase the variance in our data7,9; however, such random variation would most likely bias our findings toward a null result and lead to an underestimation of the true association. Third, albuminuria was not measured at visits 1 and 2. Fourth, although additional measurements of serum creatinine would be useful to characterize more fully the slope of change, we are limited to kidney function assessments conducted at the time of the ARIC examinations.21 Nonetheless, these data reflect a realistic clinical scenario with measurements assessed several years apart. Several years of follow-up are needed for an adequate signal-to-noise ratio in CKD progression compared with random creatinine variation. Fifth, there were relatively few participants with eGFR <60 ml/min per 1.73 m2, resulting in broad 95% CIs for HRs in the group of eGFR 30 to 59. Thus, further studies for patients with CKD are needed. Finally, despite rigorous measurement of important covariates in the ARIC Study, we cannot eliminate the possibility of residual confounding, particularly because inflammatory or oxidative stress biomarkers were not included in these analyses.
In conclusion, a steeper than average decline in eGFR (i.e., ≥5%/yr) was associated with a higher risk for all-cause mortality independent of eGFR and other known risk factors at baseline or follow-up. This was more relevant for individuals with mildly or moderately reduced eGFR (30 to 89 ml/min per 1.73 m2). Within stage 3 CKD, an increase of eGFR was also associated with higher mortality, possibly as a marker of previously more severe disease or instability. The significant association between change in kidney function and risk for clinical outcomes after adjustment for covariates at follow-up suggests the observed effect is independent of the deterioration in traditional risk factors. These data assist in the interpretation of serial serum creatinine measurements, often available in outpatient care, by indicating that change in eGFR contributes additional prognostic information beyond single eGFR measurement.
Concise Methods
Study Population
We analyzed data from the ARIC Study, a prospective, population-based sample of individuals from four US communities: Forsyth County, NC; suburban Minneapolis, MN; Washington County, MD; and Jackson, MS. Details of the design and conduct of the ARIC Study are described elsewhere.21 In brief, 15,792 men and women aged 45 to 64 yr underwent standardized clinical examinations during the baseline visit from 1987 through 1989 (visit 1). Follow-up examinations occurred at 3-yr intervals during 1990 through 1992 (visit 2), 1993 through 1995 (visit 3), and 1996 through 1998 (visit 4). In this study, we excluded participants who self-reported race other than white or black (n = 48) or who were missing serum creatinine values (n = 1598) at either visit 1 or visit 2. A total of 1256 participants were excluded because of missing data at visit 2, including 246 deaths during this interval. Additional exclusions were participants with an eGFR <15 ml/min per 1.73 m2 at visit 1 (n = 8) or a history of CHD by visit 2, on the basis of self-report, clinical examination, or hospital records, or with missing data on CHD history (n = 1109), for a final study population of 13,029.
Exposure Variables: Change in eGFR
Serum creatinine concentration was measured using a modified kinetic Jaffe method22,23 and was corrected for interlaboratory differences and calibrated to the Cleveland Clinic by subtraction of 0.24 mg/dl for visit 1 and 2 measurements and addition of 0.18 mg/dl for visit 4 measurements.7,24 GFR was estimated from serum creatinine using the simplified equation developed at the Cleveland Clinic from the MDRD Study: eGFR = 186.3 * (serum creatinine [mg/dl]−1.154) * (age−0.203) * (0.742 if female) * (1.212 if black).25
Although two indices of change in eGFR, namely the absolute difference and percentage change, have been used in the literature,4,5,9,10,20 guidelines for the management of kidney disease use annual absolute difference of eGFR to define rapid progression of kidney disease. Specifically, the Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical practice guidelines define a rapid decrease in eGFR as ≤−4 ml/min per 1.73 m2/yr,4 whereas the recent published UK National Institute for Health and Clinical Excellence (NICE) guideline adopts −5 ml/min per 1.73 m2/yr as a cutoff.5 Controversy also exists regarding whether decline in GFR tends to be linear, logarithmic, or noncontinuous (exhibits a threshold effect)4; therefore, we divided ARIC participants into quartiles of annual absolute difference and percentage annual change from visit 1 to visit 2 (Q1 to Q4, where Q1 reflects those with the largest decline in eGFR and Q4 the smallest decline or increase). Annual variations in eGFR were calculated as follows: Annual difference, (eGFR at visit 2 − eGFR at visit 1)/(years elapsed between visits) or percentage annual change assuming linear decline on the log scale, [(eGFR at visit 2/eGFR at visit 1) ^ (1/years elapsed between visits) − 1] * 100. We primarily present results on the basis of quartiles of percentage annual change, which allowed us to conduct stratified analyses across categories of eGFR. In contrast, annual differences were quite different across eGFR categories (larger at higher eGFR). We also divided the participants into three groups according to cutoff points of annual difference in eGFR used in current guidelines, namely, an increase (>0 ml/min per 1.73 m2/yr), mild decrease (≥−5 or >−4 to 0, a reference group), or rapid decrease (<−5 or ≤−4).
Assessment of Covariates
The study participants provided comprehensive demographic, risk factor, and medical history information to a trained interviewer at each clinical examination. Data from visit 1, visit 2, and visit 4 were used in this study. Blood samples were obtained from all participants following standardized procedures.26 Medication use was determined by self-report and examination of medication bottles brought to the examination. Completed years of education (<12, 12 to 16, or >16), smoking status (current, ex-, or nonsmoker), and alcohol intake (current, ex-, or nondrinker) were self-reported. Systolic and diastolic BP were measured after 5 min of rest. Three seated measurements were taken by certified technicians using a random-zero sphygmomanometer. The average of the second and third readings was recorded. Diabetes was defined as a fasting glucose of ≥126 mg/dl, nonfasting glucose of ≥200 mg/dl, self-reported physician diagnosis of diabetes, or use of oral hypoglycemic medication or insulin. Plasma cholesterol, triglycerides, and HDL cholesterol were determined using enzymatic methods, and LDL cholesterol was calculated using the Friedewald equation.22 Left ventricular hypertrophy by electrocardiogram was defined by the Cornell voltage: S amplitude in V3 + R amplitude in aVL,27 >2.8 mV for male and >2.2 mV for female. Evidence of atherosclerosis of the common carotid arteries (shadowing or plaque on either side or none) was determined by B-mode ultrasound.7,21
Outcome Assessment
ARIC investigators conduct continuous, comprehensive surveillance for all CVD-related events including hospitalizations, procedures, and deaths in the four communities. All potential coronary events are adjudicated by the ARIC Morbidity and Mortality Classification Committee using published criteria.28 In this study, we defined a CHD event as a definite or probable myocardial infarction, definite CHD death, or coronary revascularization procedure. We focused specifically on de novo CHD events among participants without prevalent CHD at visit 2.
Statistical Analysis
Continuous and categorical variables were compared across quartiles of percentage annual change in eGFR using ANOVA and χ2 tests, as appropriate. Cox proportional hazards models were used to estimate the HRs of CHD and all-cause mortality associated with the quartiles of change in eGFR. We used Q3 (which included stable eGFR) as the reference group under the assumption that Q4 includes individuals with an increase of eGFR as a result of decreased serum creatinine that may have been the result of ill health. We repeated this analysis after stratifying participants into clinical categories of eGFR: 30 to 59, 60 to 89, and ≥90. The small number of participants with eGFR <30 (n = 3) were excluded from the stratified analyses. In separate Cox proportional hazards models, we adjusted for eGFR and traditional risk factors for CHD at visit 1 or 2. We tested for an interaction between baseline eGFR level and change in eGFR from visit 1 to visit 2 by incorporating their product terms in the models.
It is possible that adjustment for baseline (visit 1) eGFR may result in bias because of regression to the mean.10,18 In contrast, adjustment for follow-up (visit 2) eGFR may also be problematic. This adjustment may disregard the notion that individuals with a rapid decline in eGFR in the past may have lower levels of eGFR at visit 2 than individuals without a rapid decrease, the so-called “horse-racing effect.”18 Thus, we also repeated our analyses of percentage annual change in eGFR from visit 1 to visit 2 without adjustment for eGFR levels at either visit. The follow-up time was defined as the period to the first CHD event, death, or loss to follow-up. Individuals who were free of these outcomes by January 1, 2006, were subject to administrative censoring. All analyses were conducted using Stata 10.0 software (Stata Corp, College Station, TX) and P < 0.05 was considered statistically significant.
Sensitivity Analysis of 9-Yr Change in eGFR and CHD and Mortality Risk
The primary analysis focuses on intermediate-term (3-yr) change in eGFR; however, information on kidney function was also collected at visit 4 (1996 through 1998), allowing us additionally to examine the association of long-term (9-yr) change in eGFR (visit 1 to visit 4) with CHD risk and all-cause mortality among the 10,068 participants of the final study population (n = 13,029), who were free of coronary events by visit 4. This analysis also allowed us to incorporate albuminuria (measured only at visit 4) into our models. Albuminuria is a known risk factor for both rapid progression of CKD and future cardiac events.29,30 In ARIC Study participants, urine albumin and creatinine concentrations were measured from a spot urine sample at visit 4. We calculated the ratio of urine albumin to urine creatinine (ACR; in mg/g) and categorized individuals as follows: Normal albuminuria ACR <30 mg/g, microalbuminuria 30 to 299 mg/g, and macroalbuminuria ACR ≥300 mg/g.31 In our analyses of 9-yr change in eGFR, we adjusted for covariates measured at visit 1 and visit 4 similarly to the 3-yr change analyses.
Disclosures
None.
Supplementary Material
Acknowledgments
The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. K.M. was supported by the Japan Society for the Promotion of Science, Japan; E.S. was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grant K01DK076595; L.D.B. was supported by National Institutes of Health/National Heart, Lung, and Blood Institute grant T32HL07024; N.F. was supported by AHA0675001N grant; and B.C.A. and J.C. were supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK076770.
We thank the staff and participants of the ARIC Study for important contributions.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 4.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 5.National Institute for Health and Clinical Excellence: Chronic Kidney Disease: Early Identification and Management of Chronic Kidney Disease in Adults in Primary and Secondary Care. Available at: http://www.nice.org.uk/nicemedia/pdf/CG073NICEGuideline.pdf Accessed July 27, 2009
- 6.Astor BC, Coresh J, Heiss G, Pettitt D, Sarnak MJ: Kidney function and anemia as risk factors for coronary heart disease and mortality: The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 151: 492–500, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, Chambless LE, Coresh J: Reduced kidney function as a risk factor for incident heart failure: The atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol 18: 1307–1315, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, Coresh J, Levey AS, Sarnak MJ: Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol 41: 47–55, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Cheng TY, Wen SF, Astor BC, Tao XG, Samet JM, Wen CP: Mortality risks for all causes and cardiovascular diseases and reduced GFR in a middle-aged working population in Taiwan. Am J Kidney Dis 52: 1051–1060, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB, Sarnak MJ: Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 168: 2212–2218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 110: 921–927, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Flack JM, Neaton JD, Daniels B, Esunge P: Ethnicity and renal disease: Lessons from the Multiple Risk Factor Intervention Trial and the Treatment of Mild Hypertension Study. Am J Kidney Dis 21: 31–40, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Yuyun MF, Dinneen SF, Edwards OM, Wood E, Wareham NJ: Absolute level and rate of change of albuminuria over 1 year independently predict mortality and cardiovascular events in patients with diabetic nephropathy. Diabet Med 20: 277–282, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG: Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease. Ann Intern Med 141: 929–937, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Neugarten J, Acharya A, Silbiger SR: Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J Am Soc Nephrol 11: 319–329, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Jafar TH, Schmid CH, Stark PC, Toto R, Remuzzi G, Ruggenenti P, Marcantoni C, Becker G, Shahinfar S, de Jong PE, de Zeeuw D, Kamper A-L, Strangaard S, Levey AS: The rate of progression of renal disease may not be slower in women compared with men: A patient-level meta-analysis. Nephrol Dial Transplant 18: 2047–2053, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM: When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol 162: 267–278, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Schiffrin EL, Lipman ML, Mann JF: Chronic kidney disease: Effects on the cardiovascular system. Circulation 116: 85–97, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH: Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: Results of a 4-year follow-up study. J Am Soc Nephrol 16: 1404–1412, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Atherosclerosis Risk in Communities (ARIC) Study: Design and objectives. The ARIC investigators. Am J Epidemiol 129: 687–702, 1989 [PubMed] [Google Scholar]
- 22.Operations Manual 7: Blood Collection and Processing, Atherosclerosis Risk in Communities (ARIC) Study, Bethesda, National Heart, Lung, and Blood Institute, 1987 [Google Scholar]
- 23.Eckfeldt JH, Chambless LE, Shen YL: Short-term, within-person variability in clinical chemistry test results: Experience from the Atherosclerosis Risk in Communities Study. Arch Pathol Lab Med 118: 496–500, 1994 [PubMed] [Google Scholar]
- 24.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS: Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis 39: 920–929, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Papp AC, Hatzakis H, Bracey A, Wu KK: ARIC hemostasis study: I. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thromb Haemost 61: 15–19, 1989 [PubMed] [Google Scholar]
- 27.Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P: Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: Validation with autopsy findings. Circulation 75: 565–572, 1987 [DOI] [PubMed] [Google Scholar]
- 28.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA: Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: Methods and initial two years' experience. J Clin Epidemiol 49: 223–233, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Astor BC, Hallan SI, Miller ER, 3rd, Yeung E, Coresh J: Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol 167: 1226–1234, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Iseki K, Ikemiya Y, Iseki C, Takishita S: Proteinuria and the risk of developing end-stage renal disease. Kidney Int 63: 1468–1474, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Eknoyan G, Hostetter T, Bakris GL, Hebert L, Levey AS, Parving HH, Steffes MW, Toto R: Proteinuria and other markers of chronic kidney disease: A position statement of the National Kidney Foundation (NKF) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Am J Kidney Dis 42: 617–622, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.