Abstract
Current techniques to morphologically characterize the processes of nephrogenesis and ureteric branching during kidney development have many limitations. Here, we used in vivo three-dimensional analysis to study renal development in mice lacking fibroblast growth factor receptor 2 in the ureteric bud (Fgfr2UB−/−) and in littermate controls. We found that Fgfr2UB−/− mice have more severe defects in ureteric branching morphogenesis than previously reported, including significantly fewer branches and tips than control mice. Furthermore, these mice had decreased ureteric volume and surface area and longer ureteric segments than control mice. We also observed previously unrecognized abnormalities in nephrogenesis, including a gradual increase in volume and surface area during maturation from renal vesicles to mature nephrons, in the mutant mice. Finally, we quantified many events of normal renal development that are either difficult or impossible to measure without this three-dimensional technique. In summary, the three-dimensional approach is a powerful and quantitative means to characterize branching morphogenesis and nephrogenesis.
Development of the permanent kidney (metanephros) occurs via reciprocal signaling between metanephric mesenchyme and the ureteric bud.1,2 The mesenchyme stimulates outgrowth of the bud from the Wolffian duct. After the bud invades the mesenchyme, it undergoes mostly dichotomous branching at the ampullary tips. In turn, ureteric tips stimulate mesenchymal tissue to differentiate into nephrons, the functional units of the kidney. The ureteric epithelium ends as a complex three-dimensional (3D) structure draining thousands (rodent) to millions (humans) of nephrons in each kidney through single ureters.3 Insufficient or inefficient ureteric branching results in an underendowment of nephrons, predisposing to kidney disease.4,5
Characterizing ureteric branching in 3D kidneys has been challenging. It is largely undertaken in cultured kidney explants that flatten, allowing for visualization of branching by whole-mount immunofluorescent staining or transgenic green fluorescence protein (GFP) expression. Branching is quantitated by counting ureteric tips or branch points in whole-mount specimens. Recently, investigators have used confocal microscopy to quantify ureteric branch lengths in cultured kidney explants.6 Despite the elegant studies, these approaches are limited in that explants are removed from their physiologic environment and fail to grow in three dimensions. There is also significant variability due to operator skill, culture conditions (media, incubator conditions), and in precise ages of the explants.
Recent reports have focused on ureteric branching in kidneys that developed in vivo. One study estimated branch lengths and tips in fluorescently labeled serial ultrathick murine cryosections.7 Despite the elegant study, the technique was limited by the need for special stains and potential sampling errors caused by the thick sections. Others have generated 3D images of fluorescently labeled ureteric trees using confocal microscopy without quantitating branching.7,8 Finally, the EuReGene database contains elegant 3D images of kidneys that can be linked to segment specific gene expression (see http://www.euregene.org/atlas/pages/section_viewer.html?project=euregene_atlas&stack=fullResKidney_substack); however, there is not an ability to measure ureteric branching. Thus, there are no published data to quantitatively describe 3D ureteric branching morphogenesis.
Concurrent with ureteric branching is nephron differentiation/maturation (nephrogenesis). Initially, nephrogenic mesenchyme condenses around ureteric tips and converts into epithelial vesicles (the most immature “nephrons”). Vesicles differentiate into comma- and then S-shaped bodies that join with the collecting ducts before maturing into functional nephrons (glomeruli and tubules).9–12 Experimentally, assessment of nephron formation in real kidneys has mostly been qualitative. Quantitative assessment has only been achieved in vitro utilizing immunohistochemical staining in cultured kidney explants. Thus relative distribution, 3D structure, and size of developing nephrons (vesicles through mature nephrons) have not been determined to our knowledge.
Given limitations of the aforementioned techniques, we developed a new 3D procedure to quantify branching morphogenesis and nephrogenesis of developing murine kidneys in vivo. We hypothesized that the technique would reveal new insights about renal defects in mice with deletion of fibroblast growth factor receptor 2 in the ureteric bud (Fgfr2UB−/−),13 and reveal new data about renal development in controls.
Results
Generating 3D Images
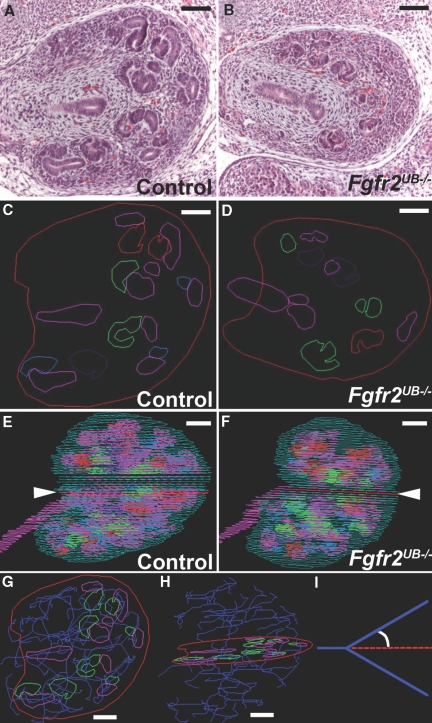
As shown (Figure 1), ureteric and nephron epithelium were traced from hematoxylin and eosin (H&E) images (A, B) and color-coded (C, D). Individual layers were projected into a stack to ensure no gross misalignments (Figure 1, E and F). Once layers were aligned and 3D images were constructed (see below), ureteric epithelium was skeletonized through each layer (Figure 1, G and H, Supplemental Movie 1). Skeletonized tree measurements included planar angles, defined as angles between the extrapolated path of reference segments and the paths of subsequent branches (Figure 1I).
Figure 1.
Representative tracing and skeletonizations in E13.5 kidneys (A and B) H&E stained sections of (A) a control and (B) a Fgfr2UB−/− kidney. (C and D) Each epithelial structure from the H&E sections was then traced in different colors, generating image layers. (E and F) Layers were aligned so that a 3D kidney could be reconstructed, with the red lines (arrowheads) representing the traced layers of (C) the control and (D) the mutant. (G and H) Representative still images (different views) demonstrating skeletonization (blue) of ureteric epithelium (pink) in a control kidney. (I) Graphic representation of a planar branch or tip angle, which is calculated from the skeletonized tree. Green, kidney capsule; pink, ureteric epithelium; dark blue, skeletonized ureteric tree; light blue, renal vesicle, red, comma-shaped body; purple, S-shaped body; green, mature glomeruli. Scale bar = 100 μm.
Macroscopic Anatomy of Mutants and Control Kidneys
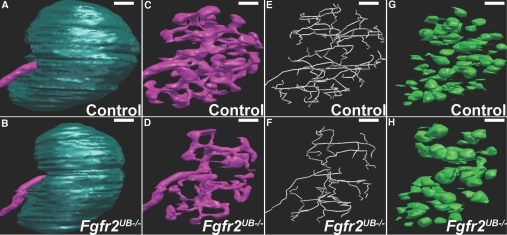
As shown (Figure 2, Supplemental Movies 2 and 3), 3D images were generated for mutants and controls. With intact capsules (Figure 2, A and B), E13.5 mutant kidneys appear smaller than controls. Without the capsule, the intact 3D branching ureteric tree, skeletonized tree, and developing nephrons are visible (Figure 2, C through H). Mutant ureteric epithelium has subjective abnormalities compared with controls, including less tissue, longer segments, and fewer tips (Figure 2, C through F). Fgfr2UB−/− embryos also appear to have fewer developing nephrons compared with controls, although some mutant nephrons appear larger than in controls (Figure 2, G and H).
Figure 2.
Representative 3D images of control and Fgfr2UB−/− kidneys reveal phenotypic abnormalities. (A and B) Compared with (A) the control, the mutant E13.5 kidney appears small. (C and D) When the capsule is subtracted, (D) the mutant ureteric epithelium appears to occupy less relative volume and to have fewer but longer ureteric segments than (C) a littermate control. (E and F) Skeletonized images (from C and D) appear to confirm the reduction in ureteric segment number and increase in individual segment lengths of (F) mutants versus (E) controls (E). (H) Fgfr2UB−/− nephrons appear to be fewer in number, but larger than (G) controls. Scale bar = 100 μm.
We next measured kidney volume and surface areas. The mean volume of Fgfr2UB−/− kidneys was significantly less than in control littermates (mutant 39.54 ± 5.51 × 106 μm3, control 61.85 ± 5.08 × 106 μm3, P < 0.05). Mean kidney surface areas were smaller in mutants than controls, but this difference did not reach statistical significance (mutant 5.31 ± 0.93 × 105 μm2, control 6.57 ± 0.53 × 105 μm2, P = 0.058). Thus E13.5 Fgfr2UB−/− kidneys were smaller than controls.
Ureteric Structures
Fgfr2UB−/− Kidneys Have Less Ureteric Tissue and Fewer Ureteric Branches and Tips.
We measured ureteric volume of Fgfr2UB−/− and control kidneys. Compared with controls, mutants had less mean total ureteric volume (Fgfr2UB−/− 3.02 ± 0.56 × 106 μm3, controls 6.97 ± 0.70 × 106 μm3, P < 0.01). Furthermore, mean relative volume of ureteric epithelium was reduced in Fgfr2UB−/− kidneys, comprising only 7.60 ± 0.39% of mutant kidneys versus 11.29 ± 0.84% in controls (P < 0.001), consistent with what was subjectively apparent in cultured explants.13
To quantify branching defects in Fgfr2UB−/− mice, we analyzed skeletonized 3D ureteric images (Figure 2, E and F). Mean lengths of the total ureteric tree, its branches (ureteric segments between 2 branch points), and its tips (terminal ureteric segments) were greatly decreased in mutants compared with controls (Table 1). Also, mean numbers of mutant branches and tips were reduced relative to controls (branches, mutant 23.0 ± 6.53, controls 55.3 ± 4.0, P < 0.001; tips, mutant 25.25 ± 6.99, controls 61.50 ± 3.11, P < 0.0001). Although differences were also observed between mutant and control tip numbers in cultured explants, the magnitude was much greater in the 3D analysis because of significantly higher tip numbers in controls.13
Table 1.
Ureteric segment length and planar anglesa
| Measurement | Branch Lengths | Tip Lengths | Combined Lengths | Branch Angles | Tip Angles | Combined Angles |
|---|---|---|---|---|---|---|
| Control mean | 80.62 (4.1) | 108.75 (6.9) | 95.45 (3.7) | 60.86 (2.9) | 87.96 (2.4) | 75.10 (1.6) |
| Mutant mean | 105.26 (5.8) | 136.37 (8.9) | 121.39 (5.2) | 65.13 (3.9) | 90.59 (3.4) | 78.28 (2.5) |
| Difference between control and mutant | 24.6 (7.1) | 27.6 (11.3) | 25.9 (6.5) | 4.26 (4.9) | 2.6 (4.1) | 3.18 (2.9) |
| P value | 0.014 | 0.050 | 0.007 | 0.417 | 0.550 | 0.316 |
aNumbers in parentheses are standard errors. Lengths and angles are measured in micrometers and degrees, respectively.
Fgfr2UB−/− Kidneys Have Increased Length of Ureteric Segments.
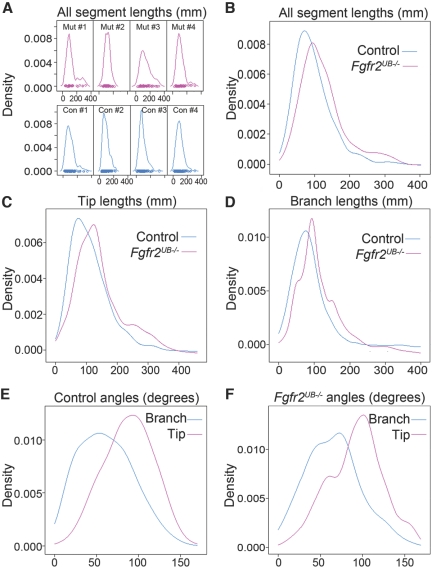
Although total ureteric segment lengths are reduced in Fgfr2UB−/− kidneys versus controls, individual segments appear larger in mutants. To quantify this observation, we determined the distribution of mutant and control ureteric segment lengths using nonparametric techniques (Figure 3). As shown, the distribution of all ureteric segments (branch and tip) in individual mutants appears shifted to the right compared with controls (Figure 3A). This was also evident when density estimates from mutant and control individuals were averaged and plotted together (Figure 3B). Grouped density plots also suggest that tip and branch lengths in mutants are each shifted to the right (Figure 3, C and D), although tip lengths appear more divergent than branch lengths. Median mutant branch lengths were 25% larger than controls (95.9 versus 76.7 μm), and mutant median tip lengths were 28% greater than controls (125.9 versus 98.0 μm). Using a mixed model approach, we detected statistically significant differences in the expected values of branch, tip, and combined ureteric segment lengths between mutant and controls (Table 1). Thus, we quantified differences in ureteric segment lengths in 3D kidneys, reflecting what was only subjectively apparent in cultured explants.13
Figure 3.
Fgfr2UB−/− ureteric segments are relatively larger as a group than controls and shifted to the right, and planar branch angles differ from tip angles irrespective of genotype. (A) Density plots of total ureteric segments for each of the mutants (pink) and controls (blue) show that the Fgfr2UB−/− peaks tend to be shifted to the right. (B) Grouped density estimates (from A) confirm that ureteric segment lengths for mutant peak to the right of the controls. (C and D) Grouped density plots of (C) ureteric tips and (D) branches demonstrate similar trends, although the tip lengths appear more divergent than branch lengths. (E and F) Density plots of planar angles demonstrate that tip angles appear larger as a group than branch angles.
To determine reproducibility and precision of ureteric segment length and number measurements, we performed a second round of skeletonizations on mutants and controls. Variability ranged from 1% to 7% in controls and from 1% to 14% in mutants (Supplemental Tables 1 through 3). Statistical comparisons of segment lengths and numbers between mutants and controls were the same between the two separate runs (not shown). Thus ureteric length and number measurements were reproducible and precise.
Planar Angles Change as Ureteric Tips become Branches.
Unlike ureteric segment lengths and numbers, there were no differences between mutant and control planar angles (branch, tip, and combined ureteric segments; Table 1). However, density plots show that planar tip angles are shifted to the right compared with planar branch angles for controls and mutants (Figure 3 E and F). Using mixed models, we estimated the expected value of control tip angles were on average 88.0° ± 1.8° compared with 60.7° ± 2.1° for branch angles (P < 0.0001). Similarly, the Fgfr2UB−/− tip angle mean was 90.3° ± 3.0° compared with mean branch angles of 65.0° ± 3.4° (P < 0.0001). Thus, as ureteric tips mature into branches, planar angles tend to decrease as a group, irrespective of genotype.
Nephron Structures
Fgfr2UB−/− Kidneys Have Reduced Number of Nephron Structures That Are Increased in Size.
Consistent with what was qualitatively apparent, mutant kidneys had 46% fewer nephrons than controls (33.0 ± 12.4 versus 61.0 ± 6.4, P < 0.01); as in the branching analysis, differences in the 3D analysis were markedly greater than in cultured explants13 because of a much higher number of nephrons in controls. Despite having nearly half of the number of nephrons, mutant total nephron volume and surface area were not statistically different than in controls (Table 2). Even more striking, relative volume of nephron structures was not different with 9.10% ± 0.20% of control kidneys consisting of nephrons versus 9.30% ± 1.07% in mutant kidneys. Taken together, individual nephron dimensions were greater in Fgfr2UB−/− mutant kidneys than controls by volume (13.57 ± 2.02 × 104 μm3 versus 9.31 ± 1.29 × 104 μm3, P < 0.01) and surface area (13.01 ± 1.55 × 103 μm2 versus 9.88 ± 1.79 × 103 μm2, P < 0.05). Thus the 3D analysis revealed marked differences in nephron number between mutants and controls and larger mean nephron size in mutants. Observations of increased nephron size were also not apparent in the earlier Fgfr2UB−/− mouse study.13
Table 2.
Nephron measurements in Fgfr2UB−/− and controls
| Measurement | Control | Fgfr2UB−/− |
|---|---|---|
| Total nephron volume (×106 μm3) | 5.63 ± 0.54 | 3.71 ± 0.95 |
| Total nephron surface area (×105 μm2) | 5.95 ± 0.71 | 4.22 ± 1.55 |
| Relative nephron volume (%) | 9.10 ± 0.20 | 9.30 ± 1.07 |
| Nephron number | 61.0 ± 6.4 | 33.0 ± 12.4b |
| Mean nephron volume (×104 μm3) | 9.31 ± 1.29 | 13.57 ± 2.02b |
| Mean nephron surface area (×103 μm2) | 9.88 ± 1.79 | 13.01 ± 1.55a |
aP < 0.05.
bP < 0.01.
Analyses of Embryonic Nephrons at Different Developmental Stages.
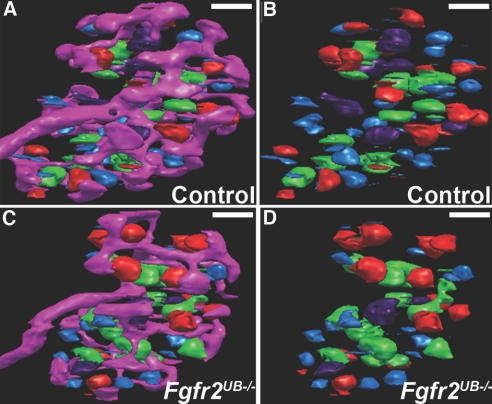
To examine distribution, shapes, and sizes of different developing nephron structures, we re-color-coded each developing nephron by developmental stage (Figure 4, Supplemental Movies 4 and 5). Although total numbers of nephrons were lower in Fgfr2UB−/− kidneys, relative distribution of different nephron types was similar between mutants and controls. At E13.5, most nephrons were vesicles (Fgfr2UB−/− 41% ± 5%, controls 46% ± 1%). Progressively fewer were comma-shaped bodies (Fgfr2UB−/− 18% ± 7%, controls 22% ± 6%) and S-shaped bodies (Fgfr2UB−/− 15% ± 9%, controls 12% ± 2%). The number of nephrons in final mature stages rebounded (Fgfr2UB−/− 26% ± 3%, controls 21% ± 5%). To our knowledge, these data have never been described in embryonic mouse kidneys.
Figure 4.
Representative 3D images of different nephron stages in E13.5 Fgfr2UB−/− and control kidneys. (A and B) Images of the developing nephrons with (A) the ureteric tree and (B) alone in controls. (C and D) Similar views in an Fgfr2UB−/− kidney with some mature glomeruli (green) appearing larger than controls. Pink, ureteric epithelium; light blue, renal vesicle; red, comma-shaped body; purple, S-shaped body; green, mature glomeruli. Scale bar = 100μ m.
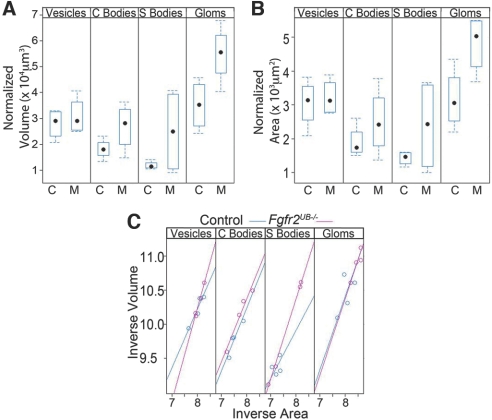
We then examined whether differences in nephron size between mutants and controls were present at all stages of nephron development. As shown (Figure 5A), vesicle volume was similar in mutants and controls. In subsequent stages, there were clear trends showing increases in nephron volume in mutants compared with controls, reaching statistical significance at the mature glomerular stage (Figure 5A). Similarly, relative surface areas of mutant and control vesicles were the same but trended up with subsequent stages in mutants until reaching statistical significance in mature glomeruli (Figure 5B). To determine whether there were any differences in shapes of maturing nephrons, we evaluated the allometric relation between volume and surface areas using a reduced major axis techniques (Figure 5C); as shown, the mutation enlarges or magnifies structures without altering them otherwise. Thus, 3D imaging reveals that developing nephrons in E13.5 Fgfr2UB−/− mice appear normal as vesicles but progressively hypertrophy as they differentiate. Such data have not been described in any system to our knowledge and were not ascertained in our prior study.13
Figure 5.
Fgfr2UB−/− nephrons become progressively larger than controls with maturity. (A) Box plots with median (dot), 25th percentile (lower box limit), and 75% percentile (upper box limit) as well as interquartile ranges (error bars) show that the volume of the developing mutant nephrons increase relative to controls, reaching significance in mature glomeruli. (B) Likewise, the surface area of Fgfr2UB−/− nephrons increases relative to controls as they differentiate, reaching significance in mature glomeruli. (C) Regression analysis does not reveal a change in the nephron shape between mutants and controls.
Discussion
The study presented here describes a method for rendering precise and reproducible 3D quantitative analysis of developing kidneys. As shown in E13.5 Fgfr2UB−/− and control mice, this method allows for quantification of kidney sizes, ureteric branching, and nephron development that are often underestimated or difficult to ascertain using other approaches.
Ureteric Branching
The 3D imaging technique represents a significant advancement in quantitative assessment of branching morphogenesis. Presently, measuring branching morphogenesis is mostly confined to cultured kidney explants. In our previous evaluation, E11.5 explants grown for 3 d (equivalent to E14.5) had an average of 42.3 ureteric tips per control and 24.6 in Fgfr2UB−/− mutants.13 (The number of control ureteric tips is consistent with other cultured explant studies.14–16) In contrast, 3D assessment revealed much higher ureteric tip numbers in E13.5 kidneys, with an average of 61.5 tips per control and 25.3 tips per mutant. Furthermore, utilizing cultured explants, Fgfr2UB−/− kidneys had a 42% decrease in numbers of tips compared with controls, whereas with the 3D technique the decrease was 59%. Thus the 3D approach reveals more accurate numbers of ureteric tips than cultured explants and increases the sensitivity of detecting differences between control and mutant kidneys. This would likely be true for other organs with branching epithelia that are often assessed as cultured explants, such as lung, prostate, salivary gland, and mammary gland.
The 3D imaging approach also allows for measurements of branching that are technically difficult in cultured explants. First the 3D E13.5 Fgfr2UB−/− kidneys had significantly less total and relative ureteric tissue than controls (ureteric tissue occupied 7.6% of the kidney volume in mutants versus 11.3% in controls). Although these observations were qualitatively apparent in cultured explants and whole kidneys, we were previously unable to quantify these findings.13 Second, the 3D method revealed that ureteric segment lengths were shifted to the right in Fgfr2UB−/− E13.5 kidneys compared with controls. Although others have characterized ureteric segment length distributions in cultured explants,6 these methods are technically challenging because of the need for labeling tissues with markers such as GFP for confocal microscopy and for complicated algorithms after generating images. These latter approaches also do not distinguish between ureteric tips and branches and are likely subject to error for the reasons described earlier (altered growth patterns, etc. in cultured explants). Finally, the 3D approach revealed that ureteric tips from mutant and control kidneys have planar angles approximating 90° (similar to the “T” shape of the very first ureteric branching event). This angle then reduces to approximately 60° degrees (producing a “Y” shape), likely maximally increasing the volume of ureteric tissue/surface area of the kidney.17 The change in planar angles with maturity from tip to branch would likely be relevant in describing other mutants with branching anomalies.
Other advantages with the 3D system for branching analysis are its ease of use, precision, and reproducibility. The MBF system works with simple H&E staining, avoiding the need for fluorescence antibody or transgenic GFP labeling to generate 3D images as in some other systems.8 The ability to render 3D images from serial sections avoids potential sampling errors from ultrathick sections.7 This system is not limited by organ size, as was the case in an elegant study in which intact embryonic lungs were whole-mount immunostained and then airway epithelial branching morphogenesis was quantified.18 Although the present system could potentially underestimate absolute organ volumes due to shrinkage from paraffin processing, relative volumes are likely preserved; for example, the relative ureteric volume of our controls is remarkably similar to what was found in equivalent rat kidneys processed into glycolmethacrylate, which tends to not shrink.19 Paraffin is also a versatile and widely utilized embedding medium including uses for measurements such as corticomedullary ratios.20 The 3D technique is also precise with variability between the two skeletonizations, only ranging from 1% to 7% in controls and from 1% to 14% in the mutants. (The latter rates were likely higher due in part to the smaller ureteric tree amplifying variability.)
Nephrogenesis
Some compelling observations generated by the 3D examination of E13.5 kidneys were regarding nephrogenesis. Counting nephrons in utero is often fraught with experimenter bias and technical difficulties including need for special labeling.21 Although whole mount staining in cultured explants with antibodies and lectins are more easily accomplished than in whole kidneys,22,23 these data are limited by the same problems with cultured explants outlined earlier. Another easy and common method for determining nephron number has been to use birth weight as a correlate; however, this provides only a crude estimate and recent studies have shown that some insults such as intrauterine glucocorticoid exposure lead to dramatic reductions in nephron number without affecting body weight.24 In this study, we identified and quantitated nephron number and sizes at all stages using simple morphologic stains. (Although identification of nephrons at different developmental stages relies on subjective judgments of the experimenter, the same person did the counts and has extensive training in recognizing the structures.) In so doing, we observed that at E13.5, most nephrons were either immature vesicles or mature glomeruli, irrespective of genotype; one interpretation is that once passing through the vesicle stage, nephrons rapidly differentiate into mature forms. The ability to determine absolute distribution of nephrons at different stages may prove important as in examples of mice with constitutive deletion of Wnt4 or mice with conditional deletion of Fgf8 from mesenchyme, both of which form early nephrons that fail to mature.25–27 Moreover, these techniques may reveal subtle differences in distribution of nephron types in mice with less severe phenotypes (e.g., as might be the case of heterozygotes for Wnt4 of Fgf8).
Other key observations about nephrogenesis were made in comparing 3D control and Fgfr2UB−/− mice. First, as with ureteric branching, the total number of nephrons in E11.5 cultured explants grown for 3 d (equivalent to E14.5) was dampened with an average 28.6 in controls and 22.3 in Fgfr2UB−/−13; 3D E13.5 controls had an average of 61 nephrons versus mutants with 33. Thus the 3D technique detected not only much higher numbers of nephrons but also a 46% decrease in mutant nephron number versus a 22% decrease in cultured explants, dramatically increasing sensitivity of the measurements. Second, the 3D system revealed that despite decreases in nephron number, relative nephron volumes in E13.5 Fgfr2UB−/− were unchanged with 9.3% of mutant kidney volume consisting of nephrons versus 9.1% in controls. Thus, we made the novel observation that embryonic Fgfr2UB−/− mice had larger nephrons than controls. By identifying nephrons at different stages of maturity, we determined that mutant vesicles are the same size as controls but gradually increase in volume and surface area, reaching statistical differences as mature glomeruli. Furthermore, given that nephron shapes were not different, it appears that augmented glomerular size (hypertrophy) in Fgfr2UB−/− mutants occurs before urine generation (which occurs at about E15.5 in mice). It is unclear exactly why Fgfr2UB−/− nephrons increase in size as they mature however, with greater distances between inducing ureteric tips (and therefore nephrons), there may be a decrease in a zone of growth inhibition. These novel observations made with this new system may have implications regarding fetal reprogramming in patients with intrauterine insults, allowing one to determine whether glomerular hypertrophy observed in these patients (or animal models) in utero and/or postnatally.5,28–30
There are other potential applications of this 3D imaging system. Embryonic sections could be stained with markers such as Foxd1 to quantify stromal mesenchyme in whole kidneys. Older embryonic/postnatal sections could be stained for specific tubular markers to quantify perturbations of proximal-distal axis patterning in nephrons. 3D images could be rendered from human fetal magnetic resonance imaging or other imaging modalities, which would affect prognosis and therapy for patients (before and/or after birth).
Concise Methods
Animals
Hoxb7creEGFPTg/+Fgfr2Lox/+ mice were bred with Fgfr2Lox/Lox mice to produce Hoxb7creEGFPTg/+Fgfr2Lox/Lox mice with conditional deletion of Fgfr2 within the ureteric epithelium (Fgfr2UB−/−) and littermate controls as described previously.13 Pregnant female mice were sacrificed at E13.5, the embryos were removed, and tissue was taken for genotyping.13 Four mutants and four controls from three separate litters were utilized. All experiments were carried out with the approval of the local Institutional Animal Care and Use Committee.
Processing and Sectioning of Tissue
Embryos were immediately placed into 4% paraformaldehyde in PBS. After overnight fixation the embryos were placed into 70% ethanol, processed into paraffin, and embedded rump down. Embryos were then serially sectioned at 4 μm through the transverse plane from the bladder all of the way through the kidneys. Slides were then stained with H&E.
3D Rendering
Generation of Individual Image Layers.
An upright Olympus microscope was utilized with a motorized stage directly linked to an image analysis computer that was configured with image tracing software (Stereoinvestigator, Microbrightfield). Using the Stereoinvestigator program, a reference point was selected in the center of the kidney. Slides were visualized at 100× and structures within the kidneys were traced, generating an individual layer from each section (as represented in Figure 1, A through F). The first section traced contained the most inferior/caudal region of the kidney and was designated as 0 μm. Every alternate section was then traced through the entire kidney under the microscope after alignment with previous sections. Within each section, different colors were used to trace individual structures including capsule, ureteric epithelium, renal vesicles, comma-shaped bodies, S-shaped bodies, and mature glomeruli (Figure 1, A through D). The total time to assemble a complete set of layers for one kidney is approximately 4 h (approximately 60 layers for controls and 50 for mutants).
Reconstructing the 3D Image.
Once all of the images were traced, they were projected into a crude 3D stack to ensure that there were no gross misalignments (Figure 1, E through F). A solid 3D rendering was generated, which could be manipulated to show any of the traced structures in three dimensions (either separately or simultaneously). Rarely, an immature nephron was misidentified in a specific layer as ureteric epithelium. This was very apparent when visualizing the 3D images. In those cases, the misidentified structure was correctly re-color-coded on the individual layer. Ultimately, movies and still images of the different 3D kidney structures were generated. From these images, the number of nephrons was counted (total and at each individual stage of development).
3D Contour Analysis.
Neurolucida Explorer (MBF) was used to analyze the 3D contours of each structure within the developing kidneys. Specifically, volume and surface area were calculated for the entire kidney, ureteric epithelium, and developing nephrons (in aggregate and in the individual stages of development including vesicles, comma-shaped bodies, S-shaped bodies, and mature glomeruli). From this the relative volume (volume density) of ureteric epithelium (ureteric volume/kidney volume) was determined. In addition, relative volume, surface area, and a shape index (inverse of volume versus inverse of surface area) were calculated for the developing nephrons. At the mature nephron stage, glomerular volume was measured (and not tubular portions of the nephron).
Skeletonizing the Ureteric Epithelium.
Using Neurolucida, the ureteric epithelium was subsequently skeletonized manually through each individual layer (as demonstrated in Figure 1, G through I and Supplemental Movie 1). First the layer containing the most distal portion of the main ureter was located and the lumen was marked. Adjacent layers containing the main ureter were identified, marked, and linked to one another, generating a skeleton of the ureter. This process continued into the kidney throughout the ureteric tree. At each branch point, the ureteric lumen was marked as a “node” and a new ureteric segment (or segments) was then generated until all ureteric tissues were skeletonized. Throughout the process, segments and nodes identified in each layer were repeatedly compared to confirm that the skeleton accurately represented the ureteric epithelial tree (Figure 1, G and H). Once the skeletonization was complete, the number of nodes (branch points) and tips were calculated. In addition, lengths of branches (ureteric segments between two nodes) and tips (ureteric segments from the last node to the outer cortex) were calculated. Also, planar branch angles (angle between the extrapolated path of the reference ureteric segment and the path of the subsequent branch; Figure 1I) for tips and branches were calculated. In calculating planar angles, the local information about the segments is disregarded and the segments are seen as lines that connect the end points (nodes and terminations). To ensure the precision of the skeletonizations, the assays were performed a second time for each ureteric tree, and segment lengths and counts were compared (the initial values were reported in this study). The total time to skeletonize an entire kidney was approximately 2 h.
Statistical Analysis
The distributions of lengths, angles, volumes, surface areas, and numbers of developing structures were graphically summarized with histograms and box and whisker plots. The total surface area and volume for each structure type was also divided by the number of the corresponding structures to generate a single structure (normalized) volume (or surface area). The relationships between genotype and normalized volume/area were tested with both univariate (ANOVA) and multivariate (multiple outcome—MANOVA) analysis of variance techniques. To examine the allometric relation between normalized volume and surface area in developing structures of mutants and controls, we applied standard major axis regression (also known as geometric mean regression31) to the logarithmically transformed (natural base) and standardized (zero mean, unit variance) measurements. Standard major axis regression is a (type II) regression method when the x and y axes are subject to measurement error and is one of the standard methods used to explore allometric relations that may be expressed as a power law.32
The histograms of branch lengths for each kidney were smoothed with nonparametric kernel density techniques (Parzen window) to generate an estimate of the distribution of lengths and angles within each individual animal. The Gaussian kernel was used as a basis for the nonparametric kernel density technique smoother and Silverman's rule was applied to calculate the optimal bandwidth for the kernel.33 Kernel density estimates from individual animals were subsequently averaged to generate the estimates for the wild-type and mutant phenotypes. Univariate responses (such as the total number of structures, the average normalized volume and surface area) in mutants and controls were compared with nonparametric techniques (two sample Wilcoxon rank sum).
For the analyses of average branch length and angle in controls and mutants, we utilized mixed effect models.34 The general methodology is used for measurements comprised of repeated subject-specific observations. These approaches are statistical methods used to study the relationship between the mean response (length or angle in this particular case) and explanatory variables (such as the genotype) while accounting for random effects due to interindividual variability. For all statistical tests we adopted a nominal level of significance (α) equal to 0.05. All analyses were performed in R for Windows version 2.8.1 (see database at http://www.R-project.org) using the libraries lmodel2 (SMA regressions), nlme (for mixed effect modeling) and lattice (for multipanel graph generation).
Disclosures
None.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. David Ornitz for the floxed Fgfr2 mice. This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, R01-DK070030 (C.M.B.). The author(s) have made the following declarations about their contributions: Conceived and designed the experiments: SSL, KM, JFB, CMB. Performed the experiments: SSL, KK. Analyzed the data: SSL, CA, RQ, CMB. Wrote the paper: SSL, CMB.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Saxen L, Sariola H: Early organogenesis of the kidney. Pediatr Nephrol 1: 385–392, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Dressler GR: The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Costantini F: Renal branching morphogenesis: Concepts, questions, and recent advances. Differentiation 74: 402–421, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Brenner BM, Garcia DL, Anderson S: Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1: 335–347, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Douglas-Denton RN, McNamara BJ, Hoy WE, Hughson MD, Bertram JF: Does nephron number matter in the development of kidney disease? Ethn Dis 16[2 Suppl 2]:S2-40–S2-45, 2006 [PubMed] [Google Scholar]
- 6.Cain JE, Nion T, Jeulin D, Bertram JF: Exogenous BMP-4 amplifies asymmetric ureteric branching in the developing mouse kidney in vitro. Kidney Int 67: 420–431, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Cebrian C, Borodo K, Charles N, Herzlinger DA: Morphometric index of the developing murine kidney. Dev Dyn 231: 601–608, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Clendenon JL, Byars JM, Hyink DP: Image processing software for 3D light microscopy. Nephron Exp Nephrol 103: e50–e54, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Clark A, Bertram J: Advances in renal development. Curr Opin Nephrol Hypertens 9: 247–251, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Clark AT, Bertram JF: Molecular regulation of nephron endowment. Am J Physiol 276: F485–F497, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Davies JA: Mesenchyme to epithelium transition during development of the mammalian kidney tubule. Acta Anat 156: 187–201, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Davies JA, Bard JB: The development of the kidney. Curr Top Dev Biol 39: 245–301, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM: Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol 276: 403–415, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yosypiv IV, El-Dahr SS: Role of the renin-angiotensin system in the development of the ureteric bud and renal collecting system. Pediatr Nephrol 20: 1219–1229, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Clarke JC, Patel SR, Raymond RM, Jr., Andrew S, Robinson BG, Dressler GR, Brophy PD: Regulation of c-Ret in the developing kidney is responsive to Pax2 gene dosage. Hum Mol Genet 15: 3420–3428, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Hartwig S, Bridgewater D, Di Giovanni V, Cain J, Mishina Y, Rosenblum ND: BMP receptor ALK3 controls collecting system development. J Am Soc Nephrol 19: 117–124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies JA: Branching Morphogenesis, Berlin, Springer, 2005 [Google Scholar]
- 18.Metzger RJ, Klein OD, Martin GR, Krasnow MA: The branching programme of mouse lung development. Nature 453: 745–750, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertram JF, Young RJ, Spencer K, Gordon I: Quantitative analysis of the developing rat kidney: Absolute and relative volumes and growth curves. Anat Rec 258: 128–135, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Cochrane AL, Kett MM, Samuel CS, Campanale NV, Anderson WP, Hume DA, Little MH, Bertram JF, Ricardo SD: Renal structural and functional repair in a mouse model of reversal of ureteral obstruction. J Am Soc Nephrol 16: 3623–3630, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Moritz KM, Wintour EM, Black MJ, Bertram JF, Caruana G: Factors influencing mammalian kidney development: Implications for health in adult life. Adv Anat Embryol Cell Biol 196: 1–78, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Gilbert T, Gaonach S, Moreau E, Merlet-Benichou C: Defect of nephrogenesis induced by gentamicin in rat metanephric organ culture. Lab Invest 70: 656–666, 1994 [PubMed] [Google Scholar]
- 23.Bates CM, Kharzai S, Erwin T, Rossant J, Parada LF: Role of N-myc in the developing mouse kidney. Dev Biol 222: 317–325, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Moritz K, Singh RR, Probyn ME, Denton KM: Developmental programming of a reduced nephron endowment: More than just a baby's birth weight. Am J Physiol Renal Physiol 296: F1–F9, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Stark K, Vainio S, Vassileva G, McMahon AP: Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372: 679–683, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Grieshammer U, Cebrian C, Ilagan R, Meyers E, Herzlinger D, Martin GR: FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development 132: 3847–3857, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M: Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 132: 3859–3871, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Hayslett JP: Functional adaptation to reduction in renal mass. Physiol Rev 59: 137–164, 1979 [DOI] [PubMed] [Google Scholar]
- 29.Johnson HA, Vera Roman JM: Compensatory renal enlargement. Hypertrophy versus hyperplasia. Am J Pathol 49: 1–13, 1966 [PMC free article] [PubMed] [Google Scholar]
- 30.Pisoni R, Aros C, Ruggenenti P, Remuzzi G: Mechanisms of progression of chronic renal disease. Saudi J Kidney Dis Transpl 13: 250–256, 2002 [PubMed] [Google Scholar]
- 31.Legendre P, Legendre L: Standard major axis regression. In: Numerical Ecology, Elsevier Science, Amsterdam, The Netherlands, 1998, pp 510–511 [Google Scholar]
- 32.Kerkhoff AJ, Enquist BJ: Multiplicative by nature: Why logarithmic transformation is necessary in allometry. J Theoret Biol 257: 519–521, 2009 [Google Scholar]
- 33.Wasserman L: Density estimation. In: All of Nonparametric Statistics Berlin, Springer, 2007, pp 125–144 [Google Scholar]
- 34.Pinheiro JC, Bates DM: Mixed-Effects Models in S and S-Plus, Springer, Heidelberg, 2000 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.