Abstract
Hypertension in patients with chronic kidney disease (CKD) strongly associates with cardiovascular events. Among patients with CKD, reducing the accumulation of uremic toxins may protect against the development of hypertension and progression of renal damage, but there are no established therapies to accomplish this. Here, overexpression of human kidney-specific organic anion transporter SLCO4C1 in rat kidney reduced hypertension, cardiomegaly, and inflammation in the setting of renal failure. In addition, SLCO4C1 overexpression decreased plasma levels of the uremic toxins guanidino succinate, asymmetric dimethylarginine, and the newly identified trans-aconitate. We found that xenobiotic responsive element core motifs regulate SLCO4C1 transcription, and various statins, which act as inducers of nuclear aryl hydrocarbon receptors, upregulate SLCO4C1 transcription. Pravastatin, which is cardioprotective, increased the clearance of asymmetric dimethylarginine and trans-aconitate in renal failure. These data suggest that drugs that upregulate SLCO4C1 may have therapeutic potential for patients with CKD.
All individuals with an estimated GFR (eGFR) <60 ml/min per 1.73 m2 are defined as having chronic kidney disease (CKD).1 The prevalence of CKD is now estimated at approximately 10% of the population and will progress to ESRD. In patients with CKD, the accumulation of uremic toxins causes difficulty in controlling BP, impairs renal function, and worsens prognosis.2,3 So far, more than 110 organic compounds have been identified as uremic toxins.4 Among these, guanidino compounds, including guanidino succinate (GSA) and asymmetric dimethylarginine (ADMA), are increased in patients with CKD and correlate with prognosis.3,5 In particular, ADMA, an inhibitor of nitric oxide synthase, is implicated in hypertension, renal damage, cardiac hypertrophy, and cardiovascular events.6,7 Currently, administration of the oral adsorbent AST-120 is the only therapy to remove uremic toxins in patients with CKD and diabetic nephropathy.8 Although AST-120 removes indoxyl sulfate, other compounds are not eliminated.9 Thus, a new approach that addresses this problem is urgently needed.
Recently, we isolated a human kidney-specific organic anion transporting polypeptide (OATP), termed SLCO4C1, and functionally characterized it as a digoxin transporter.10 The OATP family is involved in the membrane transport of bile acids, conjugated steroids, thyroid hormone, eicosanoids, peptides, cardiac glycosides (digoxin, digitoxin, and ouabain), and numerous drugs.10 Among these, in the kidney, SLCO4C1 might be a first step of transport pathway of digoxin and various compounds into urine.10 In renal failure, basolateral SLCO4C1 expression was decreased; however, the expression level of MDR1, a member of the ATP-binding cassette transporter family that mediates the tubular secretion of digoxin at the apical membrane of the proximal tubule cell, was not changed.10 This reduction of SLCO4C1 in the proximal tubules may be one of the mechanisms of impaired urinary excretion of digoxin and drugs in renal failure.10 In humans, SLCO4C1 is the only organic anion transporter in the kidney, whereas, in rodent kidney, several oatps exist at the basolateral and apical membrane of the proximal.10 This species diversity of the OATP family subtypes and the multiple locations in proximal tubules make it difficult to extrapolate from experimental studies of rodents to humans. To overcome this issues, here, we generated a transgenic (TG) rat harboring human SLCO4C1 in rat kidney and clarified physiologic and pathophysiologic roles of human SLCO4C1.
Results
Generation of TG Rat Harboring Human SLCO4C1 in the Kidney
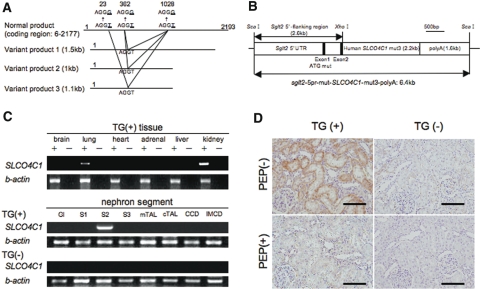
TG rat harboring human SLCO4C1 in the kidney was generated using the proximal tubule–specific promoter11 (Figure 1, A and B). In addition, to avoid unusual mRNA splicing during overexpression, we mutated three atypical splicing donor-adaptor sites in the coding region of SLCO4C1 without changing the amino acids (Figure 1A). As a result, the human SLCO4C1 mRNA was exclusively expressed in the kidney, especially in the proximal tubules of TG rats (Figure 1C). Immunohistochemical analysis also revealed that human SLCO4C1 protein was strongly detected at the basolateral side of the proximal tubules (Figure 1D).
Figure 1.
Characterization of human SLCO4C1 TG rats is shown. (A) Three different smaller sizes of mRNA by alternative splicing were found and mutated to avoid unusual splicing (AGGT to AGGG). (B) The mutated human SLCO4C1 cDNA was inserted into a plasmid under the proximal tubule–specific promoter. (C) Expression of human SLCO4C1 in rat organs and microdissected renal tubules examined by reverse transcriptase–PCR. Gl, glomerulus; S1, proximal tubule S1 segment; S2, proximal tubule S2 segment; S3, proximal tubule S3 segment; mTAL, medullary thick ascending limb; cTAL, cortical thick ascending limb; CCD, cortical collecting duct; IMCD, inner medulla collecting duct. (D) Immunohistochemical analysis. The human SLCO4C1 immunostains were abolished by peptide absorption. Bars = 100 μm.
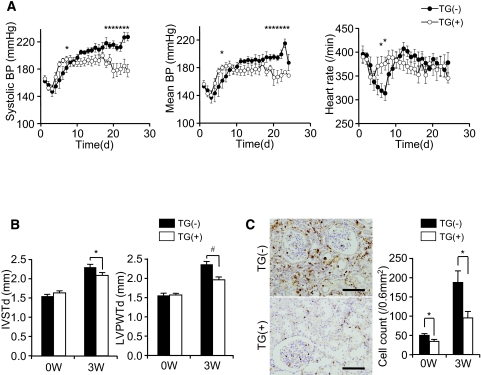
When renal mass was reduced by five-sixths nephrectomy (Nx), BP was significantly decreased in TG(+)Nx rats compared with non-TG littermate [TG(−)Nx] rats (Figure 2A). This BP reduction was seen in two independently generated lines. In TG(+)Nx rats, cardiac hypertrophy was also significantly reduced (Figure 2B).
Figure 2.
Phenotype of human SLCO4C1 TG rats. (A) BP and heart rate of TG(−)Nx and TG(+)Nx rats. *P < 0.05 versus TG(−)Nx rats (n = 4 to 6 per group). (B) Thickness of the interventricular septum (IVSTd) and left ventricular posterior wall at end-diastole (LVPWTd) were measured by echocardiogram before and 3 wk after five-sixths Nx. *P < 0.05; #P < 0.01 (n = 4 to 9 per group). (C) CD68 staining in the rat kidney before and 3 wk after five-sixths Nx. CD68+ cell number counts were performed before and 3 wk after five-sixths Nx. *P < 0.05 versus TG(−) rats (n = 6 to 9 per group). Bars = 100 μm.
The survival rate of TG(+)Nx rats was slightly increased from that of TG(−)Nx rats, but the results did not reach statistical significance (Supplemental Figure 1C). In patients with CKD, renal inflammation is also a risk factor of renal damage and morbidity and mortality.12 Immunohistochemically, mononuclear cell infiltration stained with the macrophage marker CD68 was strongly detected in TG(−)Nx rat kidneys (Figure 2C). Conversely, TG(+)Nx kidneys demonstrated less infiltration of macrophage (Figure 2C). These data indicate that expression of human SLCO4C1 in rat kidneys ameliorated not only hypertension but also inflammation in renal failure.
Elimination of Uremic Toxins in TG(+) Rats
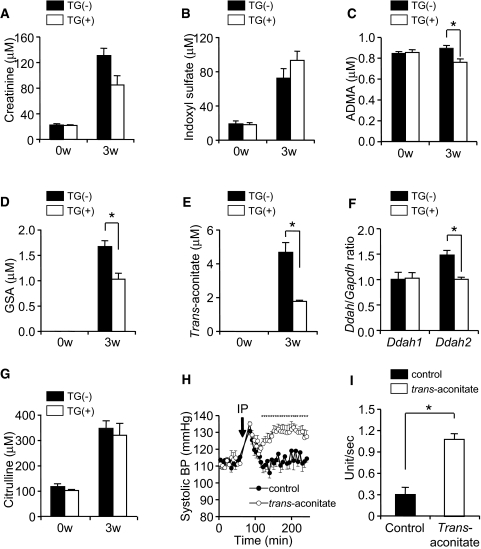
To understand the mechanism by which SLCO4C1 exerted antihypertensive and anti-inflammation effects, we performed comprehensive quantitative metabolome analysis.13 Blood and urine specimens were measured by capillary electrophoresis mass spectrometry (CE-MS) and HPLC, and 188 anions and 298 cations were identified (Supplemental Tables 1 through 4). Among these, we focused on 21 compounds for which concentration was significantly changed after Nx (Supplemental Figure 2). As a result, the plasma levels of creatinine and indoxyl sulfate were increased 3 wk after Nx as previously reported,4 but the concentrations of these compounds were not different between TG(+)Nx and TG(−)Nx rats 3 wk after Nx (Figure 3, A and B). Conversely, although the plasma concentration of ADMA, GSA, and trans-aconitate were significantly increased 3 wk after Nx, the increments were significantly decreased in TG(+)Nx rats compared with TG(−)Nx rats (Figure 3, C through E). These data suggest the facilitation of the excretion of uremic toxins in TG(+) rats.
Figure 3.
Metabolome analysis and characterization of uremic toxins are shown. (A through E and G) The plasma concentration of creatinine (A), indoxyl sulfate (B), ADMA (C), GSA (D), trans-aconitate (E), and citrulline (G) before and 3 wk after five-sixths Nx (n = 4 to 5 per group). (F) The mRNA expression level of DDAH1 and DDAH2 in the kidney 3 wk after five-sixths Nx (n = 5 per group). (H) BP after intraperitoneal injection of trans-aconitate (400 mg/kg; n = 5 per group). (I) Trans-aconitate–induced superoxide production in HK-2 cells. *P < 0.05.
To exclude the possibility of the compensative or nonspecific effects by overexpression of SLCO4C1 in the kidney, we performed microarray analysis. As a result, there was NS difference in the expression levels of other rat transporters (slco4c1, oatp1, oatp3, oatp5, abcb11, mrp2, mdr1, and mlc1).
The serum ADMA level is controlled by two pathways: (1) Enzymatic degradation by dimethylarginine dimethylaminohydrolase (DDAH) and (2) urinary excretion.14 In TG(+)Nx rats, the DDAH1 mRNA level was not different between TG(+)Nx and TG(−)Nx rats, and the DDAH2 mRNA level in TG(+)Nx rats was decreased compared with TG(−)Nx rats (Figure 3F), suggesting that the decrease of ADMA in TG(+)Nx rats was not dependent on facilitating enzymatic degradation. In addition, neither the plasma level of citrulline (Figure 3G), produced from ADMA by DDAHs, nor the mRNA level of protein arginine N-methyltransferase that generates ADMA from arginine was different between TG(−)Nx and TG(+)Nx rats. Because GSA excretion had not completely correlated with creatinine clearance,15 these data further suggest that the overexpression of SLCO4C1 at the proximal tubule facilitates guanidino compound excretion in renal failure.
Trans-aconitate is a competitive inhibitor of aconitase.16 Aconitase is a key enzyme in catalyzing citrate to isocitrate via cis-aconitate in the TCA cycle, and the accumulation of trans-aconitate inhibits TCA cycle and respiration in tissues.16 The retention compounds that are biologically/biochemically active and responsive for the uremic syndrome are called uremic toxins.4 It is widely known that he accumulation of guanidino compounds (including ADMA and GSA) and several uremic toxins generate oxidative stress, and it causes further renal damage in patients with CKD17; however, the existence in mammals, biologic effects, and the precise role of trans-aconitate in renal failure have not been clarified. When trans-aconitate was administered to rats intraperitoneally, the BP of injected rats was immediately elevated compared with controls (Figure 3H). This increase of BP was cancelled when trans-aconitate was injected into TG(+) rats compared with TG(−) rats, further suggesting the excretion through SLCO4C1 (Supplemental Figure 1D). In addition, trans-aconitate significantly induced superoxide production in human kidney proximal tubule cells (Figure 3I).
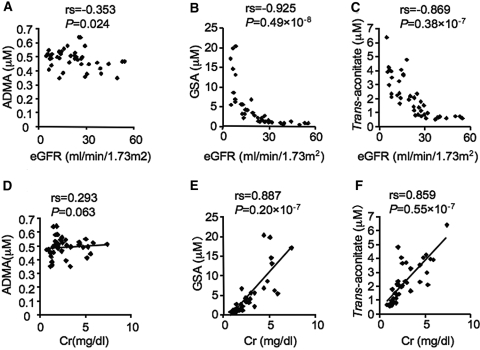
To confirm further that not only ADMA and GSA but also trans-aconitate exists in humans and the concentration is increased in accordance with CKD progression, we performed CE-MS analysis of 41 patients with CKD at various stage. The plasma level of trans-aconitate was significantly correlated with the increase of plasma creatinine, and that inversely correlated with the eGFR similar to ADMA and GSA (Figure 4). Because the plasma level of trans-aconitate in patients without CKD is low, these data suggest that trans-aconitate can be a new uremic toxin, and a newly identified biomarker for predicting the onset of renal damage and, thus, the elimination of trans-aconitate plays a beneficial role in CKD.
Figure 4.
Relation between uremic toxins and eGFR as well as plasma creatinine in 41 patients with CKD is shown. (A through C) Correlations between eGFR and the plasma ADMA (A), GSA (B), and trans-aconitate (C) in patients with CKD. (D through F) Concentrations between plasma creatinine (Cr) and plasma ADMA (D), GSA (E), and trans-aconitate (F).
Functional Analysis of SLCO4C1 Promoter and Its Modulation by Statins
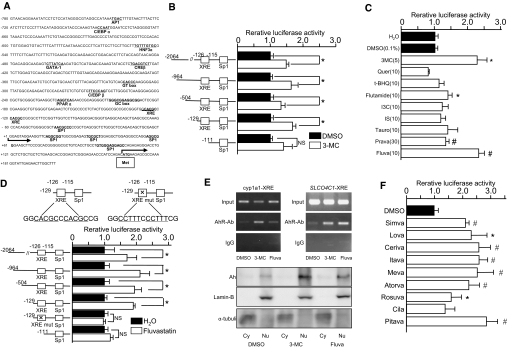
We assumed that enhancement of SLCO4C1 in the kidney may facilitate the excretion of uremic toxins and thereby ameliorate the symptoms of CKD. In this scenario, drugs that upregulate SLCO4C1 in the kidney may facilitate excretion of uremic toxins and reduce renal inflammation, decelerating progression of renal damage and entry of hemodialysis. To address this, we isolated the promoter region of human SLCO4C1. Human SLCO4C1 promoter region has a predominant transcription start site located 164 bp upstream of the ATG codon (Figure 5A). Potential cis-acting motifs for GATA-1, hepatocyte nuclear factor (HNF)-3α, CCAAT/enhancer-binding protein (C/EBP)α, C/EBPβ, cAMP response element-binding protein (CREB), and peroxisome proliferator–activated receptor α were found. We also identified tandem xenobiotic-responsive element (XRE) motifs containing the substitution-intolerant core sequence 5′-CACGC-3′ at position −126 (GGCACGCCCACGCCG). That sequence is generally recognized by AhR and AhR nuclear translocator heterodimer,18 although the flanking sequences are not typical compared with cyp1a1 XRE motifs19,20 (Supplemental Figure 3D). AhR binds “classical” ligands such as the environmental pollutants halogenated aromatic hydrocarbons (e.g., dioxin, benzo[a]pyrene, 3-methylcholanthrene [3-MC]).21
Figure 5.
Transcriptional analysis and ligand screening are shown. (A) The 5′ region of human SLCO4C1. Potential cis-acting sequences are indicated. Met, first methionine. (B) Promoter activity of human SLCO4C1. Deletion constructs of the human SLCO4C1 promoter region were analyzed with 3-MC (5 μM). *P < 0.05 (n = 3 to 4 per group). (C) Enhancement of promoter activity of human SLCO4C1 with various compounds (concentration as indicated, μM). Quer, quercetin; t-BHQ, tert-butylhydroquinone; I3C, indole-3-carbinole; IS, indoxyl sulfate; Tauro, taurocholic acid; Prava, pravastatin; Fluva, fluvastatin. *P < 0.05 versus DMSO; #P < 0.05 versus H2O (n = 3 to 4 per group). (D) Effect of fluvastatin (10 μM) on human SLCO4C1 transcription. Deletion constructs and loss-of-function mutation construct in XRE motifs of human SLCO4C1 were examined. *P < 0.05 (n = 3 to 4 per group). (E) ChIP assay and Western blotting of 3-MC or fluvastatin-treated cells. (Top) After application of 3-MC (1 μM) or fluvastatin (10 μM), fixed cell extract was analyzed by mouse cyp1a1 XRE or human SLCO4C1 XRE PCR. (Bottom) Western blotting of nuclear and cytoplasmic fractions from HEK293T cells were stained with antibodies against AhR, Lamin B, or α-tubulin antibodies. Cy, cytosolic fraction; Nu, nuclear fraction. (F) Enhancement of human SLCO4C1 promoter activity with various statins (10 μM) using the minimal promoter region (−129). *P < 0.05; #P < 0.01 (n = 3 to 4 per group).
Human SLCO4C1 promoter activity was increased 1.49-fold (−2064) and 1.68-fold (−129) by 3-MC compared with controls (Figure 5B). The −129 construct exhibited the highest activity, and this segment contained XRE core motifs. Because AhR can also bind to a structurally divergent range of chemicals,21 we next screened various compounds. The hepatic hydroxymethyl glutaryl–CoA reductase inhibitor (statin) fluvastatin (2.3-fold at 10 μM) and pravastatin (1.3-fold at 30 μM) and atypical AhR ligand flutamide (1.4-fold at 10 μM) upregulated the SLCO4C1 promoter activity (Figure 5C). Because of the comparable magnitude to 3-MC and its clinical availability, we further focused on statins. Deletion experiments showed that all constructs exerted potent promoter activation, but removal of the XRE core segment or mutation in the XRE core motifs abolished the response to fluvastatin (Figure 5D). Because there are various clinical reports on renoprotective effects of statins,22 we further examined various statins on human SLCO4C1 transcription. Simvastatin, lovastatin, cerivastatin, itavastatin, mevastatin, atorvastatin, rosuvastatin, and pitavastatin upregulated SLCO4C1 transcription (Figure 5F).
Next, we determined the ligand-dependent recruitment of the AhR-XRE system by chromatin immunoprecipitation (ChIP) assay. Application of the antibody against AhR resulted in a positive band for both 3-MC and fluvastatin (Figure 5E, top). In addition, the nuclear recruitment of AhR protein was further confirmed by Western blotting with a strong band in the nuclear extract by 3-MC and fluvastatin (Figure 5E, bottom). These data suggested that statins regulate SLCO4C1 transcription through the AhR-XRE system.
Statins Increase Tubular Uremic Toxin Excretion
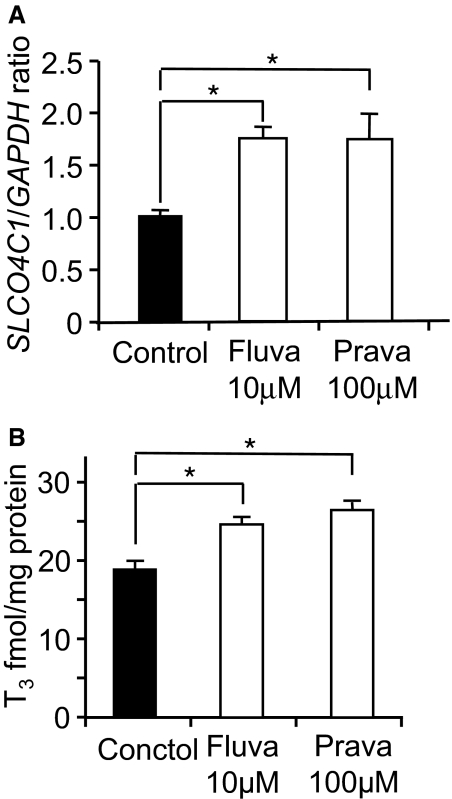
On the basis of our results, we next examined the effect of statins in renal failure. In human kidney proximal cells, application of fluvastatin and pravastatin significantly potentiated the SLCO4C1 mRNA by 1.72- and 1.73-fold, respectively (Figure 6A). The uptake of thyroid hormone T3, a representative ligand of SLCO4C1, was also significantly potentiated by fluvastatin and pravastatin by 1.3- and 1.4-fold, respectively (Figure 6B), suggesting the potentiation of SLCO4C1 function in the proximal tubules.
Figure 6.
Effects of statins on SLCO4C1 expression and function in vitro. (A) Real-time PCR of SLCO4C1 in ACHN cells with fluvastatin (10 μM) or pravastatin (100 μM; n = 3 per group). (B) The uptake of T3 by ACHN cells treated with fluvastatin (10 μM) and pravastatin (100 μM). *P < 0.05 (n = 3).
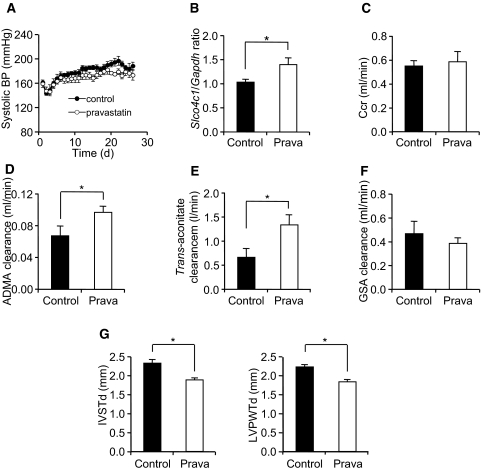
We next examined the effects of pravastatin in vivo. We and other groups reported that pravastatin reduced BP.23,24 In addition, pravastatin has been reported to modulate DDAH activity and modulate ADMA concentration.25 To avoid the effect on BP and to eliminate other pleiotropic effects of pravastatin, we administered low-dosage pravastatin to Nx Wistar rats and examined renal tubular function. After administration of pravastatin, BP was not changed but the mRNA level of rat slco4c1 was significantly increased in the kidney (Figure 7, A and B). Under this condition, the ADMA and trans-aconitate clearance were significantly increased in pravastatin-treated Nx rats without changing creatinine clearance, although the GSA clearance was not statistically significant (Figure 7, C through F). Furthermore, the mRNA level of DDAHs, protein arginine N-methyltransferases, or other transporters was not changed (data not shown). These data strongly suggested that pravastatin increased ADMA and trans-aconitate excretion in the proximal tubules. In addition, cardiac hypertrophy was decreased in the pravastatin-treated group (Figure 7G).
Figure 7.
Effects of pravastatin in vivo. (A) BP in control and pravastatin-treated (0.1 mg/ml drinking water) rats after five-sixths Nx (n = 6 to 7 per group). (B) The mRNA expression of rat slco4c1 in the kidney after pravastatin administration (n = 11 per group). (C through F) Renal clearance of creatinine (C), ADMA (D), trans-aconitate (E), and GSA (F) 3 wk after five-sixths Nx (n = 5 to 7 per group). (G) Thickness of the interventricular septum (IVSTd) and left ventricular posterior wall at end-diastole (LVPWTd) before and after five-sixths Nx (n = 6 to 7 per group). *P < 0.05.
Discussion
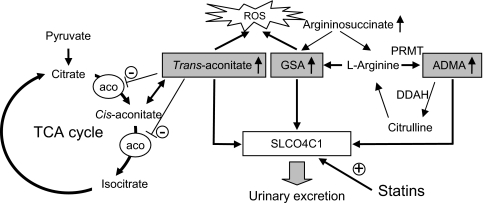
Here, we found that the plasma concentration of uremic toxins ADMA, GSA, and trans-aconitate were significantly reduced in TG(+)Nx rats. The guanidino compounds are a large group of structural metabolites of arginine, and the concentrations of GSA and ADMA are markedly increased in renal failure.2,3 GSA accumulation causes various harmful effects, such as inhibition of platelet aggregation hemolysis and convulsions.26 Likewise, ADMA is the most specific endogenous compound with inhibitory effects on NO synthesis, and it has also been implicated in the development of hypertension and adverse cardiovascular events.6,7 Trans-aconitate, known as anti-feedant in brown plant hoppers,27 is an inhibitor of aconitase and inhibits the TCA cycle16; however, its existence in mammals, especially in renal failure, was not previously known. Compounds that inhibit the TCA cycle are “poison.” It is also widely known that fluoroacetate is a “suicide” substrate for aconitase. Acute fluoroacetate poisoning in humans mainly affects the central nervous system, cardiovascular system, and kidney, and the biochemical effects include TCA cycle blockade, respiratory failure, and metabolic acidosis and lactate accumulation.28 Trans-aconitate administration also increased BP and generated oxidative stresses in rats. These data suggest that the overexpression of SLCO4C1 in the renal proximal tubules in TG(+) rats causes the beneficial effect of excretion of harmful uremic toxins such as ADMA, GSA, and trans-aconitate and proposes a new approach to decrease uremic toxins and to reduce the exacerbation of renal function in patients with CKD (Figure 8).
Figure 8.
Uremic toxins and SLCO4C1 transporter in renal failure. ADMA is formed by protein arginine N-methyltransferase (PRMT) from arginine and degrades to citrulline by DDAH. Note that SLCO4C1 facilitates the excretion of GSA, ADMA, and trans-aconitate and that statins increase the expression and the function of SLCO4C1, resulting in reductions of the uremic toxins and BP. Trans-aconitase inhibits aconitase activity and induces reactive oxygen species (ROS). Aco, aconitase.
Here we show that statins function as a nuclear receptor ligand recruiting the AhR-XRE system and upregulating SLCO4C1 transcription to facilitate the excretion of uremic toxins like a transgene phenotype. In patients with CKD, therapy with statins has the potential not only to lower cardiovascular morbidity and mortality but also to slow the progression of renal disease.22 The effects are thought to be dependent on such mechanisms as a reduction of endothelial dysfunction, inhibition of inflammatory responses, and reduction of oxidative stress.22,29 Recently, the relationship between statin administration and ADMA was examined in humans. The serum level of ADMA in metabolic syndrome was reduced by fluvastatin.30 Thus, our data provide new scientific bases for renal protection to facilitate the excretion of uremic toxins in patients with CKD by drugs including statins as “transporter potentiators” (Figure 8). Because the significantly increased levels of GSA and ADMA were reported in patients with autosomal dominant polycystic kidney disease (ADPKD),5 our data also support the clinical study and will be a new clue for further protection of renal damage in patients with ADPKD.
Cytochrome P-450 (CYP) comprises a superfamily of enzymes that catalyze oxidation of numerous xenobiotic chemicals, including drugs, toxic chemicals, and carcinogens, as well as endobiotic chemicals.31 Among these CYP enzymes, cyp1a1 is important in the metabolism of carcinogens such as dioxin and halogenated aromatic hydrocarbons.31 Because of the prominently catalyzing role, it has been believed that compounds that induce cyp1a1 activation are detrimental to humans and animals; however, it is also reported that induction of cyp1a1 is a sensitive but nonspecific indicator of AhR binding and activity, and the induction of cyp1a1 and activation of AhR are not synonymous with dioxin-like toxicity, including carcinogenesis.32 Clinically, various weak AhR ligands, such as flutamide, omeprazole, and atorvastatin, were identified32 but the Food and Drug Administration approves usage of these compounds, and in fact, they do not produce dioxin-like toxicities, including carcinogenesis in humans. Because statins have been used for a long time with a high safety and tolerability profile, induction of SLCO4C1 by statins in the kidney in patients with CKD and ADPKD may be a safe and new therapeutic tool to excrete uremic toxins and for reduction of renal inflammation.
We also found that the activation potency of the AhR-XRE system differs between cyp1a1 and slco4c1 in the kidney. In the rat liver, cyp1a1 was significantly induced by flutamide (329-fold) and omeprazole (79-fold), although renal cyp1a1 was weakly upregulated by flutamide (three- fold) and omeprazole (15-fold; Supplemental Figure 3, A and B). It is also reported that some statins significantly induced cyp1a1 in kidney but rather weakly in the liver, suggesting that statins act as AhR ligands mainly in the kidney.32 Conversely, the renal activation of slco4c1 by flutamide and omeprazole was quite weak (Supplemental Figure 3C). Thus, further exploring for drugs that upregulate human SLCO4C1 only in the kidney much more potently than statins should be a new clinical tool for patients with CKD and ADPKD to decelerate renal damage and to delay initiating hemodialysis.
Metabolomics is an emerging tool that can be used to gain insights into cellular and physiologic responses. By CE-MS, we identified various renal failure–related compounds (Supplemental Figure 2, Supplemental Tables 1 through 4). In renal failure, indoxyl sulfate, creatinine, GSA, and guanidinoacetate were reported as uremic toxins.4 Increase of citrulline and trimethyl N-oxide,33 3-methylhistidine,34 N,N-dimethylglycine.35 and allantoin36 and decrease of carnitine,37 Trp, and Tyr38 were also reported in renal failure.
On the other hand, increase of trans-aconitate, 4-acetylbutyrate, hexanoate, argininosuccinate, α-aminoadipate, and pipecolate and decrease of desethylatrazine and methionine sulfoxide so far have not been reported in renal failure (Supplemental Figure 2). Thus, our data will be useful for clarifying the metabolic pathway of renal failure.
Concise Methods
Materials
Pravastatin was provided by Daiichi-Sankyo (Tokyo, Japan). Other statins were purchased from Sequoia Sciences (St. Louis, MO).
Construction of Kidney-Specific TG Rats
The mutated coding region of human SLCO4C110 was inserted into the pGEM-sglt2–5pr-mut plasmid containing kidney-specific sglt2 promoter.11 The linear purified plasmid was injected into the pronuclei of fertilized oocytes of Wistar rats. Pups were analyzed for the genomic integration by Southern blotting and by PCR amplification of tail DNA using the following primers: Forward (mouse sglt2) 5′-tccccccacttctgtttcccagtctatgt-3′ and reverse (human SLCO4C1) 5′-acgcgatctgcagaattagcttgggctc-3′. Reverse transcriptase–PCR was carried out using the same primers that can amplify the full length of human SLCO4C1 cDNA. Resultant TG(+) rats showed normal breeding and development with no obvious phenotypic abnormalities in body weight, water and food intake, and renal functions compared with TG(−) littermates, whose genetic background is the same as that of TG(+) rats except for expression of human SLCO4C1 (Supplemental Figure 1A). All animal experiments were approved by the Tohoku University Animal Care Committee.
Immunohistochemistry
The rabbit antiserum against 107 peptides of the N-terminus of human SLCO4C1 was raised and immunopurified. Western blotting and immunohistochemistry were performed as described previously,39 and the quality was confirmed by peptide absorption (Supplemental Figure 1, B and D). The mouse mAb against CD68 was purchased from Serotec (Martinstried, Germany).
Nephrectomized Rat Model and BP Measurement
Five-sixths nephrectomized rats were generated as previously reported.10 Briefly, male TG rats were intraperitoneally anesthetized with ketamine (30 mg/kg) and xylazine (2 mg/kg) and subjected to five-sixths renal ablation. At the time of surgery, rats were prepared for telemetric monitoring of BP (Data Sciences Int., St. Paul, MN).40
Echocardiogram
Rats were anesthetized with ketamine (50 mg/kg) and xylazine (10 mg/kg) and studied with Doppler imaging by echocardiogram. The thickness of the interventricular septum and the left ventricular posterior wall at end-diastole were measured as described previously.41
CE-MS Method for Metabolome Analysis
A comprehensive and quantitative analysis of charged metabolites by CE-MS was performed.13 Metabolites were first separated by CE on the basis of charge and size and then selectively detected using MS by monitoring over a large range of m/z values. Plasma and urine ADMA were measured by HPLC. Anionic and cationic compounds that were increased or decreased after Nx in both of the generated rat lines were nominated as statistically significant and are summarized in Supplemental Figure 2 (all analyzed CE-MS data are in Supplemental Tables 1 through 4). In the human plasma analysis, the protocols conformed to the ethical guidelines and approvals of both Tohoku University and Nagasaki University. Informed consent was obtained from each participant. The eGFR was calculated with the formula42 eGFR (ml/min per 1.73 m2) = 175 × creatinine−1.154 × age−0.203 × 0.742 (if female) × 0.741.
Measurement of Reactive Oxygen Species
The free radical formation within the human kidney proximal cell line HK-2 evoked by trans-aconitate (100 μM) was monitored by measurement of the changes in fluorescence resulting from the oxidation of dihydroethidium to ethidium as the increase of ethidium production (U/s)43 using a 505-nm dichroic mirror with the 605/55-nm band-pass filter of an IX71 microscope (Olympus, Tokyo, Japan).
Transcriptional Assay
The human SLCO4C1 promoter DNA fragments were amplified by PCR, and the amplified fragments were inserted into the pGL3 basic luciferase expression vector (Promega, Madison, WI). The point mutation of two XREs was generated by PCR. Two micrograms of plasmid construct was transfected with 0.1 μg of Renilla Luciferase Reporter Vector PhRL-TK (Promega) as well as co-transfection with AhR and AhR nuclear translocator expression vector.18 Forty-eight hours after ligand treatment, reporter assay was performed using Dual Luciferase Reporter Assay System (Promega). Incubation with activators of constitutive androstane receptor (clotrimazole and TCPOBOP), pregnane X receptor (rifampicin), and peroxisome proliferator–activated receptor α (bezafibrate, fenofibrate, clofibrate, and LTB4) did not affect the SLCO4C1 transcription (data not shown).
ChIP Assay
ChIP assays were performed as described previously.44 Briefly, cells either untreated or exposed to 3-MC (mouse HepaC1C7 cells) or fluvastatin (HEK293T cells) were crossed-linked with 1% formaldehyde, and protein-DNA complexes were immunoprecipitated using rabbit polyclonal antibody against AhR (BIOMOL, Plymouth, PA) or nonspecific anti-rabbit IgG. The recovered DNA was then subjected to PCR using primers that amplify regions containing the XRE elements of the human SLCO4C1 gene (forward primer 5′-AAGGGGAGCTTATGGCCAGAGACTC-3′ and reverse primer 5′-TCGCCTCAAGGACCAACCGGAAG-3′) or mouse cyp1a1 gene (forward primer 5′-CTATCTCTTAAACCCCACCCCAA-3′ and reverse primer 5′-CTAAGTATGGTGGAGGAAAGGGTG-3′). Nuclear and cytoplasmic fraction extracts were prepared and Western blotting was performed as described previously39 using antibodies against AhR, Lamin B (Santa Cruz Biotechnology, Santa Cruz, CA), and α-tubulin (Sigma-Aldrich, St. Louis, MO).
Real-Time PCR Analysis
We performed real-time PCR analysis with probe sets from Applied Biosystems (Foster City, CA).
Statistical Analysis
The data are means ± SEM. We used an unpaired t test for comparisons between two groups. For multiple comparisons, we used two-way ANOVA with repeated measures in Figures 2A, 3H, and 7A and Supplemental Figure 1D and ANOVA on rank in Supplemental Figure 3, A through C. We derived P values for Supplemental Figure 1C using log-rank test. In Figure 4, Spearman rank correlation was calculated. P < 0.05 was considered to be significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported in part by research grants from the Miyagi Kidney Foundation; the Ministry of Education, Science and Culture of Japan; the Yokoyama Clinical Pharmacology Foundation; and Japan Science and Technology Agency.
We thank T. Shindo and H. Shima for maintaining TG rats and I. Nakamura for secretarial assistance; S. Endo, Y. Yoneki, Y. Ohsaki, T. Mori, and T. Naganuma (Tohoku University) for advice on animal experiments; N. Anzai (Kyorin University) for discussion; and S.J. Karp (Harvard Medical School) for manuscript reading.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Harnessing Transporters to Clear Uremic Toxins,” on pages 2483–2484.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Kielstein JT, Zoccali C: Asymmetric dimethylarginine: A novel marker of risk and a potential target for therapy in chronic kidney disease. Curr Opin Nephrol Hypertens 17: 609–615, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Marescau B, Nagels G, Possemiers I, De Broe ME, Becaus I, Billiouw JM, Lornoy W, De Deyn PP: Guanidino compounds in serum and urine of nondialyzed patients with chronic renal insufficiency. Metabolism 46: 1024–1031, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Vanholder R, Van Laecke S, Glorieux G: What is new in uremic toxicity? Pediatr Nephrol 23: 1211–1221, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torremans A, Marescau B, Kranzlin B, Gretz N, Billiouw JM, Vanholder R, De Smet R, Bouwman K, Brouns R, De Deyn PP: Biochemical validation of a rat model for polycystic kidney disease: Comparison of guanidino compound profile with the human condition. Kidney Int 69: 2003–2012, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Zoccali C, Mallamaci F, Maas R, Benedetto FA, Tripepi G, Malatino LS, Cataliotti A, Bellanuova I, Boger R: Left ventricular hypertrophy, cardiac remodeling and asymmetric dimethylarginine (ADMA) in hemodialysis patients. Kidney Int 62: 339–345, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Fliser D, Kronenberg F, Kielstein JT, Morath C, Bode-Boger SM, Haller H, Ritz E: Asymmetric dimethylarginine and progression of chronic kidney disease: The Mild to Moderate Kidney Disease Study. J Am Soc Nephrol 16: 2456–2461, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Sanaka T, Akizawa T, Koide K, Koshikawa S: Protective effect of an oral adsorbent on renal function in chronic renal failure: Determinants of its efficacy in diabetic nephropathy. Ther Apher Dial 8: 232–240, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Owada A, Nakao M, Koike J, Ujiie K, Tomita K, Shiigai T: Effects of oral adsorbent AST-120 on the progression of chronic renal failure: A randomized controlled study. Kidney Int Suppl 63: S188–S190, 1997 [PubMed] [Google Scholar]
- 10.Mikkaichi T, Suzuki T, Onogawa T, Tanemoto M, Mizutamari H, Okada M, Chaki T, Masuda S, Tokui T, Eto N, Abe M, Satoh F, Unno M, Hishinuma T, Inui K, Ito S, Goto J, Abe T: Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proc Natl Acad Sci U S A 101: 3569–3574, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubera I, Poujeol C, Bertin G, Hasseine L, Counillon L, Poujeol P, Tauc M: Specific Cre/Lox recombination in the mouse proximal tubule. J Am Soc Nephrol 15: 2050–2056, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Silverstein DM: Inflammation in chronic kidney disease: Role in the progression of renal and cardiovascular disease. Pediatr Nephrol 24: 1445–1452, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Soga T, Ohashi Y, Ueno Y, Naraoka H, Tomita M, Nishioka T: Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J Proteome Res 2: 488–494, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Baylis C: Arginine, arginine analogs and nitric oxide production in chronic kidney disease. Nat Clin Pract Nephrol 2: 209–220, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levillain O, Marescau B, Possemiers I, Al Banchaabouchi M, De Deyn PP: Influence of 72% injury in one kidney on several organs involved in guanidino compound metabolism: A time course study. Pflugers Arch 442: 558–569, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Saffran M, Prado JL: Inhibition of aconitase by trans-aconitate. J Biol Chem 180: 1301–1309, 1949 [PubMed] [Google Scholar]
- 17.Cachofeiro V, Goicochea M, de Vinuesa SG, Oubina P, Lahera V, Luno J: Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int SupplS4–S9, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Fujii-Kuriyama Y, Mimura J: Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun 338: 311–317, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Wu L, Whitlock JP, Jr: Mechanism of dioxin action: Receptor-enhancer interactions in intact cells. Nucleic Acids Res 21: 119–125, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nioi P, Hayes JD: Contribution of NAD(P)H:quinone oxidoreductase 1 to protection against carcinogenesis, and regulation of its gene by the Nrf2 basic-region leucine zipper and the arylhydrocarbon receptor basic helix-loop-helix transcription factors. Mutat Res 555: 149–171, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Denison MS, Nagy SR: Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol 43: 309–334, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Agarwal R: Effects of statins on renal function. Mayo Clin Proc 82: 1381–1390, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Kawano H, Yano K: Pravastatin decreases blood pressure in hypertensive and hypercholesterolemic patients receiving antihypertensive treatment. Circ J 70: 1116–1121, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Golomb BA, Dimsdale JE, White HL, Ritchie JB, Criqui MH: Reduction in blood pressure with statins: Results from the UCSD Statin Study, a randomized trial. Arch Intern Med 168: 721–727, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin QF, Xiong Y: Pravastatin restores DDAH activity and endothelium-dependent relaxation of rat aorta after exposure to glycated protein. J Cardiovasc Pharmacol 45: 525–532, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Cohen BD: Methyl group deficiency and guanidino production in uremia. Mol Cell Biochem 244: 31–36, 2003 [PubMed] [Google Scholar]
- 27.Watanabe K, Katsuhara M, Nakao H, Sato M: Detection and molecular analysis of plant- and insect-associated bacteria harboring aconitate isomerase involved in biosynthesis of trans-aconitic acid as antifeedant in brown planthoppers. Curr Microbiol 35: 97–102, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Tong WH, Rouault TA: Metabolic regulation of citrate and iron by aconitases: Role of iron-sulfur cluster biogenesis. Biometals 20: 549–564, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Campese VM, Park J: HMG-CoA reductase inhibitors and the kidney. Kidney Int 71: 1215–1222, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Oguz A, Uzunlulu M: Short term fluvastatin treatment lowers serum asymmetric dimethylarginine levels in patients with metabolic syndrome. Int Heart J 49: 303–311, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ: Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem 279: 23847–23850, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Hu W, Sorrentino C, Denison MS, Kolaja K, Fielden MR: Induction of cyp1a1 is a nonspecific biomarker of aryl hydrocarbon receptor activation: Results of large scale screening of pharmaceuticals and toxicants in vivo and in vitro. Mol Pharmacol 71: 1475–1486, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Bain MA, Faull R, Fornasini G, Milne RW, Evans AM: Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant 21: 1300–1304, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Ceballos I, Chauveau P, Guerin V, Bardet J, Parvy P, Kamoun P, Jungers P: Early alterations of plasma free amino acids in chronic renal failure. Clin Chim Acta 188: 101–108, 1990 [DOI] [PubMed] [Google Scholar]
- 35.McGregor DO, Dellow WJ, Lever M, George PM, Robson RA, Chambers ST: Dimethylglycine accumulates in uremia and predicts elevated plasma homocysteine concentrations. Kidney Int 59: 2267–2272, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Kand'ar R, Zakova P: Allantoin as a marker of oxidative stress in human erythrocytes. Clin Chem Lab Med 46: 1270–1274, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Reddy V, Bhandari S, Seymour AM: Myocardial function, energy provision, and carnitine deficiency in experimental uremia. J Am Soc Nephrol 18: 84–92, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Swendseid ME, Wang M, Vyhmeister I, Chan W, Siassi F, Tam CF, Kopple JD: Amino acid metabolism in the chronically uremic rat. Clin Nephrol 3: 240–246, 1975 [PubMed] [Google Scholar]
- 39.Abe T, Unno M, Onogawa T, Tokui T, Kondo TN, Nakagomi R, Adachi H, Fujiwara K, Okabe M, Suzuki T, Nunoki K, Sato E, Kakyo M, Nishio T, Sugita J, Asano N, Tanemoto M, Seki M, Date F, Ono K, Kondo Y, Shiiba K, Suzuki M, Ohtani H, Shimosegawa T, Iinuma K, Nagura H, Ito S, Matsuno S: LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology 120: 1689–1699, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Watanabe T, Tan N, Saiki Y, Makisumi T, Nakamura S: Possible involvement of glucocorticoids in the modulation of interleukin-1-induced cardiovascular responses in rats. J Physiol 491: 231–239, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukui S, Fukumoto Y, Suzuki J, Saji K, Nawata J, Tawara S, Shinozaki T, Kagaya Y, Shimokawa H: Long-term inhibition of Rho-kinase ameliorates diastolic heart failure in hypertensive rats. J Cardiovasc Pharmacol 51: 317–326, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S: Modification of the Modification of Diet in Renal Disease (MDRD) Study equation for Japan. Am J Kidney Dis 50: 927–937, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Bindokas VP, Jordan J, Lee CC, Miller RJ: Superoxide production in rat hippocampal neurons: Selective imaging with hydroethidine. J Neurosci 16: 1324–1336, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang S, Hankinson O: Functional involvement of the Brahma/SWI2-related gene 1 protein in cytochrome P4501A1 transcription mediated by the aryl hydrocarbon receptor complex. J Biol Chem 277: 11821–11827, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.