Abstract
Cellular lesions form in Bowman's space in both crescentic glomerulonephritis and collapsing glomerulopathy. The pathomechanism and origin of the proliferating cells in these lesions are unknown. In this study, we examined proliferating cells by lineage tracing of either podocytes or parietal epithelial cells (PECs) in the nephrotoxic nephritis model of inflammatory crescentic glomerulonephritis. In addition, we traced the fate of genetically labeled PECs in the Thy-1.1 transgenic mouse model of collapsing glomerulopathy. In both models, cellular bridges composed of PECs were observed between Bowman's capsule and the glomerular tuft. Genetically labeled PECs also populated larger, more advanced cellular lesions. In these lesions, we detected de novo expression of CD44 in activated PECs. In contrast, we rarely identified genetically labeled podocytes within the cellular lesions of crescentic glomerulonephritis. In conclusion, PECs constitute the majority of cells that compose early extracapillary proliferative lesions in both crescentic glomerulonephritis and collapsing glomerulopathy, suggesting similar pathomechanisms in both diseases.
Glomerular epithelial hyperplasia is an important characteristic of crescentic glomerulonephritis (CrGN), also known as rapidly progressive glomerulonephritis. Despite a wide variety of underlying causes, CrGN is characterized commonly by the development of cellular crescents (multilayered accumulation of cells in Bowman's space) and necrosis of glomerular capillaries.1 Loss of renal function occurs as a consequence of the obstruction of the tubular outlet by cellular crescents, so the proliferating cells present an important target for therapeutic interventions.2
Collapsing glomerulopathy (CG) is characterized by massive proteinuria and rapid progressive renal insufficiency and histologically by segmental to global collapse of the capillary tuft and pronounced epithelial cell hyperplasia.3 This pattern has been described in HIV-associated nephropathy,4 parvovirus B19 infection,5 and pamidronate toxicity6 and also as an idiopathic form.3
The pathomechanism of the development of cellular lesions remains to be established, and in both CrGN and CG the origin of the hyperplastic cells within cellular lesions has been a matter of debate. In CrGN, the cellular composition of crescents appears to change over time, with predominantly epithelial cells of unknown origin proliferating in early stages and increasing numbers of infiltrating macrophages, lymphocytes, and myofibroblasts in later stages, especially when Bowman's capsule is ruptured.7–9 Recent studies also pointed to a contribution of podocytes in the development of crescentic lesions.10–13 Collapsing glomerulopathy lesions in turn often are associated with hyperplasia of epithelial cells covering the glomerular tuft, although connections to Bowman's capsule appeared to be lacking. The visceral localization and the finding that these proliferating cells lacked expression of podocyte markers led to the concept of dysregulated podocytes, which are no longer growth restricted, causing epithelial hyperplasia.14 However, from the findings that these cells expressed markers normally expressed by PECs15 and the finding in serial sections that the cells on the tuft were connected to the PECs on Bowman's capsule,16 we and others suggested that these cells may originate from parietal epithelial cells (PECs) rather than from podocytes.16–20
In the studies described above, the origin of the proliferating cells was identified based on the expression or loss of specific markers. This approach may be misleading, given that, first, PECs and podocytes share a common embryonic origin. Only during the last stages of nephrogenesis the phenotypes of both cells diverge. Second, PECs lack specific differentiation markers, and third, proliferating cells may possibly transdifferentiate into cells with a different phenotype. Genetic cell lineage tracing is a technique that has been established recently and enables one to trace cells over prolonged times, even when the cells switch to a different phenotype due to de- or transdifferentiation.19,21 In the present study, we therefore used this technique to trace the relative contributions of PECs and podocytes in the development of cellular glomerular lesions in two murine models of CrGN, namely, nephrotoxic nephritis, and CG, namely, Thy-1.1 transgenic mice. These established murine models were chosen because both characteristically develop proliferative extracapillary lesions.
Results
Histopathology of the Crescentic Glomerulonephritis Model
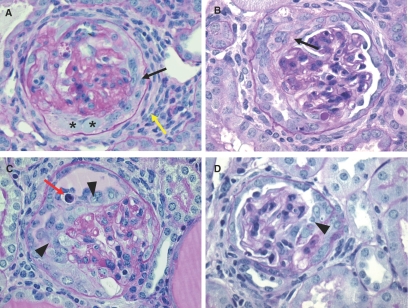
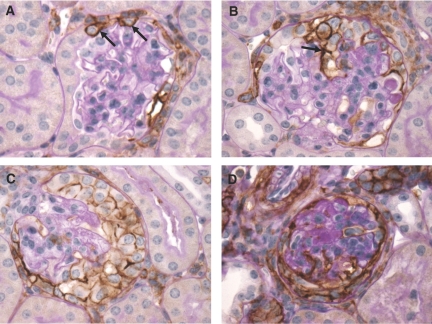
Injection of the nephrotoxic serum (NTS) serum induced proteinuria and hematuria within the first day or three days after injection, respectively. The renal histology of NTS-injected mice was examined at day 14 after the induction of CrGN. At this time point, “true” crescents (organized multilayered epithelial lesions lining Bowman's capsule) were observed (Figure 1, A and B). Between the cell layers, accumulation of extracellular matrix was present. In addition to the “true” crescents, many glomeruli contained “pseudocrescents” consisting of one or more layers of proliferating polygonal cells located close to or on the glomerular tuft (Figure 1, C and D). Within the capillaries, hyalinosis was present. Marked periglomerular fibrosis was seen in the regions surrounding affected glomeruli.
Figure 1.
Histology of the CrGN model. (A–D) PAS stainings of the CrGN model at day 14 after anti-nephrotoxic serum injection. Light microscopy revealed pronounced hyperplasia of glomerular epithelial cells, forming organized multilayered true crescentic lesions (A and B, arrows) and less organized pseudocrescents and monolayer lesions on the glomerular tuft (C and D, arrowheads). Marked periglomerular fibrosis was seen in the regions surrounding affected glomeruli (A, yellow arrow). An occasional infiltrative inflammatory cell (C, red arrow, polymorphonuclear leukocyte) or protein accumulation (A, asterisks) could be observed within the cellular lesions.
Tracing Genetically Tagged Parietal Cells in Crescentic Glomerulonephritis
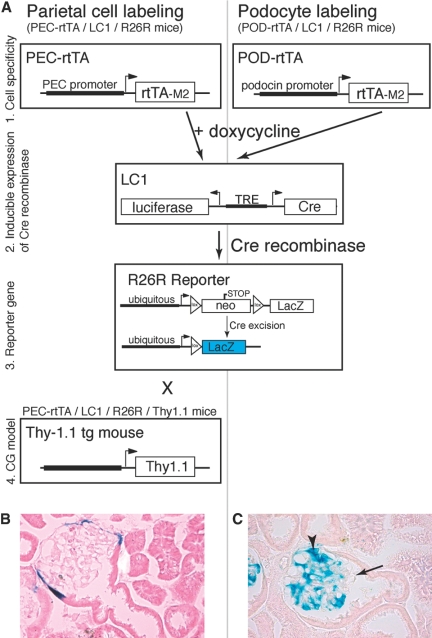
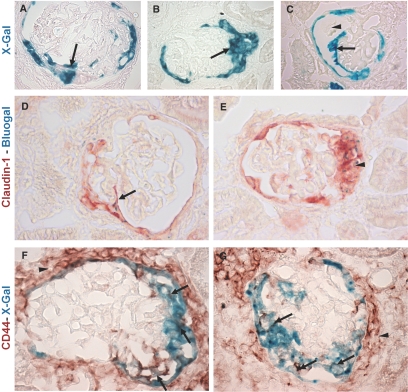
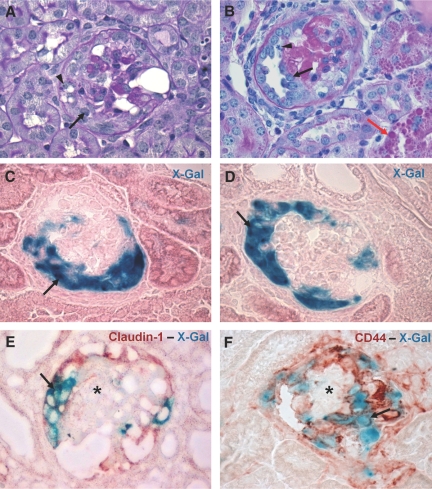
To test whether PECs contribute to the formation of cellular crescents, PECs were labeled genetically in triple transgenic PEC-Reverse Tetracycline-Transactivator (rtTA)/LC1/Rosa 26 reporter (R26R) mice by doxycycline administration for 14 d. After doxycycline administration, Cre recombination occurred in more than 70% of all PECs and could be visualized by enzymatic X-gal staining (Figure 2, A and B). After genetic labeling and a washout period of at least one week, the PEC-rtTA mice were injected with the nephrotoxic serum to induce CrGN. At day 14 after CrGN induction, X-gal staining persisted in PECs lining Bowman's capsule. In affected glomeruli, crescentic lesions contained numerous X-gal positive cells (Figure 3A). Pseudocrescents and monolayer lesions attached to or covering glomerular tuft segments were also X-gal positive (Figure 3, B and C). Monolayer lesions were associated with an adhesion between Bowman's capsule and the glomerular tuft, providing the presumptive entry site for genetically tagged parietal cells onto the capillary convolute. Single X-gal or Bluo-gal positive PECs were observed regularly that were attached to the glomerular tuft, forming cellular bridges between Bowman's capsule and the glomerular tuft (Figure 3D).
Figure 2.
Genetic tagging of podocytes or PECs in triple transgenic doxycycline-inducible mouse lines. (A) The modified rabbit podocalyxin promoter (PEC promoter) drives expression of the enhanced tetracycline reverse transactivator (rtTA-M2) as described previously.22 The podocyte-specific Pod-rtTA transgenic mice were kindly provided by Jeffrey Kopp, Massachusetts Institute of Technology, Cambridge, MA.35 Both mice were mated with LC1 mice expressing Cre recombinase (and luciferase) under the control of tetracycline responsive elements (TREs), which can be activated by the transcription factor rtTA-M2 in the presence of doxycycline. The R26R mice were used as a reporter line, which irreversibly expresses β-galactosidase (LacZ) under the control of the ubiquitously active Rosa26 locus only after Cre excision of an interposed floxed neomycin cassette (neo, acting as a stop signal) has occurred. These matings resulted in the triple transgenic PEC-rtTA/LC1/R26R (to tag parietal cells) and POD-rtTA/LC1/R26R mice (to tag podocytes). For studies in the CG model, the PEC-rtTA/LC1/R26R mice were mated to the Thy-1.1 transgenic mouse line (T6 clone).37 (B) Genetic labeling of PECs using doxycycline at the age of 4 wk in triple transgenic PEC-rtTA/LC1/R26R mice. After induction, Cre recombination occurred in more than 70% of all PECs (arrowheads, labeled PEC). Sporadic labeling was observed in some tubular cells of the renal cortex (not shown). (C) Genetic labeling of podocytes using doxycycline at the age of 4 wk in triple transgenic Pod-rtTA/LC1/R26R mice. After induction, Cre recombination occurred in more than 70% of all podocytes, similar to the PEC-rtTA mice (arrowheads, labeled podocytes; arrow, unlabeled tuft segment).
Figure 3.
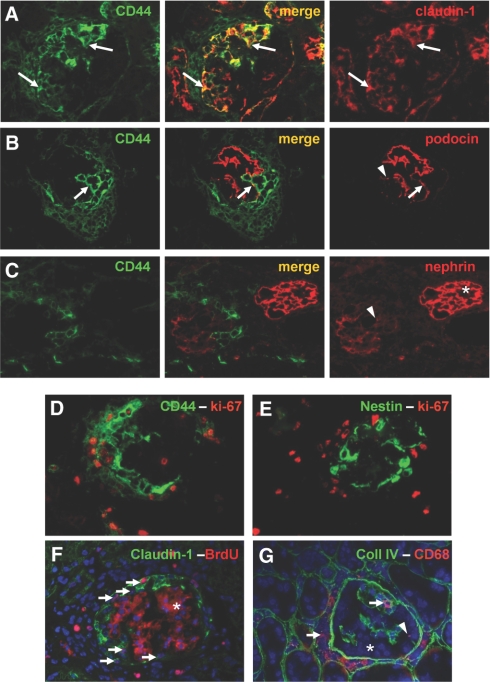
Genetically labeled PECs are present within crescentic lesions. (A–C) The PECs that were tagged genetically after doxycycline administration in PEC-rtTA/LC1/R26R mice were traced in crescentic lesions. True crescentic lesions (A), pseudocrescents (B), and monolayer lesions (C) observed at day 14 after the NTS injection stained positive with X-gal, confirming their origin from the parietal epithelial compartment (arrows). A cross section of the glomerular tuft is seen separating the monolayer lesion and Bowman's capsule in C (arrowhead). (D) β-Gal expressing cells (stained using Bluo-Gal, resulting in intracellular blue particles) frequently formed cell bridges between Bowman's capsule and the glomerular tuft (D, arrow). The Bluo-gal expressing cells showed colocalization with the PEC marker claudin-1 (D, arrow; E, arrowhead). Within monolayer (F, arrow) and crescentic lesions (G, arrows), the X-gal positive cells stained for CD44. Note that CD44 also is expressed by the cells present in the periglomerular fibrotic region (F and G, arrowheads).
De Novo Expression of CD44 in Activated Parietal Cells
First, we determined whether the PEC marker claudin-1 could still be used to identify PECs within crescentic lesions. For this purpose, we detected the β-gal expression using Bluo-gal, which typically yields only a few granular particles per stained cell, and subsequently stained the section with an antibody directed against claudin-1 (in red). In nonaffected glomeruli, claudin-1 was expressed exclusively by PECs lining Bowman's capsule.22,23 In CrGN, the crescentic lesions also stained positive for claudin-1. Many of the claudin-1 positive cells within the crescentic lesion or lining Bowman's capsule showed costaining with Bluo-gal (Figure 3, D and E). Next, we examined the expression of CD44. In control mice, we did not observe CD44 expression in the glomerulus, as described in the literature;24 however, in the CrGN model, CD44 was expressed de novo in affected glomeruli. CD44 was expressed by cells in the areas of interstitial and periglomerular fibrosis and within the crescentic lesion. CD44 colocalized with the X-gal positive cells lining Bowman's capsule and forming the crescentic lesions (Figure 3, F and G).
Tracing Genetically Tagged Podocytes in Crescentic Glomerulonephritis
To examine whether and to what extent podocytes also contribute to the formation of cellular crescents, triple transgenic POD-rtTA/LC1/R26R mice were labeled genetically by doxycycline administration. Similar to the PEC labeling in the PEC-rtTA mice, Cre recombination occurred in more than 70% of all target cells (arrow in Figure 2C shows an unlabeled tuft segment). One week after termination of the doxycycline treatment, the POD-rtTA mice were injected with the nephrotoxic serum to induce CrGN. At day 14 after CrGN induction, the morphologically normal glomeruli within the kidneys with CrGN showed normal X-gal staining exclusively of podocytes. In affected glomeruli, segmental loss of β-gal expression was observed. Specifically, the areas of the glomerular tuft involved in glomerular crescents were X-gal negative.
None or only a few cells stained positive for X-gal within the hyperplastic lesions covering the glomerular tuft or filling up Bowman's space (Figure 4, A–C, arrows). In those cases where X-gal positive cells were involved in the lesions, the cells could be observed predominantly along the periphery of the lesion (Figure 4D, arrows).
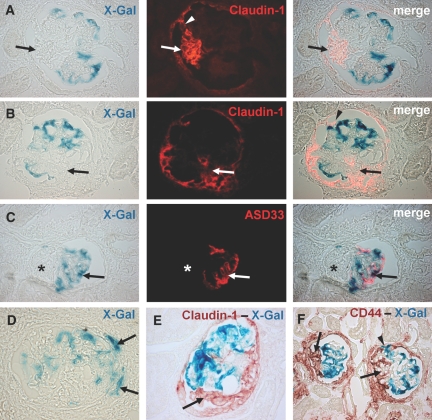
Figure 4.
Genetically labeled podocytes are excluded from crescentic lesions. (A, B) Double staining with X-gal and claudin-1 revealed no costaining in the β-gal expressing cells. Genetic labeling persisted in podocytes. But the crescentic lesions in Bowman's space that stain positive for claudin-1 were X-gal negative (A and B, arrows). Of note is the finding that the typical visceral hyperplastic lesion positive for claudin-1 is connected to the claudin-1 positive cells lining Bowman's capsule (A, arrowhead). (C) X-gal and ASD33 immunofluorescence double staining showing costaining β-gal expressing podocytes with the podocyte marker antibody ASD33. The affected segment of the glomerulus is negative for X-gal and ASD33 (C, asterisks). The merged images show a pseudocolor overlay generated with Adobe Photoshop 7.0 (Adobe Systems Incorporated, San Jose, CA). (D) X-gal staining showing rare X-gal positive cells that are present along the periphery of glomerular lesions (D, arrows). (E, F) Immunohistochemical double staining for claudin-1 and CD44 with X-gal revealed no costaining. The crescentic lesions were positive for claudin-1 and CD44 but generally negative for X-gal (E and F, arrows). Occasionally, involvement of podocytes within the lesion was observed (F, arrowhead). Interestingly, in this particular lesion the podocyte seems to be attached to Bowman's capsule, a rare finding.
To analyze the nature of the X-gal negative segments within the glomerular tuft, the slides were costained with X-gal and ASD33, an antibody directed specifically against podocytes. In these double stainings, the X-gal and podocyte marker staining overlapped (Figure 4C). Both X-gal and ASD33 were expressed in the morphologically normal tuft segments but lost in the segments that were involved in the crescentic lesions (arrow). Conversely, in a costaining of X-gal with claudin-1 and CD44, it could be established that the X-gal negative areas were covered with claudin-1 (Figure 4, A, B, and E) and CD44 (Figure 4F) positive cells, presumably representing PECs. It was concluded, that parietal cells invade and overgrow the glomerular tuft using cellular adhesions as entry sites.
To evaluate the relative contributions of tagged podocytes and PECs to the crescentic lesions, we quantified the percentages of crescentic lesions that contained either X-gal stained podocytes or PECs. However, in X-gal stained sections that were counterstained with eosin, small hyperplastic lesions in Bowman's capsule were difficult to detect and therefore could be missed. We therefore looked for a marker that can be used to also detect early crescentic lesions. To this end, we examined how CD44 expression correlated with the pathological changes in the glomeruli by performing CD44 immunostainings in combination with periodic acid–Schiff (PAS) staining. In morphologically normal glomeruli, there was either no CD44 expression or de novo expression by the PECs lining Bowman's capsule, indicating activation of PECs as shown above (Figure 5A). Strikingly, we found that 100% of the crescentic lesions (pseudo- and true crescents) visible in the PAS staining were positive for CD44 (Figure 5, B–D), indicating that CD44 is a good marker for the presence of crescentic lesions. To evaluate the contribution of tagged podocytes and PECs, we analyzed the X-gal–CD44 double staining and found that 85 ± 8% of the crescentic lesions in the PEC-rtTA mice contained X-gal positive PECs. In the POD-rtTA mice, we found that in 13 ± 9% of the crescentic lesions X-gal positive podocytes were involved. Only in the PEC-rtTA mouse intracellular colocalization of X-gal and CD44 in cells was detected.
Figure 5.
PAS–CD44 double staining. (A) A still morphologically normal glomerulus in the CrGN mouse that shows CD44 expression by PECs on Bowman's capsule (A, arrows). (B) A glomerulus containing a small pseudocrescent, which stains for CD44 (B, arrow). (C) Large crescentic lesion showing a homogenous CD44 positive cell population in Bowman's space. (D) True crescentic lesion with flattened cells that circumvent the glomerular tuft. The cells forming the crescent stain for CD44.
Phenotype of Proliferating Cells in Crescentic Glomerulonephritis
The cells involved in the crescentic lesions were examined in more detail using immunofluorescence triple stainings. First, the expression of CD44 was examined. As already observed using immunohistochemistry (Figure 5), almost all of the cells within crescentic lesions (as detected by Hoechst counterstaining) expressed CD44. Furthermore, the CD44 staining colocalized with the PEC marker claudin-1, supporting the notion that CD44 is a specific marker for activated PECs (Figure 6A). Double immunostaining for CD44 with the podocyte markers podocin and nephrin showed that the lesion, positive for CD44, was negative for both podocin and nephrin (Figure 6, B and C, respectively). Both podocin and nephrin antibodies specifically stained the podocytes. Of note, within the affected glomeruli, segmental to global reduction of the nephrin and podocin staining was observed.
Figure 6.
Phenotype of proliferating cells (A) Double immunofluorescence staining for claudin-1 (red) and CD44 (green). CD44 is de novo expressed by the cells forming the crescentic lesions. Outside of the glomerulus, CD44 is expressed by a subpopulation of the cells within the periglomerular fibrotic area and some tubular cells. Within the crescentic lesions, the CD44 positive cells costain with claudin-1 (A, arrows). (B, C) Double immunofluorescence staining for CD44 (green) and the podocyte markers podocin (B) and nephrin (C). The proliferating cells filling up Bowman's space are positive for CD44 and negative for the podocyte markers. Note that the CD44 positive cells are positioned directly against the podocin positive podocytes (B, arrow). The glomerular tufts of affected glomeruli showed segmental (B, arrowhead) to a global (C, arrowhead) reduction in podocyte marker staining. Panel C also shows a morphologically normal glomerulus showing no CD44 staining and a normal nephrin staining (C, asterisks). (D) Double immunofluorescence staining for CD44 (green) and the proliferation marker Ki-67 (red). Many CD44 positive cells in the periglomerular area and within the crescentic lesions express the proliferation marker Ki-67. (E) Double immunofluorescence staining for nestin (green) and Ki-67 (red). Only rare nestin positive cells show costaining with the proliferation marker Ki-67. The majority of the Ki-67 positive cells were detected in the area peripheral to the nestin positive cells. (F) Double immunofluorescence for the proliferation marker BrdU (red) and claudin-1 (green). The nuclei are counterstained with Hoechst to reveal nuclei (blue). Many claudin-1 positive cells forming the cellular lesion stain positive for BrdU. Together with the Hoechst staining, BrdU positive cells show purple nuclei (arrows). The secondary anti-mouse antibody used for the detection of the BrdU antibody also recognized the endogenous mouse antibodies that bind to the GBM (F, asterisks). (G) Double immunofluorescence staining for collagen IV (green) and the macrophage marker CD68 (red). The nuclei are counterstained with Hoechst (blue). The cells forming the crescentic lesion do not express CD68 (G, asterisks). Many CD68 positive cells were detected in the periglomerular areas surrounding affected glomeruli and within the glomerular tuft (G, arrows).
By double immunostaining for Ki-67 together with CD44 (Figure 6D) or with the podocyte marker nestin (Figure 6E), it could be established that the CD44 positive cells account for the majority of the proliferating cells, whereas only occasionally double staining for Ki-67 and nestin could be observed. Nestin staining specifically labeled podocytes and transitional cells (podocyte progenitors) on Bowman's capsule in normal glomeruli,22 and in glomeruli with small crescentic lesions the staining also was restricted to the glomerular tuft. In contrast, in advanced crescentic lesions, nestin staining was sometimes also present within the glomerular tuft, crescent, and periglomerular region. Therefore, the origin of the rare nestin and Ki-67 positive cells cannot be resolved entirely. Double immunostainings for bromodeoxyuridine (BrdU) and claudin-1 confirmed that the PEC-derived cells within the crescentic lesions exhibited a high proliferation rate (Figure 6F).
To test whether infiltrating macrophages contribute to the formation of the crescentic lesions as early as day 14 after CrGN induction, stainings for CD68, a membrane antigen expressed by macrophages and dendritic cells, were performed. In costainings for collagen IV, expressed within the basement membranes of the glomerulus, CD68 expressing cells were present in the periglomerular areas and also within the glomerular tuft (Figure 6G, arrows). In contrast, CD68 positive cells populating the crescentic lesions were observed rarely (Figure 6G, arrowhead). In this regard, it is important to mention that the Bowman's capsules, stained by the anti-collagen IV antibody, seemed to be still intact at this early time point, because it has been shown that breaks in the capsule correlate with an increase in the number of macrophages in the cellular crescent.7,8
Tracing Genetically Tagged Parietal Epithelial Cells in Collapsing Glomerulopathy
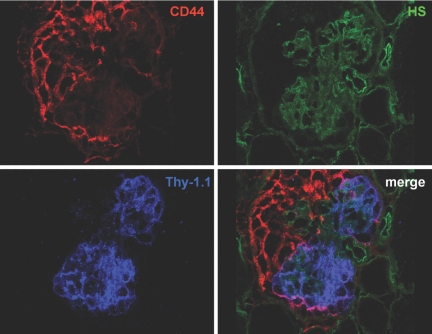
To examine the role of PECs in the development of CG, the triple transgenic PEC-rtTA/LC1/R26R mouse was crossbred with heterozygous Thy-1.1 transgenic mice. This resulted in quadruple transgenic mice, in which PECs can be marked genetically using doxycycline, and after a washout phase, CG can be induced by a single injection of anti-Thy-1.1 mAb, which binds to the Thy1.1 antigen ectopically expressed on podocytes. The histopathology of this model shows a high resemblance to that of human CG, with collapse of the glomerular tuft and development of proliferative lesions within 7 d after the antibody injection.25 Crossbreeding of the Thy-1.1 mouse with the PEC-rtTA mouse did not affect the phenotype of the Thy-1.1 model (data not shown). After injection of an anti-Thy 1.1 antibody, the PEC-rtTA/LC1/R26R/Thy-1.1 mice acutely developed proteinuria within 1 h by dipstick analysis, and CG lesions were noted within 7 d. On day 7, most of the affected glomeruli showed a high degree of hypertrophy and hyperplasia of epithelial cells in Bowman's space, and many showed segmental to global collapse of capillaries (Figure 7, A and B). The hyperplastic cells formed multilayered and monolayer pseudocrescentic lesions that covered the glomerular tuft. True crescents with flattened cells lining Bowman's capsule were observed infrequently. The histopathology resembled that of the heterozygous Thy-1.1 transgenic mice described in previous studies.25,26 Increased X-gal staining in Bowman's space was noted in most of the affected glomeruli. X-gal positive cells populated cellular lesions covering glomerular tuft segments (Figure 7, C and D). Similar to the lesions in CrGN, these cells coexpressed the PEC marker claudin-1 (Figure 7E) and showed de novo expression of CD44 (Figure 7F). CD44 was expressed by most if not all cells within the lesion. The CD44 positive cells filling up Bowman's space sometimes directly covered the Thy-1.1 expressing podocytes (Figure 8).
Figure 7.
Genetically labeled PECs are present within the CG lesions. (A, B) PAS staining of kidney sections at day 7 after the anti-Thy-1.1 mAb injection. The early lesions in the PEC-rtTA/Thy-1.1 mice showed segmental to global collapse of the glomerular tuft, hypertrophy and hyperplasia of the glomerular epithelial cells that resulted in the formation of pseudocrescents (A, arrow), and monolayer lesions on the glomerular tuft (B, arrow). The arrowhead in panel A shows vacuoles and resorption droplets, a sign of podocyte injury. The arrowhead in panel B shows a mitotic cell body in a monolayer lesion. Protein casts were observed frequently in the tubular structures. In addition, many proximal tubular cells contained protein resorption droplets (B, red arrow). (C, D) The CG lesions stained positive with X-gal (C and D, arrows). (E, F) Double staining of X-gal with claudin-1 and CD44, respectively. Within cellular lesions in Bowman's space, X-gal positive cells costain with claudin-1 (E, arrow) and CD44 (F, arrow), whereas the cells of the glomerular tuft remain negative for the PEC markers (E and F, asterisks).
Figure 8.
Triple immunofluorescence staining for CD44, Thy-1.1, and heparan sulfates (HS). The HS panel, in which the glomerular basal membranes are stained with an antibody directed against heparan sulfates, shows a glomerulus with a collapsed glomerular tuft. The epithelial cells in Bowman's space show expression of CD44. Only at the margin of the glomerular tuft we sometimes observed colocalization of CD44 and the Thy-1.1 antigen expressed by podocytes, indicating that the Thy1.1 positive podocytes are covered directly with the CD44 positive cells of the lesion.
Discussion
In this study, PECs were traced for the first time in experimental CrGN and in CG using the novel approach of tagging either of the two glomerular epithelial cell populations: PECs or podocytes. This approach overcomes the issue of altered immunohistological characteristics of both podocytes and PECs in disease situations, which has limited many studies on this topic so far. Furthermore, an inducible transgenic system was employed, ruling out any additional genetic labeling during the experimental period.
As the first major finding of this study, we firmly establish that PECs are the major cell population within cellular extracapillary lesions in the crescentic nephritis model. The low number of CD68 expressing cells within the cellular lesions indicates that in these early lesions there is a minor contribution from the macrophages. A transition of podocytes to macrophage-like cells, as observed by Bariéty et al. in idiopathic CG, posttransplantation relapse of focal segmental glomerulosclerosis, and CrGN11,27,28 were negligible in our models.
The second major finding was that PECs also constitute the predominant cell population in proliferative cellular lesions in a model of CG. The findings of X-gal positive proliferating cells in the present study provide direct evidence for the contribution of PECs in the formation of the CG lesions in the Thy-1.1 mouse model and are in line with the previously reported marker studies performed in the Thy-1.1 transgenic mouse model.25,26 In a parallel study on human biopsies,29 we also found that proliferative lesions in patients with CrGN and CG predominantly contained PECs. These hyperplastic cells were identified by their expression of the renal progenitor markers CD133 and CD24, similar to the cells on Bowman's capsule. Unfortunately, CD24 and CD133 cannot be used as a marker for renal progenitor cells on Bowman's capsule in mouse due to interspecies differences.
In contrast to the prominent role for PECs in the proliferative lesions, we observed a relatively minor contribution of genetically tagged podocytes in experimental CrGN. A similarly minor contribution of podocytes also was described recently in a study using lineage tracing of permanently tagged podocytes in a model of CG.30
Nevertheless, the pathogenetic role of podocytes within cellular lesions still remains unresolved. Podocytes might be displaced passively as innocent bystanders into cellular lesions by the proliferating cells. Podocytes also might facilitate PEC proliferation, because Le Hir et al. observed that podocytes do not form intercellular junctions with proliferating PECs, which is necessary for antiproliferative signals through “lateral contact inhibition”.2 This also would be in line with the notion that the PEC renal progenitors from Bowman's capsule proliferate in the attempt to replace injured podocytes. In the presence of extensive podocyte damage, an abnormal attempt of resident renal progenitors to regenerate injured podocytes could result in excessive proliferation, causing the formation of crescentic lesions. Whether some of these proliferating cells succeed in differentiating and replacing podocytes is unknown. In the current study, the proliferating cells in Bowman's space were lacking podocyte markers, arguing against a differentiation of the cells. Because X-gal stainings are incompatible with most immunostainings for podocyte markers, it was impossible to study whether any of the genetically tagged cells on the tuft had differentiated into podocytes. In our first report that podocytes participate in the formation of cellular crescents, podocyte-derived cells were identified in a somewhat higher percentage of glomeruli compared with that in the present study (i.e., 52% versus 14% in the present study).10 Several reasons could account for this difference. First, in our initial study, podocytes were traced genetically using a less sophisticated transgenic model: the double transgenic 2.5P-Cre/R26R mouse. In this mouse, expression of Cre recombinase was driven constitutively by the podocyte-specific podocin promoter. In this study, no significant nephrin or podocin expression was detected in cellular crescents, indicating that differentiation of transitional cells toward a podocyte phenotype did not occur. However, increased promoter activity may not always result in detectable expression levels of protein. For example, the slit-diaphragm protein podocin may be degraded, because these specialized cellular structures are absent in cellular crescents. By use of the transgenic doxycycline-inducible system in the present study (PEC-rtTA and Pod-rtTA mice), this phenomenon was excluded definitely, because additional genetic labeling during the experimental period in the absence of doxycycline is impossible.22 Second, a different (and potentially less active) nephrotoxic serum in combination with a Toll-like receptor 9 agonist was used in this study.30 Third, even although the genetic background of both transgenic mouse strains was similarly mixed C57Bl6, there could have been more C57Bl6 in the 2.5P-Cre/R26R transgenic mice. In fact, we noticed that the development of “true” crescents was more pronounced in the previously used anti-GBM model compared with that in the model used for the present study. Although genetically tagged PECs were detected in both types of crescents in the present study, our results suggested that morphological differences between pseudocrescents and true crescents correlate with differences in activity and developmental stage of the crescentic lesion.
The third major finding of this study was the striking resemblance of the marker expression and morphology of the lesions in two entirely different models of glomerular disease, the nephrotoxic nephritis and anti-Thy-1.1 CG, which closely resemble the human lesions found in CG. One of the resemblances in morphology was the development of pseudocrescents in both the model of CrGN and the CG. Pseudocrescents are typical lesions of CG and less often associated with CrGN. However, so far most studies on human and experimental CrGN make no distinction between true and pseudocrescents. Bariéty et al. showed in a study of patients with anti-GBM disease and class IV lupus glomerulonephritis that both types of crescentic lesions were present in similar percentages.11 Despite the latter study, the epithelial cells forming pseudocrescents have so far mainly been investigated in humans or experimental CG. The development of pseudocrescents is believed to result from podocyte dedifferentiation and dysregulation leading to podocyte hyperplasia. The main argument for the origin from podocytes was the visceral location of the proliferating cells forming the pseudocrescent14.15,27,28 In the present study, it now has been demonstrated unequivocally that in experimental CrGN and CG pseudocrescents including monolayer lesions located directly on top of the glomerular convolute consisted of genetically tagged PECs.
The resemblance in morphology and marker expression in CG and CrGN suggests that despite the different etiologies of both models PECs show a common response to the induced glomerular injury. Interesting in this regard is the de novo expression of CD44, a glycoprotein involved in cell–cell interactions, cell adhesion, and migration in both CG and CrGN. Also in human crescentic lesions found in biopsies of patients with IgA nephropathy, de novo expression of CD44 was described.31 The de novo expression of CD44 in CrGN and CG by PECs may point to a role for this adhesion molecule in the activation migration of PECs.
Another common feature in both experimental CrGN and CG and one of the earliest detectable changes in Bowman's space that has been reported in previous studies are cell bridges between Bowman's capsule and the glomerular convolute2,16,25,32–34 In the present study, PEC lineage tracing enabled us to identify bridging PECs that adhered to the glomerular tuft. It is tempting to speculate that bridging of the PECs is a critical process in the development of the hyperplastic lesions. In this regard, understanding the processes involved in the activation of PECs, bridge formation, and PEC proliferation might provide targets for intervention to inhibit and even prevent the formation of hyperplastic lesions and subsequent loss of renal function.
Concise Methods
Transgenic Mice
The generation of PEC-rtTA, POD-rtTA, LC1, R26R, and Thy1.1 transgenic mice was described previously.22,35–37 Animals were housed under standard specific pathogen-free conditions. All animal procedures were approved by the Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen (8.87–50.10.35.08.106; 8.87–50.10.35.08.254, and 50.203.2—AC 10/06).
Doxycycline Treatment
The PEC-rtTA, POD-rtTA, and PEC-rtTA/Thy-1.1 animals received doxycycline hydrochloride via the drinking water ad libidum for a total of 14 d (5% sucrose and 0.5 mg of doxycycline per milliliter, protected from light), which was exchanged every 2 d. To be sure that the mice are free from doxycycline during the experimental procedures, the mice received normal drinking water ad libdtum for at least 7 d.
Animal Experiments
Seven-week-old POD-rtTA (n = 10) and PEC-rtTA (n = 10) mice received a single intraperitoneal injection of 0.8 ml of sheep anti-mouse nephrotoxic30 serum spiked with 40 μg of CpG oligonucleotides. The proteinuria and hematuria were checked every 2 d by dipstick analysis (Roche, Basel, Switzerland). After 14 d, the mice were killed, and the kidneys were collected.
Seven-week-old PEC-rtTA/LC1/R26R/Thy-1.1 crossbreeds (n = 5) received an intravenous injection with 2 mg of anti-Thy-1.1 mAb (19XE5) in 0.1 ml of 0.9% saline solution. The proteinuria and hematuria were checked daily by dipstick analysis. Kidney samples were collected at day 7 after anti-Thy-1.1 mAb injection.
Perfusion Fixation
Mice were anesthetized (ketamine/rompun). The blood vessels toward and from the left kidney were clamped, and the left kidney was removed. Subsequently, the right kidney was perfused via the left heart ventricle with perfusion solution (3% paraformaldehyde, 0.2% glutaraldehyde, 2.5 mM EGTA, and 4 mM MgCl2 in 0.5× PBS, pH 7.6) for 3 min. Pieces of both kidneys were snap-frozen in liquid nitrogen or (post)fixed in 3% buffered formalin and embedded in paraffin.
Light Microscopy
For light microscopy, kidney fragments were fixed in 3% buffered formalin, dehydrated, and embedded in paraffin. Four-micrometer sections were stained with PAS. To obtain the percentage of glomeruli containing lesions, at least 60 glomeruli per mouse were evaluated for the presence of hypertrophy and hyperplasia of the glomerular epithelium, adhesions, sclerosis, or hyalinosis. Crescentic lesions were defined as cellular structures made of two or more cell layers in the urinary space. True crescents were distinguished from pseudocrescents.11 In true crescents, the epithelium lining Bowman's capsule is replaced by several cell layers, and Bowman's space often is obliterated, either segmentally or circumferentially by these proliferating, flattened cells.
Pseudocrescents consist of cuboidal, polygonal cells proliferating close to the glomerular tuft and not necessarily lining Bowman's capsule. Often, there remains some free Bowman's space.
In addition to the multilayered crescentic lesions, monolayer lesions were observed that consisted of one layer of proliferating cells located directly on the glomerular tuft. Also monolayer lesions were classified as pseudocrescents.
β-Galactosidase Detection
β-Gal activity was detected using enzymatic X-gal or Bluo-gal staining. For this purpose, 4- to 6-μm cryosections were incubated overnight at 37°C in a humidified atmosphere in staining solution (1 mg/ml X-gal or Bluo-gal, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 2 mM MgCl2 in PBS, pH 7.8). On the next day, samples were either counterstained with eosin, washed in tap water, and mounted (Immu-Mount, Thermo Scientific, Waltham, MA) or further processed for immunofluorescence or immunohistochemistry (see below).
Immunofluorescence
The immunofluorescence stainings were performed on 2- or 4-μm cryosections. The sections were fixed in −20°C acetone with the exception of the sections used for the double stainings with X-gal and stainings for BrdU (see below). For double labeling with X-gal, immunofluorescence staining was performed on the X-gal stained sections (see Concise Methods, “β-Galactosidase Detection”). The BrdU staining was performed on 3% buffered paraformaldehyde fixed cryosections. Prior to the immunostaining, the sections were incubated in 2 N HCl solution at 37°C for 30 min and subsequently neutralized in borate buffer.
For the single and double immunofluorescence staining, the sections were incubated with the following primary antibodies: rabbit anti-claudin-1, chicken anti-nestin, mouse anti-BrdU, and rabbit anti-collagen IV (Abcam, Cambridge, UK), rabbit anti-podocin P0372 (Sigma Aldrich, St. Louis, MO), rabbit anti-nephrin (kind gift of Larry Holzman, University of Pennsylvania, Philadelphia), rat anti-CD44, (R&D Systems, Minneapolis, MN), rabbit anti-mouse Ki-67 (Dianova Immundiagnostic, Hamburg, Germany), rat anti-mouse CD68 (clone FA-11, Serotec, Oxford, UK), rat anti-aminopeptidase A (ASD41),38 and rat anti-ASD33 (ASD33).38 The following secondary antibodies were used: goat anti-rabbit Dylight 549 (1:100 #35557, Pierce Biotechnology, Rockford, IL), Alexa Fluor 488-conjugated goat anti-rabbit, Alexa Fluor 488-conjugated anti-rat, Alexa Fluor 568-conjugated anti-rat IgG, and Alexa Fluor 488-conjugated anti-mouse IgG (MoBiTech, Goettingen, Germany), and carbocyanine 2-conjugated donkey anti-chicken IgG (Jackson ImmunoResearch Laboratories). All secondary antibodies, except the anti-mouse antibody, were immuno-absorbed with 4% normal mouse serum. The nuclei were stained using a Hoechst 33342 (Sigma Aldrich, St Louis, MO). Sections were evaluated with an Olympus BX 41 microscope. Images were collected with AnalySIS (Soft Imaging System, Muenster, Germany).
Triple immunofluorescence staining was performed on 2-μm acetone-fixed cryosections. The sections were incubated with the following primary antibodies: mouse anti-mouse Thy-1.1 mAb (19XE5, subclass IgG3),25 rat anti-CD44, (R&D Systems, Minneapolis, MN), and an anti-heparan sulfate single-chain antibody HS4E4.39 The following secondary antibodies were used: Alexa Fluor 647-conjugated anti-mouse IgG3, Alexa Fluor 568-conjugated anti-rat IgG (MoBiTech, Goettingen, Germany), and the single-chain antibody were detected with a carbocyanine 2-labeled rabbit antibody directed against the vesicular stomatitis virus G epitope tag (Sigma-Aldrich, St. Louis, MO). The sections were examined with a confocal laser scanning microscope (Leica Lasertechnik GmbH, Heidelberg, Germany).
Immunohistochemistry
For double labeling of claudin-1 with X-gal or Bluo-gal, 4-μm kidney X-gal or Bluo-gal stained sections (see Concise Methods, “β-Galactosidase Detection”) were processed for immunohistochemistry. The sections were blocked for endogenous avidin/biotin (Avidin/Biotin Blocking Kit, Vector Laboratories, Burlingame, CA) and peroxidase activity (3% H2O2). Subsequently, the sections were incubated with a rabbit anti-claudin-1 antibody (Abcam, Cambridge, UK) or rat anti-CD44 (BD Pharmingen, Franklin Lakes, NJ). As a secondary antibody, a biotinylated goat anti-rabbit or goat anti-rat antibody was used (Vector Laboratories, Burlingame, CA). Detection was carried out with Vectastain ABC Kit (Vector Laboratories, Burlingame, CA) with the use of peroxidase as a label and 3-amino-9-ethylcarbazole as a substrate. Images were acquired using an Olympus BX 41 microscope. Images were collected with AnalySIS.
To establish whether CD44 expression correlated with the presence of a crescentic lesion, we performed CD44 immunostaining on 4-μm paraffin sections. The sections were subjected to microwave antigen retrieval in Antigen Unmasking Solution (Vector Laboratories, Burlingame, CA) followed by the immunostaining as described above. Subsequently, the sections were stained with PAS). CD44 staining was assessed in four mice at day 14 after CrGN induction. At least 70 glomeruli with crescentic lesions were examined per mouse.
To quantify the relative contributions of podocytes and PECs to the development of the crescentic lesions, we examined double staining of X-gal and CD44 in POD-rtTA (n = 4) and PEC-rtTA (n = 4) mice. We quantified the percentage of CD44 positive crescentic lesions that contained X-gal stained podocytes or PECs. At least 30 glomeruli containing a CD44 positive crescent per mouse were examined.
Disclosures
None.
Acknowledgments
This work was supported by the German Research Foundation (DFG) (MO1082/3–1, P17 SFB/Transregio 57), the Interdisciplinary Center for Clinical Research “BIOMAT” within the Faculty of Medicine at the Aachen University of Technology (RWTH-Aachen), the Bundesministerium für Wissenschaft und Forschung (FKZ 01GN0804), the Else Kröner-Fresenius-Stiftung, the START Program of the Faculty of Medicine, RWTH-Aachen (to M.J.M.), and the NephCure Foundation (F001 to B.S.). Furthermore, this work received support from the The Netherlands Organization for Scientific Research (2007/09196/ALW to B.S.). M.J.M. is a member of the DFG research group “Mechanisms of Chronic Renal Failure” and of the SFB/Transregio 57 DFG consortium “Mechanisms of Organ Fibrosis”.
We thank Dr. L. Gnudi, King's College London, London, UK, for providing the POD-rtTA-M2 transgenic mice, and Larry Holzman, University of Pennsylvania, Philadelphia, and T. van Kuppevelt, Radboud University Nijmegen Medical Center, Nijmegen, The Netherlands, for providing the anti-nephrin and anti-heparan sulfates antibodies, respectively.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Jennette JC, Olson JL, Schwartz MM, Silva FG: Crescentic glomerulonephritis. In: Heptinstall's Pathology of the Kidney, 6th Ed., Philadelphia, Lippincott-Raven, pp 615–641, 2005 [Google Scholar]
- 2.Le Hir M, Besse-Eschmann V: A novel mechanism of nephron loss in a murine model of crescentic glomerulonephritis. Kidney Int 63: 591–599, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Detwiler RK, Falk RJ, Hogan SL, Jennette JC: Collapsing glomerulopathy: A clinically and pathologically distinct variant of focal segmental glomerulosclerosis. Kidney Int 45: 1416–1424, 1994 [DOI] [PubMed] [Google Scholar]
- 4.D'Agati V, Suh JI, Carbone L, Cheng JT, Appel G: Pathology of HIV-associated nephropathy: A detailed morphologic and comparative study. Kidney Int 35: 1358–1370, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Moudgil A, Nast CC, Bagga A, Wei L, Nurmamet A, Cohen AH, Jordan SC, Toyoda M: Association of parvovirus B19 infection with idiopathic collapsing glomerulopathy. Kidney Int 59: 2126–2133, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S, Kuhn JA, Dratch AD, D'Agati VD: Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol 12: 1164–1172, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Ophascharoensuk V, Pippin JW, Gordon KL, Shankland SJ, Couser WG, Johnson RJ: Role of intrinsic renal cells versus infiltrating cells in glomerular crescent formation. Kidney Int 54: 416–425, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Boucher A, Droz D, Adafer E, Noël LH: Relationship between the integrity of Bowman's capsule and the composition of cellular crescents in human crescentic glomerulonephritis. Lab Invest 56: 526–533, 1987 [PubMed] [Google Scholar]
- 9.Goumenos D, Tsomi K, Iatrou C, Oldroyd S, Sungur A, Papaioannides D, Moustakas G, Ziroyannis P, Mountokalakis T, El Nahas AM: Myofibroblasts and the progression of crescentic glomerulonephritis. Nephrol Dial Transplant 13: 1652–1661, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Moeller MJ, Soofi A, Hartmann I, Le Hir M, Wiggins R, Kriz W, Holzman LB: Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol 15: 61–67, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Bariéty J, Bruneval P, Meyrier A, Mandet C, Hill G, Jacquot C: Podocyte involvement in human immune crescentic glomerulonephritis. Kidney Int 68: 1109–1119, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Ding M, Cui S, Li C, Jothy S, Haase V, Steer BM, Marsden PA, Pippin J, Shankland S, Rastaldi MP, Cohen CD, Kretzler M, Quaggin SE: Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med 12: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Thorner PS, Ho M, Eremina V, Sado Y, Quaggin S: Podocytes contribute to the formation of glomerular crescents. J Am Soc Nephrol 19: 495–502, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barisoni L, Kriz W, Mundel P, D'Agati V: The dysregulated podocyte phenotype: A novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 10: 51–61, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Ohtaka A, Ootaka T, Sato H, Soma J, Sato T, Saito T, Ito S: Significance of early phenotypic change of glomerular podocytes detected by Pax2 in primary focal segmental glomerulosclerosis. Am J Kidney Dis 39: 475–485, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Dijkman H, Smeets B, van der Laak J, Steenbergen E, Wetzels J: The parietal epithelial cell is crucially involved in human idiopathic focal segmental glomerulosclerosis. Kidney Int 68: 1562–1572, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Nagata M, Horita S, Shu Y, Shibata S, Hattori M, Ito K, Watanabe T: Phenotypic characteristics and cyclin-dependent kinase inhibitors repression in hyperplastic epithelial pathology in idiopathic focal segmental glomerulosclerosis. Lab Invest 80: 869–880, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Nagata M, Hattori M, Hamano Y, Ito K, Saitoh K, Watanabe T: Origin and phenotypic features of hyperplastic epithelial cells in collapsing glomerulopathy. Am J Kidney Dis 32: 962–969, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Asano T, Niimura F, Pastan I, Fogo AB, Ichikawa I, Matsusaka T: Permanent genetic tagging of podocytes: Fate of injured podocytes in a mouse model of glomerular sclerosis. J Am Soc Nephrol 16: 2257–2262, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Dijkman HB, Weening JJ, Smeets B, Verrijp KC, van Kuppevelt TH, Assmann KK, Steenbergen EJ, Wetzels JF: Proliferating cells in HIV and pamidronate-associated collapsing focal segmental glomerulosclerosis are parietal epithelial cells. Kidney Int 70: 338–344, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ: Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohse T, Pippin JW, Vaughan MR, Brinkkoetter PT, Krofft RD, Shankland SJ: Establishment of conditionally immortalized mouse glomerular parietal epithelial cells in culture. J Am Soc Nephrol 19: 1879–1890, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouschop KM, Roelofs JJ, Claessen N, da Costa Martins P, Zwaginga JJ, Pals ST, Weening JJ, Florquin S: Protection against renal ischemia reperfusion injury by CD44 disruption. J Am Soc Nephrol 16: 2034–2043, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Smeets B, Te Loeke NA, Dijkman HB, Steenbergen ML, Lensen JF, Begieneman MP, van Kuppevelt TH, Wetzels JF, Steenbergen EJ: The parietal epithelial cell: A key player in the pathogenesis of focal segmental glomerulosclerosis in Thy-1.1 transgenic mice. J Am Soc Nephrol 15: 928–939, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Smeets B, Steenbergen ML, Dijkman HB, Verrijp KN, Te Loeke NA, Aten J, Steenbergen EJ, Wetzels JF: Angiotensin converting enzyme inhibition prevents development of collapsing focal segmental glomerulosclerosis in Thy-1.1 transgenic mice. Nephrol Dial Transplant 21: 3087–3097, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Bariéty J, Bruneval P, Hill G, Irinopoulou T, Mandet C, Meyrier A: Posttransplantation relapse of FSGS is characterized by glomerular epithelial cell transdifferentiation. J Am Soc Nephrol 12: 261–274, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Bariéty J, Nochy D, Mandet C, Jacquot C, Glotz D, Meyrier A: Podocytes undergo phenotypic changes and express macrophagic-associated markers in idiopathic collapsing glomerulopathy. Kidney Int 53: 918–925, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Smeets B, Angelotti ML, Rizzo P, Dijkman H, Lazzeri E, Mooren F, Ballerini L, Parente E, Sagrinati C, Mazzinghi B, Ronconi E, Becherucci F, Benigni A, Steenbergen E, Lasagni L, Remuzzi G, Wetzels J, Romagnani P: Renal progenitor cells form the hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J Am Soc Nephrol 20: 2593–2603, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scholz J, Lukacs-Kornek V, Engel DR, Specht S, Kiss E, Eitner F, Floege J, Groene HJ, Kurts C: Renal dendritic cells stimulate IL-10 production and attenuate nephrotoxic nephritis. J Am Soc Nephrol 19: 527–537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Florquin S, Nunziata R, Claessen N, van den Berg FM, Pals ST, Weening JJ: CD44 expression in IgA nephropathy. Am J Kidney Dis 39: 407–414, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Gibson IW, Downie TT, More IA, Lindop GB: Tuft-to-capsule adhesions and their precursors: Differences between the vascular and tubular poles of the human glomerulus. J Pathol 184: 430–435, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int 54: 687–697, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Le Hir M, Keller C, Eschmann V, Hähnel B, Hosser H, Kriz W: Podocyte bridges between the tuft and Bowman's capsule: An early event in experimental crescentic glomerulonephritis. J Am Soc Nephrol 12: 2060–2071, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Shigehara T, Zaragoza C, Kitiyakara C, Takahashi H, Lu H, Moeller M, Holzman LB, Kopp JB: Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol 14: 1998–2003, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Soriano P: Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Kollias G, Evans DJ, Ritter M, Beech J, Morris R, Grosveld F: Ectopic expression of Thy-1 in the kidneys of transgenic mice induces functional and proliferative abnormalities. Cell 51: 21–31, 1987 [DOI] [PubMed] [Google Scholar]
- 38.Mentzel S, Van Son JP, De Jong AS, Dijkman HB, Koene RA, Wetzels JF, Assmann KJ: Mouse glomerular epithelial cells in culture with features of podocytes in vivo express aminopeptidase A and angiotensinogen but not other components of the renin-angiotensin system. J Am Soc Nephrol 8: 706–719, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Dennissen MA, Jenniskens GJ, Pieffers M, Versteeg EM, Petitou M, Veerkamp JH, van Kuppevelt TH: Large, tissue-regulated domain diversity of heparan sulfates demonstrated by phage display antibodies. J Biol Chem 277: 10982–10986, 2002 [DOI] [PubMed] [Google Scholar]