Abstract
Proper localization of nephrin determines integrity of the glomerular slit diaphragm. Slit diaphragm proteins assemble into functional signaling complexes on a raft-based platform, but how the trafficking of these proteins coordinates with their signaling function is unknown. Here, we demonstrate that a raft-mediated endocytic (RME) pathway internalizes nephrin. Nephrin internalization was slower with raft-mediated endocytosis than with classic clathrin-mediated endocytosis. Ultrastructurally, the RME pathway consisted of noncoated invaginations and was dependent on cholesterol and dynamin. Nephrin constituted a stable, signaling-competent microdomain through interaction with Fyn, a Src kinase, and podocin, a scaffold protein. Tyrosine phosphorylation of nephrin triggered its own RME-mediated internalization. Protamine-induced hyperphosphorylation of nephrin led to noncoated invaginations predominating over coated pits. These results demonstrate that an RME pathway couples nephrin internalization to its own signaling, suggesting that RME promotes proper spatiotemporal assembly of slit diaphragms during podocyte development or injury.
Regulation of the leakiness and structural integrity of the slit diaphragm (SD) is a key process for maintenance of the glomerular filtration function.1 Nephrin is a core structural component of the SD, and its ectodomains interact in a homophilic manner, thereby forming a zipper-like intercellular junction.2,3 In addition to its structural component, nephrin transduces a phosphotyrosine signal through its cytoplasmic domain, which contains several phosphorylation sites for the Src-family kinases, such as Fyn. Ectodomain engagement of nephrin induces tyrosine phosphorylation via the Src-family kinases, which, in turn, leads to recruitment of an adaptor (e.g., Nck) and subsequent actin reorganization.4,5
SD proteins are organized within a specialized submicrodomain of the plasma membrane called rafts, liquid-ordered structures enriched in cholesterol and sphingolipids. They are therefore regarded as lipid–protein supercomplexes formed by a dynamic lateral assembly of the microdomains.6 Clustering of nephrin leads to sequential coalescence of preexisting small rafts, thereby forming a large-scale, stable signal platform through selective sequestration of adaptors and scaffolds.7,8 In podocyte foot processes, a raft-resident scaffold protein, podocin, and a CD2-associated protein, CD2AP, facilitate the selective recruitment of signaling molecules and strengthen nephrin-based cell–cell attachment.9–13 These scaffold proteins accommodate diverse functions of the SD complex: Cell survival, polarity, endocytosis, and cytoskeletal organization.6
More than 70 nephrin mutations have been reported in familial nephrotic syndrome.3,14,15 Several studies with humans and in animal nephrotic models showed that dysfunctional nephrin may be downregulated or mislocated in the effaced foot processes. These observations indicated that the spatiotemporal regulation of nephrin is a critical determinant of SD leakiness; however, the detailed mechanisms are still unclear. In this study, we focused on the role of endocytosis, a fundamental process that coordinates cell-surface expression and signal transduction.16 In mammalian cells, endocytosis is mediated via two principal routes: clathrin-mediated endocytosis (CME) and clathrin-independent, raft-mediated endocytosis (RME).16–19 CME targets proteins to the early endosome, a sorting station directing vesicles to either recycling or degradation. Besides this “classic” CME pathway, RME has recently been the focus of intensive research, uncovering a new concept that the microdomain itself behaves as a vehicle for internalization. RME is generally defined by its clathrin independence, cholesterol sensitivity, and a typical morphology of smooth invaginations.18,19 Several subtypes of RME pathways are differentially regulated by caveolin, dynamin, and a small GTPase, and they have been implicated in diverse biologic processes (e.g., viral infection, immunologic reactions, receptor signaling).19
Podocytes can internalize circulating proteins as well as SD constituents.20–22 In nephrotic podocytes, the number of endocytic vesicles is markedly increased and nephrin is downregulated and/or dislocated.23,24 In view of accumulating evidence that SD proteins are assembled into functional signal complexes on a raft-based platform, we reasoned that an RME pathway could play a key role in spatiotemporal regulation of nephrin; however, it is still unclear how nephrin specifies CME or RME pathways and how these trafficking routes are coordinated with the signaling function. This study is the first to demonstrate that nephrin traffics via RME in cultured cells and podocytes in vivo. The results point to the possibility that this new RME pathway is implicated in remodeling and development of foot processes; these observations should attract further research focusing on disease pathogenesis and drug discovery.
Results
Internalized Nephrin Is Sorted into Endosomal Compartments
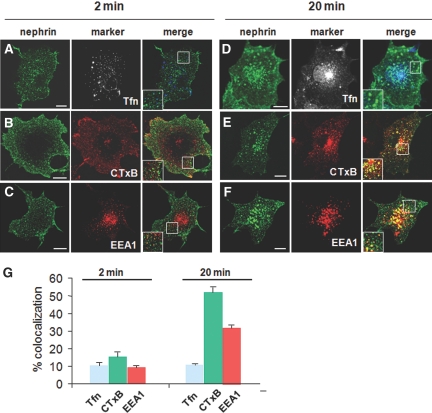
We first traced the nephrin endocytic pathway using transfected COS-7 cells,25 because they have a favorable morphology for quantification and they internalize nephrin in a similar manner to cultured podocytes (Supplemental Figure 1). At the early phase of internalization (2 min), nephrin was taken up into fine punctate structures near the cell periphery (Figure 1, A through C). The numbers of cytoplasmic vesicles at 2 min were significantly greater than those at time 0 (no temperature shift to 37°C after surface labeling; data not shown). Internalized nephrin only partially overlapped with transferrin, a CME marker (9.9 ± 1.8%), cholera-toxin B (CTxB) subunit, an RME marker (15.2 ± 2.5%), and EEA1, an early endosome marker (8.7 ± 1.2%). This suggests that these organelles represent nascent, incoming endocytic intermediates that are in transition to mature early endosomes (Supplemental Figure 2).19,26 When cells were allowed to endocytose continually for 20 min (Figure 1, D through G), nephrin became sorted into larger vesicular organelles and distributed broadly from the cell periphery to the perinuclear area. The endocytic structures at 20 min were remarkably co-localized with CTxB (52.1 ± 2.8%) and EAA1 (31.7 ± 1.7%) but only partially with the late endosome marker LAMP-2 (8.4 ± 2.7%; data not shown). This indicated that additional nephrin was trafficked to early endosomes enriched in lipid rafts. By contrast, nephrin was co-localized to a lesser degree with transferrin (10.8 ± 0.4%), which was taken up much faster and condensed in the perinuclear regions. There was little if any co-localization of nephrin with the fluid-phase marker dextran at either stage of internalization (data not shown). These observations suggest that nephrin is initially sorted into a peripheral pre-endosomal intermediate and becomes increasingly incorporated into an EEA1-positive, lipid raft–enriched compartment at later times.
Figure 1.
Nephrin is internalized in pre- and early endosomal compartments. A single nephrin transfectant of COS-7 cells (no Fyn overexpression) was surface-labeled using an anti-nephrin ectodomain antibody and was internalized at 37°C. Cells were double labeled with the endocytic markers of CME (transferrin [Tfn]; A and D), RME (CTxB; B and E), and the early endosomes (EEA1; C and F). (Insets) Magnified views of boxed areas. Bar = 10 μm. At very early times (2 min), nephrin distributes at the cell periphery and co-localizes to some extent with Tfn (A), CTxB (B), and, to a lesser degree, EEA1 (C). As endocytosis proceeds over a longer period of time (20 min), nephrin becomes sorted into a larger punctate structure that distributes evenly over the peripheral sorting endosomes. (E and F) Incoming nephrin overlaps markedly with CTxB (E) and EAA1 (F). Note that nephrin merges only slightly with Tfn (D; a CME marker taken up more rapidly than nephrin). (G) Quantification of co-localization. A proportion of merged signals in the total cytoplasmic, nephrin-positive puncta is shown. Data are means ± SEM for a total of 50 cell profiles from three independent experiments.
Ultrastructure of Early Endocytic Intermediates on the Plasma Membrane
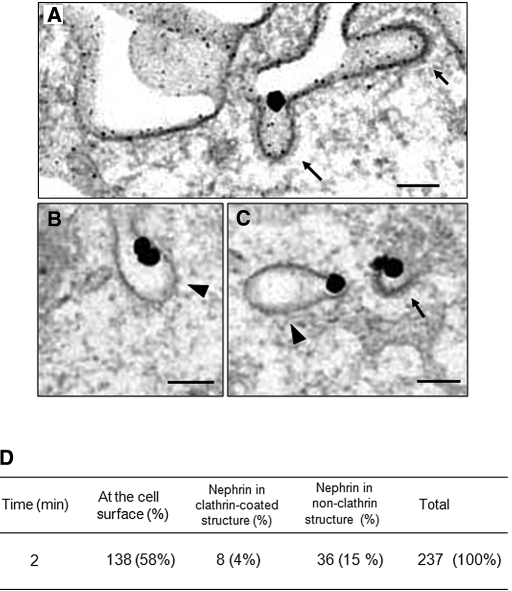
We next used electron microscopy to visualize the ultrastructure of endocytic vehicles for nephrin in transfected COS-7 cells. At 2 min, immunogold labeling showed that nephrin localized predominantly to the plasma membrane in flat regions and in invaginations, as well as to intracellular vesicles. Some nephrin also associated with coated structures at the cell surface and in the subplasmalemma zone (clathrin-coated pits or vesicles; Figure 2, A and C). In addition, we detected nephrin in 50- to 75-nm diameter noncoated, smooth membrane invaginations, which resembled caveolae, a canonical raft-based endocytic organelle (Figure 2, B and C).8,27 Quantitative analysis of a total of 237 nephrin-gold particles counted at 2 min of internalization revealed that 15% of surface-labeled nephrin was associated with nonclathrin structures, whereas 4% was with clathrin-coated structures and 58% was still at the plasma membrane (Figure 2D). The data indicated that nephrin is internalized via morphologically distinct endocytic intermediates of both coated and noncoated types.
Figure 2.
Ultrastructure of early endocytic organelles containing nephrin is shown. Representative electron micrographs of the pre-embedding immunogold labeling of nephrin in COS-7 cell transfectants. Surface nephrin was labeled at 4°C with anti-nephrin antibody and allowed to be endocytosed at 37°C for 2 min. Nephrin was detected by a gold-enhanced immunogold technique. (A) Incoming nephrin associates with the electron-dense coated membrane invaginations (clathrin coated pits, arrows). Bar = 100 nm. (B) Nephrin localizes to noncoated, flask-shaped smooth membrane invaginations (arrowhead), Bar = 100 nm. (C) Clathrin-coated pits (arrow) and noncoated invaginations (arrowhead) are observed. Bar = 100 nm. (D) Quantification of early endocytic structures. The number of nephrin immunogold labels associating with the indicated structures was counted. Results were derived from six independent experiments. Magnification, ×50,000.
Nephrin Internalizes via Two Distinct Endocytic Routes
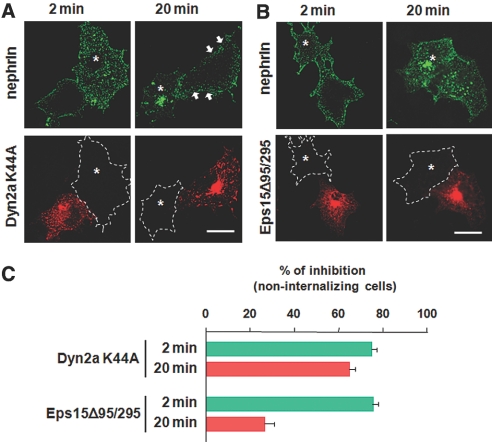
To explore the detailed mechanisms of the endocytosis, we examined the effects of dominant negative mutants on nephrin trafficking. A dynamin-2a K44A mutant inhibits most but not all endocytic pathways by perturbing scission of invaginated pits from the plasma membrane.17,19 In COS-7 cells, dynamin-2a K44A showed a marked inhibition of nephrin internalization at both 2 and 20 min by 75.9 ± 2.2% and 64.2 ± 2.5%, respectively (Figure 3, A and C). This indicated that a dynamin-based fission mechanism is required for both the RME and CME pathways. The Eps15 Δ95/295 mutant interferes with clathrin-coated pit assembly by binding to the AP-2 adaptor, thereby specifically blocking the CME pathway. Eps15 Δ95/295 significantly blocked nephrin internalization at 2 min by 77.4 ± 2.5%, whereas it only slightly inhibited nephrin uptake at 20 min by 23.3 ± 4.4% (Figure 3, B and C). Together, these results indicate that a portion of nephrin rapidly enters the cells via the CME pathway while even more nephrin is internalized by the nonclathrin RME route as endocytosis proceeds.
Figure 3.
Effects of dominant negative inhibitors on nephrin endocytosis are shown. (A and B) COS-7 cells coexpressing nephrin with either HA-Dyn2a K44A (A) or HA-Eps15 Δ95/295 (B) were surface-labeled by anti-nephrin antibody at 4°C and incubated at 37°C to allow internalization. Cells were visualized for nephrin (green) and mutants (red). (A) Dyn2a K44A inhibits nephrin uptake at both 2 and 20 min to a similar degree. The majority of nephrin remains on the cell edge or underneath the plasma membrane (arrows). (B) Eps15 Δ95/295 significantly blocked nephrin endocytosis at 2 min, whereas it only partially inhibited internalization at 20 min. White dashed lines represent the contour lines of neighboring control cells devoid of mutant expression. Bars = 10 μm. (C) Histogram denotes the percentage of cells that exhibit >10-fold reduction in nephrin internalization when compared with mutant-negative control cells. Data are means ± SEM for a total of 50 cell profiles from three independent experiments.
We next examined the effects of pharmacologic inhibitors: Potassium chloride-deficient media were used because they inhibit CME through disruption of the clathrin-lattice assembly,16 and methyl-β-cyclodextrin (MβCD), a cholesterol-depleting reagent, was used because it primarily inhibits the RME pathway but also interferes to some extent with the CME route.28,29 Potassium depletion blocked nephrin endocytosis mainly at the early time points (2 min, by 67.5 ± 1.3% compared with control) while inhibiting the later phase uptake to a lesser degree (20 min, by 46.9 ± 1.6%; Supplemental Figure 3). Conversely, inhibition of nephrin uptake was more pronounced by treatment with MβCD at later stages (20 min; 63.1 ± 1.9%) than at earlier stages (2 min; 48.8 ± 2.4%). This indicated that the later stages of endocytosis were more cholesterol dependent than the early phases. These results again support the notion that the CME pathway allows rapid internalization of nephrin, whereas the RME route mediates a much slower entry.
Nephrin Forms a Microdomain via Dynamic Lateral Raft Assembly
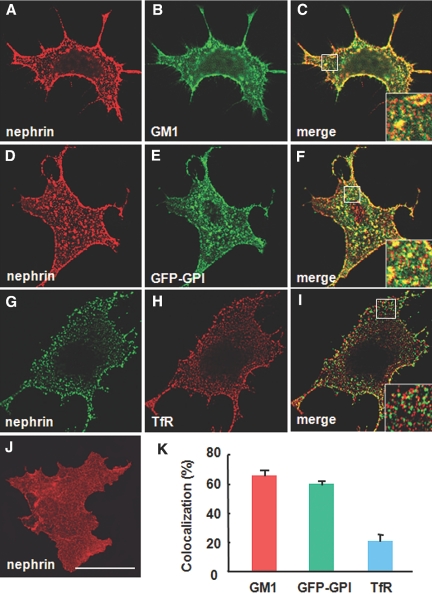
Compartmentalization of nephrin into specific membrane microdomains is potentially a key mechanism for its own selective sorting into RME pathways. To address this possibility, we performed co-patching experiments. When nephrin and GM1 were clustered on the cell surface by an anti-nephrin antibody and CTxB, respectively, the nephrin patches efficiently co-clustered with GM1 (Figure 4, A through C). Co-patching of nephrin was observed with another raft marker, GFP-fused glycosyl phosphatidylinositol (GPI-GFP; Figure 4, D through F). Notably, a nonraft marker, transferrin receptor (TfR), was spatially segregated from nephrin patches (Figure 4, G through I). The extent of nephrin co-localization with GMI and GPI-GFP (65.4 ± 3.7 and 59.5 ± 2.6%, respectively) was more remarkable than TfR (20.6 ± 5.0%; Figure 4 K). These data indicate that nephrin microdomains share a common liquid-ordered composition with the raft marker GM1 and GPI but not TfR, thereby constituting a highly specific subset of microdomains.
Figure 4.
Cross-linking induced co-patching of nephrin with the raft markers. Lateral cross-linking of raft constituents and visualization of the microdomains as discrete patches, which segregate spatially away from nonraft markers.28 Co-localization of clustered nephrin with two raft markers CTxB or GFP-GPI and nonraft marker V5-tagged TfR is examined in COS-7 cells. (A through C) Co-patching of nephrin (A) and GM1 (B). (C) Merged image. Nephrin and GM1 were cross-linked by anti-nephrin antibody and FITC-CTxB, a pentameric ligand for the ganglioside GM1, respectively. (D and E) Co-patching of nephrin (D) and GFP-GPI (E). (F) Merged image. Nephrin and GFP-GPI were cross-linked by anti-nephrin antibody and anti-GFP antibody, respectively. (G and H) Patching of nephrin (G) and TfR (H). (I) Merged image. Nephrin and TfR were cross-linked by anti-nephrin antibody and anti-V5 antibody, respectively. (Insets) Magnification of the boxed area. Bar = 10 μm. (J) appearance of nonclustered nephrin at the cell surface. (K) Quantitative analysis. A proportion of merged signals in the total of cytoplasmic, nephrin-positive puncta is shown. Data are means ± SEM from three independent experiments.
Nephrin Interacts and Co-internalizes with the Scaffold Protein Podocin
Because nephrin co-localizes with podocin at the SD in vivo, we reasoned that interactions between these two proteins may have relevance in regulation of the RME. To address this issue, we examined the biochemical basis for nephrin-podocin interactions. A sucrose gradient floating assay of COS-7 cells and isolated rat glomeruli demonstrated that nephrin co-separates with podocin into a low-buoyant, detergent-resistance membrane fraction in a cholesterol-dependent manner (Supplemental Figure 4, A through C).
We next examined how internalization of these two proteins is coordinated. At a steady state in nephrin-podocin double-transfected COS-7 cells, podocin displayed a punctate vesicle pattern scattering throughout the cytoplasm with some linear staining at cell edges, whereas nephrin exhibited a fine reticular distribution with only a few scattered puncta (data not shown). At early steps of internalization (2 min; Supplemental Figure 4, D and E), incoming nephrin showed little co-localization with podocin, indicating that nephrin initially enters cells independent of podocin. In contrast, at later times (20 min), internalized nephrin was increasingly sorted into larger, brighter endocytic structures that co-localized with podocin. Quantification analysis demonstrated that the extent of nephrin co-localization with podocin at 20 min (49.6 ± 2.9%) was four-fold greater than at 2 min (11.7 ± 1.4%). Immunoprecipitation as well as GST pull-down assays demonstrated the ability of podocin to bind nephrin and vice versa (data not shown). The data indicate that nephrin physically interacts with podocin through the rafts and is co-internalized with podocin primarily along the RME pathway.
Diffusional Mobility of Nephrin Is Restricted by Podocin
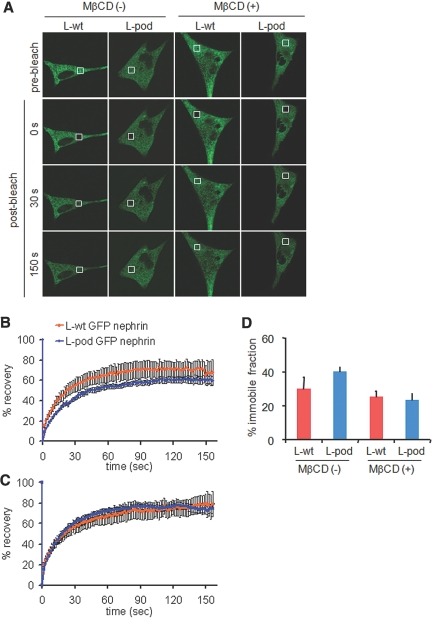
To examine the role of podocin in coordinating the nephrin microdomains, we examined diffusional mobility of GFP-nephrin in living cells by fluorescence recovery after photobleaching (FRAP) techniques.25 Wild-type L cells (L-wt) or L cells stably expressing podocin (L-pod) were transiently transfected with GFP-nephrin (L-wt GFP-nephrin and L-pod GFP-nephrin, respectively), and fluorescence recovery was compared. As shown in Figure 5, A and B, in L-wt GFP-nephrin cells, fluorescence intensity reached a plateau at 70% of the prebleached level, with a recovery time of 60 to 90 s (n = 6). In contrast, in L-pod GFP-nephrin cells, the fluorescence intensity recovered to only approximately 60% of the initial level, indicating that podocin enhanced sequestration of GFP-nephrin into the immobile fraction 1.3-fold (39.7 ± 2.9%) compared with the control (29.6 ± 7.3%; n = 8 to 10; P < 0.05; Figure 5, C and D). After treatment with 10 mM MβCD for 30 min at 37°C, both L-wt GFP-nephrin and L-pod GFP-nephrin cells recovered fluorescence to levels similar to prebleaching (25.2 ± 3.3 and 23.3 ± 3.7%, respectively; n = 6; Figure 5, C and D). The FRAP properties did not significantly differ among the bleach sites (peripheral or perinuclear portions) or cell types (i.e., COS-7 cells; data not shown). The data indicate that podocin restricts diffusive motion of nephrin in a cholesterol-dependent manner.
Figure 5.
Diffusional mobility of nephrin is restricted by the scaffold podocin. (A) FRAP was tested for a 4-μm-diameter photobleached area in living L-wt GFP-nephrin or L-pod GFP-nephrin. (B and C) Kinetics of the fluorescence recovery in the absence (B) or presence (C) of 10 mM MβCD treatment. After treatment with MβCD, fluorescence of nephrin and podocin double transfectants recovered to the same level as that of the single nephrin transfectants. Data are normalized to yield an intensity of 0 immediately after photobleaching and represent the average of 10 individual traces. (D) Quantification of cholesterol sensitivity. Immobile fractions, as estimated by fluorescence recovery levels at time points that reached a plateau, were compared. Data are means ± SEM from three independent experiments (n = 6 to 10).
Nephrin Signaling Is Functionally Linked to Endocytosis
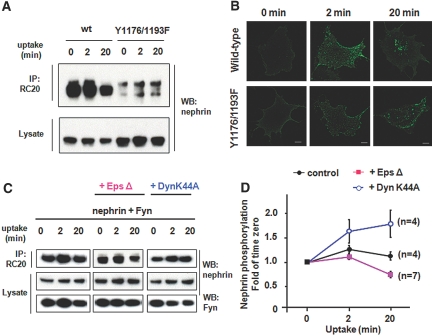
In view of recent studies showing that the RME pathway is a triggered process,8,19 we investigated whether nephrin signaling is linked to the endocytic process. Tyrosine phosphorylation of nephrin was augmented by coexpression of an active form of Fyn (Y531F) in COS-7 cells, whereas it was attenuated in the presence of the Src-family kinase inhibitor PP2 and by an inactive form of Fyn (K299M; Supplemental Figure 5A), suggesting that nephrin signaling is Fyn dependent. Antibody-mediated clustering of nephrin at the cell surface caused a dramatic change in the intracellular distribution of Fyn, inducing large discrete puncta, in contrast to the fine, evenly dispersed pattern in control cells (Supplemental Figure 5B). To test directly the role of cytoplasmic tyrosine phosphorylation in endocytic trafficking, we created a nephrin double mutant, Y1176F/Y1193F, in which two major tyrosine phosphorylation sites are mutated to phenylalanine (Supplemental Figure 6).4,5 Fyn-mediated phosphorylation of the Y1176F/Y1193F construct was significantly reduced (Figure 6A). Despite no appreciable alterations at 2 min of internalization in COS-7 cells, the underphosphorylated Y1176F/Y1193F exhibited more remarkable retention near the cell periphery at 20 min of uptake (Figure 6B). These data indicate that underphosphorylation of nephrin perturbs its own RME.
Figure 6.
Nephrin signaling is functionally coupled to its endocytic trafficking. (A) Nephrin Y1176F/Y1193F is hypophosphorylated. Lysates of a COS-7 transfectant coexpressing nephrin (wild-type or Y1176F/Y1193F) and Fyn were immunoprecipitated by the PY20 antibody and immunoblotted with an anti-nephrin antibody. (B) Reduced uptake of nephrin Y1176F/Y1193F. A COS-7 cell transfectant singly expressing either wild-type or Y1176F/Y1193F (no Fyn overexpression) was surface-labeled with anti-nephrin antibody and allowed to internalize at 37°C. The underphosphorylated nephrin Y1176F/Y1193F is poorly internalized at 20 min compared with wild-type nephrin. (C and D) Effects of pathway-specific dominant negative inhibitors on signaling. The extent of tyrosine phosphorylation was examined in nephrin-Fyn coexpressing COS-7 cells with or without inhibitors (either Dyn2a K44A or Eps-15 Δ95/295). The cells were surface-labeled with anti-nephrin antibody at 4°C and then allowed to endocytose at 37°C. Tyrosine-phosphorylated nephrin was detected by immunoblotting the PY20 antibody–derived immunoprecipitates with anti-nephrin antibody. Equivalent loading was confirmed by immunoblotting whole-cell lysates (C). The degree of nephrin phosphorylation is quantified and graphed as a change (fold) of time 0 versus time 2 and 20 min (D). Data are means ± SEM from four to seven separate experiments.
To test whether activated endocytic pathways reciprocally affect spatiotemporal signal propagation, we examined the effects of pathway-specific inhibitors on nephrin phosphorylation (Figure 6, C and D). In control COS-7 cells coexpressing nephrin and Fyn, phosphorylation of nephrin reached a maximum level at 2 min and gradually declined to the initial value during the subsequent 20 min. This suggests that the rates of assembly and breakdown of signaling-competent microdomains become equilibrated at 20 min. A CME pathway–specific inhibitor, Eps15 Δ95/295, led to a slight reduction of nephrin phosphorylation at 20 min while leaving it unaltered at 2 min. These results raised the possibility that the CME pathway is not directly implicated in the initial signal event but that its selective blockage attenuates signaling as a result of compensatory induction of the alternative RME pathway(s)16,30 A dynamin-2a K44A construct, a universal inhibitor for both RME and CME pathways, prolongs the time that nephrin is engaged in signaling at the plasma membrane. This mutant caused a slight increase in nephrin phosphorylation at 20 min while exerting little effect at 2 min (Figure 6, C and D). These data indicate that the signaling-competent microdomain enters the cells via RME at a slow rate, thereby downregulating the signaling complexes.
Uncoated Plasma Membrane Invaginations in Protamine Sulfate–Treated Podocytes
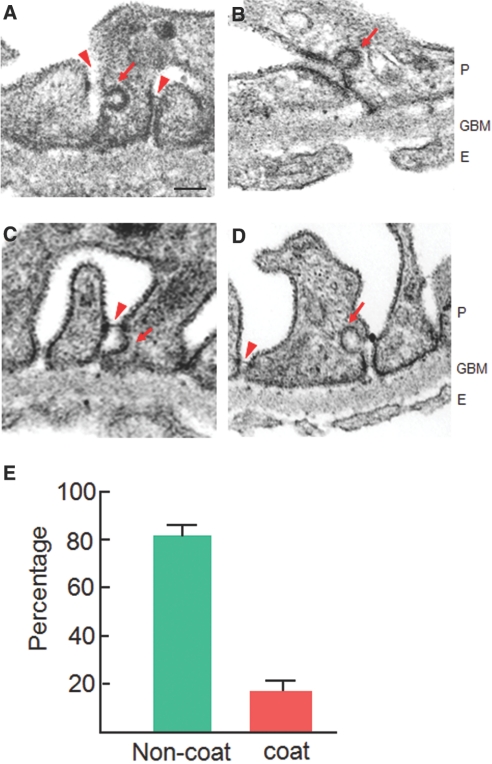
To investigate the physiologic relevance of nephrin endocytosis, we examined the ultrastructure of foot processes in a protamine sulfate (PS)-induced rat podocyte injury model.31 PS perfusion induces increased phosphorylation of the SD proteins. Moreover, it leads to a reversible foot process effacement and SD dislocation without any destructive tissue damage, thereby providing a suitable model for ultrastructural analysis of endocytic vesicles. Electron microscopy of the lateral domain in PS-treated foot processes revealed plasma membrane invaginations with diameters of 50 to 100 nm, a morphology similar to that observed in cultured cells (Figure 2). Membrane buddings in foot processes were significantly more abundant in the PS-model than in controls (no pits in >200 randomly selected foot processes). In PS-treated foot processes, the proportion of noncoated smooth invaginations was greater than that of coated pits around the SD (82 ± 4%; n = 67; Figure 7). These data suggest that a fraction of nephrin is endocytosed via a nonclathrin carrier in vivo, particularly in situations in which the podocyte is actively phosphorylated (Figure 8).
Figure 7.
Plasma membrane invaginations in PS-treated podocytes are shown. Adult rat kidneys were perfused with PS for 15 min before fixation. (A through D) Perfusion with PS induced diffuse foot process effacement and apical dislocation of the SD (arrowheads). Some buddings from the plasma membrane were lined with electron-dense coats (clathrin coated pits; arrows in A and B), whereas others lacked the coat materials (caveola-like appearance, noncoated pits; arrows in C and D). P, podocytes; GBM, glomerular basement membrane; E, endothelial cells. Bar = 100 nm. (E) Histogram represents quantification of plasma membrane invaginations at the lateral domain of the foot process membranes, where the SD complexes are most sequestered. Only structures visibly continuous with the plasma membrane are counted. Data are means ± SEM (n = 67) from three independent experiments. Magnification, ×70,000.
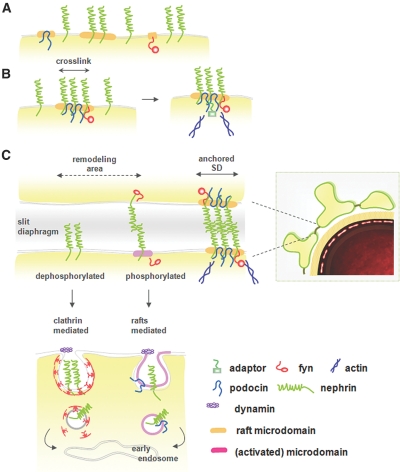
Figure 8.
A model for differential sorting of nephrin along endocytic pathways is shown. (A) The SD is composed of the backbone nephrin, signaling molecules (e.g., Fyn), and scaffold proteins (podocin), all of which reside individually in membrane microdomains. (B) Clustering of nephrin induces coalescence of microdomains that in turn leads to selective recruitment of the signaling components for its own tyrosine phosphorylation. Podocin facilitates the formation of a signaling platform and stabilizes the SD complex by linking it to the actin cytoskeleton of foot processes. (C) In the steady state, cell surface nephrin comprises two distinct membrane pools of raft and nonraft compartments. Most of the raft-resident nephrin molecules are organized into a largely immobile lipid–protein supercomplex in a dephosphorylated form, and they turn over at a slow rate in the foot processes. Clathrin-mediated machinery captures the membrane compartments spatially at random, irrespective of their raft association, and internalizes them quickly at a constant rate. β-Arrestin 2 preferentially binds to the nonraft, dephosphorylated nephrin form and directs it toward the CME pathway.38 This pathway ensures fast and continuous replacement of surface nephrin from a reservoir of its intracellular vesicles, thereby maintaining its steady-state surface expression level. Conversely, the nephrin residing within the functional signaling platform is internalized via a distinct, raft-enriched endocytic structure that traffics at a slower rate (the RME pathway). This pathway specifically internalizes the signal-activated domains containing phosphorylated nephrin, which in turn removes the signaling microdomains from the cell surface (in a “shut-off” feedback loop). The RME therefore enables a finely tuned, spatiotemporal sequestering of the SD complex by minimizing unnecessary breakdown of the signaling platforms. Such cooperation between signaling and endocytosis is particularly important when podocytes assemble a new SD complex (e.g., during development and the remodeling processes that follow injury).
Discussion
Our results demonstrate that nephrin is internalized via dynamin-dependent RME pathways, in addition to the widely known classic CME route. Vesicle-like, noncoated vesicles were morphologically distinct from those of previously reported dynamin-independent RME formations, whereby tubular/ring-like structures internalize the raft proteins (e.g., GPI-linked proteins, flotillins, fluid-phase markers; Supplemental Figure 2).19,26,32 We propose a new model of nephrin endocytosis in which the RME pathway coordinates endocytic trafficking and its signaling and may be implicated in the spatiotemporal assembly of SD complexes (Figure 8).
Role of RME in Regulation of the Lipid–Protein Supercomplex
Our results indicate that raft microdomains act as an organizing platform for an efficient coupling of signaling to endocytosis. Several features of RME seem ideally suited for such a functional interplay. First, the RME pathway allows co-internalization of high-ordered multimers, leaving the specific composition of microdomains unchanged. The RME pathway can endocytose relatively large cargo, such as viruses, bacteria, and focal adhesion complexes of detached cells, thereby suggesting that it represents a primitive form of trafficking machinery.8,33 Unlike CME, RME does not require cyclic assembly and disassembly of coated proteins during the endocytic process. This feature of RME allows the maintenance of identical constituents throughout trafficking and thus confers a “fixed shuttle” without losing or exchanging any component.8
Second, RME is an inducible process that internalizes nephrin microdomains at a slower rate relative to the classic CME pathway. This feature of RME enables effective signal transduction by increasing the surface resident time of the functioning platform and by an intimate interaction between signaling components. RME may be the most suitable machinery for shaping the foot process architecture because it could allow the finely-tuned, spatial confinement of multimeric complexes at desirable locations (e.g., the SD). Third, RME enables functional coupling of endocytosis to its own signaling. In the past several years, a functional link between endocytosis and signaling has been actively explored.16 For example, Simian virus SV40 is internalized via the RME pathway through activation of tyrosine kinase–based signaling.34 Such signal activation, in turn, recruits dynamin to endocytic sites and loosens the linkage with the cortical actin cytoskeleton, thereby facilitating the budding of noncoated vesicles from the plasma membrane. In analogy to these observations, our data suggest that tyrosine phosphorylation of nephrin represents a signal for its own endocytic trafficking and simultaneously constitutes a shutoff loop through the preferential removal of activated signaling platforms from the cell surface. On the basis of these data, we propose a model in which the RME pathway allows highly selective uptake of phosphorylated nephrin in the remodeling area while leaving nonphosphorylated nephrin undisturbed at its desirable location in the SD (Figure 8).
Our data indicate that internalized nephrin via RME is sorted into the early endosomes, but the intracellular destination of the RME pathway is still unclear. In light of previous studies with several membrane receptors,12 critical questions including whether the RME directs nephrin to recycle back to the surface or to proceed en route to the degradation pathway warrant further characterization.
Relevance of Raft-Mediated Endocytosis in Podocytes
Previous studies showed that podocytes can endocytose circulating proteins, including complement components and lipoproteins.20–23 Tracer experiments revealed that uptake occurs via clathrin-coated vesicles on both the apical and the basal surfaces, some of which are sorted in multivesicular bodies at later times.10,21 Generally, noncoated smooth pits and vesicles are rarely observed at the ultrastructural level in normal foot processes in vivo. This may be due in part to the slower kinetics of RME and/or the technical difficulty of fixing lipid-based structures, thereby making it difficult to encounter noncoated smooth pits in conventionally processed electron microscopy specimens. Several studies have proposed that podocytes play an active role in removing Ig or albumin from primary urinary ultrafiltrate. Mice lacking CD2AP or Fc receptors exhibited defective endocytosis in foot processes with glomerular immunodeposits, pointing to the importance of endocytic clearance by podocytes.10,22 The detailed mechanisms underlying these observations must await further studies.
Under in vivo conditions, nephrin molecules in podocytes interact with each other not only in cis (side by side) but also in trans (head to head) manner. The limitation of our antibody cross-linking approach in this study is that it could reflect only the lateral assembly of microdomains in the plane of the plasma membrane (cis-interaction). Another caveat of our studies is that our cultured cells all are of epithelial lineage and devoid of cell–cell junctions; therefore, other experimental models, such as Heymann nephritis, whereby the SD is actively phosphorylated, may allow better characterization of the RME process.11
Mechanisms for Sorting Nephrin into RME Pathways
Rafts are small (10 to 200 nm) and dynamic (half-life 0.1 ms to 1.0 s),29 and heterogeneous microdomains are likely captured by endocytic vesicles. In contrast to CME, in which specific adaptors recruit cargo to the coated pits, sorting adaptors for RME have not yet been fully defined. An attractive hypothesis is that microdomain assembly plays a key role in sorting nephrin into RME. Sequestration of nephrin into microdomains increases its time of residence at the plasma membrane, thereby protecting nephrin from nonspecific diffusion or internalization through the constitutively active CME pathway or passive bulk flow. Our live-cell FRAP analysis showed that nephrin microdomains are stabilized in the presence of podocin. A study of membrane dynamics demonstrated that immobilization of the microdomains occurs transiently before their budding into cells.35 In light of these observations, we speculate that the role of podocin is to organize microdomains so that RME could operate in a highly regulated manner. Podocin could allow highly site-specific, finely tuned budding at the plasma membrane by compartmentalizing membrane lipids and endocytic adaptors necessary for membrane curvature and budding and by protecting them from nonspecific diffusion or internalization.27
Previous studies showed that caveolin, a scaffold protein structurally similar to podocin, is involved in some RME pathways. Moreover, it was reported that caveolin 1 physically interacts and co-localizes with nephrin in glomeruli36; therefore, it is important to determine whether the smooth invaginations internalizing nephrin correspond to caveolin-positive “classical” caveolae or a caveolin-negative, raft-derived morphologic equivalent (Supplemental Figure 2).18,19 Our caveolin 1 silencing studies with cultured cells indicated that caveolin is not necessary for nephrin endocytosis (Supplemental Figure 7). Caveolae are highly immobile and regulated subdomains at the plasma membrane that, even after activation, are only slowly internalized (half-time >20 min). This suggests that caveolae are not involved in endocytosis in podocytes under normal circumstances.18,37 The lack of renal phenotypes in caveolin-null mice argues against its relevance in vivo.17,27 The data collectively suggest that caveolin does not necessarily play a role in nephrin endocytosis.
Relevance of the Classic CME Pathway
At early steps of internalization, nephrin enters cells mainly via CME. Clathrin-coated pits are short-lived and highly mobile structures: They form and bud from the plasma membrane within 1 to 2 min and are recycled in approximately 6 min.30,37 Such fast kinetics of CME possibly favors a constitutive trafficking mechanism that regulates the surface appearance of nephrin at a constant rate. Quack et al.38 demonstrated that a fraction of nephrin is internalized via CME in association with β-arrestin 2, a clathrin-associated sorting protein.39 β-Arrestin 2 preferentially binds to dephosphorylated nephrin that is disconnected from extracellular homophilic engagement and intracellular scaffolds, thereby targeting the “free,” nonraft fraction of nephrin into the CME pathway. Previous studies demonstrated that nephrin phosphorylation is seen to some extent even under steady-state conditions but is remarkably stimulated in particular situations in which rapid changes of cell shape and maximal cell–cell junction formation are required (i.e., developing and nephrotic podocytes).4,5,11 β-Arrestin 2–mediated CME is possibly involved in internalization of dephosphorylated nephrin during remodeling of foot processes.
In summary, we have characterized for the first time the RME pathway, which permits regulated entry of nephrin signaling microdomains into cells. We propose that cooperation between nephrin endocytosis and signaling is crucial for maintenance of SD integrity. Such coordinated functions become more important during podocyte development or injury, when signaling is activated to establish or restore cell–cell junctions. Endocytosis has been linked to a much more diverse array of cellular processes than we previously thought, including remodeling of cell shape and polarity.19,33 Elucidation of more detailed mechanisms of podocyte endocytosis will thus increase our understanding of the pathogenesis of various proteinuric disorders.
Concise Methods
Constructs, Cells, and Antibodies
These details are provided as supplemental information.
Internalization Assay
All uptake studies were performed in COS-7 cell transfectants expressing nephrin, with the exception of Supplemental Figure 1, in which cultured podocytes were used. In all but one experiment (Figure 6D), we used the COS-7/nephrin transfectants devoid of Fyn overexpression, where nephrin is phosphorylated by endogenous Fyn. To label the surface nephrin, a divalent form of anti-nephrin antibody [F(ab′)2] was used, because it gives significantly brighter signals than its monovalent Fab′ fragments without perturbing the endocytic properties. Cells were serum starved for 1 h and then incubated at 4°C for 30 min with anti-nephrin antibody and endocytic markers of Alexa Fluor 633–transferrin or Alexa Fluor 594/488-CTxB. After internalization at 37°C for the indicated times, the markers remaining on the cell surface were then stripped by a rinse with 0.2 N acetic acid and 0.5 M NaCl (pH 3.0).40 At 2 min, transferrin is present at close to steady-state levels in sorting endosomes (t½ 2 min).17,26 Cells were fixed with 4% paraformaldehyde for 15 min and were permeabilized in 0.1% Triton X-100/PBS. For potassium depletion, cells were washed with serum-free DMEM supplemented with 10 mM HEPES acid and 20 mM sodium bicarbonate (pH 7.2) and then preincubated for 5 min at 37°C. Cells were then incubated in KCL-free buffer (140 mM NaCl, 20 mM HEPES, 1 mM CaCl2, 1 mM MgCl2, 1 mg of d-glucose/ml [pH 7.4]) at 37°C for 30 min.41 For the cholesterol-depleting experiment, cells were preincubated with 10 mM MβCD in serum-free DMEM at 37°C for 30 min.29,42
Immunoelectron Microscopy
Surface-labeled nephrin by anti-nephrin antibody in COS-7 cells was internalized at 37°C for 2 min and fixed in 4% paraformaldehyde and 0.1% glutaraldehyde for 30 min. The cells were incubated with goat anti-rabbit IgG conjugated to colloidal gold (1.4-nm diameter; Nanoprobes Inc.) and intensified with a gold enhancement kit (GoldEnhance EM; Nanoprobes Inc.). Cells were postfixed in 1% OsO4 and embedded in epoxy resin.
Antibody-Induced Patching Assay
Surface nephrin and raft/nonraft markers in COS-7 cell transfectants were cross-linked according to Harder et al.28 The V5-tagged human TfR deletion mutant (hTfR del 5 to 41) was described previously,28 and GPI-anchored EGFP was a gift from G. Kondoh (Osaka University). Surface proteins on live COS-7 cells were first reacted with the primary antibodies (anti-nephrin polyclonal antibody for nephrin, anti-V5 mAb for V5-TfR, anti-GFP mAb for GFP-GPI) or CTxB-biotin conjugate (Sigma-Aldrich) at 4°C for 20 min and then further incubated at 12°C for 30 min. The secondary antibodies (or streptavidin-Texas Red conjugate for CTxB) were applied at 12°C for 1 h. Cells were then fixed with 3.7% formaldehyde in PBS on ice for 15 min and incubated in 80% methanol and 20% acetone at −20°C for 5 min.
Floatation Gradient Centrifugation
For preparation of detergent-resistant membranes, cell lysates or isolated glomeruli were homogenized by 14 strokes in a Dounce homogenizer in 3 ml of TNE buffer (250 mM NaCl, 5 mM EDTA, 10 mM Tris [pH 8.0], and proteinase inhibitors).25,43 The homogenate was centrifuged at 1000 × g for 10 min to obtain a postnuclear supernatant. The supernatant was centrifuged at 200,000 × g for 30 min and resuspended in 3 ml of homogenization buffer containing 0.2% Triton X-100. The extracts were centrifuged at 200,000 × g for 30 min and resuspended in 0.3 ml of homogenization buffer containing 0.2% Triton X-100. The extracts were adjusted to 40% sucrose and overlaid with a discontinuous sucrose gradient (1.6 ml of 35% sucrose, 0.8 ml of 15% sucrose, 0.4 ml of 5% sucrose, and 0.2 ml TNE). Gradients were centrifuged for 24 h at 250,000 × g at 4°C in a swingout rotor.
FRAP Analysis
Mouse L cells, L-wt or L-pod,25 were transiently transfected with GFP-nephrin cDNA. Forty-eight hours after transfection, cells were held in a glass-bottom dish containing 10% FCS/DMEM on the temperature-controlled stage of a Zeiss LSM PASCAL confocal microscope.25 Fluorescence of selected 4.0 × 4.0-μm square regions of interest (ROIs) was bleached at 37°C with the 488-nm laser line at full power and full transmission for 2 s. The fluorescence recovery was acquired every 1-ms interval at full laser power and 1% transmission, minimizing unnecessary phototoxicity during the scanning period. The recovery values were expressed as pixel intensity and were normalized by the average prebleach values of each region of interest (Supplemental Figures 8 and 9). The half-time required for the bleached fluorescence to rise to 50% of the full recovery value (t½) and mobile fractions were estimated by nonlinear regression to a function for lateral diffusion. For cholesterol depletion experiments, cells were treated with 10 mM MβCD for 30 min.
Immunoprecipitation and Immunoblot Analysis
Transfected COS-7 cells were lysed in RIPA buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 1% NP-40, and protease inhibitor). Lysates (500 μg) were rocked at 4°C for 2 h with the appropriate primary antibody. Forty microliters of protein G–Sepharose beads was then added and rocked at 4°C for 1 h. The bound proteins were resolved by 10% SDS-PAGE and analyzed by immunoblotting using an ECL-plus detection kit (GE Healthcare Bio-Sciences Corp.).
Phosphorylation Assay
COS-7 cells were transfected with nephrin and FynY531F (a kinase-active form) or FynK299M (a kinase-dead form). After surface labeling of nephrin with an N-terminus–specific antibody at 4°C for 30 min, cells were incubated at 37°C for the indicated periods of time. The cells were lysed in NP40 buffer (150 mM NaCl, 1% NP-40, 50 mM Tris [pH 8.0], 0.5% sodium deoxycholate, 0.1% SDS with proteinase inhibitor, 10 mM NaF, and 1 mM Na3VO4). Lysates were incubated with biotin-conjugated phosphotyrosine-PY20 at 4°C for 2 h and were precipitated with streptavidin beads (Pierce). Phosphorylated nephrin was detected by immunoblot analysis. Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d] pyrimidine) PP2, genistein, and sodium orthovanadate (Na3VO4) were purchased from Calbiochem (San Diego, CA).
PS Podocyte Injury Model
Male rats (Sprague-Dawley, 200 g) were anesthetized with Nembral (Abbott Laboratories), and the left kidneys were perfused in situ with PS (500 μg/ml in Hanks balanced salt solution).31 The kidneys were perfused with physiologic saline and subsequently with 2.5% glutaraldehyde fixative buffered with 0.1 M phosphate buffer (PB; pH 7.4) under anesthesia. The perfused kidneys were immersed in 2.5% glutaraldehyde containing 1% tannic acid, 0.1 M PB for 2 h and were successively immersed in 1% OsO4 in 0.1 M PB for 1 h and 1% uranyl acetate in 0.05 M maleate buffer for 3 h. The samples were then dehydrated with a graded series of ethanol solutions before embedding into Epon 812. Ultrathin silver-gold sections were transferred to copper grids (50 mesh) coated with Formvar membranes, then stained with both uranyl acetate and lead citrate.
Statistical Analysis
The unpaired, two-tailed t test with P < 0.05 was taken as significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by grants from Japan Society for the Promotion of Science (a Grant-in Aid for Scientific Research [C19590953 and C21591045]), the Ministry of Health, Labor and Welfare (Research on Children and Families), Kansai Medical University (Research C), the Japan Human Science Foundation (KHA 1001), Fujii-Otsuka International Exchange Fund (to X.-S.Q.), and the Yoshimitsu Otsuka Memorial Foundation (to A.S.).
Part of this article was presented in abstract form at the annual meeting of the American Society of Nephrology; San Francisco, CA; November 3, 2007.
We are grateful to Drs. K. Taguchi, N. Tanimura, K. Kawai, and Y. Taketani for assistance with the raft experiments.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Kerjaschki D: Caught flat-footed: Podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest 108: 1583–1587, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T: Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB: Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116: 1346–1359, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huber TB, Benzing T: The slit diaphragm: A signaling platform to regulate podocyte function. Curr Opin Nephrol Hypertens 14: 211–216, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Simons K, Toomre D: Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31–39, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Parton RG, Simons K: The multiple faces of caveolae. Nat Rev Mol Cell Biol 8: 185–194, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, Unanue ER, Shaw AS: CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 300: 1298–1300, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Li H, Lemay S, Aoudjit L, Kawachi H, Takano T: SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J Am Soc Nephrol 15: 3006–3015, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P: Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 108: 1621–1629, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehtonen S, Ryan JJ, Kudlicka K, Iino N, Zhou H, Farquhar MG: Cell junction-associated proteins IQGAP1, MAGI-2, CASK, spectrins, and alpha-actinin are components of the nephrin multiprotein complex. Proc Natl Acad Sci U S A 102: 9814–9819, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitamura A, Tsukaguchi H, Hiramoto R, Shono A, Doi T, Kagami S, Iijima K: A familial childhood-onset relapsing nephrotic syndrome. Kidney Int 71: 946–951, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Patrakka J, Kestila M, Wartiovaara J, Ruotsalainen V, Tissari P, Lenkkeri U, Mannikko M, Visapaa I, Holmberg C, Rapola J, Tryggvason K, Jalanko H: Congenital nephrotic syndrome (NPHS1): Features resulting from different mutations in Finnish patients. Kidney Int 58: 972–980, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Le Roy C, Wrana JL: Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol 6: 112–126, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Conner SD, Schmid SL: Regulated portals of entry into the cell. Nature 422: 37–44, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Lajoie P, Nabi IR: Regulation of raft-dependent endocytosis. J Cell Mol Med 11: 644–653, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayor S, Pagano RE: Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol 8: 603–612, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farquhar MG, Palade GE: Glomerular permeability: II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. J Exp Med 114: 699–716, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerjaschki D, Exner M, Ullrich R, Susani M, Curtiss LK, Witztum JL, Farquhar MG, Orlando RA: Pathogenic antibodies inhibit the binding of apolipoproteins to megalin/gp330 in passive Heymann nephritis. J Clin Invest 100: 2303–2309, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS: Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci U S A 105: 967–972, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weavers H, Prieto-Sanchez S, Grawe F, Garcia-Lopez A, Artero R, Wilsch-Brauninger M, Ruiz-Gomez M, Skaer H, Denholm B: The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457: 322–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG, Reponen P, Tryggvason K, Camussi G: Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol 158: 1723–1731, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shono A, Tsukaguchi H, Yaoita E, Nameta M, Kurihara H, Qin XS, Yamamoto T, Doi T: Podocin participates in the assembly of tight junctions between foot processes in nephrotic podocytes. J Am Soc Nephrol 18: 2525–2533, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Sabharanjak S, Sharma P, Parton RG, Mayor S: GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell 2: 411–423, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Parton RG: Caveolae and caveolins. Curr Opin Cell Biol 8: 542–548, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Harder T, Scheiffele P, Verkade P, Simons K: Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol 141: 929–942, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hancock JF: Lipid rafts: Contentious only from simplistic standpoints. Nat Rev Mol Cell Biol 7: 456–462, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandvig K, Torgersen ML, Raa HA, van Deurs B: Clathrin-independent endocytosis: From nonexisting to an extreme degree of complexity. Histochem Cell Biol 129: 267–276, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurihara H, Anderson JM, Kerjaschki D, Farquhar MG: The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulfate-treated rats contain the tight junction protein ZO-1. Am J Pathol 141: 805–816, 1992 [PMC free article] [PubMed] [Google Scholar]
- 32.Romer W, Berland L, Chambon V, Gaus K, Windschiegl B, Tenza D, Aly MR, Fraisier V, Florent JC, Perrais D, Lamaze C, Raposo G, Steinem C, Sens P, Bassereau P, Johannes L: Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature 450: 670–675, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Kirkham M, Parton RG: Clathrin-independent endocytosis: New insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta 1746: 349–363, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M: Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature 436: 78–86, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Frick M, Bright NA, Riento K, Bray A, Merrified C, Nichols BJ: Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr Biol 17: 1151–1156, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Sörensson J, Fierlbeck W, Heider T, Schwarz K, Park DS, Mundel P, Lisanti M, Ballermann BJ: Glomerular endothelial fenestrae in vivo are not formed from caveolae. J Am Soc Nephrol 13: 2639–2647, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Thomsen P, Roepstorff K, Stahlhut M, van Deurs B: Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol Cell 13: 238–250, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quack I, Rump LC, Gerke P, Walther I, Vinke T, Vonend O, Grunwald T, Sellin L: Beta-arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci U S A 103: 14110–14115, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haucke V: Cargo takes control of endocytosis. Cell 127: 35–37, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Naslavsky N, Weigert R, Donaldson JG: Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol Biol Cell 14: 417–431, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larkin JM, Brown MS, Goldstein JL, Anderson RG: Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell 33: 273–285, 1983 [DOI] [PubMed] [Google Scholar]
- 42.Keller P, Simons K: Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol 140: 1357–1367, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lingwood D, Simons K: Detergent resistance as a tool in membrane research. Nat Protoc 2: 2159–2165, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.