Abstract
Th1 effector CD4+ cells contribute to the pathogenesis of proliferative and crescentic glomerulonephritis, but whether effector Th17 cells also contribute is unknown. We compared the involvement of Th1 and Th17 cells in a mouse model of antigen-specific glomerulonephritis in which effector CD4+ cells are the only components of adaptive immunity that induce injury. We planted the antigen ovalbumin on the glomerular basement membrane of Rag1−/− mice using an ovalbumin-conjugated non-nephritogenic IgG1 monoclonal antibody against α3(IV) collagen. Subsequent injection of either Th1- or Th17-polarized ovalbumin-specific CD4+ effector cells induced proliferative glomerulonephritis. Mice injected with Th1 cells developed progressive albuminuria over 21 d, histologic injury including 5.5 ± 0.9% crescent formation/segmental necrosis, elevated urinary nitrate, and increased renal NOS2, CCL2, and CCL5 mRNA. Mice injected with Th17 cells developed albuminuria by 3 d; compared with Th1-injected mice, their glomeruli contained more neutrophils and greater expression of renal CXCL1 mRNA. In conclusion, Th1 and Th17 effector cells can induce glomerular injury. Understanding how these two subsets mediate proliferative forms of glomerulonephritis may lead to targeted therapies.
Although proliferative and crescentic glomerulonephritides occur in different primary renal diseases and are an important component of several systemic diseases, features of human renal biopsies suggest some common effector pathways. In most cases of rapidly progressive GN there is evidence for an important role for cellular immune effectors: T cells, macrophages, and neutrophils,1–3 a role confirmed in animal models.4–7 CD4+ T cells are key components of renal injury.4,8 When activated, CD4+ cells tend to differentiate into subsets (T helper cells—Th1, Th2 and Th17) that engage immune effectors in different ways. In proliferative forms of GN, T cells direct adaptive immune responses that drive glomerular disease, but also, in rapidly progressive GN, CD4+ cells themselves accumulate in glomeruli as effectors. These effector T helper cells activate innate effector cells, predominantly neutrophils and macrophages, and activate and damage intrinsic renal cells.
In GN, the variable Th1-Th2 predominance of responses influences the histologic patterns and severity of GN.9 Th1 cells, which secrete IFNγ and activate macrophages, are important in some forms of experimental proliferative GN. Th2 cells, characterized by IL-4 production, promote humoral immunity and are important in several forms of GN, but there is little evidence that Th2 cells play primary roles as effector cells within glomeruli in rapidly progressive GN. A binary Th1/Th2 model explains many of the differences in the patterns of immune responses in GN. However, there are discrepancies10 that might be explained by defining a role for a third major subset, Th17 cells, characterized by the production of IL-17A. Its biology has recently been comprehensively reviewed.11 Th17 subset effects potentially relevant to rapidly progressive GN include direct effects on neutrophils and stimulating the production of neutrophil chemoattractants by tissue cells. Thus, in most rapidly progressive types of GN, cell-mediated injury, a key component of injury, may be directed by the Th17 subset, the Th1 subset, or both subsets.
Although antigen-specific T cells are critical to adaptive immune responses, the cells themselves are relatively infrequent. T cell receptor (TcR) transgenic mice help define the contributions of different antigen-specific T helper cell subsets in organ-specific disease.12–14 In the studies presented here we have established a new antigen-specific model of GN. Ovalbumin (OVA)-specific OT-II TcR transgenic CD4+ cells15 are polarized ex vivo under Th1- or Th17-inducing conditions. Effector cells are transferred into recombination activating gene-1 deficient (Rag1−/−) mice with OVA planted in their glomeruli by injecting an OVA conjugate. OVA is conjugated to a mouse IgG1 mAb binding to α3(IV) collagen in murine glomerular basement membrane (GBM). The mAb-OVA conjugate dose is capable of planting significant OVA in glomeruli as an antigen to induce effector responses, but is insufficient to induce significant histologic or functional injury as an antibody. This model allows us to understand effector CD4+ T cell and Th subset-induced injury, with no effects from CD8+ cells or B cells.
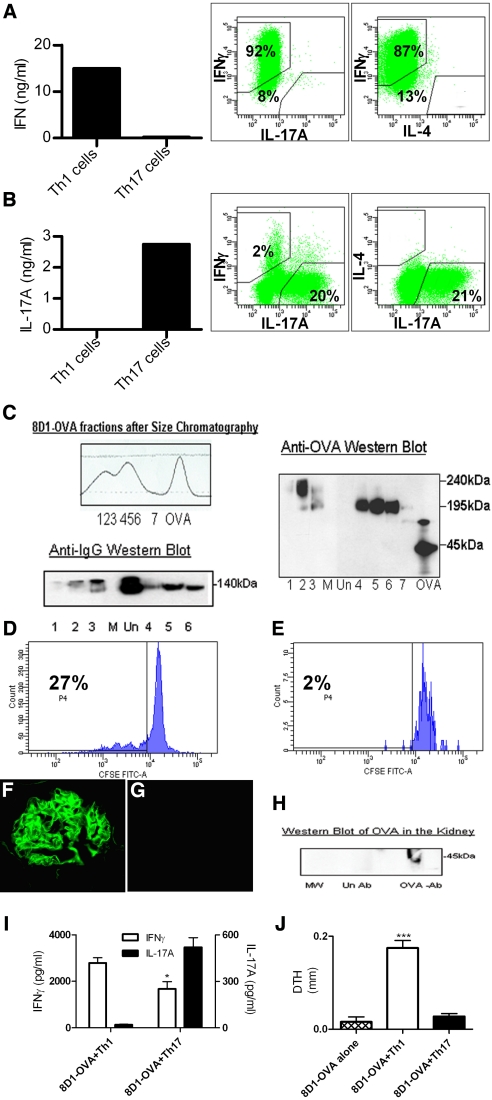
CD4+ cells, isolated from OVA-specific TcR transgenic (OT-II) mice and cultured under Th1 or Th17 priming conditions (see Concise Methods), were confirmed to be Th1 or Th17 by cytokine production before transfer. Th1 polarized cells expressed IFNγ, whereas no IL-17A or IL-4 production was detected (Figure 1A). Th17 polarized cells were strong IL-17A producers, showing only weak IFNγ production, with >20% of cells producing IL-17A, but few IFNγ or double-positive cells (Figure 1B). To plant OVA in glomeruli, the mAb 8D1, a non-nephritogenic, murine IgG1 binding to mouse α3(IV)NC1,16 was conjugated to OVA and purified by size-exclusion chromatography so that no free OVA or unconjugated 8D1 mAb remained, confirmed by Western blotting (Figure 1C). Antigen-specific CD4+ cells recognized OVA bound to the 8D1 anti-GBM mAb. Culture of CFSE-labeled naive OT-II cells incubated with the 8D1-OVA conjugate enhanced their survival (30% to 40% survival after 72 h versus 5% to 6% with unconjugated antibody) and induced OT-II cell proliferation (72 h: 27% of cells, up to 4 cycles, Figure 1D). OT-II cells incubated with unconjugated 8D1 did not proliferate (Figure 1E). After intravenous injection, 8D1-OVA conjugates bound to the GBM in a linear manner; no fluorescence signal was observed after transfer of Th1 cells without 8D1-OVA (Figure 1G). Western blotting showed OVA in the kidney after injection of 8D1-OVA conjugate (Figure 1H). Lungs from 8D1-OVA-injected mice were weakly positive for mouse IgG, whereas no IgG was detected in the spleen or liver (detecting antibody titer 1:100) 3 or 21 d after injection. Mouse IgG was not detected in sera (ELISA, dilution 1:100) at day 3 or day 21. As expected (given the transfer of only CD4+ cells to Rag1−/− mice), no anti-OVA antibodies in sera were detected in recipient mice (data not shown).
Figure 1.
Differentiation of OVA-specific OT-II Th1 and Th17 cells, antibody-OVA conjugation, glomerular IgG and intrarenal OVA detection, and recipient immune responses after cell transfer. (A) After stimulating naive OT-II cells with OVA in a Th1 environment, IFNγ was produced and intracellular cytokine staining of CD4+ cells demonstrated strong IFNγ staining with minimal IL-17A or IL-4. (B) Culturing cells in a Th17-stimulating environment led to strong IL-17A production, whereas cells stained positive for IL-17A but not IL-4, and only 2% of cells produced IFNγ. (C) Chromatographic profile of 8D1-OVA conjugation. The numbers 1 to 7 represent fractions collected for analyses by Western blotting, which confirmed that all OVA-conjugated fractions contained OVA and IgG (lanes 1–6), whereas unconjugated fractions (represented as “Un”) contained IgG alone. The lane labeled “M” contained molecular weight markers. (D and E): 8D1-OVA was recognized by OT-II cells because multiple cycles of proliferation of cultured naive OT-II cells (D) were seen with 8D1-OVA conjugate and (E) not seen with unconjugated antibody. Strong linear IgG staining of glomeruli was seen after (F) the administration of 8D1-OVA to Rag1−/− mice, but not after (G) the injection of Th1 cells without antibody. Western blotting of homogenized kidney (H) 24 h after the administration of 8D1-OVA demonstrated OVA in the kidneys (labeled as OVA-Ab); this was not seen after the administration of unconjugated antibody (labeled as Un Ab). (I) Systemic immune responses of recipient Rag1−/− mice at 21 d assessed by splenic cytokine production demonstrated enhanced IFNγ production in mice given 8D1-OVA and Th1 cells, with enhanced IL-17A production by mice receiving 8D1-OVA and Th17 cells. (J) DTH to OVA (at 21 d) was induced only in mice given 8D1-OVA and Th1 cells. *P < 0.05, ***P < 0.001.
To determine whether transfer of either Th1 or Th17 antigen-specific effector cells induces glomerular injury, 8D1-OVA conjugate was administered intravenously to Rag1−/− mice (lacking adaptive immunity). Three hours later, 5 × 106 Th1 or Th17 cells were injected intravenously. Groups of mice injected with 8D1-OVA alone (without cells) or Th1 cells alone (without 8D1-OVA) served as controls. At 21 d, the injected T cells largely maintained their initial phenotype, because host splenocytes from mice given Th1 cells showed enhanced OVA-stimulated IFNγ production whereas IL-17A production was enhanced in mice given Th17 cells (Figure 1I). Dermal-delayed-type hypersensitivity (DTH) was induced by footpad injection of OVA and measured after 24 h. Only mice that received the 8D1-OVA conjugate and Th1 polarized cells developed dermal DTH (Figure 1J), a classical Th1 response.17
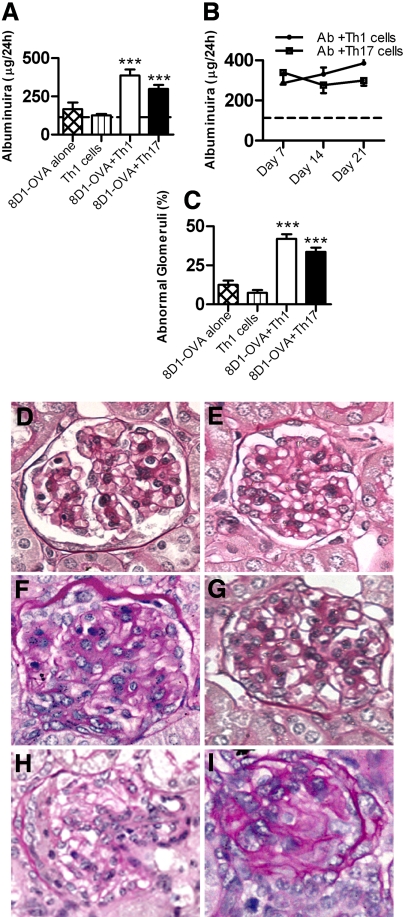
After planting OVA in glomeruli, administration of Th1 or Th17 cells induced glomerular disease. Urinary albumin excretion was not increased in mice given 8D1-OVA conjugate alone or Th1 cells alone, but Th1 or Th17 cells with 8D1-OVA induced significant albuminuria (Figure 2A). Albuminuria was consistent throughout the time course of the study in the Th17 group, whereas in the Th1 group there was a progressive increase in albuminuria until day 21 (Figure 2B). Control mice given Th1 cells alone or the 8D1-OVA conjugate alone exhibited only mild histologic changes (no crescent formation, fibrinoid necrosis, or hyalinosis). Analysis of histologic injury demonstrated substantially more abnormal glomeruli in the mice given 8D1-OVA conjugate with Th1 or Th17 cells compared with control groups (Figure 2C). Th1 and Th17 (+8D1-OVA) recipients developed proliferative GN, [glomerular hypercellularity: 8D1-OVA and Th1 cells: 32.1 ± 1.0 cells/glomerular cross section (c/gcs), 8D1-OVA and Th17 cells: 29.8 ± 1.1 c/gcs, 8D1-OVA alone: 21.3 ± 0.2 c/gcs, Th1 cells alone: 18.9 ± 2.0 c/gcs; P < 0.001]. Representative kidney sections from each group are shown (Figure 2, D through G). Crescent formation and fibrinoid necrosis, although seen in only a few glomeruli, was observed exclusively in mice given 8D1-OVA conjugate and Th1 cells (5.5 ± 0.9% at day 21; Figure 2, H and I). No crescent formation was observed in mice receiving 8D1-OVA conjugate and Th17 cells. Mice did not develop significant renal impairment (measured by BUN; data not shown).
Figure 2.
Renal injury in mice injected with 8D1-OVA conjugate, then either Th1 or Th17 cells. (A) Mice given 8D1-OVA conjugate or Th1 cells alone did not develop albuminuria above values for noninjected Rag1−/− mice (dotted line). At 21 d, albuminuria was increased in mice given 8D1-OVA and Th1 cells or 8D1-OVA and Th17 cells. (B) In mice given 8D1-OVA and Th17 cells, albuminuria had plateaued by day 7 and did not progress. In mice given 8D1-OVA and Th1 cells there was a progressive rise in albuminuria. (C) Histologic injury was significant in mice given 8D1-OVA and either Th1 or Th17 cells. Representative glomeruli from mice given (D) 8D1-OVA alone, (E) Th1 cells alone, (F) 8D1-OVA and Th1 cells, and (G) 8D1-OVA and Th17 cells are shown. (H and I) Crescentic injury and fibrinoid necrosis were only seen in mice given 8D1-OVA and Th1 cells. ***P < 0.001
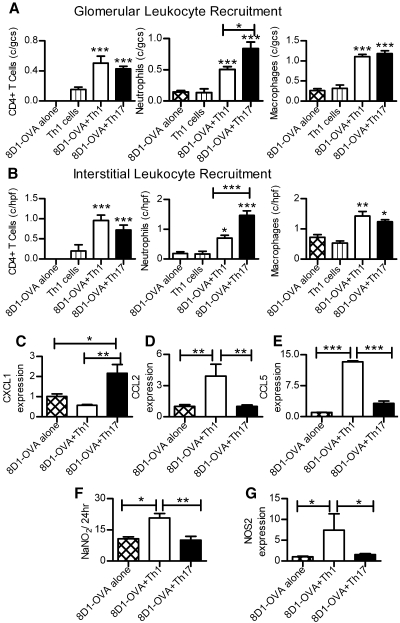
Recruitment and activation of leukocyte subpopulations differed in mice administered Th1 or Th17 cells (Figure 3A). Although glomerular CD4+ cell and macrophage numbers were similarly increased in mice given 8D1-OVA conjugate and either Th1 or Th17 cells at day 21, more neutrophils were found in mice given 8D1-OVA and Th17 cells compared with mice given 8D1-OVA and Th1 cells. Interstitial leukocyte infiltrates followed a similar pattern (Figure 3B). Consistent with the finding of increased neutrophils in kidneys of mice receiving Th17 cells, renal mRNA expression of the primary neutrophil attracting chemokine CXCL1 was elevated (Figure 3C). Th17 cells attract neutrophils18 and in vitro studies have shown that neutrophil recruitment is achieved via production of CXCL8, the human homologue of CXCL1, by Th17 cells.19 It is therefore likely that at least some of the Th17-induced renal injury is mediated by neutrophils. In mice receiving 8D1-OVA and Th1 cells, macrophages were likely to be more activated; only these mice developed dermal DTH and increased expression of mRNA for the macrophage chemoattractants CCL2 and CCL5 (Figure 3, D and E), which have been associated with experimental crescentic GN.20 Furthermore, type 2 nitric oxide synthase (NOS2/iNOS) mRNA, a marker of macrophage activation21 and urinary nitrate, a marker of intrarenal macrophage NOS2 production, were increased in this group (Figure 3, F and G).
Figure 3.
Leukocytes in kidneys of mice with either Th1- or Th17-induced injury 21 d after cell transfer. (A) Glomerular CD4+T cells, neutrophils, and macrophages were increased in mice given 8D1-OVA and Th1/Th17 cells. Neutrophil recruitment was incrementally increased in mice given 8D1-OVA and Th17 cells compared with 8D1-OVA and Th1 cells. (B) A similar pattern of recruitment was seen in the cortical interstitium. Renal chemokine mRNA expression demonstrated (C) enhanced CXCL1 mRNA in mice given 8D1-OVA and Th17 cells, whereas (D) CCL2 and (E) CCL5 were increased in mice given 8D1-OVA and Th1 cells. (F and G) NOS2 and urinary nitrate, markers of macrophage activation, were increased in mice receiving 8D1-OVA and Th1 cells. For mRNA, values for the 8D1-OVA alone group are presented as 1. *P < 0.05, **P < 0.01, ***P < 0.001.
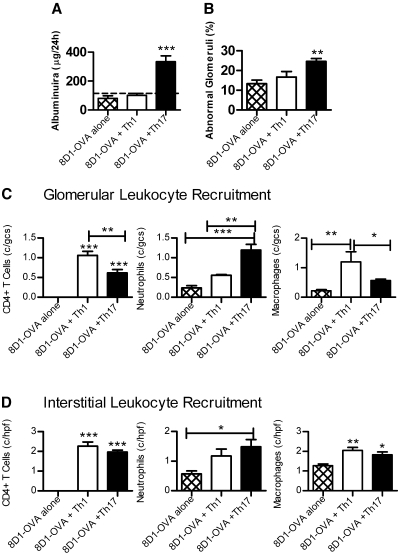
Further studies were performed 3 d after cell transfer. At this time point, albuminuria was present in mice receiving 8D1-OVA conjugate and Th17 cells, but not 8D1-OVA conjugate and Th1 cells (Figure 4A), and a higher proportion of glomeruli were abnormal in mice that had received Th17 cells (Figure 4B). Therefore, Th17-induced glomerular injury occurred earlier than Th1-induced injury. Leukocytes were present in glomeruli (Figure 4C) with increased numbers of neutrophils in glomeruli of mice receiving Th17 cells (compared with Th1 cell recipients), whereas Th1 cell recipients exhibited more macrophages. At day 3, these findings were glomerulo-specific; differences between Th1 and Th17 cell recipients were not seen in the interstitium (Figure 4D).
Figure 4.
Renal disease in mice 3 d after injection with 8D1-OVA and either Th1 or Th17 cells. (A) Pathologic albuminuria (dotted line represents values for noninjected Rag1−/− mice) and (B) increased numbers of abnormal glomeruli were evident in mice that received 8D1-OVA and Th17 cells. (C) Leukocyte recruitment to glomeruli demonstrated CD4+ cells (more in mice receiving Th1 cells), with comparatively more neutrophils in glomeruli of Th17 cell recipients and more macrophages in glomeruli of Th1 cell recipients. (D) Interstitial leukocytes were similar in Th1 and Th17 cell recipients 3 d after cell transfer. *P < 0.05, **P < 0.01, ***P < 0.001.
These studies used Rag1−/− mice as recipients of effector antigen-specific Th1 or Th17 cells. Because these mice do not possess T or B cells, OVA planted in glomeruli cannot induce CD8+ or B cell responses, and regulatory T cells are unable to influence the pattern of injury. A major advantage of this strategy is that Th1- and Th17-mediated injury can be assessed in a pure experimental system. However, T cells transferred into Rag1−/− mice can undergo homeostatic expansion, and it is possible that the transferred Th1 cells might have expanded more rapidly than Th17 cells. Recently, studies in experimental type 1 diabetes induced by transfer of cells from a TcR transgenic mouse specific for an islet autoantigen showed conversion of Th17 cells to a Th1 phenotype after transfer.22,23 Although our Th17 polarized OT-II cells, specific for a foreign antigen, showed some IFNγ production after 21 d, they were still capable of producing IL-17A. Furthermore, dermal DTH and renal disease were different in Th1 recipients compared with the Th17 recipients at 21 d, supporting the maintenance of separate phenotypes after transfer. Although the studies presented here are the first to demonstrate a role for Th17 and Th1 cells in the same experimental system, other studies24–26 have used genetically deficient mice to implicate Th17 cells in experimental renal disease.
These studies describe a novel model of cell-mediated proliferative GN for which adaptive components are only effector antigen-specific CD4+ T cells. They demonstrate that both Th1 and Th17 cells can induce proliferative GN. Th17 cells induce albuminuria early, with persistent accumulation of leukocytes. Administration of Th1 cells lead to a slower rise in albuminuria, but more macrophage activation and DTH-like injury, including, in some glomeruli, crescent formation and fibrinoid necrosis. It is likely that Th1 and Th17 responses play a role in proliferative forms of GN and both represent potential therapeutic targets.
Concise Methods
Antibody Conjugation and T Cell Polarization
The 8D1 mAb (5 mg/ml in PBS) was reacted with 0.1 mg/ml N-succinimidyl-6-maleimido-caproate (Sigma-Aldrich, St. Louis, MO) for 2 h at room temperature. OVA (10 mg/ml in PBS; Sigma-Aldrich) was reduced with 5 nM trin-butyl phosphine (Sigma-Aldrich) for 2 h at 50°C. The reduced OVA and activated antibody solutions were buffer-exchanged into degassed PBS, then mixed with OVA in a 10-fold molar excess over mAb. The conjugation reaction was allowed to proceed for 3 h at room temperature before stopping with 2 mM cysteine. The OVA-conjugated 8D1 was purified from free OVA and nonconjugated antibody by size exclusion chromatography (Superdex 200 column, GE Healthcare). Eluted fractions, which corresponded with peaks, were collected and stored at 4°C.
To generate Th1 and Th17 cells, OT-II cells from lymph nodes of OT-II transgenic mice were harvested. Initial cell preparations (containing 30% to 50% OT-II cells) were cultured with antigen presenting cells (4 × 105 OT-II+ cells/ml with 4 × 106 antigen presenting cells/ml) under Th1 or Th17 inducing conditions. For Th1, 1μM OVA323-339 peptide, 2 ng/ml rmIL-12 with 10 μg/ml of anti IL-4 (11B11, ATCC), for Th17 20 ng/ml rmIL-6, 50 ng/ml rmIL-23, 5 ng/ml rmTGF-β, 10 μg/ml anti-IL-4 mAb, and 10 μg/ml anti-IFNγ. After 48 h, cells were adjusted to a density of 4 × 105 cells/ml in fresh media, For Th1 cells, 5 μg/ml rmIL-2 was added, for Th17 cells 50 μg/ml of IL-2 and 20 ng/ml of rmIL-23; cells were incubated for 5 additional days. On day 7, cells were harvested and the proportions of IFNγ+ and IL-17A+ cells were determined by flow cytometry. (BD FACS Canto flow cytometer, BD Biosciences, North Ryde, Australia). IFNγ and IL-17A concentrations in OVA-stimulated splenocyte cultures from recipients were measured by ELISA.20 Mouse IgG and anti-OVA IgG were assessed by ELISA as described previously.25
To determine the activity and binding of the 8D1-OVA conjugate, OT-II cells from splenocytes were labeled with CFSE. Cells (5 × 105) were stimulated with 8D1-OVA or unconjugated 8D1 mAb (0.84 mg/ml) After 72 h later flow cytometric analysis was performed on the cells to determine their survival and proliferation. 8D1-OVA and unconjugated 8D1 mAb were tested for the presence of OVA and IgG using mAb to OVA (Abcam, Cambridge, MA) or sheep anti-mouse IgG (Amersham Biosciences, Rydalmere, Australia). The secondary antibody used was streptavidin horseradish peroxidise (Amersham). To detect the presence of OVA in the kidney, kidneys from Rag1−/− mice (specific pathogen-free facility, Monash Medical Centre, Melbourne Australia) were studied 24 h after receiving 8D1-OVA. Kidneys were homogenized and supernatants and cell lysates were analyzed by SDS-PAGE.
In Vivo Experiments and Assessment of Renal Injury
C57Bl/6.Rag1−/− mice were injected intravenously with 150 μg of 8D1-OVA or unconjugated 8D1 mAb. Groups that received cells were intravenously injected 3 h later with 5 × 106 Th1 or Th17 polarized cells. 24 h before the end of experiments (at 21 d after induction of disease), mice were injected with 100 μg of OVA (in 20 μl of sterile-filtered PBS) in the left hind-foot and 20 μl of sterile-filtered PBS alone in right hind-foot. The difference between footpad thicknesses was assessed using a micrometer. Studies were performed in accordance with National Health and Medical Research Council of Australia guidelines and approved by the Monash University Animal Ethics Committee. Results are expressed as mean ± SEM. Recipient group numbers were as follows: day 21 8D1-OVA alone, n = 7; Th1 cells alone, n = 3; 8D1-OVA + Th1 cells, n = 9; 8D1-OVA + Th17 cells, n = 11; day 3 8D1-OVA alone, n = 6; 8D1-OVA + Th1 cells, n = 6; 8D1-OVA + Th17 cells, n = 7. Statistical analysis was by ANOVA with post hoc analysis by Tukey's test (GraphPad Prism; Graphpad Software, San Diego, CA).
Glomerular abnormalities were assessed on Periodic acid-Schiff-stained, 3-μm-thick, paraffin-embedded sections on coded slides. The percentage of abnormal glomeruli was determined by examining a minimum of 50 glomeruli/mouse for abnormalities according to previously published protocols.27,28 Abnormalities included glomerular hypercellularity, crescent formation, fibrinoid necrosis, segmental proliferation, hyalinosis, and capillary wall thickening. Cellularity was quantified by counting total glomerular cell nuclei (minimum 20 glomeruli/mouse) expressed as c/gcs. Glomerular, lung, liver, and splenic IgG deposition was assessed on 6-μm-thick frozen sections using FITC-sheep anti-mouse Ig (1:100, Silenus, Hawthorn, Victoria, Australia). For albuminuria, 24-h urine collections were obtained on days 2 to 3, 7, 14, and 21 after injection of 8D1-OVA and/or cells. Albuminuria was measured using a Mouse Albumin ELISA Quantification Kit (Bethyl Laboratories Inc, Montgomery, TX). Glomerular leukocytes were enumerated by immunohistochemistry (minimum of 20 glomeruli/mouse),20 and interstitial leukocytes counted in a minimum of ten high-powered cortical fields. Urinary nitrate was measured using the Greiss assay, and measurement of CXCL1, CCL2, and CCL5 mRNA was by real-time PCR as described previously.20 Results for the 8D1-OVA alone group are presented as 1.
Disclosures
These studies were supported by a Program Grant from the National Health and Medical Research Council of Australia (NHMRC; A.R.K.), an NHMRC Postgraduate Research Scholarships (S.S.), and by grant DK080799 from the National Institutes of Health (D.B.B.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Neale TJ, Tipping PG, Carson SD, Holdsworth SR: Participation of cell-mediated immunity in deposition of fibrin in glomerulonephritis. Lancet 2: 421–424, 1988 [DOI] [PubMed] [Google Scholar]
- 2.Brouwer E, Huitema MG, Mulder AH, Heeringa P, van Goor H, Tervaert JW, Weening JJ, Kallenberg CG: Neutrophil activation in vitro and in vivo in Wegener's granulomatosis. Kidney Int 45: 1120–1131, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Cunningham MA, Huang XR, Dowling JP, Tipping PG, Holdsworth SR: Prominence of cell-mediated immunity effectors in “pauci-immune” glomerulonephritis. J Am Soc Nephrol 10: 499–506, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Huang XR, Tipping PG, Shuo L, Holdsworth SR: Th1 responsiveness to nephritogenic antigens determines susceptibility to crescentic glomerulonephritis in mice. Kidney Int 51: 94–103, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Bolton WK, Chandra M, Tyson TM, Kirkpatrick PR, Sadovnic MJ, Sturgill BC: Transfer of experimental glomerulonephritis in chickens by mononuclear cells. Kidney Int 34: 598–610, 1988 [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Hicks J, Borillo J, Glass WF, 2nd, Lou YH: CD4(+) T cells specific to a glomerular basement membrane antigen mediate glomerulonephritis. J Clin Invest 109: 517–524, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruth AJ, Kitching AR, Kwan RY, Odobasic D, Ooi JD, Timoshanko JR, Hickey MJ, Holdsworth SR: Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J Am Soc Nephrol 17: 1940–1949, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Radeke HH, Tschernig T, Karulin A, Schumm G, Emancipator SN, Resch K, Tary-Lehmann M: CD4+ T cells recognizing specific antigen deposited in glomeruli cause glomerulonephritis-like kidney injury. Clin Immunol 104: 161–173, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Holdsworth SR, Kitching AR, Tipping PG: Th1 and Th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int 55: 1198–1216, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Kitching AR, Turner AL, Semple T, Li M, Edgtton KL, Wilson GR, Timoshanko JR, Hudson BG, Holdsworth SR: Experimental autoimmune anti-glomerular basement membrane glomerulonephritis: A protective role for IFN-gamma. J Am Soc Nephrol 15: 1764–1774, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Korn T, Bettelli E, Oukka M, Kuchroo VK: IL-17 and Th17 cells. Ann Rev Immunol 27: 485–517, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM: IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med 205: 1535–1541, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR: Either a Th17 or a Th1 effector response can drive autoimmunity: Conditions of disease induction affect dominant effector category. J Exp Med 205: 799–810, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stummvoll GH, DiPaolo RJ, Huter EN, Davidson TS, Glass D, Ward JM, Shevach EM: Th1, Th2, and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. J Immunol 181: 1908–1916, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edgtton KL, Kausman JY, Li M, O'Sullivan K, Lo C, Hutchinson P, Yagita H, Holdsworth SR, Kitching AR: Intrarenal antigens activateCD4+ cells via co-stimulatory signals from dendritic cells. J Am Soc Nephrol 19: 515–526, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang JS, Colon S, Hellmark T, Sado Y, Hudson BG, Borza DB: Identification of noncollagenous sites encoding specific interactions and quaternary assembly of alpha 3 alpha 4 alpha 5(IV) collagen: Implications for Alport gene therapy. J Biol Chem 283: 35070–35077, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sallusto F, Lanzavecchia A, Mackay CR: Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today 19: 568–574, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Iwakura Y, Nakae S, Saijo S, Ishigame H: The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev 226: 57–79, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A: Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol 162: 2347–2352, 1999 [PubMed] [Google Scholar]
- 20.Phoon RK, Kitching AR, Odobasic D, Jones LK, Semple TJ, Holdsworth SR: T-bet deficiency attenuates renal injury in experimental crescentic glomerulonephritis. J Am Soc Nephrol 19: 477–485, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie QW, Cho HJ, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Ding A, Troso T, Nathan C: Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 256: 225–228, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A: Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest 119: 565–572, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C: Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol 39: 216–224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paust HJ, Turner JE, Steinmetz OM, Peters A, Heymann F, Holscher C, Wolf G, Kurts C, Mittrucker HW, Stahl RA, Panzer U: The IL-23/Th17 axis contributes to renal injury in experimental glomerulonephritis. J Am Soc Nephrol 20: 969–979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ooi JD, Phoon RK, Holdsworth SR, Kitching AR: IL-23, not IL-12, directs autoimmunity to the Goodpasture antigen. J Am Soc Nephrol 20: 980–989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacob N, Yang H, Pricop L, Liu Y, Gao X, Zheng SG, Wang J, Gao HX, Putterman C, Koss MN, Stohl W, Jacob CO: Accelerated pathological and clinical nephritis in systemic lupus erythematosus-prone New Zealand Mixed 2328 mice doubly deficient in TNF receptor 1 and TNF receptor 2 via a Th17-associated pathway. J Immunol 182: 2532–2541, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tesch GH, Schwarting A, Kinoshita K, Lan HY, Rollins BJ, Kelley VR: Monocyte chemoattractant protein-1 promotes macrophage-mediated tubular injury, but not glomerular injury, in nephrotoxic serum nephritis. J Clin Invest 103: 73–80, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dean EG, Wilson GR, Li M, Edgtton KL, O'Sullivan KM, Hudson BG, Holdsworth SR, Kitching AR: Experimental autoimmune Goodpasture's disease: A pathogenetic role for both effector cells and antibody in injury. Kidney Int 67: 566–575, 2005 [DOI] [PubMed] [Google Scholar]